Abstract
Objectives
The randomized Phase IIIb/IV EXTEND trial showed that extended-pulsed fidaxomicin significantly improved sustained clinical cure and reduced recurrence versus vancomycin in patients ≥60 years old with Clostridium difficile infection (CDI). Cost-effectiveness of extended-pulsed fidaxomicin versus vancomycin as first-line therapy for CDI was evaluated in this patient population.
Methods
Clinical results from EXTEND and inputs from published sources were used in a semi-Markov treatment-sequence model with nine health states and a 1 year time horizon to assess costs and QALYs. The model was based on a healthcare system perspective (NHS and Personal Social Services) in England. Sensitivity analyses were performed.
Results
Patients receiving first-line extended-pulsed fidaxomicin treatment had a 0.02 QALY gain compared with first-line vancomycin (0.6267 versus 0.6038 QALYs/patient). While total drug acquisition costs were higher for extended-pulsed fidaxomicin than for vancomycin when used first-line (£1356 versus £260/patient), these were offset by lower total hospitalization costs (which also included treatment monitoring and community care costs; £10 815 versus £11 459/patient) and lower costs of managing adverse events (£694 versus £1199/patient), reflecting the lower incidence of CDI recurrence and adverse events with extended-pulsed fidaxomicin. Extended-pulsed fidaxomicin cost £53 less per patient than vancomycin over 1 year. The probability that first-line extended-pulsed fidaxomicin was cost-effective at a willingness-to-pay threshold of £30 000/QALY was 76% in these patients.
Conclusions
While fidaxomicin acquisition costs are higher than those of vancomycin, the observed reduced recurrence rate with extended-pulsed fidaxomicin makes it a more effective and less costly treatment strategy than vancomycin for first-line treatment of CDI in older patients.
Introduction
Clostridium difficile is a leading cause of healthcare-associated infection, with a spectrum of illness ranging from mild self-limiting diarrhoea to severe potentially fatal outcomes.1 Approximately 12 800 cases of C. difficile infection (CDI) were reported in England in 2016/17,2 with a 30 day all-cause mortality rate of 15.1%.3 Known risk factors for CDI are prior exposure to antibiotics, hospitalization, comorbidities and older age;1 advanced age is also a risk factor for severe disease4 and poorer CDI outcomes.5
The CDI burden is considerable due to associated morbidity, hospital readmissions and sometimes the requirement for surgery.6 Hospitalized patients require isolation and environmental decontamination.7 In the UK, CDI episodes are associated with ∼7–16 days of additional hospitalization8–10 and total incremental costs of £6986 per case (2010 values).8 The reported mean length of stay for patients with hospital-acquired CDI in the UK is 37–47 days, versus 7–8 days for patients without hospital-acquired CDI.9,10 Reporting of CDI cases is mandatory in England.11
Fidaxomicin is a narrow-spectrum oral macrocyclic antibiotic for CDI treatment. Two randomized, double-blind, Phase III trials demonstrated fidaxomicin (200 mg twice daily for 10 days) was non-inferior to vancomycin.12,13 Furthermore, fidaxomicin significantly reduced the CDI recurrence rate12,13 and improved sustained clinical cure rates.12 A validated CDI-simulating in vitro human gut model suggests an extended-pulsed fidaxomicin (EPFX) regimen may enhance suppression of C. difficile, spare gut commensal microbiota and facilitate its recovery.14 The clinical relevance of this was tested in EXTEND, a randomized trial comparing a 25 day EPFX regimen with standard 10 day oral vancomycin in patients ≥60 years old with CDI.15 EPFX significantly increased rates of sustained clinical cure at 30 days after end of treatment (EOT) versus vancomycin (difference 10.8%; 95% CI 1.0%–20.7%; P = 0.030), the primary study endpoint, and significantly reduced CDI recurrence rates.15
The higher acquisition cost of fidaxomicin compared with vancomycin prompted cost-effectiveness studies, and a recent systematic review suggested that fidaxomicin was cost-effective versus vancomycin in 79% (11/14) of published studies.16 With EPFX demonstrating enhanced clinical benefits without additional expense compared with vancomycin, we hypothesized that cost-effectiveness would be further improved. We developed a model to evaluate cost-effectiveness of EPFX versus vancomycin as first-line therapy for CDI, based on data from EXTEND and conducted from the healthcare system perspective of the NHS and Personal Social Services (PSS) in England.
Methods
EXTEND study
The cost-effectiveness model was developed to accommodate the clinical data from the EXTEND study, the primary findings of which have been previously reported.15 Briefly, EXTEND, a Phase IIIb/IV, randomized, controlled, open-label study compared EPFX with vancomycin in hospitalized patients ≥60 years old with confirmed CDI. Patients were randomly allocated (1:1) to receive either fidaxomicin (200 mg twice daily on Days 1–5, then once daily on alternate days from Days 7–25) or vancomycin (125 mg four times daily on Days 1–10) stratified by age, baseline CDI severity, number of previous recurrences and presence/absence of cancer. The study was registered (ClinicalTrials.gov identifier, NCT02254967).
EXTEND evaluated one treatment course for CDI, following outcomes during a 90 day period.15 Clinical response and test of cure (TOC) were evaluated 2 days after EOT (Day 12 for vancomycin, Day 27 for fidaxomicin). For patients with clinical response at TOC, CDI recurrence was assessed up to Day 90. Sustained clinical cure was defined as clinical response at TOC with no CDI recurrence. All other cases were deemed treatment failures. The primary efficacy endpoint was sustained clinical cure at 30 days after EOT (Day 40, vancomycin; Day 55, fidaxomicin).
Ethics
All data analyses conducted during this research were secondary; ethics approval was previously obtained for EXTEND. Data were anonymized prior to inclusion in the model.
