Borrelia burgdorferi responds to a variety of host-derived factors and appropriately alters its gene expression for adaptation under different host-specific conditions. We previously showed that various levels of acetate, a short-chain fatty acid (SCFA), altered the protein profile of B. burgdorferi.
KEYWORDS: Borrelia burgdorferi, Lyme disease, short-chain fatty acids
ABSTRACT
Borrelia burgdorferi responds to a variety of host-derived factors and appropriately alters its gene expression for adaptation under different host-specific conditions. We previously showed that various levels of acetate, a short-chain fatty acid (SCFA), altered the protein profile of B. burgdorferi. In this study, we determined the effects of other physiologically relevant SCFAs in the regulation of metabolic/virulence-associated proteins using mutant borrelial strains. No apparent increase in the synthesis of outer surface protein C (OspC) was noted when a carbon storage regulator A (csrA of B. burgdorferi, or csrABb) mutant (mt) was propagated within dialysis membrane chambers implanted within rat peritoneal cavity, while the parental wild type (wt; B31-A3 strain) and csrABb cis-complemented strain (ct) had increased OspC with a reciprocal reduction in OspA levels. Growth rates of wt, mt, ct, 7D (csrABb mutant lacking 7 amino acids at the C terminus), and 8S (csrABb with site-specific changes altering its RNA-binding properties) borrelial strains were similar in the presence of acetate. Increased levels of propionate and butyrate reduced the growth rates of all strains tested, with mt and 8S exhibiting profound growth deficits at higher concentrations of propionate. Transcriptional levels of rpoS and ospC were elevated on supplementation of SCFAs compared to those of untreated spirochetes. Immunoblot analysis revealed elevated levels of RpoS, OspC, and DbpA with increased levels of SCFAs. Physiological levels of SCFAs prevalent in select human and rodent fluids were synergistic with mammalian host temperature and pH to increase the levels of aforementioned proteins, which could impact the colonization of B. burgdorferi during the mammalian phase of infection.
INTRODUCTION
Borrelia burgdorferi, the agent of Lyme disease, is transmitted to vertebrate hosts via the bite of infected Ixodes scapularis ticks. This spirochetal pathogen adapts to highly disparate environmental conditions that exist in the tick vector and the vertebrate hosts by altering its gene expression profile (1–3). B. burgdorferi has limited metabolic and regulatory capabilities, although it can funnel multiple host-derived signals in the form of nutrients/metabolites to modulate its host-specific adaptation (4, 5). Previous studies have shown the significance of a variety of signals, such as temperature (6–8), pH (9), dissolved gases (10, 11), host-specific stressors (12), and nutrients (13–15), among others, to influence appropriate expression and synthesis of key borrelial determinants enabling survival and colonization of B. burgdorferi in different hosts. These signals can, therefore, be modulated to reduce fitness of the spirochetes and thereby interfere with the pathogen survival in the tick or mammalian phases of infection. Since levels of these nutrients/signals vary under different host-specific microenvironments, a greater understanding of how environmental cues are perceived to alter the physiology and virulence capabilities of B. burgdorferi will add to our strategies to reduce the pathogen burden in the transmission and reservoir hosts.
We previously showed that acetate, a short-chain fatty acid (SCFA), is a key nutrient utilized by B. burgdorferi for its cell wall biogenesis and to modulate its gene expression profile, favoring adaptation to the vertebrate host (16). Increased levels of acetate consistently induced RpoS and rpoS-regulated lipoproteins, such as outer surface protein C (OspC), decorin binding protein A (DbpA), and the fibronectin binding protein, BBK32. A reduction in the levels of RpoS and rpoS-regulated lipoproteins was observed when BBA34, encoding oligopeptide permease AV (OppAV), which is induced on shifting the spirochetes to conditions mimicking those of the tick midgut after a blood meal, was deleted (17). However, the oppAV mutant was capable of surviving in the C3H/HeN mice up to 14 days, suggesting that additional host-derived signals can facilitate initial stages of adaptation to the vertebrate host, although the phenotype of this mutant following long-term infection in the mouse models of Lyme disease remains to be determined (17). When the oppAV mutant was propagated with increasing concentrations of acetate, RpoS and rpoS-regulated proteins were synthesized at levels similar to those of the parental strain (17). These observations demonstrated that acetate is a key signaling molecule and that the formation of acetyl-phosphate due to the actions of the enzyme acetate kinase (AckA) serves as an essential substrate for the mevalonate pathway critical for the biogenesis of the cell wall of B. burgdorferi (16, 18). Recently, it has been shown that SCFAs act as weak acids that influence pH-dependent gene expression in B. burgdorferi (19).
Additional studies from our laboratory focused on the regulation of the enzyme immediately downstream of AckA in the mevalonate pathway, namely, phosphate acetyltransferase (Pta) (16, 20–22). We deleted an RNA-binding protein, termed carbon storage regulator A of Borrelia burgdorferi (CsrABb), and observed that RpoS and rpoS-regulated proteins were downregulated in the csrABb mutant (21). The csrABb mutant was shown to be incapable of colonization of C3H/HeN mice (22–24), although a recent study showed that the csrABb mutant was capable of colonization of C3H/HeN mice (25). Previous studies suggested that signals and growth conditions regulating the levels of specific mRNAs wholly or partly regulated by CsrABb play a role in the phenotype of the csrABb mutant. These results also revealed the effects of CsrABb in regulating the translational levels of mRNA with known or putative CsrABb-binding sites and in modulating the overall metabolic and virulence-related fitness of the spirochetes during different phases of infection (22). Additional in vitro studies using the csrABb mutant propagated under conditions mimicking those of the fed ticks revealed that a reduction in the levels of select virulence-related proteins, such as OspC, DbpA, and BBK32, among others, presumably impacted the colonization of the csrABb mutant in a mammalian host. It is critical to point out that the in vitro and in vivo phenotype of B. burgdorferi is reflected in its growth conditions that result in variable levels of mRNA specifically bound by CsrABb.
Previously, we showed that external nutrients/signals and key residues of CsrABb contribute to the phenotypic effects by influencing both the levels of target mRNA and avidity/affinity of binding to CsrABb binding domains present in these transcripts (22). Replacement of 8 critical residues of CsrABb (8S) with alanines and deletion of 7 residues that are unique to spirochetal homologs of CsrABb (7D) resulted in mutants that provided insights into the role of specific residues critical for the functions of CsrABb, notably in regulating a key enzyme (Pta) of the mevalonate pathway (16). While the 8S strain produced CsrABb that was stable and bound the 5′-untranslated region (UTR) of mRNA of pta avidly, the 7D strain had a phenotype very similar to that of the csrABb mutant. By regulating the translational levels of Pta, CsrABb contributes to the flux of acetyl-phosphate and acetyl-coenzyme A (CoA), which are essential for cell wall biogenesis (22). Mutants lacking ackA and pta were rescued with exogenous addition of mevalonate, suggesting the relevance of the substrates generated by these enzymes in cell wall biogenesis (18). However, acetyl-phosphate, the substrate generated via AckA, appears to be a minor high-energy phospho-substrate contributing to the activation of the response regulatory protein 2 (Rrp2), the response regulator of a two-component regulatory system, involved in modulating the mammalian host phase of B. burgdorferi infection, although the acetyl-CoA generated from acetyl-phosphate due to the enzymatic functions of Pta is critical for the cell wall biogenesis (18). It is also possible that CsrABb has pleiotropic regulatory effects impacting the levels of translation of many mRNA species, leading to an adaptive response in B. burgdorferi consistent with the environmental cues encountered under different host-specific conditions (18, 26).
We further examined the effect of additional SCFAs, such as propionate and butyrate, on the adaptive capabilities of B. burgdorferi to build on our previous findings on the role of acetate in modulating both the key enzymes of the mevalonate pathway and the levels of virulence-associated antigens of B. burgdorferi. Bioinformatic analysis of the genome of B. burgdorferi revealed no apparent homologs of propionate and butyrate kinases, while there are instances of a single acetate kinase using propionate and butyrate as alternative substrates (27–30), suggesting the AckA homolog of B. burgdorferi undertakes the phosphorylation of both of these substrates. Moreover, these SCFAs can function as weak acids contributing to the acid stress response of B. burgdorferi (19). Although a large body of information is available on the role of gut microbiota in contributing to serum levels of acetate, propionate, and butyrate in various mammalian hosts, the significance of the levels of these SCFAs in the dissemination and colonization of a vector-borne pathogen such as B. burgdorferi is unclear.
We therefore initiated studies to determine the role of SCFAs on the pathophysiological responses of B. burgdorferi. We focused on defining the effects of different concentrations of SCFAs under a single temperature and pH condition (pH 7.6 and 32°C), where the levels of RpoS and rpoS-inducible genes are minimal to dissociate the contributions of SCFAs in modulating the transcriptional and translational levels of different metabolic/virulence-related genes of B. burgdorferi. The effect of SCFAs on mutants either lacking csrABb or with site-specific alterations in key residues within CsrABb were also used in this study. In addition, the combined effect of the physiological levels of SCFAs prevalent in select biological fluids from humans and rodents at mammalian temperature and pH was also explored using the parental strain. The findings from these studies underscore the importance of host-derived nutrients/signals and reveal the role of SCFAs in influencing the levels of molecular determinants critical for the colonization of B. burgdorferi, notably in the reservoir hosts and during its transmission to and from the tick vector. These studies also provide avenues to explore the influence of pathogen metabolism on the pathogenesis of Lyme disease.
RESULTS
Reduced levels of OspC in csrABb mutant following mammalian host adaptation.
We implanted dialysis membrane chambers (DMCs) containing wt, csrABb mt, or ct strains within the rat peritoneal cavity and compared the protein profiles of mammalian host-adapted spirochetes with that of in vitro-cultivated B. burgdorferi at day 11 postimplantation (31, 32). As shown in Fig. 1, OspC levels were not apparent in the DMC-grown csrABb mutant (Fig. 1, mt, lane 2) compared to those of wt and ct strains (Fig. 1, wt, ct, lane 2). Moreover, OspA was apparent in the in vitro-propagated spirochetes (Fig. 1, wt, mt, and ct, lane 1) and was not apparent in the DMC-grown spirochetes, indicating OspA to OspC switch following adaptation to a mammalian host. Reduced levels of OspC induction in csrABb mutant were previously observed following propagation under in vitro conditions mimicking fed ticks (pH 6.8 and 37°C) and presumably contribute to lack of infectivity of the csrABb mutant in C3H/HeN mice (21, 22). It is also possible that the levels of translational products from several mRNA species recognized by CsrABb via its RNA-binding domain contribute to the phenotypic response following mammalian host adaptation of the csrABb mutant.
FIG 1.
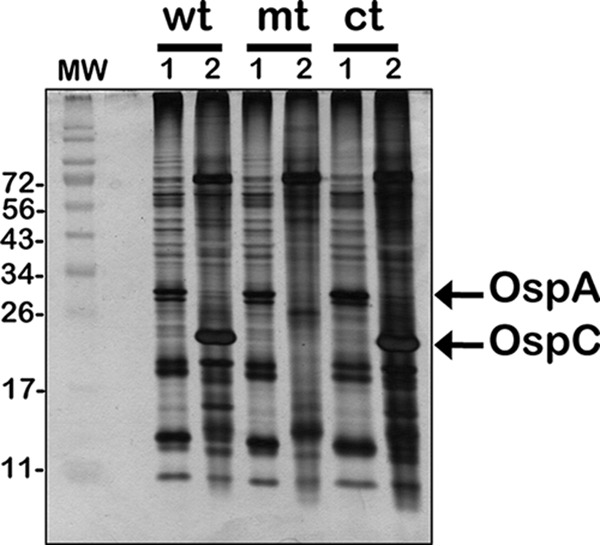
Mammalian host adaptation of csrABb mutant. Total protein profile of lysates from wild-type (wt), csrABb mutant 1 (mt), and cis-complemented (ct) strains propagated under in vitro conditions (lane 1) compared to strains grown within dialysis membrane chambers (DMCs) implanted in rat peritonea (lane 2). Proteins were separated on 4 to 20% gradient SDS-PAGE gel and visualized by silver stain. Molecular weight markers (MW; in kDa) are indicated to the left.
