Repurposing drugs may be useful as an add-on in the treatment of Mycobacterium abscessus pulmonary infections, which are particularly difficult to cure. M. abscessus naturally produces a β-lactamase, BlaMAb, which is inhibited by avibactam.
KEYWORDS: avibactam, β-lactamase inhibitor, cystic fibrosis, imipenem, Mycobacterium abscessus, rifabutin
ABSTRACT
Repurposing drugs may be useful as an add-on in the treatment of Mycobacterium abscessus pulmonary infections, which are particularly difficult to cure. M. abscessus naturally produces a β-lactamase, BlaMAb, which is inhibited by avibactam. The recommended regimens include imipenem, which is hydrolyzed by BlaMAb and used without any β-lactamase inhibitor. Here, we determine whether the addition of rifabutin improves the activity of imipenem alone or in combination with avibactam against M. abscessus CIP104536. Rifabutin at 16 μg/ml was only bacteriostatic (MIC of 4 μg/ml) and was moderately synergistic in combination with imipenem (fractional inhibitory concentration [FIC] index of 0.38). Addition of rifabutin (16 μg/ml) moderately increased killing by a low (8 μg/ml) but not by a high (32 μg/ml) concentration of imipenem. Addition of avibactam (4 μg/ml) did not further increase killing by the former combination. In infected macrophages, rifabutin (16 μg/ml) increased the activity of imipenem at 8 and 32 μg/ml, achieving 3- and 100-fold reductions in the numbers of intracellular bacteria, respectively. Avibactam (16 μg/ml) improved killing by imipenem at 8 μg/ml. A 5-fold killing was obtained for a triple combination comprising avibactam (16 μg/ml) and therapeutically achievable doses of imipenem (8 μg/ml) and rifabutin (1 μg/ml). These results indicate that the imipenem-rifabutin combination should be further considered for the treatment of M. abscessus pulmonary infections in cystic fibrosis patients and that addition of a β-lactamase inhibitor might improve its efficacy. Mechanistically, the impact of BlaMAb inhibition by avibactam on antibiotic activity was assessed by comparing CIP104536 and a β-lactamase-deficient derivative.
INTRODUCTION
Mycobacterium abscessus, a rapidly growing mycobacterium, has emerged as a significant pathogen responsible for chronic pulmonary infections in cystic fibrosis patients (1–5). Among the nontuberculous mycobacteria, M. abscessus is the predominant species isolated in these patients (40 to 50%) (6, 7). The treatment is particularly difficult since M. abscessus is intrinsically resistant to a broad range of antibiotics, including those used for the treatment of tuberculosis, such as rifampin (8, 9).
The recommended treatment of lung infections due to M. abscessus is not yet well standardized. For cystic fibrosis patients, it consists of an initial phase with the combination of a macrolide (azithromycin), an aminoglycoside (amikacin), a glycylcycline (tigecycline), and a carbapenem (imipenem) for 3 months (10). The continuation phase (at least 12 months) relies on four per os-administered drugs (azithromycin, minocycline, clofazimine, and moxifloxacin) (10). Since resistance to macrolides compromises the efficacy of this treatment schedule (11), there is an urgent need to identify additional therapeutic options. This could be achieved in the short term by repurposing existing drugs and in the longer term by the discovery of new antimycobacterial agents.
Infections due to M. abscessus are treated by β-lactams in the absence of any β-lactamase inhibitor although all M. abscessus isolates produce the broad-spectrum β-lactamase BlaMAb. This enzyme effectively hydrolyzes most β-lactams except cefoxitin and imipenem, the latter being slowly inactivated (12, 13). BlaMAb is not inhibited by classical inhibitors (clavulanate, tazobactam, and sulbactam) (14). In contrast, complete and rapid inactivation of BlaMAb is obtained with avibactam (12), which has been developed and approved for the treatment of infections due to multidrug-resistant Enterobacteriaceae (15–17). Deletion of the gene encoding BlaMAb (ΔblaMAb) or chemical inhibition of the β-lactamase by avibactam extends the spectrum of β-lactams active against M. abscessus (12, 18) and improves the efficacy of imipenem in both the macrophage and zebrafish embryo models (19).
The rifampin derivative rifabutin is used as a first-line treatment for infections due to members of the Mycobacterium avium complex (8). Recently, screening of 2,700 approved drugs against M. abscessus identified rifabutin as an active drug (MIC and minimum bactericidal concentration [MBC] of 3 and 6 μg/ml, respectively) (20). Rifabutin was active in vitro against reference strains belonging to the three subspecies of M. abscessus (M. abscessus subsp. abscessus, M. abscessus subsp. bolletii, and M. abscessus subsp. massiliense), as well as against a collection of clinical isolates (20).