Model design
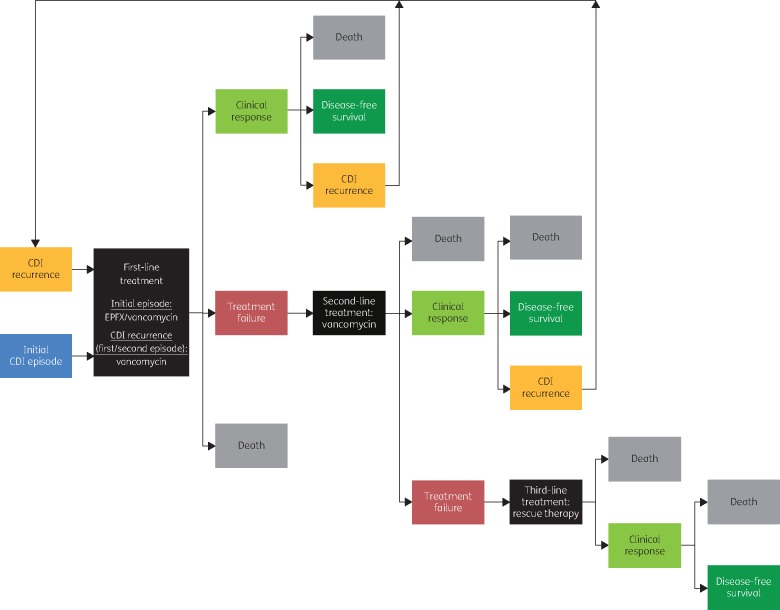
To evaluate cost-effectiveness of CDI therapy, it is necessary to consider the initial episode and subsequent treatments for CDI recurrence. A cohort-based, semi-Markov treatment-sequence model based on EXTEND data was developed in ExcelTM 2010 (Microsoft Corp., Redmond, WA, USA), evaluating up to three treatment courses. The model assesses outcomes every 5 days during a 365 day period. An analysis using the model was conducted from the healthcare system perspective of a third-party payer (NHS and PSS in England) and considered direct medical costs only. Figure 1 shows a schematic representation of the clinical pathway in the model.
Figure 1.
Overview of clinical pathway used in the semi-Markov model. Hypothetical patients entered the model in the ‘initial CDI episode’ health state and received either EPFX or vancomycin, with possible outcomes of ‘clinical response’, ‘treatment failure’, or ‘death’. Patients in the ‘clinical response’ state were considered to be at risk of CDI recurrence for ≤90 days after treatment initiation: up to two recurrence episodes were permitted. If a recurrence occurred, patients transitioned to the (first or second) ‘CDI recurrence’ state and received treatment, whereas if no recurrence occurred within 90 days, patients moved to a ‘disease-free survival’ state, where they either remained or moved to the ‘death’ state. Patients who had treatment failure initiated another course of therapy, with the same possible outcomes of ‘clinical response’, ‘treatment failure’, or ‘death’. Those failing the second course of therapy received a third course (rescue therapy) and transitioned either to ‘death’ or ‘clinical response’ followed by the ‘disease-free survival’ state after 90 days, as third-line therapy was assumed to provide 100% response with no risk of further recurrences to keep the model tractable. CDI, Clostridium difficile infection; EPFX, extended-pulsed fidaxomicin.
Markov models are a decision analytical model in which a disease is divided into mutually exclusive states and transition probabilities are assigned for movement between states over a discrete period of time (cycle).17 In a semi-Markov model, the risk of moving to the next health state depends on time spent in the current health state. Health outcomes and cost data are ascribed to the health states, enabling outcome and cost estimations over a predetermined number of cycles associated with a particular intervention.17 Weights in the form of utilities, which represent quality of life on a standard scale of 0 (dead) to 1 (full health), are ascribed to the model states allowing QALYs to be estimated.17
The model comprises nine health states describing the CDI episode, clinical outcome of treatment and treatment line [initial CDI episode (first-, second-, third-line), clinical response, CDI recurrence (first-, second-, third-line), disease-free survival, death; Figure 1]. In the base-case analysis, it is assumed that the initial CDI episode was treated with either fidaxomicin or vancomycin using the EXTEND regimens. Vancomycin was the assumed second-line treatment, if initial treatment with fidaxomicin failed, and first- and second-line treatment for all CDI recurrences. Patients were assumed to receive a full course of every treatment. Other model assumptions are summarized in Table S1 (available as Supplementary data at JAC Online).
Model inputs
Clinical
Two efficacy outcomes from EXTEND were used in the analysis: clinical response and CDI recurrence. Clinical response, assessed 2 days after EOT, was taken directly from the EXTEND findings and evaluated in the modified full analysis set—the primary analysis set for efficacy analyses. CDI recurrence rates (at Days 40, 55 and 90) were obtained from the subgroup of patients in the modified full analysis set who achieved a clinical response 2 days after EOT. The rates of clinical response or CDI recurrence for vancomycin were utilized directly; for fidaxomicin, risks relative to vancomycin were derived and applied. The probabilities of CDI recurrence were transformed to a 5 day probability to reflect the cycle length of the model (see Supplementary data); the probability of clinical cure was applied at EOT.
Two safety outcomes from EXTEND were utilized in the analysis: incidence of all-grade treatment-emergent adverse events (AEs) reported in ≥5% of patients, and all-cause mortality rates up to 90 days post-randomization, which included CDI-attributable deaths (Table 1 and Table S2). For disease-free patients beyond Day 90, mortality was assumed to be 0%. This assumption was made based on the short time horizon (<1 year) and because the risk of mortality for disease-free patients beyond Day 90 was expected to be equal between the cohorts.
Table 1.