Growth rates of borrelial strains in the presence of SCFAs.
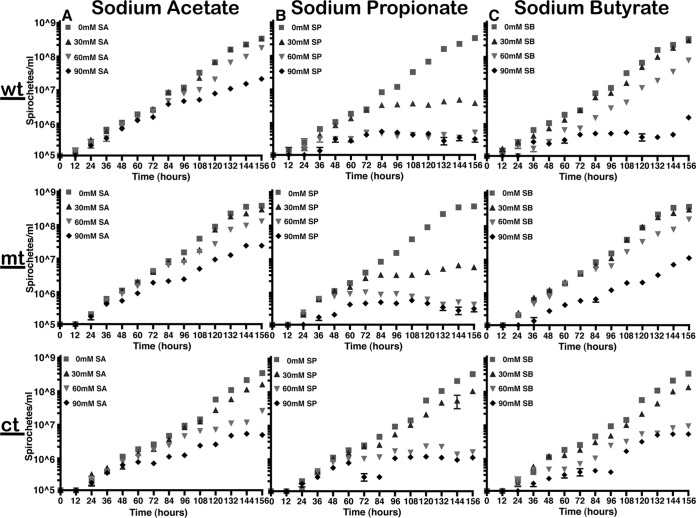
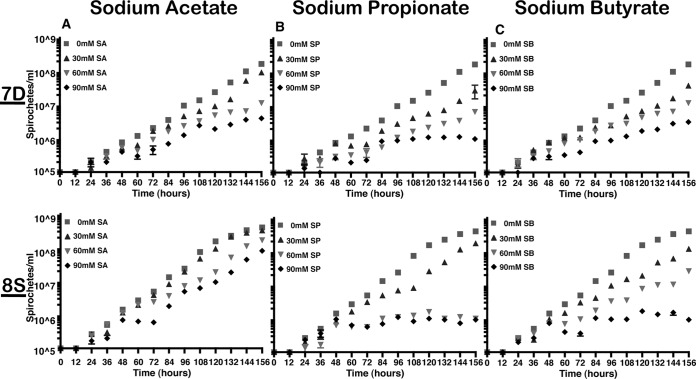
We propagated several borrelial mutants in the presence of various concentrations of SCFA at pH 7.6 and 32°C to minimize the effects of pH and temperature and prevent other signals from compounding the effects of SCFA on the growth, transcriptional, and translational levels of Lyme spirochetes (20, 33). We found that all the borrelial strains tested had a reduced growth rate at 90 mM acetate compared to growth at a lower concentration of acetate at 156 h (Fig. 2 and 3A). Sodium propionate at 90 mM concentration had the most drastic impact on the growth of all borrelial strains tested compared to untreated spirochetes, with the 7D strain exhibiting a significant growth deficit even at 60 mM concentration (Fig. 3B). Moreover, a more pronounced growth deficit was observed with csrABb mt and 8S strains in the presence 90 mM propionate, and the spirochetes were unable to grow past 96 h to be processed for transcriptional and translational analysis (Fig. 2 and 3B; see also Fig. 5). A drastic difference was noted in the growth rate of all strains in the presence of 90 mM butyrate compared to growth in control medium (Fig. 2 and 3C), although the csrABb mutant was unaffected at 60 mM sodium butyrate (Fig. 2C). Among all SCFAs, increased concentrations of propionate reduced growth rates of all B. burgdorferi strains tested, suggesting that increased levels of propionate serve to reduce the metabolic and growth phenotypes of the spirochetes. It should be pointed out that the effects of SCFAs were tested under laboratory growth conditions (pH 7.6 and 32°C), where these borrelial strains had maximal growth rates, and in the presence of antibiotics, consistent with the resistance markers used initially to derive these strains. Statistical analysis was done to compare the growth rates between untreated and treated samples. Significant differences (P value of <0.001) in growth rates were noted in the wt (i) from 108 h with 60 mM and 90 mM but not with 30 mM sodium acetate (Fig. 2A); (ii) from 108 h with all concentrations of sodium propionate, while the growth rates were less significant (P value of <0.01) at 96 h with 60 and 90 mM sodium propionate (Fig. 2B); and (iii) from 108 to 156 h with 60 and 90 mM sodium butyrate (Fig. 2C). Growth rates were less significant (P value of <0.01) from 108 to 120 h but were highly significant at later time intervals (P value of <0.001, 132 to 156 h) with 30 mM sodium butyrate. Statistical analysis revealed significant differences (P < 0.001) in growth rates of mt (i) from 132, 120, and 108 h with 30, 60, and 90 mM sodium acetate, respectively (Fig. 2A); (ii) from 108 h with different concentrations of sodium propionate, while growth rates were less significant (P value of <0.01) at 96 h for 60 mM and 90 mM sodium propionate (Fig. 2B); and (iii) from 132 h, 120 h, and 108 h with 30, 60 and 90 mM sodium butyrate, respectively (Fig. 2C). Significant differences were observed in the growth rates of the ct strain (P value of <0.001) from 120 h with 30, 60, and 90 mM sodium acetate (Fig. 2A), sodium propionate (Fig. 2B), and sodium butyrate (Fig. 2C) compared to those of untreated samples. Significant differences (P value of <0.001) were also observed in growth rates of 7D strain at 96 h for the highest concentration (90 mM) of each SCFA-treated group (Fig. 3A, B, and C). Growth rates were significantly different, with a P value of <0.001 at 156 h for all treated samples. Significant differences (P value of <0.001) in growth rates were noted with 8S strain at 96 h for sodium acetate (90 mM) and at 84 h for both sodium propionate and butyrate (Fig. 3A, B, and C). Growth rates were significantly different, with a P value of <0.001 at 156 h for all treated samples compared to untreated samples.
FIG 2.
Effects of SCFA on in vitro growth phenotype of B. burgdorferi B31-A3 (wt), csrABb mutant (mt), and cis-complemented (ct) strains. The borrelial strains were propagated in medium supplemented with 0, 30, 60, or 90 mM sodium acetate (SA) (A), sodium propionate (SP) (B), or sodium butyrate (SB) (C) at pH 7.6 and 32°C. Motile spirochetes were enumerated every 12 h for a period of 156 h in triplicate by dark microscopy. Statistical significance in growth rates was determined between untreated and treated spirochetes using a two-way analysis of variance (ANOVA) with α of 95%.
FIG 3.
Effects of SCFA on in vitro growth phenotype of 7D and 8S strains. Cis-complemented csrABb strains lacking the 7 C-terminal residues (7D) or with 8 critical residues of CsrABb replaced with alanines (8S) were propagated with 0, 30, 60, or 90 mM sodium acetate (A), sodium propionate (B), or sodium butyrate (C) at pH 7.6 and 32°C. Motile spirochetes were enumerated every 12 h for a period of 156 h in triplicate by dark microscopy. Statistical significance in growth rates was determined between untreated and treated spirochetes using a two-way ANOVA with α of 95%.
FIG 5.
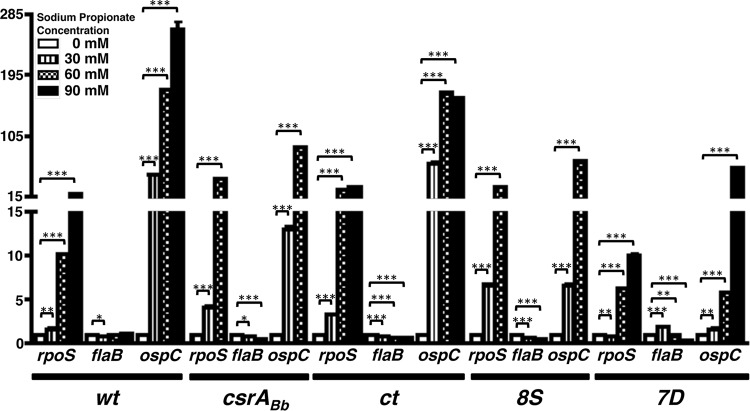
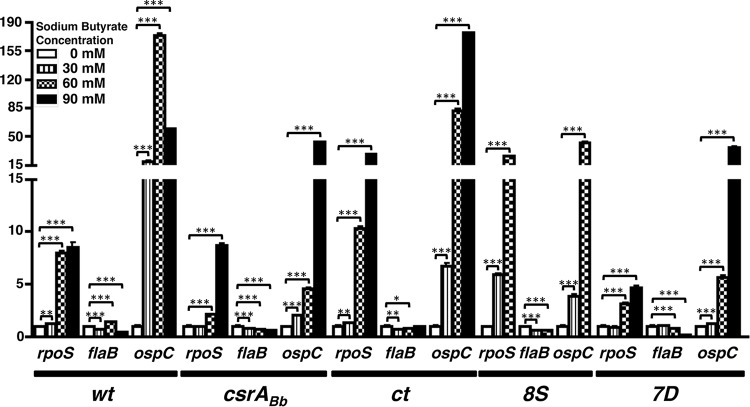
Transcriptional levels of select ORFs in response to supplementation with propionate. Real-time reverse transcription-PCR to detect levels of rpoS, flaB, and ospC in different borrelial strains is shown. Total RNA was extracted from wt, csrABb mutant, ct, 8S, and 7D strains propagated at pH 7.6 and 32°C in BSKII medium supplemented with 0, 30, 60, or 90 mM sodium propionate and converted to cDNA, followed by quantitative real-time PCR using primers specific to rpoS, flaB, and ospC as described in Materials and Methods. The values for all samples were normalized relative to the values for recA, and the change in the CT values for each transcript was obtained as an average of the values determined for each sample analyzed in triplicate. The 2−ΔΔCT values for each transcript from various strains are shown as fold difference on the y axis with the corresponding error bars. The ΔCT values obtained for all strains were subjected to an unpaired Student t test implemented in Prism software. The asterisks indicate levels of significance: ***, P < 0.001; **, P < 0.01; *, P < 0.05.
Transcriptional levels of select borrelial determinants in response to SCFAs.