In this study, we investigated the possibility of repurposing rifabutin for M. abscessus infections in combination with imipenem or with imipenem and avibactam. We report the in vitro and intracellular antibacterial activities of various combinations of these three drugs. We have also compared the activity of the drug combinations against a smooth variant of M. abscessus CIP104536 and a derivative obtained by deletion of the gene encoding BlaMAb in order to assess the impact of β-lactamase production.
RESULTS
Bacteriostatic activity of rifabutin alone or combined with imipenem and synergy between these drugs.
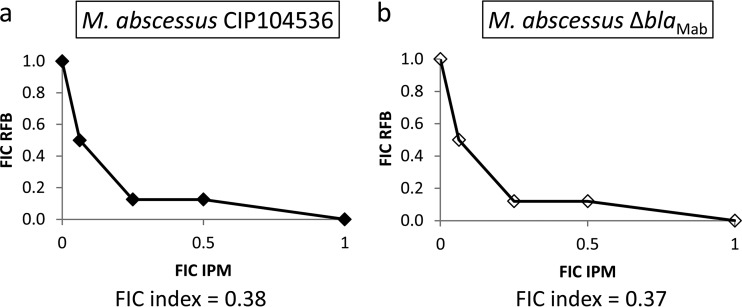
The MIC of rifabutin was 4 μg/ml against both M. abscessus CIP104536 and its β-lactamase-deficient (ΔblaMAb) derivative (Table 1). The MICs of imipenem were 4 μg/ml and 2 μg/ml against CIP104536 and its ΔblaMAb derivative. The isobolograms for the interaction between rifabutin and imipenem are presented in Fig. 1. Against CIP104536 (Fig. 1a), the combination of rifabutin with imipenem showed a moderate synergistic effect, with a fractional inhibitory concentration (FIC) index of 0.38 at 1/8× the MIC of imipenem and 1/4× the MIC of rifabutin. Against the ΔblaMAb derivative (Fig. 1b), the FIC index was 0.37 at 1/8× the MIC of imipenem and 1/4× the MIC of rifabutin.
TABLE 1.
MIC of antibiotics against M. abscessus
| M. abscessus strain | MIC (μg/ml) |
|
|---|---|---|
| Imipenem | Rifabutin | |
| CIP104536 | 4 | 4 |
| CIP104536 ΔblaMAb | 2 | 4 |
FIG 1.
Isobolograms showing the interaction between rifabutin (RFB) and imipenem (IPM) against M. abscessus CIP104536 (a) and its ΔblaMAb derivative (b). The fractional inhibitory concentration (FIC) index was determined by the checkerboard method.
In vitro killing of M. abscessus by rifabutin alone or in combination with imipenem.
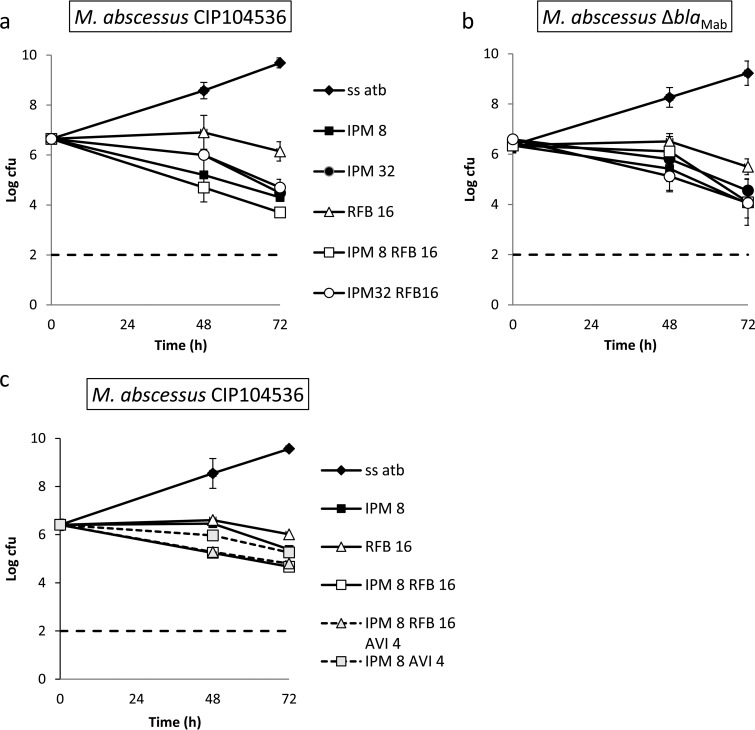
Rifabutin was tested at 4-fold the MIC (16 μg/ml), and imipenem was tested at 2- and 8-fold the MIC (8 and 32 μg/ml). Against CIP104536, rifabutin alone led to a 0.4-log10 reduction in CFU count (Fig. 2a; see Table S1 in the supplemental material for statistical analysis). Higher reductions in the log10 CFU count were observed for imipenem alone at 8 μg/ml (2.2-log10 reduction) and 32 μg/ml (2.0-log10 reduction). The addition of rifabutin moderately increased bacterial killing for imipenem at 8 μg/ml but not at 32 μg/ml. Against CIP104536, none of the tested regimens showed bactericidal activity defined by a reduction of more than 99.9% of the CFU present in the inoculum. Similar results were obtained for the ΔblaMAb derivative of CIP104536 except that rifabutin did not improve the activity of imipenem (Fig. 2a and statistical analysis in Table S2). Thus, the production of the β-lactamase compromised the activity of imipenem only if this antibiotic was tested at a low dose, and this effect was not observed if the carbapenem was associated with rifabutin.
