LETTER
Newly approved Food and Drug Administration (FDA) β-lactam–β-lactamase inhibitor (βL-βLI) combinations, such as ceftazidime-avibactam and meropenem-vaborbactam, are potent inhibitors of class A carbapenemases (typified by Klebsiella pneumoniae carbapenemase-like [KPC-like] genotypes) (1, 2) but are ineffective against carbapenemase-producing Enterobacteriaceae (CPE)-producing class B metallo-β-lactamases (MβLs), such as New Delhi metallo-β-lactamases (NDMs) (1–4). Hence, these βL-βLI combinations are expected to have less utility in geographical regions where NDMs predominate, namely, the Asian continent, which has an estimated abundance of 60% of global NDM producers (5, 6).
Locally in Singapore, NDM, KPC, and OXA-48-like are the predominant circulating carbapenemase genotypes (5). The carriage of dual carbapenemases, typically NDM cooccurring with KPC or OXA-48-like carbapenemases, is not uncommon (7). MβLs such as those of the IMP and VIM genotypes are infrequently encountered in our epidemiologic context (5). The monobactam aztreonam is refractory to MβL hydrolysis (2, 8, 9); hence, combining aztreonam with a βLI can restore the activity of aztreonam due to inhibition of the cocarried non-MβL. The aztreonam-avibactam combination has been demonstrated to be inhibitory to MβLs (NDM, IMP, or VIM MβLs) cocarrying a KPC or OXA-48-like carbapenemase (10–13). The largest test set of isolates in these studies contained 47 isolates (12). Here, we present in vitro susceptibility testing data on, to our knowledge, one of the largest collections of NDMs in combination with KPC or OXA-48-like carbapenemases.
A total of 70 phenotypically carbapenem-resistant, dual-carbapenemase Enterobacteriaceae isolates (13 Escherichia coli, 44 Klebsiella pneumoniae, 7 Citrobacter freundii, and 6 Enterobacter cloacae complex isolates) were obtained from the reference National Public Health Laboratory, Singapore, and their carbapenemase genotypes were confirmed by PCR and sequencing (14) (see Table S1 in the supplemental material). MICs were determined using the reference broth microdilution method (15). All antibiotic powders were purchased from MedChemExpress (Princeton, NJ, USA). Aztreonam and meropenem were tested alone and in combination with avibactam. The final concentration of aztreonam and meropenem tested ranged from 0.06 mg/liter to 64 mg/liter. For combination testing, avibactam concentrations were fixed at 4 mg/liter. Quality control isolates E. coli ATCC 25922 and K. pneumoniae ATCC 700603 were used for susceptibility testing, and the results were considered valid when they fell within the expected ranges for aztreonam and meropenem (ATCC 25922) and aztreonam-avibactam (ATCC 700603). The testing was performed in duplicate. Susceptibility was interpreted based on Clinical and Laboratory Standards Institute (CLSI) breakpoints for aztreonam and meropenem (15). For aztreonam, susceptibility was indicated by a MIC of ≤4 mg/liter, intermediate susceptibility by 8 mg/liter, and resistance by ≥16 mg/liter. For meropenem, susceptibility was indicated by a MIC of ≤1 mg/liter, intermediate susceptibility by 2 mg/liter, and resistance by ≥4 mg/liter.