Model inputs
| Parameter | Value | Reference(s) | |
|---|---|---|---|
| Clinical inputs | EPFX | Vancomycin | |
| Clinical response 2 days after EOT (%) | 78.0 | 82.1 | Guery et al. 201715 |
| RR (95% CI)a | 0.95 (0.86‒1.05) | derived from Guery et al. 201715 | |
| Recurrence at Day 40 (%) | 1.4* | 19.7 | derived from Guery et al. 201715 |
| RR (95% CI)a | 0.07 (0.02–0.30) | calculated | |
| Recurrence at Day 55 (%) | 4.3* | 21.1 | derived from Guery et al. 201715 |
| RR (95% CI)a | 0.21 (0.09–0.48) | calculated | |
| Recurrence at Day 90 (%) | 7.2* | 22.4 | derived from Guery et al. 201715 |
| RR (95% CI)a | 0.32 (0.17–0.63) | calculated | |
| AEs (all-grade), (%)b | |||
| anaemia | 2.8 | 5.5 | Guery et al. 201715 |
| cardiac failure | 2.2 | 5.5 | Guery et al. 201715 |
| clostridial infectionc | 3.9 | 13.3 | Guery et al. 201715 |
| constipation | 5.5 | 2.8 | Guery et al. 201715 |
| diarrhoea | 5.5 | 6.6 | Guery et al. 201715 |
| pneumonia | 2.8 | 5.5 | Guery et al. 201715 |
| pyrexia | 3.9 | 6.6 | Guery et al. 201715 |
| sepsis | 0.6 | 5.0 | Guery et al. 201715 |
| urinary tract infection | 3.3 | 6.6 | Guery et al. 201715 |
| Deaths, n (%) | |||
| Days 1–12 | 12 (3.3) | Guery et al. 201715 | |
| Days 13–27 | 13 (3.6) | Guery et al. 201715 | |
| Days 28–90 | 40 (11.0) | Guery et al. 201715 | |
| Probability of mortality (%) | |||
| Days 0 to <10 | 1.39 | see Table S2 | |
| Days 10 to <15 | 1.28 | see Table S2 | |
| Days 15 to <25 | 1.20 | see Table S2 | |
| Days 25 to <30 | 1.03 | see Table S2 | |
| Days 30 to <90 | 0.92 | see Table S2 | |
| Days 90+ | 0 | see Table S2 | |
| Costs (£) | Fidaxomicind | Vancomycine | |
| Drug acquisition | |||
| per pack | 1350.00 | 132.47 | BNF 201625 |
| per course | 1350.00 | 189.24 | BNF 201625 |
| Hospitalization for CDI episode | |||
| per 10 day admittance | 8214.00 | DoH 201226; ONS 201624 | |
| rescue treatment | 4107.00 | assumption | |
| AEsf | |||
| anaemia | 46.35 | ONS 201624; Curtis & Burns 201527 | |
| cardiac failure | 7305.97 | ONS 201624; NICE 201536 | |
| clostridial infection | 0 | assumption | |
| constipation | 1414.96 | DoH 201526; ONS 201624 | |
| diarrhoea | 1414.96 | DoH 201526; ONS 201624 | |
| pneumonia | 1992.84 | DoH 201526; ONS 201624 | |
| pyrexia | 1026.66 | DoH 201526; ONS 201624 | |
| sepsis | 2215.78 | DoH 201526; ONS 201624 | |
| urinary tract infection | 0 | assumption | |
| Utilities | |||
| Health state utilities | |||
| CDI initial episodeg | 0.33 | derived from Slobogean et al. 201018 | |
| clinical failure (first recurrence)g | 0.30 | derived from Slobogean et al. 201018 | |
| clinical failure (second recurrence)g | 0.27 | derived from Slobogean et al. 201018 | |
| clinical response (initial episode) | 0.78 | derived from Slobogean et al. 201018 | |
| clinical response (recurrence) | 0.56 | derived from Slobogean et al. 201018 | |
| disease-free | 0.78 | derived from Slobogean et al. 201018 | |
| AE decrements | |||
| anaemia | −0.081 | NICE 201020 | |
| cardiac failure | −0.108 | NICE 201522 | |
| clostridial infection | 0 | assumption | |
| constipation | −0.007 | NICE 201020 | |
| diarrhoea | −0.007 | NICE 201020 | |
| pneumonia | −0.008 | Marti et al. 201323 | |
| pyrexia | −0.001 | NICE 201020 | |
| sepsis | −0.171 | NICE 201521 | |
| urinary tract infection | −0.00282 | NICE 201522 | |
BNF, British National Formulary; CDI, Clostridium difficile infection; DoH, Department of Health; EOT, end of treatment; EPFX, extended-pulsed fidaxomicin; RR, relative risk.
Asterisks indicate P<0.001 according to Cochran–Mantel–Haenszel test adjusted for stratificiation factors.
EPFX versus vancomycin.
Treatment-emergent AEs reported in ≥5% of patients in either the EPFX or vancomycin arm.
All CDI recurrences.
A pack includes 20 × 200 mg tablets and a course requires 20 × 200 mg tablets.
A pack includes 28 × 125 mg capsules and a course requires 40 × 125 mg capsules.
Anaemia, assumed to be the cost of one general practitioner visit; clostridial infections, assumed to be captured in the hospitalization costs; constipation, sum of weighted NHS reference costs for gastrointestinal infections (currency codes, FZ36G, FZ36H, FZ36J, FZ36K, FZ36L, FZ36M, FZ36N, FZ36P, FZ36Q); diarrhoea, assumed to be the same as for constipation; pneumonia, sum of weighted NHS reference costs for lobar, atypical or viral pneumonia (currency codes, DZ11K–V); pyrexia, sum of weighted NHS reference costs for fever of unknown origin (currency codes, WJ07A–D); sepsis, sum of weighted NHS reference costs for sepsis (currency codes, WJ06A–H, and WJ06J); urinary tract infection, assumed to be captured in the hospitalization costs.
Utility value applies for first-, second- and third-line therapies.
All clinical inputs were assumed to be the same for the first, second and third treatment courses.
Utilities
Health-related quality-of-life weights (or utilities) on a scale of 0 (dead) to 1 (full health) were ascribed to each health state in the model; utility decrements were applied on treatment initiation to account for the impact of AEs. Health utilities for CDI were derived from a previously published cost-effectiveness study.18 A (weighted) value of 0.33 was applied to the ‘CDI initial episode’ state according to published data.18,19 For first and second recurrence, a progressive 10% decrease in the utility values from the initial CDI episode (i.e. 0.30 and 0.27, respectively) was assumed. The utilities for ‘clinical response’ after the initial CDI episode and the ‘disease-free’ state were assumed to be the same (0.78); a utility of 0.56 (i.e. midpoint of the utilities for an initial CDI episode and clinical response) was assumed for clinical response following a CDI recurrence.
Reductions in utilities (disutilities) were applied to capture the impact of AEs on quality of life (Table 1).20–23 AE-related reductions were applied at the start of the analysis to the proportion of patients who experienced an AE to ensure that the full impact of events was captured.