FIG 2.
Time-kill curves. Rifabutin (RFB) was tested alone or in combination with imipenem (IPM) and the β-lactamase inhibitor avibactam (AVI) against M. abscessus CIP104536 and its derivative ΔblaMAb. (a) Time-kill curves of rifabutin at 16 μg/ml alone or in combination with imipenem (8 and 32 μg/ml) against M. abscessus CIP104536. (b) Time-kill curves of rifabutin at 16 μg/ml alone or in combination with imipenem (8 and 32 μg/ml) against the ΔblaMAb derivative. (c) Time-kill curves of rifabutin at 1 and 8 μg/ml alone or in combination with imipenem (8 μg/ml) with or without avibactam (4 μg/ml) against M. abscessus CIP104536. The number of CFU was determined after 0, 48, and 72 h of exposure to antibiotics. Values are means of three independent experiments. Bars represent the standard deviations.
Impact of avibactam on in vitro killing of M. abscessus by rifabutin and imipenem.
Since the addition of rifabutin to imipenem led to a moderate increase in the activity of imipenem at 8 μg/ml against M. abscessus CIP104536 (Fig. 2a), we investigated the benefit of adding avibactam (4 μg/ml) to this combination (Fig. 2c and statistical analysis in Table S3). The triple combination of imipenem-rifabutin-avibactam was not more active than imipenem-rifabutin. Avibactam did not potentiate the killing by imipenem alone.
To summarize the in vitro activity of the drugs, rifabutin alone was bacteriostatic against M. abscessus. Against M. abscessus CIP104536, rifabutin moderately increased the activity of imipenem at 8 μg/ml but not at 32 μg/ml. Avibactam did not improve the activity of imipenem or imipenem-rifabutin. None of the combinations was bactericidal.
Intramacrophage activity of rifabutin alone or in combination with imipenem.
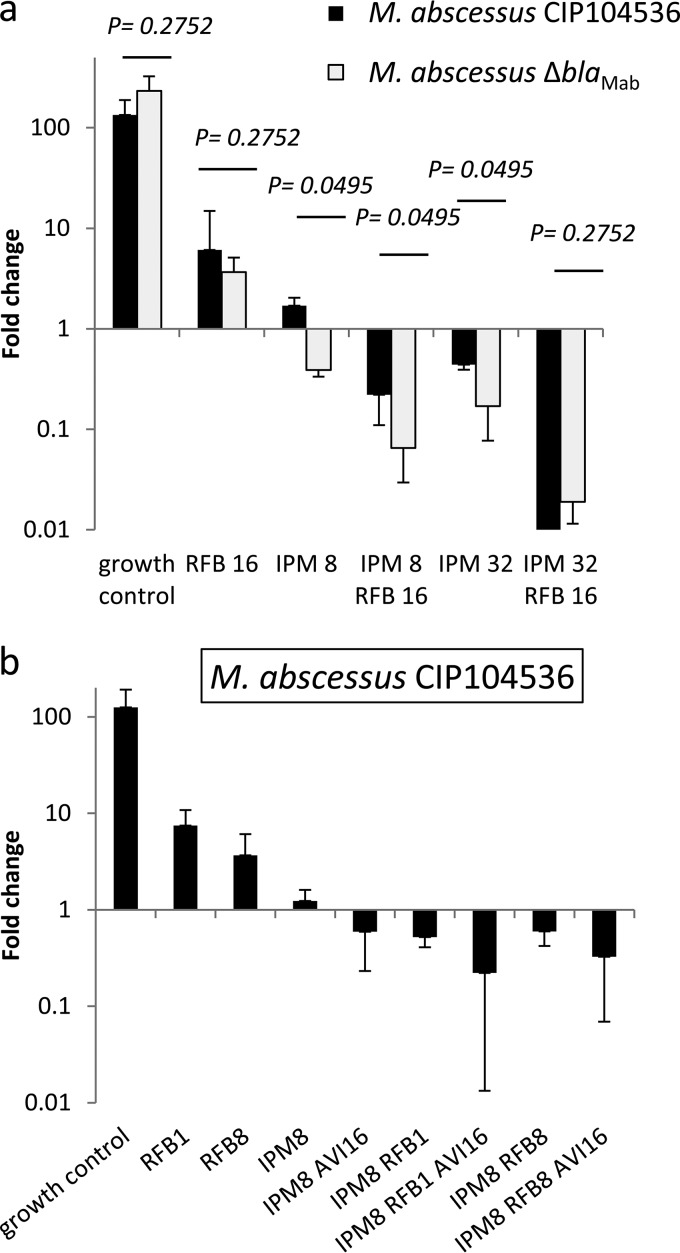
THP-1-derived macrophages were infected with M. abscessus CIP104536 and its ΔblaMAb derivative and exposed to various drugs for 48 h, and surviving bacteria were enumerated by plating serial dilutions of macrophage lysates (Fig. 3 and statistical analysis in Table S4). In the absence of antibiotic, M. abscessus CIP104536 grew in the macrophages, leading to a 130-fold increase in the number of CFU in 48 h (Fig. 3a). Rifabutin at 16 μg/ml partially prevented intramacrophage growth (6.1- versus 134-fold increase in absence of antibiotic in the number of CFU; P < 0.05). Imipenem at 8 μg/ml was more active than rifabutin (1.7- versus 6.1-fold increase in CFU count, respectively; P < 0.05). Increasing the concentration of imipenem from 8 μg/ml to 32 μg/ml improved the activity of this drug, leading to a minor decrease in the intracellular number of bacteria (1.7- versus 0.44-fold change in CFU count, respectively; P < 0.05). The combination of rifabutin and imipenem (8 μg/ml) was more active than that imipenem alone (0.22- versus 1.7-fold change in CFU count, respectively; P < 0.05). Increasing the concentration of imipenem from 8 μg/ml to 32 μg/ml improved the activity of the combination (0.01- versus 0.22-fold increase in CFU count, respectively; P < 0.05). Of note, the combination of imipenem (32 μg/ml) and rifabutin (16 μg/ml) achieved 99% killing of M. abscessus in macrophages.
FIG 3.
Intracellular activity of rifabutin (RFB) alone, in combination with imipenem (IPM), or in combination with imipenem (IPM) and avibactam (AVI). (a) Rifabutin (16 μg/ml) alone or in combination with imipenem (8 μg/ml and 32 μg/ml) against M. abscessus CIP104536 and its ΔblaMAb derivative. (b) Rifabutin (1 and 8 μg/ml) alone, in combination with imipenem (8 μg/ml), or in combination with imipenem (8 μg/ml) and avibactam (16 μg/ml) against M. abscessus CIP104536. Intracellular bacteria were enumerated, and the fold change in CFU counts was determined between days 0 and 2 postinfection. Values are means of three independent experiments. Bars represent standard deviations. A Mann-Whitney U test was used to compare M. abscessus CIP104536 and its ΔblaMAb derivative.
Rifabutin (16 μg/ml) and imipenem (8 and 32 μg/ml) were also tested against the isogenic strain M. abscessus CIP104536 ΔblaMAb to evaluate the impact of the production of the β-lactamase BlaMAb on the intracellular activity of the combination (Fig. 3a and statistical analysis in Table S5). As expected, rifabutin alone was similarly active against CIP104536 and its ΔblaMAb derivative (6.1- versus 3.7-fold change, respectively, P = 0.27). Deletion of blaMAb significantly improved the activity of imipenem alone at the two concentrations tested (1.7- versus 0.39-fold change at 8 μg/ml and 0.44- versus 0.17-fold change at 32 μg/ml for CIP104536 and the ΔblaMAb strain, respectively; P < 0.05 for both comparisons). As observed for the parental strain, the addition of rifabutin improved the activity of imipenem against the ΔblaMAb derivative, leading to 93% and 98% killing at 8 and 32 μg/ml, respectively (P < 0.05 for both comparisons). At a low dose of imipenem, production of BlaMAb reduced the activity of imipenem-rifabutin since the fold reduction was higher for the ΔblaMAb derivative than for the parental strain (93% versus 78% killing, respectively; P < 0.05). However, this difference was not observed for the combination involving a high dose of imipenem (98% and 99% killing for the ΔblaMAb derivative and the parental strain, respectively; P = 0.27).
Impact of avibactam on intramacrophage killing of M. abscessus by rifabutin and imipenem.