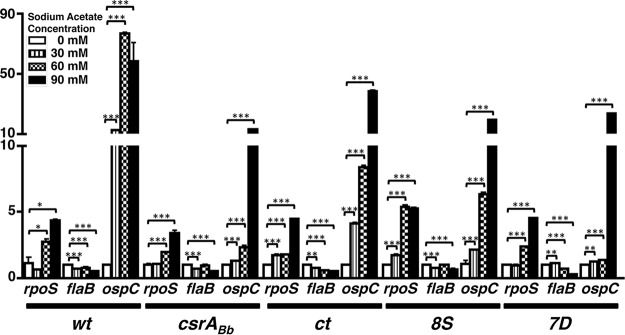
We performed transcriptional analysis on a select set of genes (rpoS, flaB, and ospC) using cDNA generated from wt, csrABb mt, ct, and 8S strains propagated at 0, 30, 60, and 90 mM acetate (Fig. 4), propionate (Fig. 5), and butyrate (Fig. 6). Compared to other strains, the ospC levels were lower for the csrABb and 7D mutant strains at 30, 60, and 90 mM acetate (Fig. 4). The transcriptional levels of rpoS were relatively comparable in all strains, although the 8S strain had higher levels at 60 mM acetate. The transcriptional levels of flaB were lower with higher concentrations of acetate in all strains, and the differences were significant in the 7D strain. The most significant increase in the transcriptional levels of rpoS and ospC were observed when the different strains were propagated in the presence of increasing concentrations of propionate (Fig. 5). There was no reliable isolation of mRNA from mt and 8S strains due to low numbers of spirochetes that were viable when propagated at 90 mM propionate, hence there are no transcriptional data for these two strains (Fig. 5, mt and 8S). However, there was maximal expression of ospC in wt and ct strains at 90 mM propionate. This suggested that the metabolic response of B. burgdorferi in the absence (mt) or stable levels (8S) of CsrABb contribute to increased sensitivity with higher concentrations of propionate in the growth medium. All strains except 8S had higher levels of ospC at 90 mM butyrate, even though the wt strain had significantly higher levels of ospC at 60 mM butyrate (Fig. 6). These transcriptional analyses clearly demonstrate that both rpoS and ospC are induced in response to supplementation of SCFAs. The transcriptional levels of rpoS and ospC were reflective of the increased levels of the corresponding translational products observed using specific antisera for these determinants in immunoblot assays, as shown in Fig. 7 to 14. It should be pointed out that all of the mutant strains were grown in the presence of antibiotics used for their counterselection (22).
FIG 4.
Transcriptional levels of select open reading frames (ORFs) in response to supplementation with acetate. Real-time reverse transcription-PCR to detect levels of rpoS, flaB, and ospC in different borrelial strains is shown. Total RNA was extracted from wt, csrABb mutant, ct, and 8S strains propagated at 32°C and pH 7.6 in BSKII medium supplemented with 0, 30, 60, or 90 mM sodium acetate and converted to cDNA, followed by quantitative real-time PCR using primers specific to rpoS, flaB, and ospC as described in Materials and Methods (Table 2). The values for all samples were normalized to the values for recA, and the change in the CT values for each transcript was obtained as an average of the values determined for each sample analyzed in triplicate. The 2−ΔΔCT values for each transcript from different strains are shown as fold difference on the y axis with the corresponding error bars. The ΔCT values obtained for all strains were subject to an unpaired Student t test implemented in Prism software. The asterisks indicate levels of significance: ***, P < 0.001; **, P < 0.01; *, P < 0.05.
FIG 6.
Transcriptional levels of select ORFs in response to supplementation with butyrate. Real-time reverse transcription-PCR to detect levels of rpoS, flaB, and ospC in different borrelial strains is shown. Total RNA was extracted from wt, csrABb mutant, ct, and 8S strains propagated at pH 7.6 and 32°C in BSKII medium supplemented with 0, 30, 60, or 90 mM sodium butyrate and converted to cDNA, followed by quantitative real-time PCR using primers specific to rpoS, flaB, and ospC as described in Materials and Methods. The values for all samples were normalized relative to the values for recA, and the change in the CT values for each transcript was obtained as an average of the values determined for each sample analyzed in triplicate. The 2−ΔΔCT values for various strains are shown as fold difference on the y axis with the corresponding error bars. The ΔCT values obtained for all strains were subjected to an unpaired Student t test implemented in Prism software. The asterisks indicate levels of significance: ***, P < 0.001; **, P < 0.01; *, P < 0.05.
FIG 7.
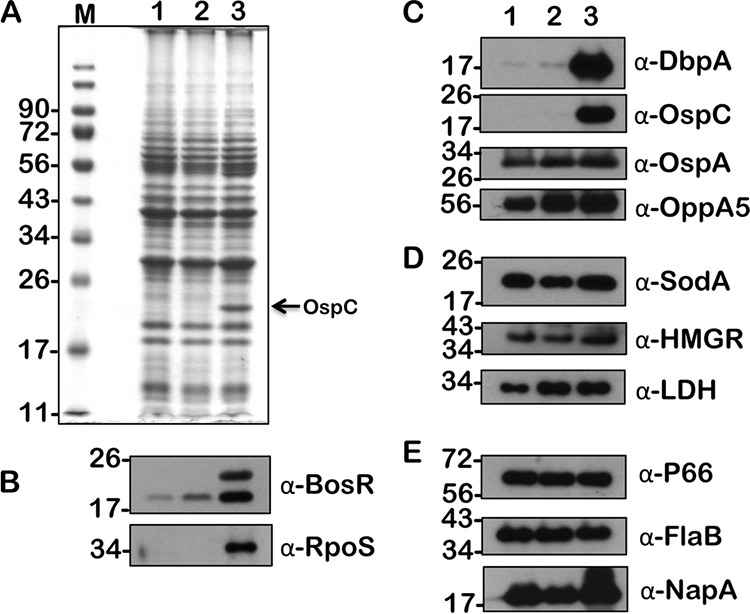
Immunoblot analysis of metabolic and virulence-associated proteins of B. burgdorferi strain B31-A3 (wt) with increased levels of acetate. B. burgdorferi was propagated in BSKII plus 6% normal rabbit serum supplemented with 0 (lane 1), 30 (lane 2), and 90 mM (lane 3) acetate. (A) Total stain of protein lysates separated using SDS–12.5% polyacrylamide gel and stained with Coomassie blue. Note the presence of increased levels of OspC upon growth with a 90 mM (lane 3) concentration of acetate. (B to E) Immunoblot analysis using antisera specific to borrelial regulatory proteins (B), lipoproteins and oligopeptide permease (C), enzymes (D), and proteins (E) serving as loading controls. Molecular weights (M), in kDa, are indicated to the left.
FIG 8.
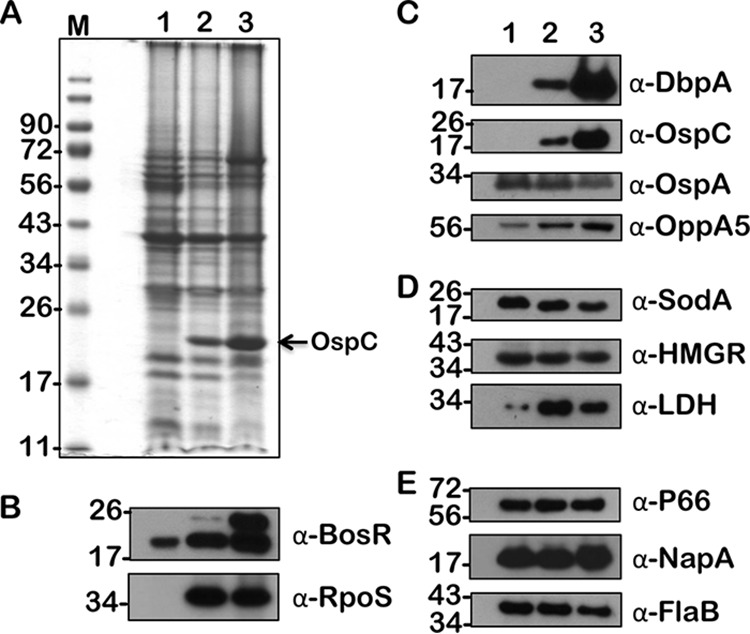
Immunoblot analysis of metabolic and virulence-associated proteins of B. burgdorferi strain B31-A3 (wt) with increased levels of propionate. B. burgdorferi was propagated in BSKII plus 6% normal rabbit serum supplemented with 0 (lane 1), 30 (lane 2), and 90 mM (lane 3) propionate. (A) Total stain of protein lysates separated using SDS–12.5% polyacrylamide gel and stained with Coomassie blue. Note the presence of increased levels of OspC with 90 mM (lane 3) acetate. (B to E) Immunoblot analysis using antisera specific to borrelial regulatory proteins (B), lipoproteins and oligopeptide permease (C), enzymes (D), and proteins (E) serving as loading controls. Molecular weights, in kDa, are indicated to the left.
FIG 9.
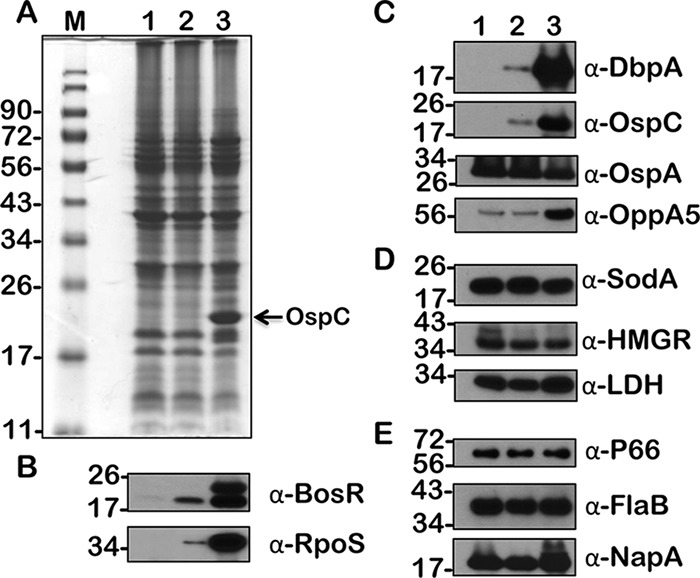
Immunoblot analysis of metabolic and virulence-associated proteins of B. burgdorferi strain B31-A3 (wt) with increased levels of butyrate. B. burgdorferi was propagated in BSKII plus 6% normal rabbit serum supplemented with 0 (lane 1), 30 (lane 2), and 90 mM (lane 3) butyrate. (A) Total stain of protein lysates separated using SDS–12.5% polyacrylamide gel and stained with Coomassie blue. Note the presence of increased levels of OspC at 90 mM concentration of acetate (lane 3). (B to E) Immunoblot analysis using antisera specific to borrelial regulatory proteins (B), lipoproteins and oligopeptide permease (C), enzymes (D), and proteins (E) serving as loading controls. Molecular weights, in kDa, are indicated to the left.
FIG 10.
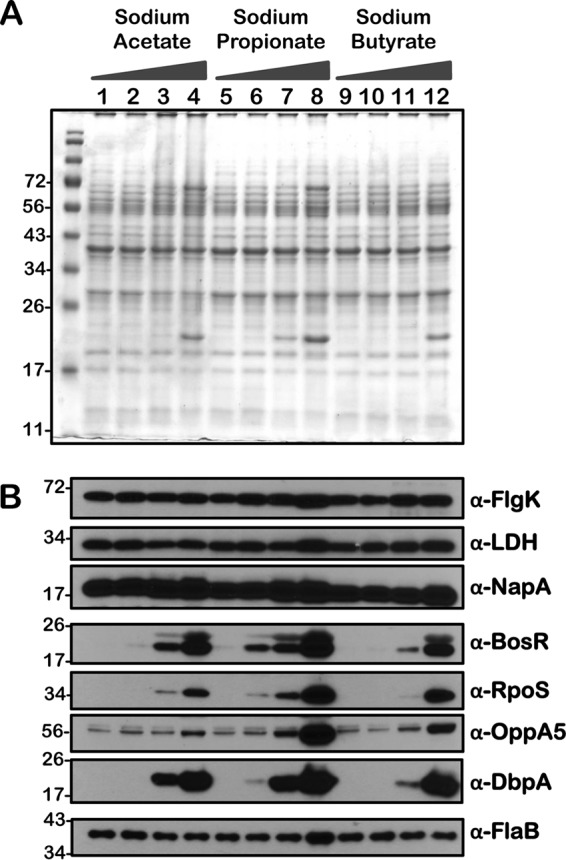
Immunoblot analysis of metabolic and virulence-associated proteins of B. burgdorferi strain B31-A3 (wt) with increased levels of three SCFAs. B. burgdorferi B31-A3 strain was propagated in BSKII plus 6% normal rabbit serum supplemented with 0 (lanes 1, 5, and 9), 30 (lanes 2, 6, and 10), 60 (lanes 3, 7, and 11), and 90 (lanes 4, 8, and 12) mM sodium acetate, sodium propionate, or sodium butyrate as indicated above the SDS-PAGE gel. (A) Total stain of protein lysates separated using SDS–12.5% polyacrylamide gel and stained with Coomassie blue. Note the presence of increased levels of OspC at 90 mM concentration of each of the SCFAs (lane 3). (B) Immunoblot analysis using antisera specific to a variety of borrelial determinants are indicated to the right of each blot. Molecular masses, in kDa, are indicated to the left.
