Acinetobacter baumannii is a Gram-negative organism that is a cause of hospital-acquired multidrug-resistant (MDR) infections. A. baumannii has a unique cell surface compared to those of many other Gram-negative pathogens in that it can live without lipopolysaccharide (LPS) and it has a high content of cardiolipin in the outer membrane.
KEYWORDS: antibiotic resistance, drug efflux
ABSTRACT
Acinetobacter baumannii is a Gram-negative organism that is a cause of hospital-acquired multidrug-resistant (MDR) infections. A. baumannii has a unique cell surface compared to those of many other Gram-negative pathogens in that it can live without lipopolysaccharide (LPS) and it has a high content of cardiolipin in the outer membrane. Therefore, to better understand the cell envelope and mechanisms of MDR A. baumannii, we screened a transposon library for mutants with defective permeability barrier function, defined as a deficiency in the ability to exclude the phosphatase chromogenic substrate 5-bromo-4-chloro-3-indolylphosphate (XP). We identified multiple mutants with mutations in the ABUW_0982 gene, predicted to encode a permease broadly present in A. baumannii isolates with increased susceptibility to the ribosome-targeting antibiotic chloramphenicol (CHL). Moreover, compared to other known CHL resistance genes, such as chloramphenicol acyltransferase genes, we found that ABUW_0982 is the primary determinant of intrinsic CHL resistance in A. baumannii strain 5075 (Ab5075), an important isolate responsible for severe MDR infections in humans. Finally, studies measuring the efflux of chloramphenicol and expression of ABUW_0982 in CHL-susceptible Escherichia coli support the conclusion that ABUW_0982 encodes a single-component efflux protein with specificity for small, hydrophobic molecules, including CHL.
INTRODUCTION
Acinetobacter baumannii is a Gram-negative bacterium that is an important cause of serious nosocomial infections. Antibiotic resistance and desiccation tolerance facilitate the survival of A. baumannii in susceptible patients and health care settings, respectively (1–3). According to the U.S. Centers for Disease Control and Prevention, more than 60% of A. baumannii clinical isolates are resistant to at least three different classes of antibiotics (4). Multidrug-resistant (MDR) isolates colonize the skin and nasopharynx of hospitalized patients, and treatment can require last-resort therapy with antimicrobial peptide (AMP) antibiotics (2, 5). Similar to the case for other Gram-negative opportunistic pathogens, such as Pseudomonas aeruginosa and Klebsiella spp., individuals with breached skin or respiratory tract mucosa are the most vulnerable to A. baumannii infection (6). Infections with high morbidity and mortality include intractable wound infections, ventilator-associated pneumonia, skeletal and central nervous system infections, and septicemia (7). Recently, the World Health Organization declared that the need for new drugs to combat A. baumannii infection is of critical importance (8). The emergence of MDR Gram-negative pathogens represents a major public health concern, and a more thorough understanding of resistance mechanisms is critical to treatment decisions and antimicrobial development.
Treating MDR A. baumannii is complicated by the bacterial cell envelope, which contributes to survival in the presence of antibiotics (9). Our understanding of the Gram-negative bacterial cell envelope is largely through study of the Enterobacteriaceae Escherichia coli and Salmonella enterica serovar Typhimurium. All Gram-negative bacteria have similar arrangements and compositions of the dual membrane which separates the cell's interior from the extracellular environment. The inner membrane (IM) of the cell envelope is a bilayer made of membrane-spanning proteins and glycerophospholipids, while the outer membrane (OM) is asymmetrical, with a glycerophospholipid inner leaflet and an outer leaflet composed primarily of lipopolysaccharide (LPS) (10). A periplasmic space defined by a peptidoglycan cell wall demarcates the IM and OM. The peptidoglycan is bound to the OM by lipoproteins and forms a sacculus that encases the IM, providing rigidity and cell shape (11). Porins, commonly called outer membrane proteins (OMPs), are embedded within the OM and are composed of β-sheets that fold into cylinders (12). The individual components of the cell envelope, i.e., proteins, phospholipids, and LPS, are dynamically regulated to accommodate changes in the extracellular environment (10). A. baumannii derivatizes lipid A to resist polymyxins and desiccation (13–15). Unlike Salmonella, A. baumannii lacks the two-component regulatory system PhoPQ but encodes the PmrAB system, known to modify lipid A, and it likely has a unique outer membrane structure, since lipid A synthesis is not essential and the organism has a high content of cardiolipin in its outer membrane (16–18). In addition to the physical barrier provided by the cell envelope, multiple antibiotic resistance factors can be found in A. baumannii, including β-lactamases, aminoglycoside-inactivating enzymes, and MDR efflux pumps (1, 19).
The current state of the art in A. baumannii research utilizes strains that are representative of contemporary clinical isolates, such as strain 5075 (called Ab5075 here) (20). The experimental reference strain Ab5075 was selected from a set of 33 genetically diverse clinical isolates because of the high virulence observed in mouse lung and Galleria mellonella infection models relative to that of other A. baumannii strains (21). Ab5075 possess an 80-kbp plasmid that contains 4 genes for aminoglycoside resistance (aacA4, aadA2, aadB, and strAB) and is absent in strains 17978 and 19606 (22). This plasmid is likely responsible for the MDR observed for Ab5075. To further our understanding of the genetic basis of antibiotic resistance and pathogenesis in Ab5075, Gallagher et al. generated a comprehensive transposon mutant library (22). To construct the transposon library, a large pool of Ab5075 mutants was initially generated by random transposon mutagenesis. From this initial pool, an arrayed library of mutants with transposon insertions in most nonessential genes was constructed. The Ab5075 genome carries approximately 3,500 nonessential genes. For each nonessential gene, three mutants with unique transposon insertion sites were selected and sequence verified. In total, the “three-allele library” contains approximately 10,800 unique mutants arrayed in 96-well format.
To identify mutants in the three-allele library with compromised cell envelope function, we utilized the chromogenic compound 5-bromo-4-chloro-3-indolylphosphate (XP). When XP is exposed to alkaline phosphatase, the phosphate group is liberated and the remaining molecule forms a blue precipitate that is visible to the naked eye. Functional alkaline phosphatase is localized in the periplasm in Gram-negative bacteria, such as E. coli (23). We hypothesize that disruption of a gene that is necessary for permeability barrier function by transposon insertion will result in an XP-positive phenotype when mutants from the three-allele library are cultured in the presence of XP.
RESULTS
Use of XP to identify Ab5075 mutants with compromised barrier function.