Since production of BlaMAb reduced the efficacy of the imipenem-rifabutin combination using a low dose of imipenem (above), we tested whether inhibition of the β-lactamase by avibactam at 16 μg/ml could improve the intramacrophage activity of the combination (Fig. 3b and statistical analysis in Table S6). For these experiments, we used low doses of rifabutin (1 and 8 μg/ml) since these concentrations are achievable in the plasma (21) and in lung tissue (22), respectively. For the lowest concentration of rifabutin (1 μg/ml), the combination with imipenem (8 μg/ml) led to 0.5-fold change in the number of CFU. This fold change is similar to that observed for imipenem (8 μg/ml) combined with avibactam (16 μg/ml). Increasing the concentration of rifabutin from 1 μg/ml to 8 μg/ml did not improve the activity of the imipenem-rifabutin combination (1.5- versus 0.6-fold change in CFU, respectively; P = 0.65). The triple combinations comprising rifabutin (1 or 8 μg/ml), imipenem (8 μg/ml), and avibactam (16 μg/ml) were the more active regimens, leading to a 0.22- or 0.33-fold change in the number of CFU, respectively. However, the addition of avibactam did not significantly increase the activity of the imipenem-rifabutin combinations.
DISCUSSION
Since rifabutin has recently been reported to display promising in vitro activity against M. abscessus (20), we investigated the in vitro and intramacrophage activity of a combination comprising this drug and imipenem, which is the recommended β-lactam for the treatment of pulmonary infections in cystic fibrosis patients (10). Drugs were tested at 4-fold the MIC and at therapeutically achievable concentrations. The impact of imipenem hydrolysis by the β-lactamase BlaMAb on the activity of the combinations was evaluated based on the use of the β-lactamase inhibitor avibactam and a mutant lacking BlaMAb.
In vitro, the combination of imipenem and rifabutin was moderately synergistic (Fig. 1) and moderately active in the time-kill curve assay (Fig. 2). Comparison of M. abscessus CIP104536 and its BlaMAb-deficient derivative revealed that production of BlaMAb had a minor impact on the activity of imipenem only when the drug was tested alone. In agreement with this finding, avibactam increased the activity of imipenem alone but not that of the rifabutin combination.
In macrophages, the rifabutin-imipenem combination was significantly more active than either drug tested alone (Fig. 3). This was observed for rifabutin used at 4-fold the MIC (16 μg/ml) (Fig. 3a) as well as at concentrations achievable in the lung (8 μg/ml) and in serum (1 μg/ml) (Fig. 3b) (21, 22). A 9-fold intracellular accumulation of rifabutin has been reported in human polymorphonuclear leukocytes (23). Thus, intramacrophage accumulation may account for the observed activity of rifabutin in our study at doses similar to or lower than the MIC (4 μg/ml).
Comparisons of M. abscessus CIP104536 and its ΔblaMAb derivative in the macrophage model indicated that production of the β-lactamase impaired the activity of imipenem at 8 μg/ml and 32 μg/ml as well as the combination of rifabutin at 16 μg/ml and a low dose of imipenem (8 μg/ml). The impact of production of the β-lactamase, therefore, appears to be more important in macrophages than in vitro. This may be accounted for by a difference in the levels of production of the β-lactamase since we have recently reported that the blaMAb transcript was 20-fold less abundant in M. abscessus proliferating in planktonic cultures than in infected macrophages (19). This prompted us to determine whether inhibition of BlaMAb by avibactam could improve the intracellular killing activity of imipenem. This was the case for imipenem alone tested at 8 μg/ml. Although the triple combinations of avibactam (16 μg/ml), imipenem (8 μg/ml), and rifabutin (1 and 8 μg/ml) were the most active, the extents of killing were not statistically improved by the addition of avibactam.
Our results raise the possibility of replacing amikacin by rifabutin in the recommended treatment of lung infections due to M. abscessus. The gains from this replacement include the low toxicity and oral administration of rifabutin. In addition, imipenem combined with amikacin, as evaluated in a previous study in the same macrophage model (18, 19), was less active than the imipenem-rifabutin combination. For example, amikacin at 8 μg/ml combined with imipenem at 8 μg/ml led to a 5.0-fold change (19), in contrast to the 0.6-fold change for rifabutin (8 μg/ml) combined with imipenem (8 μg/ml) (this study).