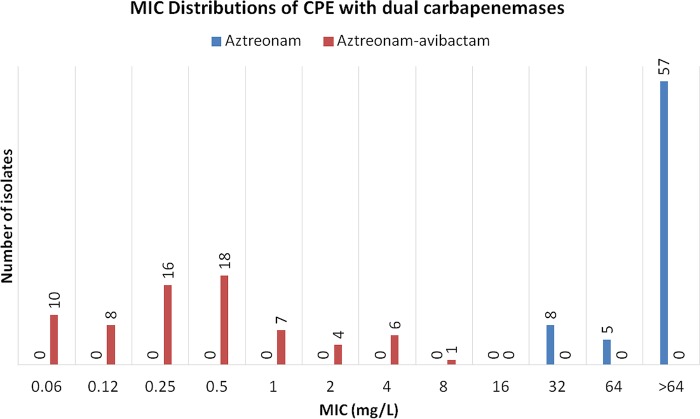
Susceptibility testing was initially performed for various βL-βLI combinations (aztreonam [or meropenem] with avibactam [n = 23]), followed by testing of the remaining isolates (n = 47) with aztreonam-avibactam only. Initially, 23 dual-carbapenemase producers were evaluated against combinations of aztreonam and meropenem with avibactam. Six isolates cocarried blaKPC-2 and blaNDM-1, while 17 isolates cocarried either blaNDM-1 or blaNDM-5 in combination with blaOXA-48-like. All isolates were nonsusceptible to meropenem and aztreonam, with the MIC90s for them being >64 mg/liter. Avibactam restored the susceptibility of 22 (95.7%) isolates to aztreonam and of one (4.3%) isolate to meropenem. Following the promising initial results for the aztreonam and avibactam combination, the remaining 48 dual-carbapenemase-producing Enterobacteriaceae were tested against aztreonam and avibactam, alone and in combination (Table 1; Fig. 1). All isolates were resistant to aztreonam but had their susceptibility restored by avibactam. The combined MIC90s of all 70 isolates were >64 mg/liter for aztreonam and 2 mg/liter for aztreonam-avibactam (Fig. 1). The aztreonam-avibactam combination was largely inhibitory to our collection of dual-carbapenemase CPE, with 98.6% (69/70) of isolates having their susceptibility to aztreonam restored by the addition of avibactam.
TABLE 1.
Susceptibilities of CPE carrying dual carbapenemases to aztreonam and avibactam singly and in combination
| Carbapenemase genes (isolates) | Avibactam (mg/liter) |
Aztreonam (mg/liter) |
Aztreonam-avibactama (mg/liter) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC or range | MIC50 | MIC90 | MIC or range | MIC50 | MIC90 | MIC or range | MIC50 | MIC90 | |
| NDM-1 + IMP-4 (1 K. pneumoniae isolate) | >64 | 32 | 0.25 | ||||||
| NDM + KPC-2 (11 K. pneumoniae isolates, 2 E. cloacae complex isolates, 1 C. freundii isolate) | 16 to >64 | 64 | >64 | 32 to >64 | 64 | >64 | ≤0.06 to 4 | 0.12 | 1 |
| NDM + OXA-48-like (32 K. pneumoniae, 13 E. coli, 4 E. cloacae complex, 6 C. freundii isolates) | 4 to >64 | 32 | >64 | 32 to >64 | >64 | >64 | ≤0.06 to 8 | 0.5 | 4 |
| All isolates (n = 70) | 4 to >64 | 64 | >64 | 32 to >64 | >64 | >64 | ≤0.06 to 8 | 0.5 | 2 |
The avibactam concentration was constant at 4 mg/liter.
FIG 1.
Aztreonam and aztreonam-avibactam MIC distribution of CPE carrying dual carbapenemases.
Avibactam is currently marketed in combination with ceftazidime and has demonstrated high rates of activity against non-MβL carbapenemases, such as KPC and OXA-48-like carbapenemases (3). However, the overall effectiveness of ceftazidime-avibactam is affected by the local epidemiology of CPE. Molecular identification of MβL carbapenemases precludes the use of ceftazidime-avibactam alone. Where MβLs and CPE predominate, aztreonam-avibactam rather than ceftazidime-avibactam should prove to be more effective therapeutically, and its efficacy further extends to CPE possessing KPC or OXA-48-like carbapenemases. The administration of ceftazidime-avibactam in combination with aztreonam separately may be useful for treating MβL-CPE infections and can be guided by phenotypic susceptibility testing results.
Supplementary Material
ACKNOWLEDGMENTS
We thank the National Public Health Laboratory (NPHL), Singapore, for providing carbapenemase-producing Enterobacteriaceae isolates, in particular, Felicia Ong for performing the carbapenemase screening assays and Siti Zulaina for preparing plate cultures. We also thank the Carbapenemase-Producing Enterobacteriaceae in Singapore (CaPES) study group.
We received no funding for this work. We have no conflicts of interest to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00414-18.