Costs
The analysis considered only direct medical costs (i.e. drug acquisition, hospitalization and AE management). These were used as the total cost of CDI treatment, applied to all patients, and assumed to be a proxy for hospitalization costs. Costs were expressed in British pounds (£) and inflated to 2016 values using the consumer price index for health, where applicable.24 There was no discounting of costs or outcomes as the time horizon was 1 year.
The drug acquisition cost for the initial course of fidaxomicin was £1350.00,25 equivalent to one pack of 20 × 200 mg tablets [sufficient for the standard (200 mg twice daily for 10 days) or the extended-pulsed regimen]. The acquisition cost for each vancomycin course (for initial CDI or CDI recurrence) was £189.24, based on a pack (28 × 125 mg capsules) cost of £132.47 (a course requires 40 × 125 mg capsules).25
Hospitalization costs for the initial CDI episode were based on the estimated cost of treating a CDI episode in the UK (£8214 per 10 day admittance)26 and were assumed to be the same regardless of treatment with fidaxomicin or vancomycin (i.e. patients receiving fidaxomicin were discharged to continue treatment at home after a 10 day hospitalization period). Hospitalization costs for rescue treatment (hypothetical cure treatment) were assumed to be £4107 (half the cost for initial and recurrent episodes, applied in one cycle only). Treatment monitoring and community care costs were assumed to be fully captured within the hospitalization costs.
The costs of treating AEs were based on published estimates for grade 3/4 events or assumed if no published data were available (Table 1). For some AE costs, only the 2015 PSS Research Unit/NHS27 reference costs were available at the time of data sourcing. These costs were inflated to 2016 values using the consumer price index for health.24 AE-related costs were ascribed in the first cycle of the analysis. The cost of treating each AE was multiplied by the incidence, and the total cost for each treatment was the sum of the costs for all AEs.
Model outputs
Clinical model outputs were clinical response, disease-free status and mortality, although the assumptions used in the analysis ensured that both disease-free status and mortality reached the same level by the end of the time horizon, regardless of initial treatment. The impact on health outcomes (QALYs) and costs over the 1 year time horizon were estimated.
The incremental cost-effectiveness ratio (ICER), expressed as cost per QALY/patient, was calculated as follows:
A cost-effectiveness threshold range of £20 000–30 000/QALY, recommended by NICE,28 was used to interpret ICERs, i.e. values less than £30 000/QALY signified that first-line fidaxomicin was cost-effective compared with vancomycin.
Sensitivity analyses
The analysis had uncertainties because of underlying assumptions and model input variability; two types of sensitivity analysis were performed to appraise these uncertainties. A one-way (or deterministic) sensitivity analysis was performed, in which each variable was adjusted individually to observe its impact on model results. With the exception of drug acquisition costs, all parameters were varied by ±20% of the base-case value or by using 95% CI where available.
A probabilistic sensitivity analysis was performed in which predefined distributions for input parameters (Table S3) were tested by employing recurrent Monte Carlo simulations for 1000 iterations. The probabilistic sensitivity analysis estimated the probability of fidaxomicin being cost-effective at a threshold of £30 000/QALY. Results were presented as a cost-effectiveness acceptability curve.
Results
EXTEND study clinical findings
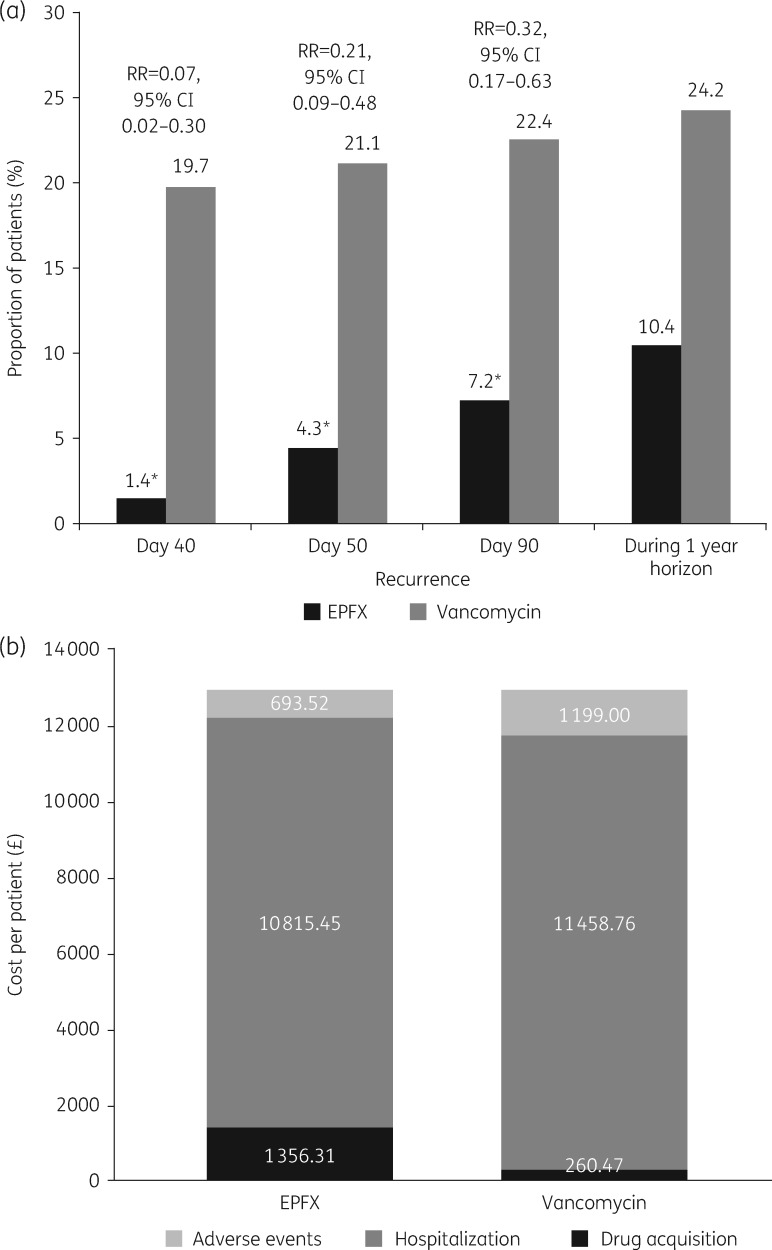
The clinical response rates at 2 days after EOT and recurrence rates at Days 40, 55 and 90 have been published previously (Table 1).15 Treatment with fidaxomicin relative to vancomycin reduced the risk of CDI recurrence by 93% at Day 40, 79% at Day 55 and 68% at Day 90 (Figure 2a). Applying the derived clinical response and recurrence data in the model over a time horizon of 1 year, the overall incidence of recurrent CDI episodes was 10.4% with first-line fidaxomicin compared with 24.2% with first-line vancomycin (Figure 2a). As anticipated, the overall proportions of patients who were either disease-free (83.0%) or who had died over 1 year (17.0%) were identical for both treatment arms because the analysis assumed that second- and third-line treatment courses used only vancomycin and that rescue therapy resolved all remaining CDI cases. Of the most common treatment-emergent AEs occurring in ≥5% of patients in any treatment group in the EXTEND study (Table 1), treatment-related serious AEs were reported in the vancomycin group: one event each of cardiac failure, Clostridium spp. infection and sepsis.15 Disaggregated data from the base-case analysis are summarized in Tables S4–S6.