In conclusion, the assessment of the efficacy of drug combinations in macrophages indicates that the combination of imipenem and rifabutin should be clinically evaluated, particularly in infections due to macrolide-resistant M. abscessus, which are often not cured by the recommended treatments (9, 24–26).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
M. abscessus CIP104536 (ATCC 19977) with a smooth (S) phenotype and its β-lactamase-deficient derivative (ΔblaMAb strain) (12) were grown in Middlebrook 7H9 broth (BD-Difco, Le Pont de Claix, France) supplemented with 10% (vol/vol) oleic acid, albumin, dextrose, and catalase (OADC; BD-Difco) and 0.05% (vol/vol) Tween 80 (Sigma-Aldrich) (7H9sB) at 30°C with shaking (150 rpm) (19).
Antibiotics.
Imipenem, rifabutin, and avibactam were purchased from Mylan (Saint-Priest, France), Serb Laboratories (Paris, France), and Advanced ChemBlocks, Inc. (Burlingame, CA). Water was the solvent for preparing stock solutions for imipenem and avibactam. Rifabutin was solubilized in 96% ethanol. The stock solutions were freshly prepared for each experiment and filtered using a sterilized 0.22-μm-pore-size polycarbonate syringe filter (Millipore, Saint-Quentin-en-Yvelines, France).
MIC determination.
MICs were determined in 96-well round-bottom microplates using the microdilution method, except that resazurin (Sigma-Aldrich, Saint-Quentin Fallavier, France) was used to facilitate reading. Briefly, approximately 5 × 105 CFU/ml was inoculated into 7H9sB containing 2-fold dilutions of antibiotics (concentration range, 0.12 to 64 μg/ml). After 2 days of incubation at 30°C, 10 μl of a 0.05% (wt/vol) resazurin solution was added to each well (200 μl), and incubation was continued for 24 h at 30°C. The MIC was defined as the lowest drug concentration preventing the resazurin color change from blue to pink or violet. The experiments were performed in quintuplet. Data are the medians from five experiments.
Synergy testing.
Synergic activities were tested by the two-dimensional dilution checkerboard method. Briefly a total of 80 μl of 7H9sB was distributed into each well of the microdilution plates. Ten microliters of imipenem was serially diluted along the abscissa, while 10 μl of rifabutin was serially diluted along the ordinate. Each well was inoculated with 100 μl of a bacterial inoculum containing 5 × 105 CFU/ml. The plates were incubated at 30°C for 3 days. All experiments were performed in quintuplet. For all of the wells that corresponded to a MIC, ∑FIC was calculated with the equation ∑FIC = FICA + FICB = (CA/MICA) + (CB/MICB), where MICA and MICB are the MICs of drugs A and B alone, and CA and CB are the concentrations of the drugs in combination. The smallest ∑FIC determined for the association is considered the FIC index of the combination. A combination with an FIC index of ≤0.5 is considered synergistic, an FIC index between 0.5 and 4 is considered indifferent, and an FIC index of >4 indicated an antagonist association, according to the American Society for Microbiology.
Time-kill assays.
Bottles of 20 ml of 7H9sB containing imipenem (8 or 32 μg/ml), rifabutin (16 μg/ml), and avibactam (4 μg/ml) alone or in combination were inoculated with exponentially growing bacteria of M. abscessus CIP104536 S or its ΔblaMAb derivative strain (5 × 106 CFU/ml) and incubated with shaking (150 rpm) at 30°C for 72 h. Imipenem (8 or 32 μg/ml) was added every 24 h due to its instability. Bacteria were enumerated at 0, 48, and 72 h by plating serial dilutions prepared in sterile water on lysogeny broth (LB) plates. Plates were incubated for 4 days at 30°C. The detection limit was 2 log10 CFU/ml. The experiments were performed in triplicate.
Activity of rifabutin alone or in combination with imipenem and avibactam in THP-1 macrophages.
The activity of antibiotics was studied on a THP-1 macrophage infection model as previously described (19). Briefly, THP-1 cells were seeded into 24-well plates (5 × 105 cells per 1-ml well), differentiated for 24 h, and infected with M. abscessus CIP104536 S or its ΔblaMAb derivative at a multiplicity of infection of 10 for 3 h. After three washes in phosphate-buffered saline (PBS), imipenem (8 and 32 μg/ml), rifabutin (1, 8, and 16 μg/ml), and avibactam (16 μg/ml), alone or in combination, were added to each well. Plates were incubated with 5% CO2 at 37°C for 2 days. For imipenem, drug was added every 24 h due to its instability. After three washes in PBS, macrophages were lysed with deionized water. Dilutions were plated onto LB agar plates, and CFU were enumerated after 4 days of incubation at 30°C. The experiments were performed in triplicate.
Statistical analysis.
A Mann-Whitney U test was used to compare the intracellular activity of antibiotics. All statistical analyses were performed with EPI Info software, version 7.1.3 (Centers for Disease Control and Prevention, Atlanta, GA).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Vaincre la Mucoviscidose and l'Association Grégory Lemarchal (RF20160501637 to J.-L.M.).