FIG 11.
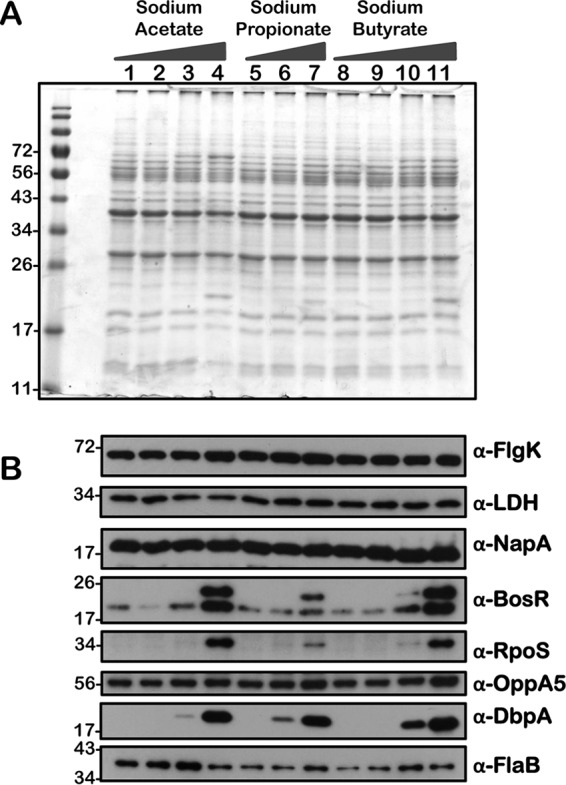
Immunoblot analysis of metabolic and virulence-associated proteins of csrABb mutant with increased levels of three SCFAs. B. burgdorferi was propagated in BSKII plus 6% normal rabbit serum supplemented with 0 (lanes 1, 5, and 8), 30 (lanes 2, 6, and 9), 60 (lanes 3, 7, and 10), and 90 (lanes 4 and 11) mM sodium acetate, sodium propionate, or sodium butyrate as indicated above the SDS-PAGE gel. (A) Total stain of protein lysates separated using SDS–12.5% polyacrylamide gel and stained with Coomassie blue. Note the presence of increased levels of OspC at 90 mM concentration of each of the SCFAs (lane 3). (B) Immunoblot analysis using antisera specific to a variety of borrelial determinants are indicated to the right of each blot. Molecular masses, in kDa, are indicated to the left.
FIG 12.
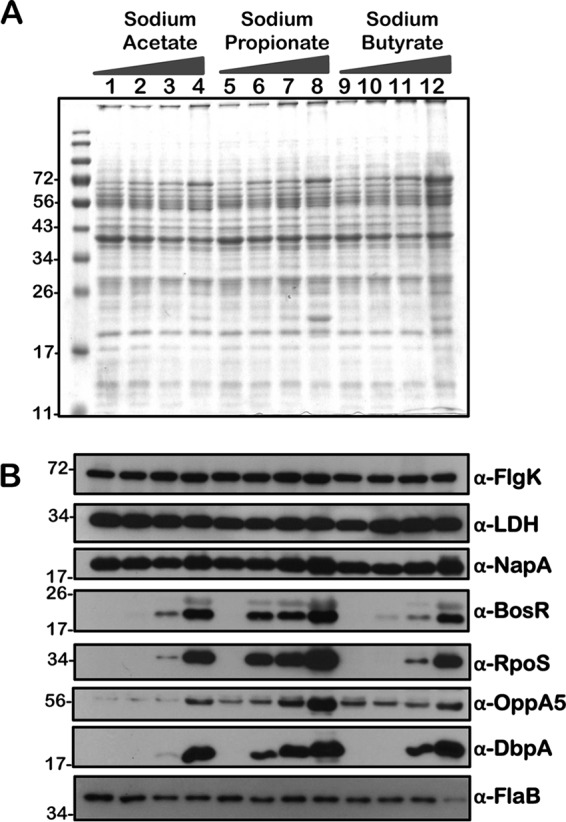
Immunoblot analysis of metabolic and virulence-associated proteins of cis-complemented strain (ct) with increased levels of three SCFAs. Cis-complemented strain of csrABb mutant was propagated in BSKII plus 6% normal rabbit serum supplemented with 0 (lanes 1, 5, and 9), 30 (lanes 2, 6, and 10), 60 (lanes 3, 7, and 11), and 90 (lanes 4, 8, and 12) mM sodium acetate, sodium propionate, or sodium butyrate as indicated above the SDS-PAGE gel. (A) Total stain of protein lysates separated using SDS–12.5% polyacrylamide gel and stained with Coomassie blue. Note the presence of increased levels of OspC at 90 mM concentration of each of the SCFAs (lane 3). (B) Immunoblot analysis using antisera specific to a variety of borrelial determinants are indicated to the right of each blot. Molecular masses, in kDa, are indicated to the left.
FIG 13.
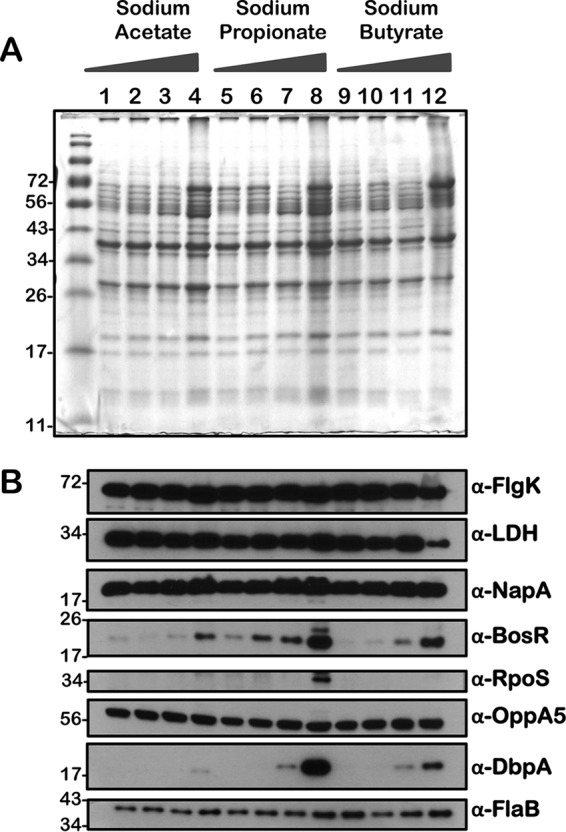
Immunoblot analysis of metabolic and virulence-associated proteins of 7D strain with increased levels of three SCFAs. 7D strain was propagated in BSKII plus 6% normal rabbit serum supplemented with 0 (lanes 1, 5, and 9), 30 (lanes 2, 6, and 10), 60 (lanes 3, 7, and 11), and 90 (lanes 4, 8, and 12) mM sodium acetate, sodium propionate, or sodium butyrate as indicated above the SDS-PAGE gel. (A) Total stain of protein lysates separated using SDS–12.5% polyacrylamide gel and stained with Coomassie blue. Note the presence of increased levels of OspC at 90 mM (lane 3) concentration of each of the SCFAs. (B) Immunoblot analysis using antisera specific to a variety of borrelial determinants, indicated to the right of each blot. Molecular masses, in kDa, are indicated to the left.
FIG 14.
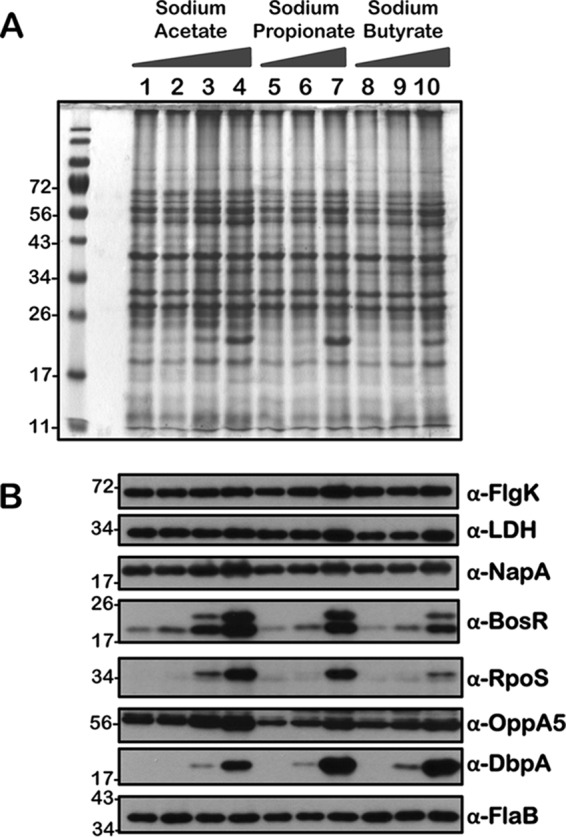
Immunoblot analysis of metabolic and virulence-associated proteins of 8S strain with increased levels of three SCFAs. 8S strain was propagated in BSKII plus 6% normal rabbit serum supplemented with 0 (lanes 1, 5, and 8), 30 (lanes 2, 6, and 9), 60 (lanes 3, 7, and 10), and 90 (lane 4) mM sodium acetate, sodium propionate, or sodium butyrate as indicated above the SDS-PAGE gel. (A) Total stain of protein lysates separated using SDS–12.5% polyacrylamide gel and stained with Coomassie blue. Note the presence of increased levels of OspC at 90 mM (lane 3) concentration of each of the SCFAs. (B) Immunoblot analysis using antisera specific to a variety of borrelial determinants that play key roles in the metabolism and pathogenesis of B. burgdorferi (indicated to the right of each blot). Molecular masses, in kDa, are indicated to the left.
Effects of SCFAs on wild-type B. burgdorferi B31 strain.