Figure 2.
Model inputs and outputs relating to (a) Clostridium difficile infection recurrence and relative risk of recurrence and (b) costs (base-case analysis). EPFX, extended-pulsed fidaxomicin; RR, relative risk. An asterisk indicates P < 0.001 according to the Cochran–Mantel–Haenszel test adjusted for stratification factors.
Model findings
Health outcomes
Patients who received first-line fidaxomicin therapy had a QALY gain of 0.02 compared with first-line vancomycin (0.6267 versus 0.6038 QALYs/patient), resulting from more health-state QALYs (0.6400 versus 0.6332) and fewer AE-related disutilities (−0.0133 versus −0.0294) with fidaxomicin versus vancomycin, respectively.
Costs
Over a 1 year time horizon, total drug acquisition costs were more than five times higher when fidaxomicin was used as first-line therapy compared with vancomycin (£1356 versus £260/patient). However, these costs were offset by lower total hospitalization costs (£10 815 versus £11 459/patient) and lower costs of managing AEs (£694 versus £1199/patient) with fidaxomicin compared with vancomycin (Figure 2b).
Cost-effectiveness
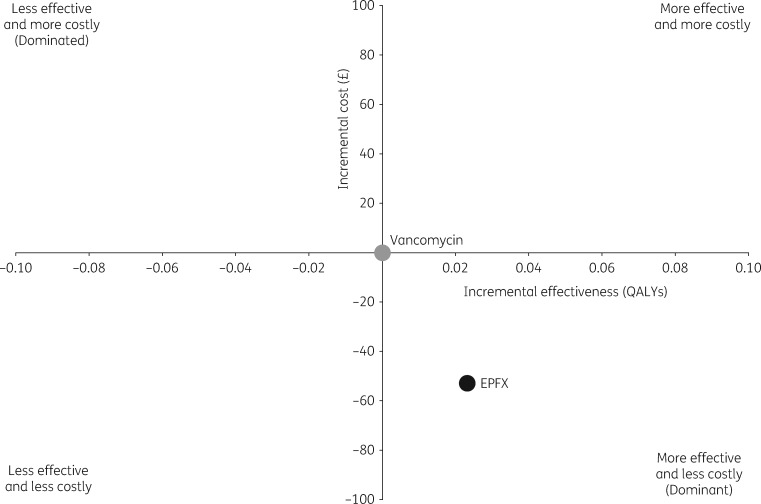
Overall, treatment of the initial CDI episode with fidaxomicin was associated with a cost-saving of £53/patient and a gain of 0.0229 QALYs/patient over 1 year compared with initial vancomycin treatment (Figure 3).
Figure 3.
Model cost-effectiveness plane (base-case analysis). A cost-effectiveness plane consists of four quadrants, where the x-axis represents the incremental level of effectiveness of a new intervention (first-line EPFX in the present model) and the y-axis represents the additional total cost of introducing the new intervention. Standard of care (first-line vancomycin in the current model) occupies the origin of the graph. Depending on the incremental cost-effectiveness ratio, the new intervention will be located to the right or left of the origin if it is more or less effective than standard of care and above or below the origin if it is more or less costly. When a new intervention is both clinically superior and cost saving, it is referred to as an economically ‘dominant’ strategy. The opposite is a ‘dominated’ strategy. EPFX, extended-pulsed fidaxomicin.
Sensitivity analyses
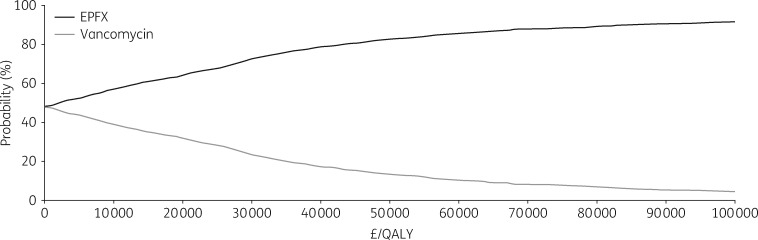
The one-way sensitivity analysis showed that the inputs with the greatest impact on the results were hospitalization costs and the probability of clinical response (Table 2). Reducing the estimated hospitalization cost during vancomycin treatment or increasing the cost during fidaxomicin treatment resulted in ICERs slightly above the willingness-to-pay threshold of £30 000/QALY (£32 833–£37 964). Reducing the estimated clinical response with fidaxomicin treatment also resulted in an ICER slightly above the willingness-to-pay threshold per QALY (£36 935). For all other parameters, fidaxomicin was either more effective and less costly (i.e. dominant) or cost-effective (i.e. ICER less than £30 000/QALY) compared with vancomycin. The probabilistic sensitivity analysis showed that first-line administration of fidaxomicin had a 76% probability of being a cost-effective treatment strategy compared with first-line vancomycin at a willingness-to-pay threshold of £30 000/QALY (Figure 4).
Table 2.
