Piperaquine is an important partner drug used in artemisinin-based combination therapies (ACTs). An increase in the plasmepsin 2 and 3 gene copy numbers has been associated with decreased susceptibility of Plasmodium falciparum to piperaquine in Cambodia.
KEYWORDS: PET-PCR, piperaquine, copy number, malaria
ABSTRACT
Piperaquine is an important partner drug used in artemisinin-based combination therapies (ACTs). An increase in the plasmepsin 2 and 3 gene copy numbers has been associated with decreased susceptibility of Plasmodium falciparum to piperaquine in Cambodia. Here, we developed a photo-induced electron transfer real-time PCR (PET-PCR) assay to quantify the copy number of the P. falciparum plasmepsin 2 gene (PfPM2) that can be used in countries where P. falciparum is endemic to enhance molecular surveillance.
TEXT
Malaria is one of the most common and serious tropical diseases in the world, with roughly 216 million cases and an estimated 445,000 malaria deaths globally (1). Malaria is caused by intracellular parasitic protozoa of the genus Plasmodium. Plasmodium falciparum is the most virulent among the human malarial parasites and causes disease with high morbidity and mortality (1, 2). The first-line therapy recommended by the World Health Organization for uncomplicated malaria caused by P. falciparum is artemisinin-based combination therapy (ACT) (4, 5), which consists of an artemisinin derivative combined with a partner drug of another class of antimalarial. However, resistance to artemisinin and partner drugs has been reported in Southeast Asia and confirmed by therapeutic efficacy studies (6–10). Dihydroartemisinin and piperaquine combination therapy is considered one of the remaining effective therapies against P. falciparum malaria; however, with the recent emergence of resistance to piperaquine, along with artemisinin in Southeast Asia, molecular surveillance to detect piperaquine-resistant parasites is now of utmost importance (7, 11–14). Recently, an increase in the copy number of the plasmepsin 2–3 gene cluster in P. falciparum (PfPM2–3) was demonstrated to be associated with piperaquine resistance in Cambodia (11, 15, 16). This gene amplification was determined using genome-wide analysis of copy number variation and a singleplex TaqMan probe-based real-time-PCR (16). This real-time PCR method requires two separate singleplex PCRs (to amplify the single-copy housekeeping P. falciparum β-tubulin gene and the PfPM2 gene of interest) and the use of a TaqMan probe or DNA intercalating dye. We previously demonstrated that the self-quenching photo-induced electron transfer (PET) fluorogenic primers used in the PET-PCR assay provide a more convenient to use and less expensive real-time PCR assay than conventional probe-based real-time PCR formats, as no internal probes (e.g., TaqMan probes) or intercalating dyes are required (17, 18). We designed and evaluated a multiplex PET-PCR assay (results obtained using a single PCR) to determine the number of copies of PfPM2 gene in P. falciparum. This novel assay was compared with the previously published singleplex TaqMan probe-based real-time PCR assay (16).
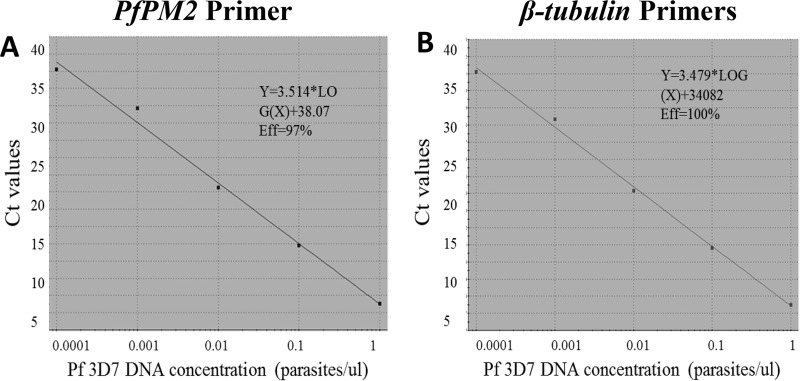
The PfPM2 and P. falciparum β-tubulin gene primers used in this study were previously reported by Witkowski and collaborators (16). In our multiplex PET-PCR assay, the reverse primers of both PfPM2 and the P. falciparum β-tubulin gene were modified with the PET tag and labeled with 6-carboxyfluorescein (FAM; PfPM2) and HEX (P. falciparum β-tubulin gene) fluorophores, as previously described by Lucchi et al. (17) (Table 1). The PET-PCR was carried out in a 20-μl volume containing 2× TaqMan Environmental master mix 2.0 (Applied Biosystems, Foster City, CA, USA), 0.5 μM each forward and reverse primer for PfPM2, 0.25 μM for the P. falciparum β-tubulin gene primers, and 3 μl of template DNA. The amplifications were performed under the following conditions: 95°C for 15 min, 45 cycles of 95°C for 15 s, 58°C for 1 min, and 72°C for 20 s. All samples were tested in triplicate, and the assays were performed using Agilent Mx3005pro thermocyclers (Agilent Technologies, Santa Clara, CA, USA). Evaluation of PET-PCR amplification efficiency and reproducibility was