We have previously shown the effects of increased levels of acetate on B. burgdorferi strain B31 by determining the expression/synthesis of several proteins critical for its metabolism and virulence (16, 34). We initially examined if the supplementation of propionate and butyrate will have similar effects on the wild-type strain and analyzed the changes in the wild-type strain supplemented with acetate. As shown previously (16), we were able to observe increased levels of BosR, RpoS, and several rpoS-regulated proteins, such as OspC, DbpA, and OppA5, at higher concentrations of acetate (Fig. 7, lane 3, 90 mM). Compared to the effects of acetate, the levels of BosR, RpoS, and rpoS-regulated proteins were elevated drastically at 30 mM propionate, suggesting that propionate is effective at inducing vertebrate host-specific adaptation at a lower concentration (Fig. 8, lane 2, 30 mM). The levels of lactate dehydrogenase (LDH) were also elevated at 30 and 90 mM propionate compared to those of the untreated sample (Fig. 8, lanes 2 and 3, α-LDH). Similar to acetate, the levels of induction of BosR, RpoS, and rpoS-regulated proteins were elevated in wild-type B. burgdorferi in the presence of butyrate (Fig. 9). The levels of OspA remained relatively unchanged in spirochetes grown under different concentrations of SCFAs (Fig. 7, 8, and 9, α-OspA). Moreover, an interesting observation is the level of a higher form of BosR noted when B31-A3 is propagated at 90 mM each of the three SCFAs used in this study (Fig. 7, 8, and 9, lane 3, α-BosR). These initial studies clearly demonstrated that different SCFAs have similar and distinct effects on the induction of proteins critical for the pathophysiology of B. burgdorferi. We expanded this analysis to include other borrelial mutants, notably one that either lacked csrABb or carried site-specific alterations in this allele.
Effects of SCFAs on csrABb mutant strains of B. burgdorferi.
We performed a comprehensive analysis of the levels of synthesis of a variety of borrelial proteins by propagating the wild type (wt), csrABb mutant (mt), cis-complemented strain (ct), and two additional mutants, 7D (lacking 7 amino acids at the C terminus of CsrABb) and 8S (with 8 critical amino acids of CsrABb replaced with alanines), under similar growth conditions in the presence of various SCFAs (Fig. 10 to 14). Since the borrelial growth medium is complex and relatively undefined, all borrelial strains were propagated using the same batch of growth medium in the presence of antibiotics, consistent with the counterselectable markers used to generate the mutants. The spirochetes were harvested at less than 5 × 107 cells per ml, minimizing the effects of cell density/pH of the growth media on the protein profiles of the spirochetes. Increased concentrations of propionate increased the levels of BosR, RpoS, DbpA, OppA5 (Fig. 10B and 12B), and OspC (Fig. 10A and 12A) in wt and ct strains. Although the csrABb mutant was unable to grow to a sufficient density at 90 mM propionate, we were able to detect higher levels of BosR, RpoS, OppA5, and DbpA in the presence of 60 mM propionate than that in the untreated control (Fig. 11B). However, the levels of the aforementioned proteins were relatively lower in the csrABb mutant than in the wt and ct strains and were similar to those of the 7D strain in the presence of all of the SCFAs (Fig. 10 to 13). The 8S strain, similar to the mt strain, was unable to grow in the presence of 90 mM sodium propionate or sodium butyrate to sufficient density, although there were higher levels of BosR, RpoS, and several rpoS-regulated lipoproteins in the presence of 60 mM concentrations of these SCFAs compared to those of untreated sample (Fig. 14, lanes 1, 5, and 8). Moreover, the 8S strain had higher levels of these proteins than the csrABb mutant and 7D strain, suggesting the role of the site-specific changes in the CsrABb resulting in these phenotypic responses to select environmental signals. The effects of propionate and butyrate on the gene and protein expression profiles of borrelial strains were similar to the effect of acetate, although the concentration of propionate needed to induce RpoS and select members of the rpoS regulon was lower (Fig. 10 to 14). Moreover, the levels of key virulence-related proteins were lower in the csrABb and 7D mutants than in the wt, ct, and 8S strains, consistent with previous studies using temperature and pH mimicking the midgut of fed and unfed ticks (22). The csrABb mutant did not survive with 90 mM propionate, while the 8S strain had a growth defect with 90 mM butyrate compared to other strains, although all strains tested had similar growth rates with 90 mM acetate. The wt, ct, and 7D strains did survive in the BSKII medium supplemented with 90 mM propionate, indicating growth, transcriptional, and translational differences among these strains. Therefore, it is possible that transient increases in some of the SCFAs in the serum/blood meal or in other host tissues could result in changes in the metabolic and virulence attributes of B. burgdorferi.
Synergistic effects of SCFAs under conditions mimicking midguts of fed ticks.
Several studies have shown the need for shifting the in vitro growth conditions of B. burgdorferi from RpoS-noninducing to RpoS-inducing conditions (such as pH and temperature, among others) to modulate the transcript/protein profiles that mimic the midgut of ticks before and after a blood meal (33, 35). In order to dissociate or limit the effects of pH and temperature on the induction of RpoS and rpoS-regulated genes from the effects of individual SCFAs on B. burgdorferi, we propagated the spirochetes at pH 7.6 and 32°C in the presence of various, albeit higher, concentrations compared to levels of SCFAs present in various biological fluids, such as serum and cerebrospinal fluid (CSF) of human or rodents (Fig. 4 to 6 and 10 to 14). Immunoblot analysis of borrelial lysates propagated under conditions mimicking the midgut of fed ticks (pH 6.8 and 37°C) supplemented with all three SCFAs at levels similar to those of human blood (30.4 μM sodium acetate, 1 μM sodium propionate, and 1 μM sodium butyrate) (Fig. 15B, lane 3), human cerebrospinal fluid (58 μM sodium acetate, 2.8 μM sodium propionate, and 1.4 μM sodium butyrate) (Fig. 15B, lane 4), and rat blood (350 μM sodium acetate; 5 μM sodium propionate and 5 μM sodium butyrate) (Fig. 15B, lane 5) showed increased levels of RpoS compared to that in lysates of B. burgdorferi grown without supplementation with SCFAs under conditions mimicking those of the midgut after (pH 6.8 and 37°C) (Fig. 15B, lane 1) and before (pH 7.6/23°C) (Fig. 15B, lane 2) a blood meal. Consistent with the increased levels of RpoS, there were increased levels of OspC and DbpA in borrelial lysates at pH 6.8 and 37°C, and the physiological levels of all three SCFAs were similar to those in human blood, CSF, and rat blood. There was a detectable increase in the levels of OspC at pH 6.8 and 37°C with all three SCFAs (Fig. 15A, OspC), indicating the contributions of pH and temperature (along with all three SCFAs) in modulating key determinants of B. burgdorferi critical for the mammalian phase of infection. These studies add to the growing number of host-derived factors that alter the adaptive capabilities of B. burgdorferi to facilitate its survival and transmission between the mammalian and tick phases of infection.
FIG 15.
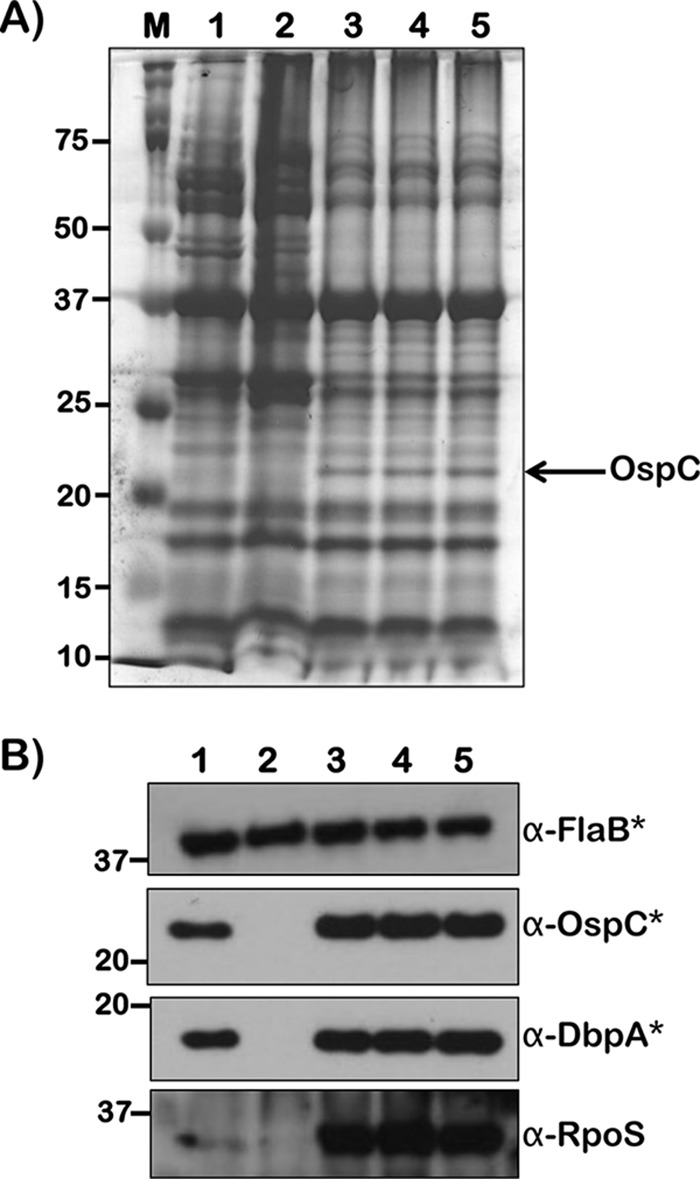
Immunoblot analysis of B. burgdorferi B31-A3 propagated at pH 6.8 and 37°C with physiological levels of SCFAs mimicking that of human blood, cerebrospinal fluid, or rat blood. B. burgdorferi strain B31-A3 was propagated in BSKII plus 6% normal rabbit serum at pH 6.8 and 37°C, mimicking midgut of fed ticks (lane 1), pH 7.6 and 23°C, mimicking midgut of unfed ticks (lane 2), pH 6.8 and 37°C plus SCFA levels reflecting human blood (lane 3), pH 6.8 and 37°C plus SCFA levels reflecting human cerebrospinal fluid (lane 4), and pH 6.8 and 37°C plus SCFA levels reflecting rat blood (lane 5). (A) Total stain of protein lysates separated using SDS–12.5% polyacrylamide gel and stained with Coomassie blue. Note the presence of increased levels of OspC in borrelial samples propagated with physiological levels of SCFAs reflecting human blood, CSF, or rat blood. (B) Immunoblot analysis using antisera specific to FlaB, OspC, DbpA, and RpoS. Asterisks indicate that the protein samples loaded on membranes probed with anti-FlaB, -OspC, and -DbpA were diluted 10-fold for all samples due to the high reactivity of specific antisera. Molecular masses, in kDa, are indicated to the left.
DISCUSSION
The adaptation of the agent of Lyme disease is dependent on its ability to sense and respond to different environmental cues present in various microenvironments of its tick vector and vertebrate hosts. Since B. burgdorferi has limited metabolic and regulatory capabilities, its fitness to survive is dependent on fine-tuning its adaptation in response to a multitude of signals prevalent in highly disparate hosts. In addition, the response to these host-derived signals, such as carbohydrates, fatty acids, nucleotides, and other essential biomolecules, is two-pronged: (i) appropriate alteration in the pathogen metabolism and (ii) modulation of virulence-associated proteins that contribute to the attachment, invasion, and persistence of the spirochetes in the transmission, reservoir, and dead-end hosts.
Analysis of the growth/transcriptional/translational features of parental and mutant strains of B. burgdorferi in the presence of various concentrations of SCFAs has opened avenues to determine if these signals/weak acids can be altered in the infected hosts to modulate the survival and virulence phenotype of the spirochetes. Since antigens such as OspC and DbpA are critical for colonization, they are also the target of antibody-mediated clearance of the spirochetes (36). Therefore, it is possible that the levels of some of these antigens and their mechanisms of regulation are transiently altered, resulting in a rapid and complete clearance of the pathogen and limiting persistence of B. burgdorferi in different vertebrate host tissues for prolonged periods of time. Persistence of spirochetes in low numbers or sustained levels of spirochetal antigens have been hypothesized to induce a prolonged inflammatory response in the joint tissues, possibly leading to antibiotic-resistant arthritis (37, 38).