To study the A. baumannii permeability barrier, we devised a screen to identify mutants in the three-allele library with defective barrier function. LB-agarose plates were supplemented with the chromogenic periplasmic phosphatase substrate and antibiotic analogue 5-bromo-4-chloro-3-indolylphosphate (XP). Preliminary experiments found that XP does not access the interior of wild-type A. baumannii, as colonies are the same color on plates with and without XP. Treatment of cells with polymyxin B (PMB) resulted in XP-positive bacteria (Fig. 1A). Challenge of Ab5075 with antibiotics that are active against intracellular targets, such as chloramphenicol and tetracycline (TET), did not result in XP processing (data not shown). We successfully established parameters for an XP-based assay that allows us to specifically recognize bacteria with a damaged permeability barrier. We screened 10,371 mutants from the three-allele library and identified multiple mutants with mutation of the ABUW_0982 gene that were XP positive (Fig. 1B). We also identified notable genes known to contribute to barrier function, including ABUW_0384 (mlaE), encoding part of the Mla ABC-type phospholipid transporter, and the K-locus polysaccharide export protein genes ABUW_3831 (wza) and ABUW_3832 (ptp) (24–26). Interestingly, an analogous transposon mutagenesis screen using A. baumannii strain 17978 also identified the Mla system, the K locus, and ABUW_0982, suggesting that similar mechanisms of XP resistance may be utilized by multiple strains of A. baumannii (Cassandra Kamischke and Samuel I. Miller, unpublished data).
FIG 1.
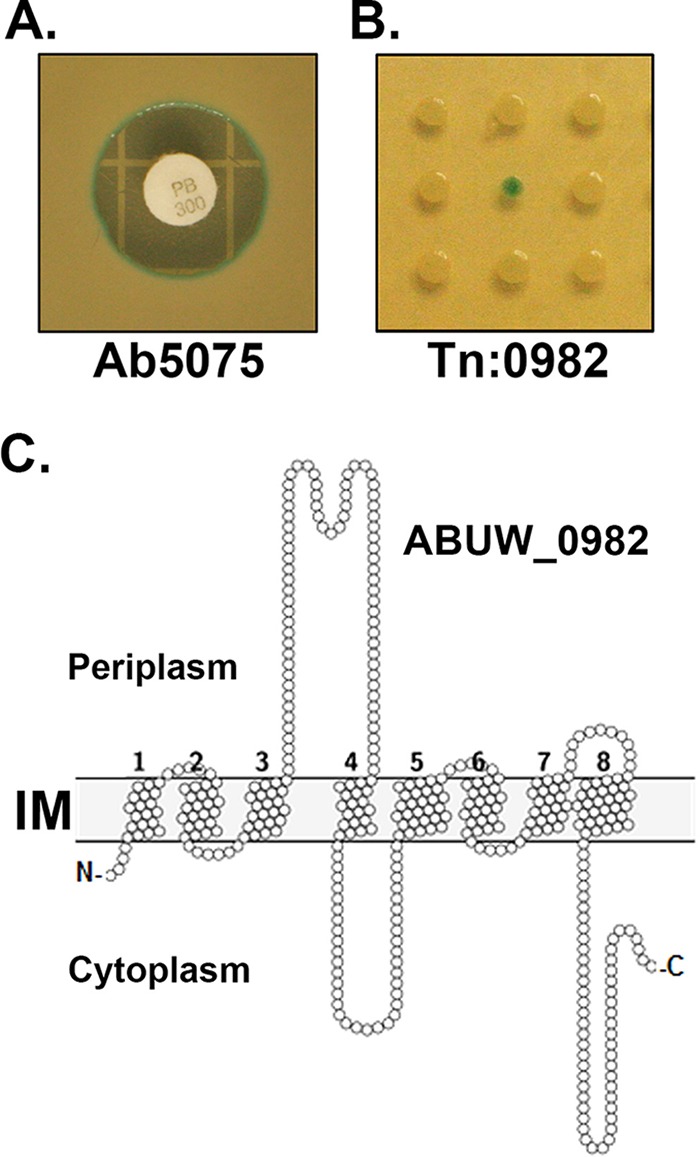
Identification of an A. baumannii barrier-deficient mutant, the ABUW_0982 mutant, by use of a chromogenic antibiotic analogue. (A) LB-agarose plates supplemented with 5-bromo-4-chloro-3-indolylphosphate (XP) can identify bacteria with membrane damage. Wild-type Ab5075 was plated on LB-agarose plates containing XP and challenged with antibiotics by disc diffusion testing. The image shown is a challenge by PMB. Note that the bacteria on the edge of the zone of inhibition are XP positive. PB 300, 300 U PMB. (B) Representative image of a resulting LB-XP plate following 18 h of incubation with a 96-well plate (kr121205p06) from the three-allele library. Each spot represents a unique mutant. The XP-positive colony in the middle is the kr121205p06q163 mutant (Tn:0982). (C) Prediction of ABUW_0982 protein transmembrane domains and loops. We used Protter (29) to generate a structural model of the ABUW_0982 protein. The ABUW_0982 protein is predicted to have 8 transmembrane domains, with a loop of approximately 70 amino acids between TMD 3 and 4 that extends into the periplasm. A cytoplasmic loop is observed between TMD 4 and 5, and the C-terminal tail extends into the cytoplasm. IM, inner membrane.
Analysis of ABUW_0982 protein structure and function.
Bioinformatics analysis by use of the Prokaryotic Genome Analysis Tool (PGAT) and the Phyre2 web portal indicated that the protein encoded by ABUW_0982 is 397 amino acids long, is inserted into the membrane with 8 transmembrane domains (TMD) of 18 to 21 residues, and is found in the genomes of other A. baumannii strains, including ATCC 17978 and 19606 (27, 28). An extended loop of 70 residues between TMD 3 and 4 is a notable feature of the ABUW_0982 protein structure. A loop between TMD 4 and 5 and a segment of the carboxy terminus that extends into the cytoplasm are predicted (29) (Fig. 1C). The transporter classification database indicates that the ABUW_0982 protein is homologous to E. coli PerM, a permease, and other members of the autoinducer-2 exporter family (AI-2E) (30, 31). One AI-2 member, E. coli TqsA, shares 23% amino acid identity with the ABUW_0982 protein and is predicted to assume the same transmembrane topology as that of the ABUW_0982 protein (32).
ABUW_0982 is a novel chloramphenicol resistance gene in Acinetobacter baumannii strain Ab5075.
Next, the ABUW_0982 transposon mutants were screened for antibiotic susceptibility by the disc diffusion method. The antibiotics we selected are from distinct classes and have unique targets, including the cell wall and the ribosome. The antimicrobial peptide PMB resulted in similar zones of clearing (13 to 15 mm) for all three strains. We also measured tetracycline sensitivity to control for transposon insertion (Table 1). For Ab5075, a 17-mm zone of clearing was observed. For the Tn:0982 mutants, the zones of clearing around tetracycline were 12 mm. This result indicates the presence of the transposon insertion in our gene of interest, as a tetracycline resistance marker is part of the transposon (22). Disruption of ABUW_0982 by the transposon does not influence intrinsic rifampin (RIF) resistance, as all strains exhibited zones of inhibition of 14 mm. Wild-type Ab5075 is resistant to chloramphenicol (CHL), as no clearing was observed (Table 1). A striking result was the appearance of a zone of clearing of >20 mm around CHL for each Tn:0982 mutant (Table 1). We also tested Tn:0982 mutants for sensitivity to ceftazidime, carbenicillin, amikacin, gentamicin, trimethoprim, imipenem, and kanamycin, with no significant difference in phenotypes noted relative to those of wild-type Ab5075 (data not shown).
TABLE 1.