performed using a 10-fold dilution of a P. falciparum control strain (3D7), with parasite densities of 2,000 parasites/μl to 2 parasites/μl. The amplification efficiencies of the PfPM2 and the P. falciparum β-tubulin gene PET-primers were 97% and 100%, respectively (Fig. 1A and B). The PfPM2 copy number was determined by the 2−ΔΔCT method (ΔCT = CT PfPM2 − CT P. falciparum β-tubulin gene; CT, threshold cycle) using the 3D7 P. falciparum strain, known to have a single copy of the PfMD2 gene, as a calibrator and two additional controls (control 2 [Ctrl-2] and Ctrl-3 obtained from Cambodia with one copy and three copies, respectively). To validate the accuracy of the PET-PCR for copy number variation determination, six additional clinical samples, all obtained from Cambodia, with known PfMD2 copy numbers were tested in a blinded fashion using both the novel PET-PCR and the previously reported singleplex TaqMan probe-based assay. These specimens were kindly provided by R. M. Fairhurst's (samples MB-PPQ-001 to MB-PPQ-004) and D. Fidock's (samples MB-PPQ-005 and MB-PPQ-006) laboratories and were previously evaluated for PfMD2 copy numbers using probe-based real-time PCR assays and/or whole-genome sequencing. The PET-PCR multiplex PfPM2 copy number results had 100% agreement with the previously published results of the singleplex TaqMan probe-based assay (16) (Table 2).
TABLE 1.
Oligonucleotide primer sequences used in the PET-PCR PfPM2 copy number assaya
| PET-PCR primer | PET-PCR sequence (5′ to 3′) | Tm (°C) | Product size (bp) | Melting temp range (°C) |
|---|---|---|---|---|
| PfPM2_F | TGGTGATGCAGAAGTTGGAG | 59.8 | 79 | 76.8–77.2 |
| PfPM2_R_FAM | aggcgcatagcgcctggTGGGACCCATAAATTAGCAGA | 59.4 | ||
| Pf β-tubulin_F | TGATGTGCGCAAGTGATCC | 61.9 | 87 | 79.0–79.2 |
| Pf β-tubulin_R_HEX | aggcgcatagcgcctggTCCTTTGTGGACATTCTTCCTC | 60.5 |
The PET-tag (lowercase letters in bold) attached to the 5′ end of the target-specific sequences does not show any homology to Plasmodium species. The PfPM2 (gene accession no. PF3D7_1408000-811659) and β-tubulin gene (gene accession no. PF3D7_1008700) primers were previously reported in the singleplex probe-based real-time PCR assay (16).
FIG 1.
Amplification efficiencies of the novel PET-PCR primers. The amplification efficiency (Eff) of the PfPM2 primers were 97% (A), and that of the P. falciparum β-tubulin gene primers was 100% (B).
TABLE 2.
Comparison between PET-PCR and TaqMan probe-based real-time PCR assaya
The PET-PCR multiplex PfPM2 copy number results had 100% agreement with previously published singleplex real-time PCR assay results (16). Ctrl, control samples; MB-PPQ-001 to -006, clinical samples that were kindly provided by R. M. Fairhurst and D. Fidock.
ID, identification number.
Simple molecular tools are valuable for conducting molecular surveillance of drug resistance markers. The PET-PCR is a molecular diagnostic assay with performance characteristics that are similar to those of commonly used real-time PCR methods, but the PET-PCR is less expensive, easy to use, and can be used for large-scale surveillance studies, even in developing country settings (17). The novel multiplex PET-PCR assay for PfPM2 copy number determination has performance characteristics similar to those of a singleplex TaqMan probe-based assay but without the need for running two independent PCRs. The novel assay provides a useful cost-effective alternative for the evaluation of the PfPM2 copy number. As monitoring for piperaquine resistance is gaining momentum and urgency, it is hoped that this new method will help enhance molecular surveillance in a timely manner even in reference laboratories in countries endemic for the disease. In addition, the PET-PCR format described here provides an alternative real-time PCR format that can be utilized for the determination of any gene amplification of interest.
ACKNOWLEDGMENTS
We thank the Malaria Branch, CDC, for supporting this project, and David Fidock, Leila Ross, Rick M. Fairhurst, and Didier Menard for proving the clinical samples and controls used in this study. Also, we give a special thank you to Ira Goldman for reviewing the article.
We acknowledge funding support for this study from the CDC Antimicrobial Resistance Working Group.