We previously reported the lack of colonization of csrABb mutant in C3H/HeN mice, while the parental and cis-complemented strains were recovered from all tissues from mice challenged via needle inoculation (22). Since CsrABb is an RNA-binding protein and has properties to posttranscriptionally regulate levels of several borrelial proteins by interacting with the 5′UTRs of their mRNA, we determined its contribution to the ability of B. burgdorferi to adapt to the mammalian host (20–23). The csrABb mutant was able to survive within the DMCs implanted within the rat peritoneal cavity, although the levels of OspC induced were not apparent compared to the levels in wt and ct strains from DMCs. This suggested that the csrABb mutant had a defect in the induction of OspC under conditions of mammalian host adaptation and could therefore serve as a genetic tool to determine the effects of select host-derived nutrients such as SCFAs on the levels of select borrelial lipoproteins in comparison to the control strains.
We also employed two additional mutants strains of csrABb, one lacking the terminal 7 amino acids (7D) unique to borrelial csrA homologs and the other with 8 critical amino acids replaced with alanines (8S), resulting in stable levels of CsrABb with a concomitant increase in the levels of RpoS and other rpoS-regulated lipoproteins, such as OspC and DbpA (22). It is critical to point out that the lack of csrABb resulted in reduced levels of OspC following mammalian host adaptation that could impact its colonization of mammalian host. While two previous studies showed the lack of colonization of csrABb mutant in mouse models of infection (22, 23), another study did not observe a defect in colonization of csrABb mutant (25). It is unclear if in vitro or in vivo growth conditions could induce select mRNAs (bound by CsrABb) at levels drastically higher than levels of CsrABb present to regulate their posttranscriptional effects. It is therefore possible that the phenotypic effects mediated by CsrABb are a result of both the levels of CsrABb and the levels of its target mRNA transcribed at start sites encompassing the CsrABb-binding sites. Alternatively, csrABb mutant expressing select mRNA species at levels above the titratable levels of CsrABb in response to variations in in vitro or in vivo culture conditions can be hypothesized to have a phenotype similar to that of the control strains. It is also possible that there are single/multiple nucleotide changes in the CsrABb binding sites of select mRNA or within csrABb itself that have the potential to alter the translation of proteins critical for colonization of mice or for optimal binding properties of CsrABb. Therefore, the effects of SCFAs on csrABb mutants would provide insights into how the expression of select borrelial determinants can be manipulated at both transcriptional and posttranscriptional levels to alter the pathogen-host interactions of B. burgdorferi. These observations also add to the unique pleiotropic effects of CsrABb in determining the adaptive capabilities of Lyme spirochetes with features that are conserved and divergent from those of CsrA homologs present in other bacterial systems (39).
We undertook a comprehensive analysis of growth rates and transcriptional and translational levels of key borrelial determinants in response to SCFAs using 5 borrelial strains (wt, csrABb mt, ct, 7D, and 8S). All strains tested had a lower growth rate at higher concentrations of SCFAs, with sodium propionate exerting the most significant effect at lower concentration and during the earlier stages of growth (Fig. 2 and 3). The rationale for using these higher concentrations of SCFAs was to determine the maximum levels of SCFAs that can be tolerated by various borrelial strains under in vitro growth conditions in BSKII medium at pH 7.6 and 32°C (buffered with HEPES). This approach was to minimize the effects of other environmental cues that induce increased levels of RpoS and its regulon instead of the RpoS-inducible growth conditions used in our laboratory obtained by shifting B. burgdorferi from pH 7.6 and 23°C to pH 6.8 and 37°C (16, 33). It is reasonable to expect that the accumulation of membrane-permeable, nonionized organic acids within the cytoplasm of spirochetes in response to higher concentrations of SCFAs result in a variety of physiological effects leading to inhibition of bacterial growth. Many studies on the effects of SCFAs on gut microbes have been carried out under low pH conditions reflective of the physiological pH of the gut (40, 41). However, it is possible that the spirochetes are exposed to variations in external pH under different microenvironments depending on the feeding status of the ticks or the cellular profile within the mammalian tissues colonized by Lyme spirochetes.
Growth rates of different borrelial mutants in the presence of various concentrations of SCFAs under in vitro conditions provided insights into physiological effects that eventually could be exploited for modulating in vivo growth/survival of B. burgdorferi, notably if altered concentration of serum levels of SCFAs will impact survival/transmission between the tick and mammalian hosts. While the in vitro concentrations of the SCFAs used to demonstrate increased levels of RpoS and rpoS-regulated lipoproteins are much higher than the normal serum concentrations of these acids (100 to 400 μM range for humans), it is important to realize that this effect was observed in medium at pH 7.6 and 32°C (18, 42). The levels of acetate in the tick midguts were determined to be around 40 mM following feeding of ticks to repletion on a rabbit, reflecting the physiological relevance of millimolar concentrations of acetate in regulating the pathophysiology of B. burgdorferi (19). In the vertebrate host, the spirochetes are exposed to other stimulatory signals, which can synergize with the effects of much lower concentrations of SCFAs in the serum and other vertebrate tissues (9, 10, 33, 43). Consistent with this premise, levels of RpoS and two key lipoproteins, OspC and DbpA, were higher when B. burgdorferi B31-A3 (wild-type strain) was shifted from pH 7.6 and 23°C to pH 6.8 and 37°C (pH-temperature combination prevalent under fed-tick conditions) with all three SCFAs (acetate, propionate, and butyrate together) at levels present in human blood, human cerebrospinal fluid (CSF), or rat blood compared to those of B. burgdorferi grown under conditions mimicking the tick midgut before (pH 7.6 and 23°C) and after (pH 6.8 and 37°C) a blood meal without supplementation with SCFAs (Fig. 15). These observations underscore the combined effects of multiple environmental cues that drive the adaptive response of B. burgdorferi that influence its virulence/colonization attributes.
One possible mechanism for the regulatory effects mediated by SCFAs is the acid stress response in B. burgdorferi, although the borrelial genome does not encode a variety of genes similar to those contributing to this phenomenon in Escherichia coli (43–45). For example, 5 genes, glutamate decarboxylase B (gadB), glutamate-GABA antiporter (gadC), outer membrane lipoprotein (slp), periplasmic chaperone of acid-denatured proteins A (hdeA), and glutamate decarboxylase (gadA), involved in glutamate decarboxylase-dependent acid stress response are induced in E. coli following the addition of polyamines (45, 46). Since there are no apparent homologs of these genes in B. burgdorferi except for a glutamate transporter (GltP, BB0401), it is possible that the spirochetes possess enzymes that carry out similar functions but share little to no similarity to the aforementioned homologs. Among three types of acid resistance systems (AR), the presence of known (and unknown) homologs of RpoS-dependent response (AR1), and the apparent lack of homologs of glutamate decarboxylase (AR2) and arginine decarboxylase (AR3) indicate that B. burgdorferi has a limited acid stress response mechanism, likely due to its microenvironments being at near neutral pH, unlike the dramatic pH alterations encountered by enteric pathogens (41). The specific ability of spermidine and spermine to induce increased levels of RpoS and proteins of rpoS-regulated genes is indicative of an AR1-type acid stress response in B. burgdorferi, although all of the key players of this system have yet to be functionally characterized (33). Alternatively, it is possible that there are functional redundancies distributed over a wide array of hypothetical proteins that could mediate the acid stress response in B. burgdorferi.
Transcriptional levels of rpoS and ospC were significantly upregulated in the presence of higher concentrations of acetate (Fig. 3), propionate (Fig. 4), and butyrate (Fig. 5) compared to those of untreated samples in all strains tested, suggesting that the levels of these SCFAs induce expression of these genes in B. burgdorferi and could serve as signals present in the incoming blood meal that is known to upregulate RpoS and members of the rpoS regulon. While the levels of rpoS and ospC were similar in wt, ct, and 8S strains, their transcriptional levels were lower in the csrABb mt and 7D strains, notably at 60 mM acetate (Fig. 4). The transcriptional levels of rpoS and ospC in the presence of propionate was maximal in the wt strain, while the ct and 8S strains had higher levels than the csrABb mt and 7D strains in the presence of 60 mM propionate. It should be pointed out that we were unable to obtain sufficient numbers of csrABb and 8S mutant spirochetes at 90 mM propionate, although the growth media/conditions employed to propagate all strains were similar. The transcriptional changes in rpoS and ospC following addition of butyrate were similar to those of acetate, except that the 8S strain was unable to survive at the higher concentration of butyrate. A noteworthy observation from these studies is that the levels of SCFAs are capable of inducing transcriptional and translational changes (Fig. 7 to 14) in key borrelial determinants that permit the colonization of the mammalian host by the spirochetes independent of the temperature (all strains were grown under pH 7.6 and 32°C).
Consistent with the transcriptional changes noted with increasing levels of SCFAs, the levels of RpoS and several rpoS-regulated gene products were elevated in all strains tested compared to those of untreated spirochetes (Fig. 7 to 14). These findings were consistent with our previous report (16) and underscore the possibility of driving increased levels of proteins, such as OspC, DbpA, and presumably other rpoS-regulated lipoproteins, to facilitate a rapid, immune-mediated clearance of B. burgdorferi in reservoir hosts, since these lipoproteins are targets of the adaptive immune system. One notable observation was the relatively similar levels of OppA5 (BBA34), one of the five oligopeptide permeases encoded by linear plasmid 54 of B. burgdorferi, in the csrABb and 7D mutants with or without SCFAs (Fig. 11 and 13, α-OppA5), in contrast to wt, ct, and 8S strains, where the OppA5 levels were elevated with increasing concentrations of SCFAs (Fig. 10, 12, and 14). This difference could be attributed to unknown regulatory effects of CsrABb or to relatively lower levels of RpoS induced in csrABb and 7D mutants leading to minimal changes in OppA5 with increasing concentrations of SCFAs. These observations are consistent with our previous report using these strains, although there are differences in the growth conditions between these studies (22). However, these observations reveal phenotypic differences between strains expressing CsrABb versus those that either lack CsrABb or have changes in terms of their response to increasing levels of SCFAs (Fig. 11 to 14). The levels of several proteins, such as FlgK, Ldh, NapA, and FlaB, were similar or showed minor variations between all the strains tested in response to supplementation with SCFAs. It is therefore possible to speculate that levels of SCFAs in the incoming blood meal or in the reservoir hosts influence the transcriptional and translational levels of proteins critical for colonization of the mammalian hosts and thereby play a role in the clearance/persistence of spirochetes in different hosts.