Evaluation of Tn:0982 antibiotic susceptibility by disc diffusion testinga
| Antibiotic | Zone of inhibition (diam [mm]) for A. baumannii strain |
||||
|---|---|---|---|---|---|
| Ab5075 | Tn:0982 (1) | Tn:0982 (2) | Tn:0982(pEmpty) | Tn:0982(p0982) | |
| PMB | 13 ± 0.5 | 13.5 ± 0.5 | 13.5 ± 0.5 | 16 ± 1.4 | 13 ± 0.5 |
| TET | 17 ± 0.5 | 12 ± 0.5 | 12 ± 0.5 | 15 ± 0 | 13 ± 0.5 |
| RIF | 14 ± 0.5 | 14.5 ± 0.5 | 14.5 ± 0.5 | 0 | 0 |
| CHL | 0 | 21 ± 0.8 | 23.5 ± 0.8 | 24 ± 1.4 | 0 |
Antibiotic susceptibilities of Acinetobacter baumannii 5075-based strains were evaluated using a disc diffusion method with LB-agarose plates. The mutants are described in Materials and Methods. PMB, polymyxin B; TET, tetracycline; RIF, rifampin; CHL, chloramphenicol.
In an effort to rigorously characterize the phenotype of the ABUW_0982 mutant, we tested for antibiotic susceptibility on Mueller-Hinton (MH) agar, the preferred medium for testing clinical specimens according to the Clinical Laboratory Standards Institute (CLSI). Wild-type Ab5075 was not susceptible to CHL on LB or Mueller-Hinton agar (Fig. 2A). We observed a similar phenotype for Tn:0982 cultured on LB or Mueller-Hinton agar. The zone of clearing for Tn:0982 around CHL was 27 mm on Mueller-Hinton agar and approximately 22 mm on LB agar (Table 1 and Fig. 2A). To ensure that the multidrug susceptibility phenotype was due to the specific disruption of ABUW_0982 function by transposon insertion and not because of some anomaly of the transposon mutagenesis process, we constructed strains using the Tn:0982 background to express either a pMMB-RIF vector with the ABUW_0982 nucleotide sequence inserted (p0982) or an empty vector (pEmpty). The mutants expressing the pMMB-RIF vector were resistant to RIF (Table 1). Expression of ABUW_0982 in trans restored the CHL resistance phenotype observed in wild-type Ab5075 (Fig. 2B and Table 1). The zone of clearing observed around CHL went from 24 mm for the cells expressing pEmpty to no clearing for the cells expressing p0982. A quantitative determination of the CHL MIC found that transposon mutation of ABUW_0982 reduced the CHL MIC from 128 μg/ml to 4 μg/ml (Table 2). Expression of ABUW_0982 in the Tn:0982 background restored the CHL MIC to wild-type levels (Table 2). Our ability to identify multiple, unique transposon mutants within the same gene that have identical phenotypes and to successfully complement this phenotype by use of an extrachromosomal expression system proves that ABUW_0982 is necessary for intrinsic CHL resistance in A. baumannii strain Ab5075.
FIG 2.
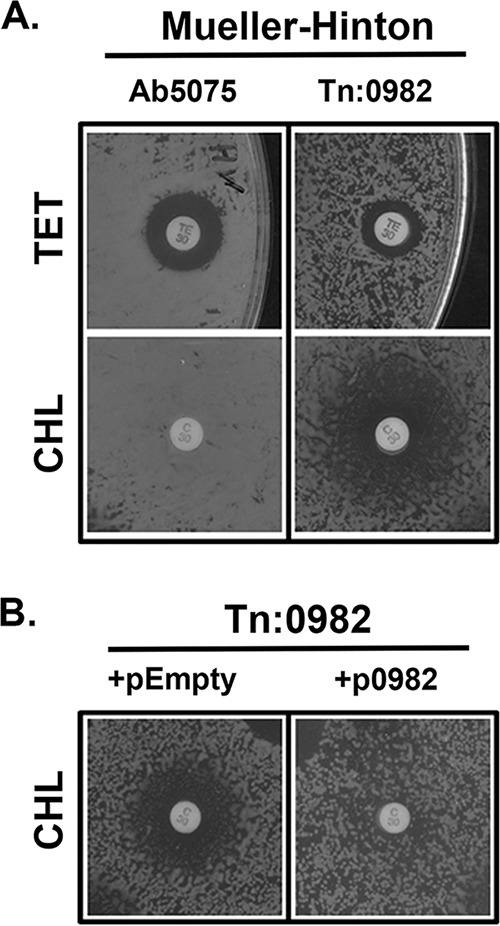
Expression of ABUW_0982 in trans restores intrinsic multidrug resistance. (A) Analysis of Tn:0982 antibiotic susceptibility on Mueller-Hinton (MH) plates. Overnight cultures of bacteria were diluted 1:100 in fresh MH broth and allowed to grow for 2 h at 37°C with shaking. Cultures were normalized to an OD600 of 0.2 and spread on MH-agarose plates. Antibiotic discs were then placed, and plates were incubated at 37°C for 18 h. Note that Ab5075 is more sensitive to TET (zone of inhibition, 17 mm) than the Tn:0982 mutant (12 mm). CHL does not effectively kill Ab5075, as no zone of inhibition is observed; for the Tn:0982 mutant, a zone of inhibition of >20 mm is observed. TE30, 30 μg TET; C30, 30 μg CHL. (B) The ABUW_0982 nucleotide sequence was inserted into the pMMB-RIF vector. The vector was then transferred into the Tn:0982 mutant by conjugation. The resulting bacteria (+p0982) as well as controls expressing an empty vector (+pEmpty) were tested for antibiotic susceptibility as described for panel A, but with LB medium plus 50 μg/ml rifampin instead of Mueller-Hinton medium. A zone of inhibition around CHL of >20 mm is observed for Tn:0982(pEmpty). Note that expression of full-length ABUW_0982 restores intrinsic CHL resistance, as no zone of inhibition is observed around Tn:0982(p0982) cells.
TABLE 2.
Chloramphenicol MICs for A. baumannii 5075-based strainsa
| Strain | MIC (μg/ml) |
|---|---|
| Ab5075 | 128 ± 0 |
| Tn:0982 | 4 ± 3.5 |
| Tn:0982(pEmpty) | 6.25 ± 5.6 |
| Tn:0982(p0982) | 128 ± 0 |
| Tn:AdeB | 80 ± 18 |
| Tn:AdeJ | 36 ± 8 |
| Tn:AdeS | 128 ± 0 |
| Tn:0337 | 112 ± 16 |
Determination of chloramphenicol MICs was done by using the Etest CHL susceptibility strip quantitative technique for determining antimicrobial susceptibility.
Cell-associated chloramphenicol is increased when ABUW_0982 is disrupted by transposon insertion.