J.-L.M. has received consulting fees (scientific advisor for ceftaroline) and reimbursement of travel expenses (attendance at the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, 2014, and attendance at the 27th European Congress of Clinical Microbiology and Infectious Diseases, 2017) from AstraZeneca and Merck Sharp and Dohme. Other authors have no conflicts of interest to report.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00623-18.
REFERENCES
- 1.Sermet-Gaudelus I, Le Bourgeois M, Pierre-Audigier C, Offredo C, Guillemot D, Halley S, Akoua-Koffi C, Vincent V, Sivadon-Tardy V, Ferroni A, Berche P, Scheinmann P, Lenoir G, Gaillard JL. 2003. Mycobacterium abscessus and children with cystic fibrosis. Emerg Infect Dis 9:1587–1591. doi: 10.3201/eid0912.020774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esther CR Jr, Esserman DA, Gilligan P, Kerr A, Noone PG. 2010. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J Cyst Fibros 9:117–123. doi: 10.1016/j.jcf.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olivier KN, Weber DJ, Wallace RJ Jr, Faiz AR, Lee JH, Zhang Y, Brown-Elliot BA, Handler A, Wilson RW, Schechter MS, Edwards LJ, Chakraborti S, Knowles MR. 2003. Nontuberculous mycobacteria. I: multicenter prevalence study in cystic fibrosis. Am J Respir Crit Care Med 167:828–834. doi: 10.1164/rccm.200207-678OC. [DOI] [PubMed] [Google Scholar]
- 4.Levy I, Grisaru-Soen G, Lerner-Geva L, Kerem E, Blau H, Bentur L, Aviram M, Rivlin J, Picard E, Lavy A, Yahav Y, Rahav G. 2008. Multicenter cross-sectional study of nontuberculous mycobacterial infections among cystic fibrosis patients, Israel. Emerg Infect Dis 14:378–384. doi: 10.3201/eid1403.061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roux AL, Catherinot E, Ripoll F, Soismier N, Macheras E, Ravilly S, Bellis G, Vibet MA, Le Roux E, Lemonnier L, Gutierrez C, Vincent V, Fauroux B, Rottman M, Guillemot D, Gaillard JL. 2009. Multicenter study of prevalence of nontuberculous mycobacteria in patients with cystic fibrosis in France. J Clin Microbiol 47:4124–4128. doi: 10.1128/JCM.01257-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qvist T, Gilljam M, Jonsson B, Taylor-Robinson D, Jensen-Fangel S, Wang M, Svahn A, Kotz K, Hansson L, Hollsing A, Hansen CR, Finstad PL, Pressler T, Hoiby N, Katzenstein TL, Scandinavian Cystic Fibrosis Study Consortium (SCFSC) . 2015. Epidemiology of nontuberculous mycobacteria among patients with cystic fibrosis in Scandinavia. J Cyst Fibros 14:46–52. doi: 10.1016/j.jcf.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bar-On O, Mussaffi H, Mei-Zahav M, Prais D, Steuer G, Stafler P, Hananya S, Blau H. 2015. Increasing nontuberculous mycobacteria infection in cystic fibrosis. J Cyst Fibros 14:53–62. doi: 10.1016/j.jcf.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Brown-Elliott BA, Nash KA, Wallace RJ Jr. 2012. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin Microbiol Rev 25:545–582. doi: 10.1128/CMR.05030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novosad SA, Beekmann SE, Polgreen PM, Mackey K, Winthrop KL, Team MaS. 2016. Treatment of Mycobacterium abscessus Infection. Emerg Infect Dis 22:511–514. doi: 10.3201/eid2203.150828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Floto RA, Olivier KN, Saiman L, Daley CL, Herrmann JL, Nick JA, Noone PG, Bilton D, Corris P, Gibson RL, Hempstead SE, Koetz K, Sabadosa KA, Sermet-Gaudelus I, Smyth AR, van Ingen J, Wallace RJ, Winthrop KL, Marshall BC, Haworth CS. 2016. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax 71(Suppl 1):i1–i22. doi: 10.1136/thoraxjnl-2015-207360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bastian S, Veziris N, Roux AL, Brossier F, Gaillard JL, Jarlier V, Cambau E. 2011. Assessment of clarithromycin susceptibility in strains belonging to the Mycobacterium abscessus group by erm(41) and rrl sequencing. Antimicrob Agents Chemother 55:775–781. doi: 10.1128/AAC.00861-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubee V, Bernut A, Cortes M, Lesne T, Dorchene D, Lefebvre AL, Hugonnet JE, Gutmann L, Mainardi JL, Herrmann JL, Gaillard JL, Kremer L, Arthur M. 2015. β-Lactamase inhibition by avibactam in Mycobacterium abscessus. J Antimicrob Chemother 70:1051–1058. doi: 10.1093/jac/dku510. [DOI] [PubMed] [Google Scholar]