Diets that are rich in complex fibers/carbohydrates, which are subjected to microbial fermentation in the gut, may not only lead to other metabolic advantages to the host but also could provide signals to modulate the pathogen response during infection. These host-derived signals/mechanisms can be manipulated to induce sustained levels of borrelial antigens that are targets of the adaptive immune response of the host to facilitate a reduction in pathogen burden, even though the spirochetes have the ability to evade the immune system of the mammalian hosts (47, 48). Even if the clearance of the spirochetes is incomplete in the reservoirs of hosts fed on diets rich in complex carbohydrates with concomitant changes in the gut microbiota, a gradual reduction in the pathogen burden could have significant cumulative impact on the transmission kinetics, leading to a reduction in the incidence of Lyme disease. These studies are bound to spawn new avenues to modulate host microbiome in different tissues (gut and skin) of reservoir hosts to influence the pathogen survival both in the mammalian hosts and in the tick vector, which is dependent on a mammalian blood meal to support spirochetes to survive during its enzootic cycle (49). Currently, the effects of high-fiber diets that lead to an increase in specific SCFAs are being tested using immunocompetent C3H/HeN and immunodeficient SCID mice infected with B. burgdorferi to determine the kinetics of pathogen transmission in the tick-mouse-tick cycle of infection that will enable connecting these in vitro findings to metabolic and infectious processes of Lyme spirochetes within its divergent hosts. These aforementioned studies will expand the role of SCFAs in contributing to the virulence manifestations of spirochetes and offer avenues to deregulate critical borrelial determinants that are known to significantly impact the pathogen-host interactions, resulting in reduced pathogen burden in the reservoir hosts or altered survival of B. burgdorferi within ticks, leading to strategies to reduce incidence of human Lyme disease.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
A clonal, infectious isolate of B. burgdorferi strain B31-A3 (wt) (Table 1), which has all the infection-associated plasmids, was used for the deletion of csrABb and restoration of a functional copy of the wild-type csrABb allele (ct strain) (22). Site-specific changes with 8 critical residues replaced with alanines (8S strain) or deletion of 7 amino acids at the C-terminal region of CsrABb (7D), as described previously, were used in this study (22, 50–56). All B. burgdorferi cultures were grown in 1% CO2 at 32°C in Barbour-Stoenner-Kelly II (BSK-II) liquid medium (pH 7.6) with 6% normal rabbit serum and supplemented with different concentrations (0, 30, 60, or 90 mM) of sodium acetate, sodium propionate, or sodium butyrate (10). Spirochetes were also grown to a density of 5 × 107 cells/ml in BSK-II growth medium that mimicked the tick midgut before (pH 7.6 and 23°C) and after (pH 6.8 and 37°C) a blood meal (35). In order to determine the combined effects of SCFAs at physiological levels, the wild-type strain was also shifted from conditions mimicking the midgut before (pH 7.6 and 23°C) to those after (pH 6.8 and 37°C) the ingestion of a blood meal in the presence of SCFAs at levels present in the human blood (30.4 μM sodium acetate, 1 μM sodium propionate, and 1 μM sodium butyrate), human cerebrospinal fluid (58 μM sodium acetate, 2.8 μM sodium propionate, and 1.4 μM sodium butyrate), or rat blood (350 μM sodium acetate, 5 μM sodium propionate, and 5 μM sodium butyrate) based on the data available at www.hmdb.ca or reported previously (57). Spirochetes were harvested at a density of 5 × 107 cells/ml for protein profile analysis or for RNA extractions. Escherichia coli TOP10 (Invitrogen, Carlsbad, CA) and Rosetta (DE3) pLysS (Novagen, Madison, MI) strains were used for all procedures involving cloning and overexpression of recombinant proteins, respectively. E. coli strains were cultured in Luria-Bertani (LB) broth supplemented with appropriate concentrations of antibiotics. These recombinant proteins were used to generate specific antibodies in BALB/c mice as needed for immunoblot analysis (16).
TABLE 1.
Borrelia burgdorferi strains used in this study
| B. burgdorferi strains | Description | Reference or source |
|---|---|---|
| B31-A3 (wt) | B31, low passage, virulent isolate | 50 |
| A3-ES10 (mt) | B31-A3, mutated in csrABb, Strr | 22 |
| A3-SR50 (ct) | A3-ES10, csrABb complemented, Genr | 22 |
| A3-RR59 (8S) | A3-ES10 complemented with RR59, 8 critical residues replaced with alanines, Genr | 22 |
| A3-RR66 (7D) | A3-ES10 complemented with RR66, 7-amino-acid C-terminal deletion strain, Genr | 22 |
Determination of growth rate of borrelial strains in the presence of SCFAs.
B. burgdorferi strains were propagated in BSK-II medium at pH 7.6 and 32°C with appropriate antibiotics and in the presence of various concentrations of SCFAs in triplicate. Growth was measured by enumerating viable spirochetes every 12 h for a period of 156 h, at which time the parental strain reached a density of 2 × 108 cells per ml (33). All experiments were repeated thrice.
RNA extraction and quantitative real-time PCR analysis.
Transcriptional analysis of key genes relevant to this study was done using quantitative real-time PCR analysis. Total RNA was extracted as previously described from B. burgdorferi cultures propagated under normal laboratory growth conditions (pH 7.6 and 32°C) and grown to a density of 2 × 106 to 3 × 106 spirochetes per ml in the presence of different concentrations of SCFAs (21). The total RNA was treated twice at 37°C for 45 min with DNase I to remove any contaminating DNA and quantified spectrophotometrically. Purity of the RNA sample was assessed using real-time PCR with recA primers (recAFq and recARq) to rule out the presence of contaminating DNA. RNA samples were reverse transcribed to cDNA using TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA). Real-time PCRs were set up with SYBR green PCR master mix with various oligonucleotide primers (Table 2) at a final concentration of 100 nM, and quantitative real-time PCR was done using an ABI Prism 7300 system (Applied Biosystems) as described previously (21). The cycle numbers of the detection threshold (CT) of each of the genes were averaged following normalization, and the levels of induction were determined with the ΔΔCT method where the quantity of each transcript was determined by the equation 2−ΔΔCT, as described previously. The normalized CT values obtained from cDNA samples from different borrelial strains were subjected to unpaired Student's t test implemented in Prism. Statistical significance was accepted when the P values were less than 0.05. Primers specific to rpoS, flaB, and ospC were used in this analysis along with recA-specific primers for normalization of the CT values.
TABLE 2.
Oligonucleotides used in this study
| Name | Sequence |
|---|---|
| recAFq | ATGCTCTTGATCCTGTTTATGCAA |
| recARq | GGTATCAGGCTGACTAAGCCAAA |
| flaBFq | CAGCTAATGTTGCAAATCTTTTCTCT |
| flaBRq | TTCCTGTTGAACACCCTCTTGA |
| rpoSFq | AGATATGCGGGTAAAGGGTTAAAA |
| rpoSRq | CAGCAGCTCTTATTAATCCCAAGTT |
| ospCFq | AATCAGTAGAGGTCTTGTCAAAAGCA |
| ospCRq | CCACAACAGGGCTTGTAAGCT |
SDS-PAGE and immunoblot analysis.
B. burgdorferi whole-cell lysates were prepared and separated on SDS–12.5% PAGE as described previously (16). The separated proteins were either visualized by Coomassie brilliant blue staining or transferred onto a polyvinylidene difluoride (PVDF) membrane (Amersham Hybond-P; GE Healthcare, Buckinghamshire, UK) and subjected to immunoblot analysis as described previously (21). The membranes were probed with monoclonal antibodies or monospecific serum against a variety of borrelial proteins. The blots were developed following incubation with appropriate dilutions of horseradish peroxidase (HRP)-conjugated anti-mouse, anti-rabbit, or anti-rat secondary antibodies using ECL Western blotting reagents (GE Healthcare).
Growth of spirochetes in DMCs implanted in rat peritoneal cavity.
B. burgdorferi strain B31-A3 (wt), csrABb mutant (mt), and the csrABb cis-complemented strain (ct) were propagated from frozen stocks in BSK-II liquid medium supplemented with 6% filter-sterilized normal rabbit serum (Pel-Freez Biological, Rogers, AR) at pH 7.6 and 32°C with 1% CO2. Once the spirochetes reached a density of 1 × 106 spirochete/ml, the cultures were further diluted to 5 × 104 spirochete/ml, and 10 ml of each diluted culture was transferred to a sterile dialysis membrane tube (6,000 to 8,000 molecular weight cutoff, 32-mm width; Spectra/Pro 6; Spectrum Labs) and implanted into the abdominal cavity of female Sprague-Dawley rats for 10 to 12 days (31). Three separate DMCs for each borrelial strain were pelleted by centrifugation at 4°C at 3,220 × g for 20 min and washed thrice with Hanks balanced salt solution (HBSS; Thermo Scientific HyClone, Logan, UT). Spirochetes propagated under in vitro conditions (pH 7.6 and 32°C) were also processed as described above, and total protein profiles were analyzed by silver-stained SDS–15% PAGE as described previously (31).
ACKNOWLEDGMENTS
This study was partly supported by Public Health Service grant AI123837 from the National Institute of Allergy and Infectious Diseases, the Army Research Office of the Department of Defense, under contract no. W911NF-11-1-0136, predoctoral fellowships from the South Texas Center for Emerging Infectious Diseases (Y.H.L. and S.L.R.K.), and the Center of Excellence in Infection Genomics (T.C.S.). T.C.S. was supported by a predoctoral fellowship from The Brown Foundation. Preliminary studies were supported by a 2016 PEP Award from the UTSA Office of the Vice-President of Research and funds from the Global Lyme Alliance.
We thank Sarah Helm, Brian Moy, and Taylor MacMackin for critical reading of the manuscript. We thank Patti Rosa for B. burgdorferi B31-A3 strain and Frank Gherardini for anti-RpoS serum.