REFERENCES
- 1.Lomovskaya O, Sun D, Rubio-Aparicio D, Nelson K, Tsivkovski R, Griffith DC, Dudley MN. 2017. Vaborbactam: spectrum of beta-lactamase inhibition and impact of resistance mechanisms on activity in Enterobacteriaceae. Antimicrob Agents Chemother 61:e01443-17. doi: 10.1128/AAC.01443-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bush K. 2015. A resurgence of beta-lactamase inhibitor combinations effective against multidrug-resistant Gram-negative pathogens. Int J Antimicrob Agents 46:483–493. doi: 10.1016/j.ijantimicag.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Vasoo S, Cunningham SA, Cole NC, Kohner PC, Menon SR, Krause KM, Harris KA, De PP, Koh TH, Patel R. 2015. In vitro activities of ceftazidime-avibactam, aztreonam-avibactam, and a panel of older and contemporary antimicrobial agents against carbapenemase-producing Gram-negative bacilli. Antimicrob Agents Chemother 59:7842–7846. doi: 10.1128/AAC.02019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castanheira M, Rhomberg PR, Flamm RK, Jones RN. 2016. Effect of the β-lactamase inhibitor vaborbactam combined with meropenem against serine carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 60:5454–5458. doi: 10.1128/AAC.00711-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marimuthu K, Venkatachalam I, Khong WX, Koh TH, Cherng BPZ, La MV, De PP, Krishnan PU, Tan TY, Choon RFK, Pada SK, Lam CW, Ooi ST, Deepak RN, Smitasin N, Tan EL, Lee JJ, Kurup A, Young B, Sim NTW, Thoon KC, Fisher D, Ling ML, Ang BSP, Teo Y-Y, Hsu LY, Lin RTP, Ong RTH, Teo J, Ng OT. 2017. Clinical and molecular epidemiology of carbapenem-resistant Enterobacteriaceae among adult inpatients in Singapore. Clin Infect Dis 64:S68–S75. doi: 10.1093/cid/cix113. [DOI] [PubMed] [Google Scholar]
- 6.Khan AU, Maryam L, Zarrilli R. 2017. Structure, genetics and worldwide spread of New Delhi metallo-beta-lactamase (NDM): a threat to public health. BMC Microbiol 17:101. doi: 10.1186/s12866-017-1012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balm MND, La M-V, Krishnan P, Jureen R, Lin RTP, Teo JWP. 2013. Emergence of Klebsiella pneumoniae co-producing NDM-type and OXA-181 carbapenemases. Clin Microbiol Infect 19:E421–E423. doi: 10.1111/1469-0691.12247. [DOI] [PubMed] [Google Scholar]
- 8.Queenan AM, Bush K. 2007. Carbapenemases: the versatile β-lactamases. Clin Microbiol Rev 20:440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans BA, Amyes SGB. 2014. OXA β-lactamases. Clin Microbiol Rev 27:241–263. doi: 10.1128/CMR.00117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kazmierczak KM, Biedenbach DJ, Hackel M, Rabine S, de Jonge BLM, Bouchillon SK, Sahm DF, Bradford PA. 2016. Global dissemination of blaKPC into bacterial species beyond Klebsiella pneumoniae and in vitro susceptibility to ceftazidime-avibactam and aztreonam-avibactam. Antimicrob Agents Chemother 60:4490–4500. doi: 10.1128/AAC.00107-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sader HS, Mendes RE, Pfaller MA, Shortridge D, Flamm RK, Castanheira M. 2018. Antimicrobial activities of aztreonam-avibactam and comparator agents against contemporary (2016) clinical Enterobacteriaceae isolates. Antimicrob Agents Chemother 62:e01856-. doi: 10.1128/AAC.01856-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlowsky JA, Kazmierczak KM, de Jonge BLM, Hackel MA, Sahm DF, Bradford PA. 2017. In vitro activity of aztreonam-avibactam against Enterobacteriaceae and Pseudomonas aeruginosa isolated by clinical laboratories in 40 countries from 2012 to 2015. Antimicrob Agents Chemother 61:e00472-17. doi: 10.1128/AAC.00472-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biedenbach DJ, Kazmierczak K, Bouchillon SK, Sahm DF, Bradford PA. 2015. In vitro activity of aztreonam-avibactam against a global collection of Gram-negative pathogens from 2012 and 2013. Antimicrob Agents Chemother 59:4239–4248. doi: 10.1128/AAC.00206-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teo J, Ngan G, Balm M, Jureen R, Krishnan P, Lin R. 2012. Molecular characterization of NDM-1 producing Enterobacteriaceae isolates in Singapore hospitals. West Pacific Surveill Response 3:19–24. doi: 10.5365/wpsar.2011.2.4.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CLSI. 2018. Performance standards for antimicrobial susceptibility testing, 28th ed, M100-S28E CLSI, Wayne, PA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.