Model input data for hospitalization costs, clinical outcomes and utilities associated with EPFX and vancomycin treatment. Fidaxomicin was considered more effective and less costly (‘dominant’ or cost-effective) when ICER was less than £30 000 per QALY compared with vancomycin treatment
| ICERb (£/QALY) |
||
|---|---|---|
| Parametersa | low valuec | high valuec |
| Hospitalization costs | ||
| vancomycin: Days 0–5 | 37 964 | dominant |
| vancomycin: Days 5–10 | 33 878 | dominant |
| EPFX: Days 0–5 | dominant | 33 327 |
| EPFX: Days 5–10 | dominant | 32 833 |
| Clinical outcomes | ||
| EPFX: clinical response (RR versus vancomycin) | 36 935 | dominant |
| EPFX: recurrence at Day 90 (RR versus vancomycin) | dominant | 28 927 |
| Vancomycin: recurrence at Day 90 | 19 960 | dominant |
| Vancomycin: clinical response | 2577 | dominant |
| Vancomycin: recurrence at Day 40 | dominant | dominant |
| Vancomycin: recurrence at Day 55 | dominant | dominant |
| EPFX: recurrence at Day 55 (RR versus vancomycin) | dominant | dominant |
| EPFX: recurrence at Day 40 (RR versus vancomycin) | dominant | dominant |
| Utilities | ||
| initial episode: disease-free health state | dominant | dominant |
| first recurrence: disease-free health state | dominant | dominant |
| EPFX: initial episode, clinical response/Days 10–25 on treatment | dominant | dominant |
| EPFX: first recurrence, clinical response/Days 10–25 on treatment | dominant | dominant |
| second recurrence: disease-free health state | dominant | dominant |
EPFX, extended-pulsed fidaxomicin; ICER, incremental cost-effectiveness ratio; RR, relative risk.
Only parameters that were deemed to have a key impact on the ICER are shown (absolute change >£120).
A threshold of £30 000 per QALY was used to interpret ICERs; ‘dominant’ indicates that EPFX was more effective and less costly than vancomycin.
Parameters varied by ±20% of the base-case value or by using 95% CIs.
Figure 4.
Probabilistic sensitivity analysis: cost-effectiveness acceptability curve. EPFX, extended-pulsed fidaxomicin.
Discussion
The EXTEND study demonstrated that compared with standard vancomycin an EPFX regimen significantly improved sustained clinical cure rates of CDI in patients aged ≥60 years and significantly reduced CDI recurrence rates up to 90 days after starting treatment.15 Of particular note, the recurrence rate at 30 days in the fidaxomicin arm was lower than that reported in randomized controlled studies of fidaxomicin, vancomycin or metronidazole regimens.15 Our base-case analysis shows that over a 1 year period first-line fidaxomicin treatment is cost-effective and associated with health benefits compared with vancomycin in this older patient population.
The high acquisition cost of fidaxomicin is a known barrier to therapy.29,30 EPFX was hypothesized to enhance clinical benefits without increasing acquisition costs by extending the delivery of the standard (20 dose) regimen over 25 days. By applying health-economic modelling techniques, we were able to go beyond the confines of the EXTEND study trial design and consider the consequences of multiple treatment lines and CDI recurrence. Further, we were able to couple health outcomes with medical costs and capture the longer-term health-economic impact of CDI treatment. The base-case analysis from our model showed that fidaxomicin was more effective and less costly (dominant) compared with vancomycin as first-line therapy for the treatment of CDI. Over 1 year, a treatment strategy of first-line fidaxomicin therapy saved £53/patient in direct medical costs and had accompanying health benefits (i.e. 0.02 QALYs gained per patient). The higher acquisition cost of fidaxomicin was completely offset by savings in hospitalization costs and, to a lesser extent, the costs of managing AEs. The probability that first-line treatment with fidaxomicin was cost-effective at the NICE-specified willingness-to-pay threshold of £30 000/QALY was 76%. These findings suggest that when efficacy and safety outcomes from the EXTEND study are modelled over 1 year, fidaxomicin is cost-effective versus standard of care in the treatment of initial episodes of CDI and may have public health implications for the treatment of this at-risk population in England. Further, an additional assessment of the impact of hospitalization costs on our economic model (data not shown) determined that in the extreme scenario in which no hospitalization costs are included, first-line fidaxomicin remains a cost-effective treatment option.
The model structure and assumptions led to conservative estimates of the economic benefits of fidaxomicin versus vancomycin. The treatment sequence adopted in our base-case analysis considered EPFX or vancomycin for first-line therapy, with vancomycin as first- or second-line therapy for subsequent CDI episodes or recurrences. While this is supported by current European treatment guidelines for CDI,31 other recommended and relevant treatment options were not considered and this represents a recognized limitation of the model. Alternative treatments include metronidazole for first-line therapy of non-severe cases (64% of the EXTEND study population had non-severe CDI), faecal transplantation after multiple recurrences or standard fidaxomicin after first-line therapy.31 In our analysis, patients were also assumed to receive a full course of antibiotics before switching to another treatment option, whereas in clinical practice patients may change treatments if little or no response is observed after 7 days. Previous findings have shown higher associated costs for recurrent compared with initial CDI episodes.32 However, owing to the absence of data on recurrent CDI costs in the UK, our model assumed that initial and recurrent CDI episodes incurred the same hospitalization costs, which may underestimate the economic impact of fidaxomicin treatment. We do acknowledge that using hospitalization costs (which encompass drug acquisition, treatment care monitoring and community care costs) as a proxy for CDI recurrence costs may have resulted in some aspects being counted more than once. Additionally, data on the incidence of second recurrences were not available in the EXTEND study and this limitation likely led to conservative estimates of the impact of fidaxomicin treatment, as we assumed similar incidences of first and second recurrences in both treatment arms.
To our knowledge, this is the first cost-effectiveness analysis of EPFX in the treatment of CDI. Vancomycin was selected as the comparator as it is the standard of care for the initial treatment of CDI,31 although the standard fidaxomicin regimen would provide a relevant comparator and be of interest for future analyses. However, there are currently no direct comparisons of EPFX and standard fidaxomicin to support such an analysis. An indirect comparison (or network meta-analysis) of available studies, while possible, could not include comparative data on late recurrence because of the different follow-up durations in the Phase III trials (30–40 days versus 90 days in EXTEND).12,13,15 Of relevance are several cost-effectiveness analyses comparing standard fidaxomicin with vancomycin as first-line therapy for CDI,33,34 severe CDI35 or CDI in at-risk populations.19 These studies, which were performed using different models, reported favourable ICERs for standard fidaxomicin versus vancomycin according to local willingness-to-pay thresholds. Further, a systematic review identified that fidaxomicin was cost-effective compared with vancomycin in 79% of 14 published analyses.16 Because EPFX appears to be associated with even lower recurrence rates than standard fidaxomicin (30 day recurrence rates: EPFX, 4.0%;15 standard fidaxomicin, 12.7%12 and 15.4%13), the extended-pulsed regimen may offer health-economic benefits over and above those observed with the standard regimen.