Using the transporter classification database, we found that the ABUW_0982 protein is homologous to members of the autoinducer-2 exporter (AI-2E) family (30, 31). Based on homology to other known small-molecule transport proteins, we hypothesize that the ABUW_0982 protein directly transports CHL across the IM by efflux. Our hypothesis expects that cells lacking functional ABUW_0982 will be deficient in the ability to rid the cell of CHL. To test our hypothesis, we designed an experimental system to directly measure cell-associated CHL in bacterial cells that is based on a previously described protocol that was optimized to measure cell-associated [14C]chloramphenicol in E. coli (33). Briefly, bacterial cells are suspended in buffer and incubated with [14C]chloramphenicol. At predetermined time points, an aliquot of cells is removed and cells are harvested by centrifugation. Harvested cells are added to liquid scintillation cocktail, and the amount of cell-associated [14C]chloramphenicol is determined. We saw trends in our control experiments with E. coli similar to those of previous studies (33). Pretreatment of cells with an exogenous energy source, glucose, reduced the amount of cell-associated CHL relative to that for untreated cells. Also, pretreatment of E. coli with carbonyl cyanide m-chlorophenylhydrazone (CCCP), an inhibitor of oxidative phosphorylation and ATP-dependent activities, including drug efflux, resulted in elevated levels of cell-associated CHL relative to those for untreated wild-type cells (Fig. 3A). These results indicate that E. coli controls the amount of cell-associated CHL by an energy-dependent process. To measure cell-associated CHL in A. baumannii, we used a similar approach, but with succinate instead of glucose as a carbon source. For experiments with A. baumannii, we saw a rapid cell association at 1 min and a decrease over time (Fig. 3B). In contrast to the E. coli data, pretreatment of A. baumannii cells with an exogenous energy source, succinate, did not reduce cell-associated CHL relative to that for untreated cells (Fig. 3B). Similar to what was observed for E. coli, CCCP pretreatment resulted in higher levels of cell-associated CHL over time (Fig. 3B). We next measured the levels of cell-associated CHL in the Tn:0982 mutant expressing pEmpty or p0982. We observed increased cell-associated CHL over time in cells expressing empty vector relative to that in cells expressing wild-type ABUW_0982 (Fig. 3C). In support of our hypothesis, we concluded that ABUW_0982 is necessary for optimal CHL efflux in A. baumannii strain Ab5075.
FIG 3.

Expression of ABUW_0982 reduces chloramphenicol burden in A. baumannii. (A) Analysis of chloramphenicol uptake in E. coli MG1655 over time. Overnight cultures were diluted in fresh LB medium and grown to exponential phase. Cells were harvested by centrifugation and resuspended in PBS to an OD of 2.0. Cells were either left untreated or incubated with 20 mM glucose or 20 μM CCCP for 15 min at 37°C with shaking. After the initial incubation, 2.5 μM [14C]chloramphenicol was added to each sample. At each time point, cells were removed and collected by centrifugation, and recovered cells were added to liquid scintillation cocktail. Cell-associated chloramphenicol was measured, and results are displayed as counts per minute (cpm). Notice the increase in cell-associated CHL following CCCP treatment and the decrease in cell-associated CHL after glucose pretreatment relative to that for untreated cells. These results are representative of multiple independent experiments. (B) Analysis of chloramphenicol uptake by wild-type Ab5075. The experimental protocol was the same as that for panel A. Cells were either left untreated or incubated with 20 mM sodium succinate or 20 μM CCCP. Note that the addition of succinate does not reduce the levels of cell-associated CHL. Preincubation with CCCP increases cell-associated CHL. These results are representative of multiple independent experiments. (C) ABUW_0982 is necessary for optimal CHL efflux in A. baumannii cells. The Tn:0982 mutant expressing empty vector (pEmpty) or p0982 was evaluated for chloramphenicol efflux as described above. Over time, less cell-associated chloramphenicol or more efflux is observed for the mutant cells expressing p0982. The error bars represent the standard deviations for three independent experiments. *, P < 0.1; **, P < 0.05. Significance was determined using Student's t test.
ABUW_0982 is sufficient for chloramphenicol resistance.
We have shown that ABUW_0982 is necessary for CHL resistance in Ab5075. To determine if ABUW_0982 is sufficient for CHL resistance, we engineered E. coli MG1655 to express ABUW_0982. If the ABUW_0982 protein directly exports CHL by efflux, its expression in a CHL-susceptible E. coli strain should result in a gain of resistance. We challenged MG1655 expressing pEmpty or p0982 with a dilution series of CHL. We observe increased resistance to CHL when ABUW_0982 was expressed in susceptible E. coli relative to that of cells expressing pEmpty (Fig. 4). Using a 2-fold dilution series of CHL, we observed that MG1655(p0982) achieved significantly more growth for four of the highest concentrations tested. This indicates that expression of ABUW_0982 in E. coli MG1655 resulted in an approximately 8-fold increase in CHL resistance.
FIG 4.
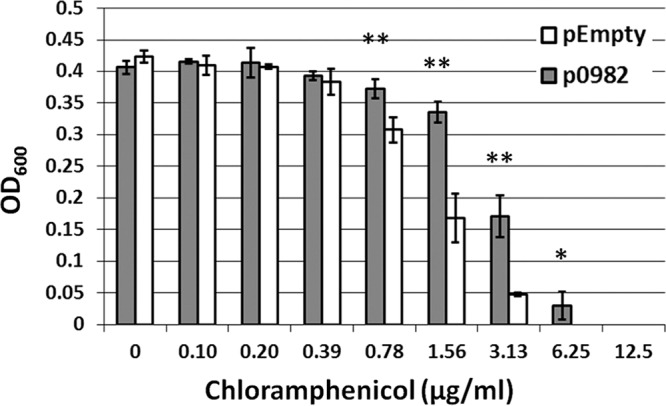
Expression of ABUW_0982 in E. coli is sufficient for chloramphenicol resistance. For evaluation of E. coli susceptibility to chloramphenicol, E. coli MG1655 was engineered to express ABUW_0982 under the control of an IPTG-inducible promoter. E. coli expressing pEmpty or p0982 was grown in 2× serial dilutions of chloramphenicol in the presence of IPTG (1 mM). The OD600 was measured after 22 h of growth at 37°C with shaking. Note that E. coli expressing ABUW_0982 gained the ability to grow at higher concentrations of chloramphenicol. The error bars represent the standard deviations for three independent experiments. *, P < 0.05; **, P < 0.01. Significance was determined using Student's t test.
ABUW_0982 is a major determinant of intrinsic chloramphenicol resistance in A. baumannii strain Ab5075.
Global genomic analysis indicates that A. baumannii has multiple genes encoding putative chloramphenicol resistance factors in addition to those characterized in the literature (22). Next, we wanted to determine if ABUW_0982 is the major determinant of intrinsic CHL resistance in Ab5075. We used mutants from the three-allele library that have transposon insertions in genes that encode bioinformatically predicted or experimentally derived CHL resistance factors.