REFERENCES
- 1.Groshong AM, Blevins JS. 2014. Insights into the biology of Borrelia burgdorferi gained through the application of molecular genetics. Adv Appl Microbiol 86:41–143. doi: 10.1016/B978-0-12-800262-9.00002-0. [DOI] [PubMed] [Google Scholar]
- 2.Samuels DS. 2011. Gene regulation in Borrelia burgdorferi. Annu Rev Microbiol 65:479–499. doi: 10.1146/annurev.micro.112408.134040. [DOI] [PubMed] [Google Scholar]
- 3.Radolf JD, Caimano MJ, Stevenson B, Hu LT. 2012. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol 10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, Gwinn M, Dougherty B, Tomb JF, Fleischmann RD, Richardson D, Peterson J, Kerlavage AR, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams MD, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fuji C, Cotton MD, Horst K, Roberts K, Hatch B, Smith HO, Venter JC. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 5.Corona A, Schwartz I. June. 2015. Borrelia burgdorferi: carbon metabolism and the tick-mammal enzootic cycle. Microbiol Spectr doi: 10.1128/microbiolspec.MBP-0011-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci U S A 92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevenson B, Schwan TG, Rosa PA. 1995. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun 63:4535–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ojaimi C, Brooks C, Casjens S, Rosa P, Elias A, Barbour A, Jasinskas A, Benach J, Katona L, Radolf J, Caimano M, Skare J, Swingle K, Akins D, Schwartz I. 2003. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect Immun 71:1689–1705. doi: 10.1128/IAI.71.4.1689-1705.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll JA, Cordova RM, Garon CF. 2000. Identification of 11 pH-regulated genes in Borrelia burgdorferi localizing to linear plasmids. Infect Immun 68:6677–6684. doi: 10.1128/IAI.68.12.6677-6684.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seshu J, Boylan JA, Gherardini FC, Skare JT. 2004. Dissolved oxygen levels alter gene expression and antigen profiles in Borrelia burgdorferi. Infect Immun 72:1580–1586. doi: 10.1128/IAI.72.3.1580-1586.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyde JA, Trzeciakowski JP, Skare JT. 2007. Borrelia burgdorferi alters its gene expression and antigenic profile in response to CO2 levels. J Bacteriol 189:437–445. doi: 10.1128/JB.01109-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caimano MJ, Drecktrah D, Kung F, Samuels DS. 2016. Interaction of the Lyme disease spirochete with its tick vector. Cell Microbiol 18:919–927. doi: 10.1111/cmi.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iyer R, Caimano MJ, Luthra A, Axline D Jr, Corona A, Iacobas DA, Radolf JD, Schwartz I. 2015. Stage-specific global alterations in the transcriptomes of Lyme disease spirochetes during tick feeding and following mammalian host adaptation. Mol Microbiol 95:509–538. doi: 10.1111/mmi.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bugrysheva JV, Pappas CJ, Terekhova DA, Iyer R, Godfrey HP, Schwartz I, Cabello FC. 2015. Characterization of the RelBbu regulon in Borrelia burgdorferi reveals modulation of glycerol metabolism by (p)ppGpp. PLoS One 10:e0118063. doi: 10.1371/journal.pone.0118063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drecktrah D, Lybecker M, Popitsch N, Rescheneder P, Hall LS, Samuels DS. 2015. The Borrelia burgdorferi RelA/SpoT homolog and stringent response regulate survival in the tick vector and global gene expression during starvation. PLoS Pathog 11:e1005160. doi: 10.1371/journal.ppat.1005160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Laar TA, Lin YH, Miller CL, Karna SL, Chambers JP, Seshu J. 2012. Effect of levels of acetate on the mevalonate pathway of Borrelia burgdorferi. PLoS One 7:e38171. doi: 10.1371/journal.pone.0038171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raju BV, Esteve-Gassent MD, Karna SL, Miller CL, Van Laar TA, Seshu J. 2011. Oligopeptide permease A5 modulates vertebrate host-specific adaptation of Borrelia burgdorferi. Infect Immun 79:3407–3420. doi: 10.1128/IAI.05234-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richards CL, Lawrence KA, Su H, Yang Y, Yang XF, Dulebohn DP, Gherardini FC. 2015. Acetyl-phosphate is not a global regulatory bridge between virulence and central metabolism in Borrelia burgdorferi. PLoS One 10:e0144472. doi: 10.1371/journal.pone.0144472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dulebohn DP, Richards CL, Su H, Lawrence KA, Gherardini FC. 2017. Weak organic acids decrease Borrelia burgdorferi cytoplasmic pH, eliciting an acid stress response and impacting RpoN- and RpoS-dependent gene expression. Front Microbiol 8:1734. doi: 10.3389/fmicb.2017.01734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanjuan E, Esteve-Gassent MD, Maruskova M, Seshu J. 2009. Overexpression of CsrA (BB0184) alters the morphology and antigen profiles of Borrelia burgdorferi. Infect Immun 77:5149–5162. doi: 10.1128/IAI.00673-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karna SL, Sanjuan E, Esteve-Gassent MD, Miller CL, Maruskova M, Seshu J. 2011. CsrA modulates levels of lipoproteins and key regulators of gene expression critical for pathogenic mechanisms of Borrelia burgdorferi. Infect Immun 79:732–744. doi: 10.1128/IAI.00882-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karna SL, Prabhu RG, Lin YH, Miller CL, Seshu J. 2013. Contributions of environmental signals and conserved residues to the functions of carbon storage regulator A of Borrelia burgdorferi. Infect Immun 81:2972–2985. doi: 10.1128/IAI.00494-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sze CW, Li C. 2011. Inactivation of bb0184, which encodes carbon storage regulator A, represses the infectivity of Borrelia burgdorferi. Infect Immun 79:1270–1279. doi: 10.1128/IAI.00871-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sze CW, Morado DR, Liu J, Charon NW, Xu H, Li C. 2011. Carbon storage regulator A (CsrA(Bb)) is a repressor of Borrelia burgdorferi flagellin protein FlaB. Mol Microbiol 82:851–864. doi: 10.1111/j.1365-2958.2011.07853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ouyang Z, Zhou J, Norgard MV. 2014. CsrA (BB0184) is not involved in activation of the RpoN-RpoS regulatory pathway in Borrelia burgdorferi. Infect Immun 82:1511–1522. doi: 10.1128/IAI.01555-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu H, Caimano MJ, Lin T, He M, Radolf JD, Norris SJ, Gheradini F, Wolfe AJ, Yang XF. 2010. Role of acetyl-phosphate in activation of the Rrp2-RpoN-RpoS pathway in Borrelia burgdorferi. PLoS Pathog 6:e1001104. doi: 10.1371/journal.ppat.1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chittori S, Savithri HS, Murthy MR. 2011. Preliminary X-ray crystallographic studies on acetate kinase (AckA) from Salmonella typhimurium in two crystal forms. Acta Crystallogr Sect F Struct Biol Cryst Commun 67:1658–1661. doi: 10.1107/S1744309111043740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chittori S, Savithri HS, Murthy MR. 2012. Structural and mechanistic investigations on Salmonella typhimurium acetate kinase (AckA): identification of a putative ligand binding pocket at the dimeric interface. BMC Struct Biol 12:24. doi: 10.1186/1472-6807-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chittori S, Simanshu DK, Banerjee S, Murthy AM, Mathivanan S, Savithri HS, Murthy MR. 2013. Mechanistic features of Salmonella typhimurium propionate kinase (TdcD): insights from kinetic and crystallographic studies. Biochim Biophys Acta 1834:2036–2044. doi: 10.1016/j.bbapap.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 30.Huang Y, Suyemoto M, Garner CD, Cicconi KM, Altier C. 2008. Formate acts as a diffusible signal to induce Salmonella invasion. J Bacteriol 190:4233–4241. doi: 10.1128/JB.00205-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caimano MJ. 2005. Cultivation of Borrelia burgdorferi in dialysis membrane chambers in rat peritonea. Curr Protoc Microbiol Chapter 12:Unit 12C-13. [DOI] [PubMed] [Google Scholar]
- 32.Akins DR, Bourell KW, Caimano MJ, Norgard MV, Radolf JD. 1998. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J Clin Investig 101:2240–2250. doi: 10.1172/JCI2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin YH, Romo JA, Smith TC II, Reyes AN, Karna SL, Miller CL, Van Laar TA, Yendapally R, Chambers JP, Seshu J. 2017. Spermine and spermidine alter gene expression and antigenic profile of Borrelia burgdorferi. Infect Immun 85:e00684-. doi: 10.1128/IAI.00684-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Laar TA, Hole C, Rajasekhar Karna SL, Miller CL, Reddick R, Wormley FL, Seshu J. 2016. Statins reduce spirochetal burden and modulate immune responses in the C3H/HeN mouse model of Lyme disease. Microbes Infect 18:430–435. doi: 10.1016/j.micinf.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang X, Goldberg MS, Popova TG, Schoeler GB, Wikel SK, Hagman KE, Norgard MV. 2000. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol Microbiol 37:1470–1479. doi: 10.1046/j.1365-2958.2000.02104.x. [DOI] [PubMed] [Google Scholar]
- 36.Belperron AA, Bockenstedt LK. 2001. Natural antibody affects survival of the spirochete Borrelia burgdorferi within feeding ticks. Infect Immun 69:6456–6462. doi: 10.1128/IAI.69.10.6456-6462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bockenstedt LK, Gonzalez DG, Haberman AM, Belperron AA. 2012. Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy. J Clin Investig 122:2652–2660. doi: 10.1172/JCI58813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Embers ME, Barthold SW, Borda JT, Bowers L, Doyle L, Hodzic E, Jacobs MB, Hasenkampf NR, Martin DS, Narasimhan S, Phillippi-Falkenstein KM, Purcell JE, Ratterree MS, Philipp MT. 2012. Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PLoS One 7:e29914. doi: 10.1371/journal.pone.0029914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vakulskas CA, Potts AH, Babitzke P, Ahmer BM, Romeo T. 2015. Regulation of bacterial virulence by Csr (Rsm) systems. Microbiol Mol Biol Rev 79:193–224. doi: 10.1128/MMBR.00052-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lambert RJ, Stratford M. 1999. Weak-acid preservatives: modelling microbial inhibition and response. J Appl Microbiol 86:157–164. doi: 10.1046/j.1365-2672.1999.00646.x. [DOI] [PubMed] [Google Scholar]
- 41.Sun Y, O'Riordan MX. 2013. Regulation of bacterial pathogenesis by intestinal short-chain fatty acids. Adv Appl Microbiol 85:93–118. doi: 10.1016/B978-0-12-407672-3.00003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. 1987. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roe AJ, McLaggan D, Davidson I, O'Byrne C, Booth IR. 1998. Perturbation of anion balance during inhibition of growth of Escherichia coli by weak acids. J Bacteriol 180:767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chattopadhyay MK, Tabor H. 2013. Polyamines are critical for the induction of the glutamate decarboxylase-dependent acid resistance system in Escherichia coli. J Biol Chem 288:33559–33570. doi: 10.1074/jbc.M113.510552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foster JW. 2004. Escherichia coli acid resistance: tales of an amateur acidophile. Nat Rev Microbiol 2:898–907. doi: 10.1038/nrmicro1021. [DOI] [PubMed] [Google Scholar]
- 46.Richard HT, Foster JW. 2003. Acid resistance in Escherichia coli. Adv Appl Microbiol 52:167–186. doi: 10.1016/S0065-2164(03)01007-4. [DOI] [PubMed] [Google Scholar]
- 47.Palmer GH, Bankhead T, Seifert HS. February. 2016. Antigenic variation in bacterial pathogens. Microbiol Spectr doi: 10.1128/microbiolspec.VMBF-0005-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmer GH, Bankhead T, Lukehart SA. 2009. “Nothing is permanent but change”–antigenic variation in persistent bacterial pathogens. Cell Microbiol 11:1697–1705. doi: 10.1111/j.1462-5822.2009.01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baumler AJ, Sperandio V. 2016. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535:85–93. doi: 10.1038/nature18849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elias AF, Stewart PE, Grimm D, Caimano MJ, Eggers CH, Tilly K, Bono JL, Akins DR, Radolf JD, Schwan TG, Rosa P. 2002. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect Immun 70:2139–2150. doi: 10.1128/IAI.70.4.2139-2150.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samuels DS. 1995. Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol Biol 47:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maruskova M, Esteve-Gassent MD, Sexton VL, Seshu J. 2008. Role of the BBA64 locus of Borrelia burgdorferi in early stages of infectivity in a murine model of Lyme disease. Infect Immun 76:391–402. doi: 10.1128/IAI.01118-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maruskova M, Seshu J. 2008. Deletion of BBA64, BBA65, and BBA66 loci does not alter the infectivity of Borrelia burgdorferi in the murine model of Lyme disease. Infect Immun 76:5274–5284. doi: 10.1128/IAI.00803-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Esteve-Gassent MD, Elliott NL, Seshu J. 2009. sodA is essential for virulence of Borrelia burgdorferi in the murine model of Lyme disease. Mol Microbiol 71:594–612. doi: 10.1111/j.1365-2958.2008.06549.x. [DOI] [PubMed] [Google Scholar]
- 55.Seshu J, Boylan JA, Hyde JA, Swingle KL, Gherardini FC, Skare JT. 2004. A conservative amino acid change alters the function of BosR, the redox regulator of Borrelia burgdorferi. Mol Microbiol 54:1352–1363. doi: 10.1111/j.1365-2958.2004.04352.x. [DOI] [PubMed] [Google Scholar]
- 56.Seshu J, Esteve-Gassent MD, Labandeira-Rey M, Kim JH, Trzeciakowski JP, Hook M, Skare JT. 2006. Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol Microbiol 59:1591–1601. doi: 10.1111/j.1365-2958.2005.05042.x. [DOI] [PubMed] [Google Scholar]
- 57.Hoverstad T, Midtvedt T. 1986. Short-chain fatty acids in germfree mice and rats. J Nutr 116:1772–1776. doi: 10.1093/jn/116.9.1772. [DOI] [PubMed] [Google Scholar]