A strength of our analysis was that all clinical inputs were derived from a large European multicentre, Phase IIIb/IV trial, although the generalizability of our findings to patients who fall outside the EXTEND study population, e.g. younger patients with fewer comorbidities, is currently unknown. In the absence of published utility data for patients with CDI, we used values from other populations with the underlying assumption that these data were representative of the CDI population and have been used in a previous cost-effectiveness analysis of fidaxomicin.19 However, quality-of-life data were collected as part of the EXTEND trial and, once available, will provide alternative health-state utility estimates. Our analysis did not consider the costs of severe CDI complications, e.g. colectomy, or costs relevant from a societal perspective, e.g. lost productivity. Through necessity, the analysis included several assumptions, some of which were made in the absence of clear-cut evidence on some parameters. Where possible, assumptions were conservative (e.g. EPFX or standard fidaxomicin were not assumed to be second-line therapy, all CDI episodes were treated in hospital, the costs of managing AEs were assumed to be those for grade 3/4 events, etc.), although we acknowledge that these inputs should be updated and validated as pertinent data become available.
In conclusion, this analysis suggests that a treatment strategy with EPFX as first-line therapy for CDI in older patients improves outcomes and saves costs compared with vancomycin. Taken together with the findings from the EXTEND study, these data provide strong clinical and economic evidence to support the use of EPFX in this at-risk patient population.
Supplementary Material
Acknowledgements
Nicholas Adomakoh, Andreas Karas and Chris Longshaw, all of Astellas Pharma, Inc., at the time of the clinical study, were instrumental in designing the EXTEND study.
Funding
This work was supported by Astellas Pharma, Inc., who funded PAREXEL Access Consulting to perform the analyses. Medical writing support, provided by Harriet Lamb, BSc (Hons) of Bioscript Medical, and Rhian Harper Owen, PhD for Cello Health MedErgy, was funded by Astellas Pharma, Inc. Simon Goldenberg is supported by the NIHR Collaboration for Leadership in Applied Health Research and Care South London at King's College Hospital NHS Foundation Trust.
Transparency declarations
O. A. C. has received research grants from Actelion, Aranis, Astellas, AstraZeneca, Basilea, Bayer, Cidara, Duke University, F2G, Gilead, GSK, Leeds University, MedPace, Melinta Therapeutics, Merck/MSD, Miltenyi, Pfizer, Rempex, Roche, Sanofi Pasteur, Scynexis, Seres Therapeutics and The Medicines Company; and personal fees from Actelion, Amplyx, Astellas, Basilea, Cidara, Da Volterra, F2G, Gilead, Janssen Pharmaceuticals, Matinas, Menarini Richerche, Merck/MSD, Paratek Pharmaceuticals, Scynexis, Seres Therapeutics, Summit, Vical and Vifor. M. W. was a full-time employee of Astellas Pharma, Inc., during the conduct of the study. E. D. is a full-time employee of Astellas Pharma, Inc. C. M. is an employee of PAREXEL Access Consulting, which received a consultancy fee from Astellas Pharma, Inc., to support the cost-effectiveness analysis. S. D. G. has received grants from Astellas Pharma Europe Ltd, and personal fees from Astellas Pharma Europe Ltd, Pfizer and MSD. The development of the manuscript, editing and submission assistance for this manuscript was provided by Harriet Lamb of Bioscript Medical and Rhian Harper Owen for Cello Health MedErgy.
Author contributions
O. A. C. was involved in the conception of the model, provided clinical model input and was involved in interpretation of the data. M. W. was involved in the conception and planning, analysis and interpretation of data, drafting and critical input to manuscript. C. M. developed and executed the model and was involved in the analysis and interpretation of the data. All authors had substantial input to the drafting and critical review of the manuscript, reviewed all versions and approved the final version prior to publication.
References
- 1. Smits WK, Lyras D, Lacy DB. et al. Clostridium difficile infection. Nat Rev Dis Primers 2016; 2: 16020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Public Health England. Clostridium difficile infection: annual data. https://www.gov.uk/government/statistics/clostridium-difficile-infection-annual-data.
- 3. Public Health England. Thirty-day all-cause fatality subsequent to MRSA, MSSA and E. coli bacteraemia and C. difficile infection, 2015/16. 2016. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/581282/HCAI_Fatality_report_2015_2016.pdf.
- 4. Shin JH, High KP, Warren CA.. Older is not wiser, immunologically speaking: effect of aging on host response to Clostridium difficile infections. J Gerontol A Biol Sci Med Sci 2016; 71: 916–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Louie TJ, Miller MA, Crook DW. et al. Effect of age on treatment outcomes in Clostridium difficile infection. J Am Geriatr Soc 2013; 61: 222–30. [DOI] [PubMed] [Google Scholar]
- 6. Kwon JH, Olsen MA, Dubberke ER.. The morbidity, mortality, and costs associated with Clostridium difficile infection. Infect Dis Clin North Am 2015; 29: 123–34. [DOI] [PubMed] [Google Scholar]
- 7. Caroff DA, Yokoe DS, Klompas M.. Evolving insights into the epidemiology and control of Clostridium difficile in hospitals. Clin Infect Dis 2017; 65: 1232–8. [DOI] [PubMed] [Google Scholar]
- 8. Wiegand PN, Nathwani D, Wilcox MH. et al. Clinical and economic burden of Clostridium difficile infection in Europe: a systematic review of healthcare-facility-acquired infection. J Hosp Infect 2012; 81: 1–14. [DOI] [PubMed] [Google Scholar]
- 9. Eckmann C, Wasserman M, Latif F. et al. Increased hospital length of stay attributable to Clostridium difficile infection in patients with four co-morbidities: an analysis of hospital episode statistics in four European countries. Eur J Health Econ 2013; 14: 835–46. [DOI] [PubMed] [Google Scholar]
- 10. van Kleef E, Green N, Goldenberg SD. et al. Excess length of stay and mortality due to Clostridium difficile infection: a multi-state modelling approach. J Hosp Infect 2014; 88: 213–7. [DOI] [PubMed] [Google Scholar]
- 11. Public Health England. Mandatory enhanced MRSA, MSSA and Escherichia coli bacteraemia, and Clostridium difficile infection surveillance. Protocol version 4.0. 2016. https://hcaidcs.phe.org.uk/ContentManagement/LinksAndAnnouncements/HCAIDCS_Mandatory_Surveillance_Protocol_v4.0.pdf? AspxAutoDetectCookieSupport=1.