Mutants in genes encoding the chloramphenicol resistance pump (CraA; ABUW_0337), the drug resistance transporter BRF/CflA subfamily (ABUW_0701), chloramphenicol acetyltransferase (CAT; ABUW_0891), a major facilitator superfamily drug transporter (ABUW_1003), a chloramphenicol resistance protein (CmlA; ABUW_4059), the Acinetobacter drug efflux resistance-nodulation-cell division (RND) system (AdeB [ABUW_1775] and AdeJ [ABUW_0843]), and the regulator of the Ade system (AdeS; ABUW_1972) (22, 34–36) were chosen for comparison and evaluated for drug resistance against a panel of antibiotics by disc diffusion (data not shown). We were able to confirm the presence of the transposon insertion by monitoring TET resistance, and of the mutants tested, Tn:CraA, Tn:AdeB, and Tn:AdeJ displayed increased susceptibility to CHL. For the mutants that showed CHL susceptibility in our initial experiments, we next determined the MIC of CHL (Table 2). The CHL MIC measured for Ab5075 was 128 μg/ml, and this was reduced to 4 μg/ml when ABUW_0982 was disrupted by transposon insertion (Table 2). Similar MICs were observed when Tn:0982 cells expressing pEmpty were compared to Tn:0982 cells expressing p0982 (Table 2). By evaluating the contribution of tripartite efflux pumps, we found that disruption of the AdeJ gene reduced the MIC to 36 μg/ml and disruption of AdeB reduced the MIC to 80 μg/ml. Tn:CraA had an MIC of 112 μg/ml. Transposon mutants in AdeS did not show measurable sensitivity to CHL relative to that of Ab5075. After a head-to-head comparison of CHL resistance factor mutants from the three-allele library, we determined that mutation to ABUW_0982 had the most deleterious effect on CHL resistance of all the genes we tested.
DISCUSSION
The aim of our study was to screen a three-allele library to identify previously unknown genes that contribute to cell envelope function in Ab5075. Our screen is based on the hypothesis that a transposon mutation in a gene necessary for barrier function will allow XP to accumulate within the cell and be processed by periplasmic phosphatases. We identified multiple mutants with mutation of the ABUW_0982 gene by using our screening approach and subsequently found that Tn:0982 mutants are CHL sensitive and deficient in CHL efflux (Fig. 5A). Expression of ABUW_0982 in E. coli is sufficient for CHL resistance, in support of our conclusion that ABUW_0982 encodes an efflux protein with specificity for CHL (Fig. 4). By comparing three-allele library mutants with transposon insertions in known and putative CHL resistance genes, we determined that ABUW_0982 is the most significant contributor to intrinsic CHL resistance in Ab5075 among all the genes we tested (Table 2). Using the three-allele library, we identified a novel and previously uncharacterized gene, ABUW_0982, that encodes a small, hydrophobic molecular efflux pump with specificity toward XP and CHL.
FIG 5.
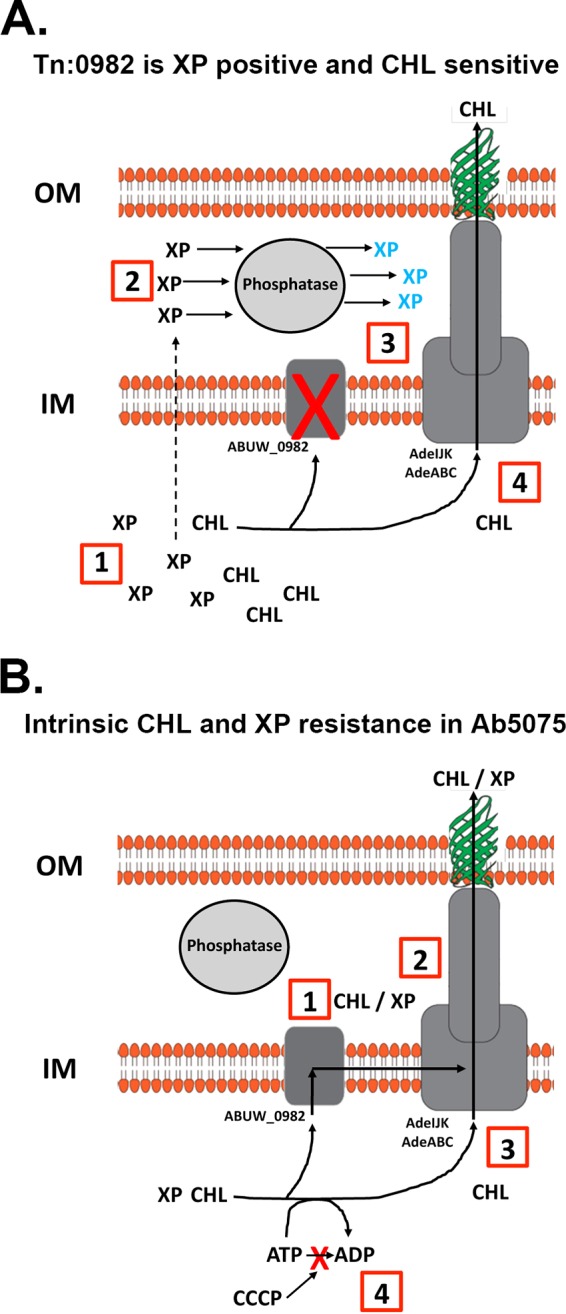
ABUW_0982 is necessary for intrinsic chloramphenicol resistance in Ab5075. Schematics of A. baumannii cell envelope organization are shown. OM, outer membrane; IM, inner membrane. (A) The Tn:0982 mutant is XP positive and CHL sensitive. (1) In the absence of a functional ABUW_0982 protein, XP and CHL accumulate in the cell (decreased CHL MIC). (2 and 3) XP enters the periplasm and is processed, resulting in blue colonies (Fig. 1B). (4) Functional AdeIJK and AdeABC continue to rid the cells of CHL in the absence of ABUW_0982 protein (Fig. 3C). (B) Mechanism of intrinsic CHL and XP resistance in Ab5075. (1) XP and CHL leave the cytoplasm, facilitated by the ABUW_0982 protein. (2) XP and CHL may be exported across the OM and into the medium by a multicomponent efflux pump, such as AdeIJK or AdeABC. (3) AdeIJK or AdeABC can pump CHL directly from the cytoplasm across the OM without the ABUW_0982 protein. (4) CHL efflux is ATP dependent and can be blocked by CCCP treatment.
Resistance to beta-lactamases and aminoglycosides appears to vary among strains, while resistance to CHL is a property observed globally among A. baumannii strains (37, 38). Previous studies found that deletion of CraA in A. baumannii strain 19606 resulted in a decrease in the CHL MIC from 256 μg/ml to 2 μg/ml (34). We also noticed that CraA contributes to intrinsic CHL resistance (Table 2) (34). We did not observe a 128-fold decrease in CHL susceptibility, as noted previously when CraA was disrupted in strain ATCC 19606 (34). This discrepancy may be attributed to the different strains used in the studies, but our study has a distinct advantage in this case because we experimentally identified ABUW_0982 after an unbiased screen of over 10,000 mutants as opposed to a biased reverse genetics approach revolving around in silico predictions. Also, our findings are in line with previous A. baumannii studies that found that AdeJ had a greater contribution to intrinsic CHL resistance than that by AdeB (Table 2) (35). Efflux systems, potentially AdeABC and AdeIJK, appear to be active in the absence of functional ABUW_0982, as the levels of cell-associated CHL decreased over time, even in the Tn:0982 mutant, highlighting the multifaceted nature of intrinsic CHL resistance in A. baumannii (Fig. 3C). By comparing the CHL susceptibilities of mutants from the three-allele library, we found that efflux, specifically ABUW_0982-mediated efflux, is a more significant contributor to intrinsic CHL resistance in Ab5075 than enzymatic alteration by CAT (38).