- 13.Soroka D, Dubee V, Soulier-Escrihuela O, Cuinet G, Hugonnet JE, Gutmann L, Mainardi JL, Arthur M. 2014. Characterization of broad-spectrum Mycobacterium abscessus class A beta-lactamase. J Antimicrob Chemother 69:691–696. doi: 10.1093/jac/dkt410. [DOI] [PubMed] [Google Scholar]
- 14.Soroka D, Ourghanlian C, Compain F, Fichini M, Dubée V, Mainardi J, Hugonnet J, Arthur M. 2017. Inhibition of β-lactamases of mycobacteria by avibactam and clavulanate. J Antimicrob Chemother 72:1081–1088. doi: 10.1093/jac/dkw546. [DOI] [PubMed] [Google Scholar]
- 15.Falcone M, Paterson D. 2016. Spotlight on ceftazidime/avibactam: a new option for MDR Gram-negative infections. J Antimicrob Chemother 71:2713–2722. doi: 10.1093/jac/dkw239. [DOI] [PubMed] [Google Scholar]
- 16.Bush K. 2015. A resurgence of beta-lactamase inhibitor combinations effective against multidrug-resistant Gram-negative pathogens. Int J Antimicrob Agents 46:483–493. doi: 10.1016/j.ijantimicag.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Papp-Wallace KM, Bonomo RA. 2016. New beta-lactamase inhibitors in the clinic. Infect Dis Clin North Am 30:441–464. doi: 10.1016/j.idc.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lefebvre AL, Dubee V, Cortes M, Dorchêne D, Arthur M, Mainardi J-L. 2016. Bactericidal and intracellular activity of β-lactams against Mycobacterium abscessus. J Antimicrob Chemother 71:1556–1563. doi: 10.1093/jac/dkw022. [DOI] [PubMed] [Google Scholar]
- 19.Lefebvre AL, Le Moigne V, Bernut A, Veckerle C, Compain F, Herrmann JL, Kremer L, Arthur M, Mainardi JL. 2017. Inhibition of the β-lactamase BlaMab by avibactam improves the in vitro and in vivo efficacy of imipenem against Mycobacterium abscessus. Antimicrob Agents Chemother 61:e02440-16. doi: 10.1128/AAC.02440-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aziz DB, Low JL, Wu ML, Gengenbacher M, Teo JWP, Dartois V, Dick T. 2017. Rifabutin is active against Mycobacterium abscessus complex. Antimicrob Agents Chemother 61:e00155-17. doi: 10.1128/AAC.00155-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Ingen J, Egelund EF, Levin A, Totten SE, Boeree MJ, Mouton JW, Aarnoutse RE, Heifets LB, Peloquin CA, Daley CL. 2012. The pharmacokinetics and pharmacodynamics of pulmonary Mycobacterium avium complex disease treatment. Am J Respir Crit Care Med 186:559–565. doi: 10.1164/rccm.201204-0682OC. [DOI] [PubMed] [Google Scholar]
- 22.Blaschke TF, Skinner MH. 1996. The clinical pharmacokinetics of rifabutin. Clin Infect Dis 22(Suppl 1):S15–S21. doi: 10.1093/clinids/22.Supplement_1.S15. [DOI] [PubMed] [Google Scholar]
- 23.Van der Auwera P, Matsumoto T, Husson M. 1988. Intraphagocytic penetration of antibiotics. J Antimicrob Chemother 22:185–192. doi: 10.1093/jac/22.2.185. [DOI] [PubMed] [Google Scholar]
- 24.Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, Park YK, Kim CK, Shin SJ, Huitt GA, Daley CL, Kwon OJ. 2011. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med 183:405–410. doi: 10.1164/rccm.201003-0395OC. [DOI] [PubMed] [Google Scholar]
- 25.Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. 2011. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis 52:565–571. doi: 10.1093/cid/ciq237. [DOI] [PubMed] [Google Scholar]
- 26.Harada T, Akiyama Y, Kurashima A, Nagai H, Tsuyuguchi K, Fujii T, Yano S, Shigeto E, Kuraoka T, Kajiki A, Kobashi Y, Kokubu F, Sato A, Yoshida S, Iwamoto T, Saito H. 2012. Clinical and microbiological differences between Mycobacterium abscessus and Mycobacterium massiliense lung diseases. J Clin Microbiol 50:3556–3561. doi: 10.1128/JCM.01175-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.