- 12. Cornely OA, Crook DW, Esposito R. et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis 2012; 12: 281–9. [DOI] [PubMed] [Google Scholar]
- 13. Louie TJ, Miller MA, Mullane K. et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364: 422–31. [DOI] [PubMed] [Google Scholar]
- 14. Chilton CH, Crowther GS, Todhunter SL. et al. Efficacy of alternative fidaxomicin dosing regimens for treatment of simulated Clostridium difficile infection in an in vitro human gut model. J Antimicrob Chemother 2015; 70: 2598–607. [DOI] [PubMed] [Google Scholar]
- 15. Guery B, Menichetti F, Anttila V. et al. Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection in patients 60 years and older (EXTEND): a randomised, controlled, open-label, phase 3b/4 trial. Lancet Infect Dis 2017; 18: 296–307. [DOI] [PubMed] [Google Scholar]
- 16. Burton HE, Mitchell SA, Watt M.. A systematic literature review of economic evaluations of antibiotic treatments for Clostridium difficile infection. Pharmacoeconomics 2017; 35: 1123–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Briggs A, Sculpher M.. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics 1998; 13: 397–409. [DOI] [PubMed] [Google Scholar]
- 18. Slobogean GP, O’Brien PJ, Brauer CA.. Single-dose versus multiple-dose antibiotic prophylaxis for the surgical treatment of closed fractures. Acta Orthop 2010; 81: 256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Watt M, McCrea C, Johal S. et al. A cost-effectiveness and budget impact analysis of first-line fidaxomicin for patients with Clostridium difficile infection (CDI) in Germany. Infection 2016; 44: 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Institute for Health and Care Excellence. Single technology appraisal TA215. Pazopanib (Votrient®) for the first-line treatment of patients with advanced renal cell carcinoma. 2010. https://www.nice.org.uk/guidance/TA215/documents/renal-cell-carcinoma-first-line-metastatic-pazopanib-manufacturer-submission-submission2.
- 21. National Institute for Health and Care Excellence. Bortezomib for previously untreated mantle cell lymphoma. Technol Apprais Guid 2015. https://www.nice.org.uk/guidance/ta370/resources/bortezomib-for-previously-untreated-mantle-cell-lymphoma-pdf-82602782522053.
- 22. National Institute for Health and Care Excellence. Empagliflozin in combination therapy for treating type 2 diabetes. 2015. https://www.nice.org.uk/guidance/ta336/resources/empagliflozin-in-combination-therapy-for-treating-type2diabetes-82602550735045.
- 23. Martí SG, Colantonio L, Bardach A. et al. A cost-effectiveness analysis of a 10-valent pneumococcal conjugate vaccine in children in six Latin American countries. Cost Eff Resour Alloc 2013; 11: 21.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Office for National Statistics. UK consumer price inflation: Oct 2016. https://www.ons.gov.uk/releases/ukconsumerpriceindicesoct2016.
- 25. Joint Formulary Committee. British National Formulary, 72nd edn London: BMJ Group and Pharmaceutical Press, 2016. [Google Scholar]
- 26. Department of Health. Updated guidance on the diagnosis and reporting of Clostridium difficile 2012. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/215135/dh_133016.pdf.
- 27. Curtis L, Burns A. Unit costs of health and social care 2015. Personal Social Services Research Unit: University of Kent, Canterbury, UK. http://www.pssru.ac.uk/project-pages/unit-costs/2015/.
- 28. National Institute for Health and Care Excellence. The guidelines manual. Process and methods [PMG6]. 2012. https://www.nice.org.uk/process/pmg6/chapter/introduction. [PubMed]
- 29. Gallagher JC, Reilly JP, Navalkele B. et al. Clinical and economic benefits of fidaxomicin compared to vancomycin for Clostridium difficile infection. Antimicrob Agents Chemother 2015; 59: 7007–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patel D, Goldman-Levine JD.. Fidaxomicin (Dificid) for Clostridium difficile infection. Am Fam Physician 2013; 87: 211–2. [Google Scholar]
- 31. Debast S, Bauer M, Kuijper E.. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect 2014; 20: 1–26. [DOI] [PubMed] [Google Scholar]
- 32. Dubberke ER, Schaefer E, Reske KA. et al. Attributable inpatient costs of recurrent Clostridium difficile infections. Infect Control Hosp Epidemiol 2014; 35: 1400–7. [DOI] [PubMed] [Google Scholar]
- 33. Stranges PM, Hutton DW, Collins CD.. Cost-effectiveness analysis evaluating fidaxomicin versus oral vancomycin for the treatment of Clostridium difficile infection in the United States. Value Health 2013; 16: 297–304. [DOI] [PubMed] [Google Scholar]
- 34. Watt M, Dinh A, Le Monnier A. et al. Cost-effectiveness analysis on the use of fidaxomicin and vancomycin to treat Clostridium difficile infection in France. J Med Econ 2017; 1–9. [DOI] [PubMed] [Google Scholar]
- 35. Nathwani D, Cornely OA, Van Engen AK. et al. Cost-effectiveness analysis of fidaxomicin versus vancomycin in Clostridium difficile infection. J Antimicrob Chemother 2014; 69: 2901–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shyangdan D, Jacob RP, Connock M et al. Empagliflozin for the treatment of type 2 diabetes: a single technology appraisal. Warwick Evidence: University of Warwick Medical School, July 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.