Previous work in E. coli found that efflux pumps of different structural types (single component and RND) confer additive resistance when coexpressed relative to those of the components expressed individually (39). Transporters in the IM facilitate movement of toxic compounds away from cytoplasmic targets, while multicomponent pumps transport toxic molecules out of the cell (40). The 8-fold increase in CHL resistance that we found when ABUW_0982 was expressed in MG1655 was the same as the 8-fold gain in CHL resistance observed when MdfA was expressed in E. coli lacking the AcrAB multicomponent efflux pump (39). When MdfA and the complementary multicomponent efflux pump were coexpressed, CHL resistance increased significantly (39). The presence of a complementary, multicomponent pump from Ab5075, such as AdeIJK or AdeABC, may significantly increase the gain of CHL resistance in MG1655(p0982). The A. baumannii core genome encodes at least 7 multicomponent systems and more than 30 single-component membrane transport proteins, and since ABUW_0982 is present in all sequenced A. baumannii genomes, it may be an attractive candidate for drug design (41).
Our finding that PMB treatment and disruption of the ABUW_0982 gene both yielded XP-positive bacteria suggests that in wild-type cells the physical OM barrier and an additional mechanism involving the IM permease encoded by ABUW_0982 inhibit XP access to the periplasm. How XP can cross the OM and IM to reach the cytosol and become a substrate for the ABUW_0982 protein without interacting with periplasmic phosphatases is currently unknown. To begin to understand efflux in Ab5075, we propose the hypothesis that tripartite efflux systems cooperate with the ABUW_0982 protein to facilitate efflux of XP and CHL. In our model, the ABUW_0982 protein may be localized near or in a functional complex with tripartite efflux pumps to detoxify the cytoplasm by pumping XP and CHL directly from the cytoplasm into the medium, bypassing the periplasm (Fig. 5B). We predict that XP and CHL are exported by overlapping pathways, since the ABUW_0982 protein has specificity for both XP and CHL. Further studies are needed to describe the structure-function properties of the ABUW_0982 protein and to test the hypothesis that a multicomponent efflux system(s) cooperates with the ABUW_0982 protein to expel toxic small molecules from the cell. Taken together, the results of our work have identified a novel efflux protein, encoded by ABUW_0982 in A. baumannii 5075, that is a major contributor to intrinsic CHL resistance and a potential drug target.
MATERIALS AND METHODS
Chemical and reagents.
Chemicals and reagents were purchased from Sigma unless otherwise noted, including glucose, sodium succinate, carbonyl cyanide m-chlorophenylhydrazone (CCCP), 5-bromo-4-chloro-3-indolylphosphate (XP), isopropyl-β-D-1-thiogalactopyranoside (IPTG), and dimethylformamide (DMF). All antibiotic discs used for susceptibility testing were BBL Sensi-Disc susceptibility test discs from BD. [14C]chloramphenicol was from PerkinElmer. Chloramphenicol Etest susceptibility strips were purchased from bioMérieux (France). Opti-Fluor scintillation liquid was purchased from PerkinElmer.
Bacterial strains used in this study.
Mutants with transposon insertions were obtained from the comprehensive three-allele library (Table 3) (22). Each mutant in the three-allele library is given a unique identifier, which is reported in the table so that readers may access these exact strains from the Manoil laboratory's Acinetobacter baumannii mutant library if desired.
TABLE 3.
Bacterial strains used in this study
| Strain | Relevant gene product(s) | Identifier or reference |
|---|---|---|
| E. coli Mach1 | ||
| E. coli MG1655 | ||
| E. coli helper strain | ||
| A. baumannii 5075 (Ab5075) | 21 | |
| A. baumannii 17978 | ATCC 17978 | |
| A. baumannii 19606 | ATCC 19606 | |
| Tn:ABUW_0982 (Tn:0982) (1) | kr130909p02q143 | |
| Tn:ABUW_0982 (Tn:0982) (2) | kr121205p06q163 | |
| Tn:ABUW_0337 (1) | CraA, MdfA | kr130913p06q136 |
| Tn:ABUW_0337 (2) | CraA, MdfA | kr130913p07q137 |
| Tn:ABUW_0891 | Cat | kr121204p03q152 |
| Tn:ABUW_0701 (1) | kr121203p06q105 | |
| Tn:ABUW_0701 (2) | kr130909p02q187 | |
| Tn:ABUW_1003 (1) | kr121203p06q152 | |
| Tn:ABUW_1003 (2) | kr121205p04q167 | |
| Tn:ABUW_4059 | CmlA | kr121127p01q191 |
| Tn:ABUW_1975 (1) | AdeB | kr121210p02q150 |
| Tn:ABUW_1975 (2) | AdeB | kr121119p04q181 |
| Tn:ABUW_0843 (1) | AdeJ | kr130913p04q121 |
| Tn:ABUW_0843 (2) | AdeJ | kr130913p10q178 |
| Tn:ABUW_1972 (1) | AdeS | kr130913p06q132 |
| Tn:ABUW_1972 (2) | AdeS | kr121127p04q136 |
Bacterial culture conditions.
All experiments were conducted by culturing bacteria at 37°C. Luria-Bertani (LB) medium was the primary medium used unless otherwise noted. When necessary, bacteria were cultured in the presence of rifampin (50 μg/ml). Plates composed of LB-agarose or Mueller-Hinton broth-agarose were used for bacterial growth at 37°C. Plates were supplemented with rifampin (50 μg/ml) or ampicillin (100 μg/ml) when appropriate. To screen the three-allele library, XP (40 mg/ml in DMF) was added to LB-agarose.
Generation of the pMMB-RIF vector and complemented A. baumannii strains.
The extrachromosomal expression vector pMMB-RIF was made by inserting the rifampin resistance marker arr-2 into the DraI site of the bla gene of pMMB67EH (42). The nucleotide sequence of ABUW_0982 was amplified by PCR, with SacI and XbaI restriction sites engineered into the 5′ and 3′ ends of the PCR product, respectively (ABUW_0982_Forward, 5′-ATATATGAGCTCTTATAACGATTAAATCAAAAAAGGCTAGATTTTATG-3′ [SacI linker underlined]; and ABUW_0982_Reverse, 5′-AATAATATCTAGATGACGCATATAATGAATTGGCCTTG-3′ [XbaI linker underlined]). The PCR insert was subcloned into pMMB-RIF digested with the SacI and XbaI enzymes. E. coli Mach1 cells were transformed by electroporation (1-mm cuvette; 1.8 kV; capacitance = 25 µF; resistance = 200 Ω), and the resulting transformants were incubated overnight on LB plates with 50 μg/ml rifampin to select for pMMB-RIF-expressing bacteria. The Mach1 cells expressing pMMB-RIF (p0982 or pEmpty) were combined with helper E. coli and A. baumannii Tn:0982 for conjugation. The resulting mixture was plated on LB plates with ampicillin (100 μg/ml) and rifampin (50 μg/ml) to kill E. coli and to select for pMMB-RIF-expressing A. baumannii. Subsequent culture and experiments with Tn:0982(pMMB-RIF) were done in the presence of rifampin (50 μg/ml).
Antibiotic susceptibility testing by disc diffusion and Etest CHL susceptibility strip tests.