The use of trade names and names of commercial sources is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention or the U.S. Department of Health and Human Services. The findings and conclusions in this presentation are those of the authors and do not necessarily represent those of the Centers for Disease Control and Prevention.
REFERENCES
- 1.WHO. 2016. World malaria report. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Bousema T, Drakeley C. 2011. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev 24:377–410. doi: 10.1128/CMR.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reference deleted.
- 4.Ashley EA, White NJ. 2005. Artemisinin-based combinations. Curr Opin Infect Dis 18:531–536. doi: 10.1097/01.qco.0000186848.46417.6c. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. 2006. Guidelines for the treatment of malaria. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 6.Dondorp AM, Fairhurst RM, Slutsker L, Macarthur JR, Breman JG, Guerin PJ, Wellems TE, Ringwald P, Newman RD, Plowe CV. 2011. The threat of artemisinin-resistant malaria. N Engl J Med 365:1073–1075. doi: 10.1056/NEJMp1108322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fairhurst RM. 2015. Understanding artemisinin-resistant malaria: what a difference a year makes. Curr Opin Infect Dis 28:417–425. doi: 10.1097/QCO.0000000000000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Packard RM. 2014. The origins of antimalarial-drug resistance. N Engl J Med 371:397–399. doi: 10.1056/NEJMp1403340. [DOI] [PubMed] [Google Scholar]
- 9.Pickard AL, Wongsrichanalai C, Purfield A, Kamwendo D, Emery K, Zalewski C, Kawamoto F, Miller RS, Meshnick SR. 2003. Resistance to antimalarials in Southeast Asia and genetic polymorphisms in pfmdr1. Antimicrob Agents Chemother 47:2418–2423. doi: 10.1128/AAC.47.8.2418-2423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price RN, Cassar C, Brockman A, Duraisingh M, van Vugt M, White NJ, Nosten F, Krishna S. 1999. The pfmdr1 gene is associated with a multidrug-resistant phenotype in Plasmodium falciparum from the western border of Thailand. Antimicrob Agents Chemother 43:2943–2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amato R, Lim P, Miotto O, Amaratunga C, Dek D, Pearson RD, Almagro-Garcia J, Neal AT, Sreng S, Suon S, Drury E, Jyothi D, Stalker J, Kwiatkowski DP, Fairhurst RM. 2017. Genetic markers associated with dihydroartemisinin-piperaquine failure in Plasmodium falciparum malaria in Cambodia: a genotype-phenotype association study. Lancet Infect Dis 17:164–173. doi: 10.1016/S1473-3099(16)30409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Menard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale JC, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Menard D. 2014. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han KT, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, et al. . 2014. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 371:411–164 423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parobek CM, Parr JB, Brazeau NF, Lon C, Chaorattanakawee S, Gosi P, Barnett EJ, Norris LD, Meshnick SR, Spring MD, Lanteri CA, Bailey JA, Saunders DL, Lin JT, Juliano JJ. 2017. Partner-drug resistance and population substructuring of artemisinin-resistant Plasmodium falciparum in Cambodia. Genome Biol Evol 9:1673–1686. doi: 10.1093/gbe/evx126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dondorp AM. 2017. New genetic marker for piperaquine resistance in Plasmodium falciparum. Lancet Infect Dis 17:119–121. doi: 10.1016/S1473-3099(16)30414-5. [DOI] [PubMed] [Google Scholar]
- 16.Witkowski B, Duru V, Khim N, Ross LS, Saintpierre B, Beghain J, Chy S, Kim S, Ke S, Kloeung N, Eam R, Khean C, Ken M, Loch K, Bouillon A, Domergue A, Ma L, Bouchier C, Leang R, Huy R, Nuel G, Barale JC, Legrand E, Ringwald P, Fidock DA, Mercereau-Puijalon O, Ariey F, Menard D. 2017. A surrogate marker of piperaquine-resistant Plasmodium falciparum malaria: a phenotype-genotype association study. Lancet Infect Dis 17:174–183. doi: 10.1016/S1473-3099(16)30415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucchi NW, Narayanan J, Karell MA, Xayavong M, Kariuki S, DaSilva AJ, Hill V, Udhayakumar V. 2013. Molecular diagnosis of malaria by photo-induced electron transfer fluorogenic primers: PET-PCR. PLoS One 8:e56677. doi: 10.1371/journal.pone.0056677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talundzic E, Ndiaye YD, Deme AB, Olsen C, Patel DS, Biliya S, Daniels R, Vannberg FO, Volkman SK, Udhayakumar V, Ndiaye D. 2017. Molecular epidemiology of Plasmodium falciparum kelch13 mutations in Senegal determined by using targeted amplicon deep sequencing. Antimicrob Agents Chemother 61:e02116-16. doi: 10.1128/AAC.02116-16. [DOI] [PMC free article] [PubMed] [Google Scholar]