Disc diffusion assays were conducted on 150-mm disposable tissue culture plates containing LB-agarose and XP. Overnight cultures of bacteria were diluted 1:100 in fresh LB medium and grown with shaking at 37°C to an optical density at 600 nm (OD600) of 0.6 to 0.8. Cells were then harvested by centrifugation and resuspended in phosphate-buffered saline (PBS). Cultures were normalized to an OD600 of 0.2, and 20 μl of bacterial culture was mixed with 400 μl of LB medium and spread on a plate. BD Sensi-Discs or Etest CHL susceptibility strips were placed, and the plate was incubated overnight at 37°C. After 18 h of incubation, zones of inhibition or MICs were measured. For analysis of pMMB-RIF-expressing mutants, 50 μg/ml rifampin was added to the culture medium and plates. For experiments featuring wild-type cells or Tn:0982 cells, the numbers reported represent the averages for at least two independent experiments. For experiments with mutants in putative CHL resistance genes, the numbers reported are composite averages obtained from testing two unique mutants twice.
Assay to measure cell-associated chloramphenicol.
The method to study the efflux of [14C]CHL in A. baumannii cells was based on previous studies with E. coli (33). Bacterial cells were cultured overnight in LB medium. The next day, cells were diluted 1:100 in fresh LB medium and grown to an OD600 of 0.6 to 0.8. Cells were harvested by centrifugation and resuspended in 50 mM KPO4-1 mM MgCl2 buffer. Samples were normalized to an OD600 of 2.0. The samples were incubated with 2.5 μM [14C]CHL at 37°C with shaking. The amount of CHL needed to observe energy-dependent efflux was based on previous studies with E. coli and determined empirically with A. baumannii. One, 5, and 10 min after CHL addition, equivalent volumes of cells were collected and harvested by centrifugation, and the cells were added to liquid scintillation cocktail. A scintillation counter was used to measure cell-associated radioactivity (LS6500; Beckman Coulter). For experiments attempting to modulate CHL uptake, 20 mM glucose was used for E. coli and 20 mM sodium succinate was used for A. baumannii. For experiments attempting to block ATP synthesis, 20 μM CCCP was used for both E. coli and A. baumannii. Cells were incubated with efflux-stimulating or -blocking agents at 37°C with shaking for 15 min prior to [14C]CHL addition. Data were analyzed and graphed using Excel (Microsoft, Redmond, WA). Statistical analysis was conducted with paired Student's t test.
Analysis of E. coli chloramphenicol susceptibility.
The pMMB-RIF vectors were cloned into and amplified in E. coli Mach1 cells. Plasmid DNAs were isolated from overnight Mach1 cultures by use of miniprep kits (Qiagen). E. coli MG1655 was transformed with pMMB-RIF_Empty (pEmpty) or pMMB-RIF_0982 (p0982) by electroporation. Transformants were selected by overnight incubation on LB-RIF plates. Liquid cultures were made from single RIF-resistant colonies. After 18 h of overnight incubation, the cells were diluted 1:100 in fresh LB-RIF and grown for 2 h at 37°C with shaking. Cells were then spun, washed, and normalized to an OD600 of 0.2. A final inoculum at an OD600 of 0.01 was used to inoculate a 96-well plate containing a 2-fold dilution series of CHL in LB-RIF. The wells of the 96-well plate were supplemented with 1 mM IPTG to induce ABUW_0982 expression. The plates were covered and incubated for 22 h at 37°C with shaking. The OD600 was determined using a plate reader (Envision; PerkinElmer, Waltham, MA). Data were analyzed and graphed using Excel (Microsoft, Redmond, WA). Statistical analysis was conducted with paired Student's t test.
Accession number(s).
The ABUW_0982 protein sequence is available at GenBank under accession number WP_001153980.1. The E. coli TqsA sequence is available at GenBank under accession number ETJ22394.1.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health, National Institute of Allergy and Infectious Diseases (NIH/NIAID) grant 5U19AI107775.
REFERENCES
- 1.Garnacho-Montero J, Amaya-Villar R. 2010. Multiresistant Acinetobacter baumannii infections: epidemiology and management. Curr Opin Infect Dis 23:332–339. doi: 10.1097/QCO.0b013e32833ae38b. [DOI] [PubMed] [Google Scholar]
- 2.Dijkshoorn L, Nemec A, Seifert H. 2007. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol 5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 3.Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. 2017. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev 30:409–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC. 2013. Antibiotic resistance threats to the United States. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 5.Olaitan AO, Morand S, Rolain JM. 2014. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front Microbiol 5:643. doi: 10.3389/fmicb.2014.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbo A, Navon-Venezia S, Hammer-Muntz O, Krichali T, Siegman-Igra Y, Carmeli Y. 2005. Multidrug-resistant Acinetobacter baumannii. Emerg Infect Dis 11:22–29. doi: 10.3201/eid1101.040001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garnacho-Montero J, Ortiz-Leyba C, Jimenez-Jimenez FJ, Barrero-Almodovar AE, Garcia-Garmendia JL, Bernabeu-Wittel IM, Gallego-Lara SL, Madrazo-Osuna J. 2003. Treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia (VAP) with intravenous colistin: a comparison with imipenem-susceptible VAP. Clin Infect Dis 36:1111–1118. doi: 10.1086/374337. [DOI] [PubMed] [Google Scholar]
- 8.WHO. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery and development of new antibiotics. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 9.Nikaido H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev 67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silhavy TJ, Kahne D, Walker S. 2010. The bacterial cell envelope. Cold Spring Harb Perspect Biol 2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruiz N, Kahne D, Silhavy TJ. 2006. Advances in understanding bacterial outer-membrane biogenesis. Nat Rev Microbiol 4:57–66. doi: 10.1038/nrmicro1322. [DOI] [PubMed] [Google Scholar]
- 12.Nikaido H. 1993. Transport across the bacterial outer membrane. J Bioenerg Biomembr 25:581–589. [DOI] [PubMed] [Google Scholar]
- 13.Qureshi ZA, Hittle LE, O'Hara JA, Rivera JI, Syed A, Shields RK, Pasculle AW, Ernst RK, Doi Y. 2015. Colistin-resistant Acinetobacter baumannii: beyond carbapenem resistance. Clin Infect Dis 60:1295–1303. doi: 10.1093/cid/civ048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelletier MR, Casella LG, Jones JW, Adams MD, Zurawski DV, Hazlett KR, Doi Y, Ernst RK. 2013. Unique structural modifications are present in the lipopolysaccharide from colistin-resistant strains of Acinetobacter baumannii. Antimicrob Agents Chemother 57:4831–4840. doi: 10.1128/AAC.00865-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boll JM, Tucker AT, Klein DR, Beltran AM, Brodbelt JS, Davies BW, Trent MS. 2015. Reinforcing lipid A acylation on the cell surface of Acinetobacter baumannii promotes cationic antimicrobial peptide resistance and desiccation survival. mBio 6:e00478-15. doi: 10.1128/mBio.00478-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopalco P, Stahl J, Annese C, Averhoff B, Corcelli A. 2017. Identification of unique cardiolipin and monolysocardiolipin species in Acinetobacter baumannii. Sci Rep 7:2972. doi: 10.1038/s41598-017-03214-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moffatt JH, Harper M, Harrison P, Hale JD, Vinogradov E, Seemann T, Henry R, Crane B, St Michael F, Cox AD, Adler B, Nation RL, Li J, Boyce JD. 2010. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother 54:4971–4977. doi: 10.1128/AAC.00834-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arroyo LA, Herrera CM, Fernandez L, Hankins JV, Trent MS, Hancock RE. 2011. The pmrCAB operon mediates polymyxin resistance in Acinetobacter baumannii ATCC 17978 and clinical isolates through phosphoethanolamine modification of lipid A. Antimicrob Agents Chemother 55:3743–3751. doi: 10.1128/AAC.00256-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YJ, Kim SI, Kim YR, Hong KW, Wie SH, Park YJ, Jeong H, Kang MW. 2012. Carbapenem-resistant Acinetobacter baumannii: diversity of resistant mechanisms and risk factors for infection. Epidemiol Infect 140:137–145. doi: 10.1017/S0950268811000744. [DOI] [PubMed] [Google Scholar]
- 20.Harding CM, Hennon SW, Feldman MF. 2018. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat Rev Microbiol 16:91–102. doi: 10.1038/nrmicro.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs AC, Thompson MG, Black CC, Kessler JL, Clark LP, McQueary CN, Gancz HY, Corey BW, Moon JK, Si Y, Owen MT, Hallock JD, Kwak YI, Summers A, Li CZ, Rasko DA, Penwell WF, Honnold CL, Wise MC, Waterman PE, Lesho EP, Stewart RL, Actis LA, Palys TJ, Craft DW, Zurawski DV. 2014. AB5075, a highly virulent isolate of Acinetobacter baumannii, as a model strain for the evaluation of pathogenesis and antimicrobial treatments. mBio 5:e01076-14. doi: 10.1128/mBio.01076-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallagher LA, Ramage E, Weiss EJ, Radey M, Hayden HS, Held KG, Huse HK, Zurawski DV, Brittnacher MJ, Manoil C. 2015. Resources for genetic and genomic analysis of emerging pathogen Acinetobacter baumannii. J Bacteriol 197:2027–2035. doi: 10.1128/JB.00131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kendall DA, Bock SC, Kaiser ET. 1986. Idealization of the hydrophobic segment of the alkaline phosphatase signal peptide. Nature 321:706–708. doi: 10.1038/321706a0. [DOI] [PubMed] [Google Scholar]
- 24.Geisinger E, Isberg RR. 2015. Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog 11:e1004691. doi: 10.1371/journal.ppat.1004691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russo TA, Luke NR, Beanan JM, Olson R, Sauberan SL, MacDonald U, Schultz LW, Umland TC, Campagnari AA. 2010. The K1 capsular polysaccharide of Acinetobacter baumannii strain 307-0294 is a major virulence factor. Infect Immun 78:3993–4000. doi: 10.1128/IAI.00366-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henry R, Vithanage N, Harrison P, Seemann T, Coutts S, Moffatt JH, Nation RL, Li J, Harper M, Adler B, Boyce JD. 2012. Colistin-resistant, lipopolysaccharide-deficient Acinetobacter baumannii responds to lipopolysaccharide loss through increased expression of genes involved in the synthesis and transport of lipoproteins, phospholipids, and poly-beta-1,6-N-acetylglucosamine. Antimicrob Agents Chemother 56:59–69. doi: 10.1128/AAC.05191-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brittnacher MJ, Fong C, Hayden HS, Jacobs MA, Radey M, Rohmer L. 2011. PGAT: a multistrain analysis resource for microbial genomes. Bioinformatics 27:2429–2430. doi: 10.1093/bioinformatics/btr418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. 2015. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omasits U, Ahrens CH, Muller S, Wollscheid B. 2014. Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics 30:884–886. doi: 10.1093/bioinformatics/btt607. [DOI] [PubMed] [Google Scholar]
- 30.Rettner RE, Saier MH Jr. 2010. The autoinducer-2 exporter superfamily. J Mol Microbiol Biotechnol 18:195–205. doi: 10.1159/000316420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saier MH Jr, Reddy VS, Tsu BV, Ahmed MS, Li C, Moreno-Hagelsieb G. 2016. The Transporter Classification Database (TCDB): recent advances. Nucleic Acids Res 44:D372–D379. doi: 10.1093/nar/gkv1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herzberg M, Kaye IK, Peti W, Wood TK. 2006. YdgG (TqsA) controls biofilm formation in Escherichia coli K-12 through autoinducer 2 transport. J Bacteriol 188:587–598. doi: 10.1128/JB.188.2.587-598.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMurry LM, George AM, Levy SB. 1994. Active efflux of chloramphenicol in susceptible Escherichia coli strains and in multiple-antibiotic-resistant (Mar) mutants. Antimicrob Agents Chemother 38:542–546. doi: 10.1128/AAC.38.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roca I, Marti S, Espinal P, Martinez P, Gibert I, Vila J. 2009. CraA, a major facilitator superfamily efflux pump associated with chloramphenicol resistance in Acinetobacter baumannii. Antimicrob Agents Chemother 53:4013–4014. doi: 10.1128/AAC.00584-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon EJ, Chabane YN, Goussard S, Snesrud E, Courvalin P, De E, Grillot-Courvalin C. 2015. Contribution of resistance-nodulation-cell division efflux systems to antibiotic resistance and biofilm formation in Acinetobacter baumannii. mBio 6:e00309-15. doi: 10.1128/mBio.00309-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marchand I, Damier-Piolle L, Courvalin P, Lambert T. 2004. Expression of the RND-type efflux pump AdeABC in Acinetobacter baumannii is regulated by the AdeRS two-component system. Antimicrob Agents Chemother 48:3298–3304. doi: 10.1128/AAC.48.9.3298-3304.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Civljak R, Giannella M, Di Bella S, Petrosillo N. 2014. Could chloramphenicol be used against ESKAPE pathogens? A review of in vitro data in the literature from the 21st century. Expert Rev Anti Infect Ther 12:249–264. doi: 10.1586/14787210.2014.878647. [DOI] [PubMed] [Google Scholar]
- 38.Vila J, Marcos A, Marco F, Abdalla S, Vergara Y, Reig R, Gomez-Lus R, Jimenez de Anta T. 1993. In vitro antimicrobial production of beta-lactamases, aminoglycoside-modifying enzymes, and chloramphenicol acetyltransferase by and susceptibility of clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother 37:138–141. doi: 10.1128/AAC.37.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee A, Mao W, Warren MS, Mistry A, Hoshino K, Okumura R, Ishida H, Lomovskaya O. 2000. Interplay between efflux pumps may provide either additive or multiplicative effects on drug resistance. J Bacteriol 182:3142–3150. doi: 10.1128/JB.182.11.3142-3150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tal N, Schuldiner S. 2009. A coordinated network of transporters with overlapping specificities provides a robust survival strategy. Proc Natl Acad Sci U S A 106:9051–9056. doi: 10.1073/pnas.0902400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coyne S, Courvalin P, Perichon B. 2011. Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob Agents Chemother 55:947–953. doi: 10.1128/AAC.01388-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furste JP, Pansegrau W, Frank R, Blocker H, Scholz P, Bagdasarian M, Lanka E. 1986. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene 48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]


