Vivax malaria remains one of the most serious and neglected tropical diseases, with 132 to 391 million clinical cases per year and 2.5 billion people at risk of infection. A vaccine against Plasmodium vivax could have more impact than any other intervention, and the use of a vaccine targeting multiple antigens may result in higher efficacy against sporozoite infection than targeting a single antigen.
KEYWORDS: Plasmodium vivax, VLP, adenoviruses, malaria, vaccines
ABSTRACT
Vivax malaria remains one of the most serious and neglected tropical diseases, with 132 to 391 million clinical cases per year and 2.5 billion people at risk of infection. A vaccine against Plasmodium vivax could have more impact than any other intervention, and the use of a vaccine targeting multiple antigens may result in higher efficacy against sporozoite infection than targeting a single antigen. Here, two leading P. vivax preerythrocytic vaccine candidate antigens, the P. vivax circumsporozoite protein (PvCSP) and the thrombospondin-related adhesion protein (PvTRAP) were delivered as a combined vaccine. This strategy provided a dose-sparing effect, with 100% sterile protection in mice using doses that individually conferred low or no protection, as with the unadjuvanted antigens PvTRAP (0%) and PvCSP (50%), and reached protection similar to that of adjuvanted components. Efficacy against malaria infection was assessed using a new mouse challenge model consisting of a double-transgenic Plasmodium berghei parasite simultaneously expressing PvCSP and PvTRAP used in mice immunized with the virus-like particle (VLP) Rv21 previously reported to induce high efficacy in mice using Matrix-M adjuvant, while PvTRAP was concomitantly administered in chimpanzee adenovirus and modified vaccinia virus Ankara (MVA) vectors (viral-vectored TRAP, or vvTRAP) to support effective induction of T cells. We examined immunity elicited by these vaccines in the context of two adjuvants approved for human use (AddaVax and Matrix-M). Matrix-M supported the highest anti-PvCSP antibody titers when combined with Rv21, and, interestingly, mixing PvCSP Rv21 and PvTRAP viral vectors enhanced immunity to malaria over levels provided by single vaccines.
INTRODUCTION
Vivax malaria remains one of the world's most neglected tropical diseases (1, 2), and it is considered to be responsible for 132 to 391 million clinical cases per year with 2.5 billion people at risk of infection (3), mainly in Southeast Asia and Latin America (4). An efficacious vaccine against Plasmodium vivax or Plasmodium falciparum could have more impact in reducing or eliminating this disease than any other intervention (5, 6). However, parasite diversity and a complex life cycle are major obstacles hampering vaccine development, and it is considered that an efficacious vaccine may require harnessing cellular and humoral immune responses against multiple antigens (7). This view has prompted an evaluation of the efficacy of multiple antigens using various vaccine platforms against infection by Plasmodium berghei (8) and P. falciparum (9).
The leading vaccine candidates against P. vivax are the preerythrocytic antigen P. vivax circumsporozoite protein (PvCSP) (2, 10, 11) and the blood stage antigen Duffy binding protein (12, 13), both having completed clinical trials (https://clinicaltrials.gov/show/NCT01816113) (10). The low levels of protective efficacy of the PvCSP vaccine candidate VMP001 has prompted the development of improved strategies consisting of a particulate circumsporozoite antigen delivered as a virus-like particle (VLP), CSV-S,S (14), and Rv21 (15). The thrombospondin-related adhesion protein (TRAP) is another preerythrocytic vivax malaria antigen that has shown promise in a recent preclinical study (16). Effective preerythrocytic vivax vaccines, unlike blood stage approaches, would have the potential to prevent or eliminate the quiescent liver stage forms known as hypnozoites, which cause relapse long after a primary infection (17) and are among the greatest obstacles to malaria eradication (2).
For P. falciparum malaria, similar vaccine candidates based on CSP (PfCSP) and PfTRAP have a longer track record of development than their P. vivax counterparts, but, unfortunately, both have reported suboptimal protection in humans (18). The leading RTS,S/AS01 falciparum vaccine candidate, Mosquirix, has completed phase III clinical trials, but despite its approval by the European Medicines Agency for childhood immunization, it did not achieve an endorsement by the WHO, thus limiting its future integration into existing malaria control programs (19). The most recent results indicate that three vaccine doses of RTS,S/AS01 protected 37% of infants (20) and 47% of children (21) against severe malaria. In a recent phase IIa clinical trial, PfTRAP delivered in a chimpanzee adenovirus 63 and modified virus Ankara (ChAd63-MVA) prime-boost regime induced sterile protection in 21% of human volunteers (22).
Efforts to improve the efficacy of malaria vaccines have led to the development of vaccine approaches in which not a single antigen is targeted; instead multiple antigens, either from the same life cycle stage or from multiple stages, are targeted. In this regard, vaccine approaches targeting the two leading preerythrocytic vaccine candidates, CSP and TRAP, show enhanced protection in animal models of malaria (8, 23) compared to vaccines targeting a single antigen. A recent phase IIa clinical trial evidenced an enhancement in protective efficacy against challenge with P. falciparum using a combination of RTS,S/AS01B with recombinant viral vectors ChAd63 and MVA expressing ME-TRAP (where ME is multiepitope) (9), thus supporting this combination strategy.
A model of enhanced protection using a vaccine targeting CSP and TRAP was suggested by Bauza et al. (8), using a two-stage approach, whereby antibodies (Abs) to P. berghei CSP (PbCSP) limited the number of P. berghei sporozoites reaching the liver, followed by PbTRAP-specific CD8+ T cells destroying a reduced number of infected hepatocytes. Viral vectors encoding the target antigen have been shown to elicit high numbers of CD8+ T cells, particularly when adenovirus-modified vaccinia virus Ankara (Ad-M or viral-vectored TRAP [vvTRAP]) is used in heterologous prime-boost regimens (16, 22, 24). Thus, viral vectors are used here to deliver the PvTRAP antigen (vvTRAP) to stimulate cytotoxic T-lymphocyte (CTL) responses, whereas the VLP Rv21 was used to generate high titers of anti-CSP antibodies (Abs). Rv21 VLP is based on the hepatitis B surface antigen (HepBsAg), and it has been shown to be highly efficacious against a sporozoite challenge, providing complete, sterile protection following two doses of Rv21 in Matrix-M adjuvant (15).
While vaccination using two antigens would be expected to provide enhanced protection against infection, the possibility remains of inducing antigenic interference due to the use of adjuvants or platforms to deliver the antigens. The phenomenon of antigenic interference may hamper the success of any combination vaccine due to the decrease in antibody or cytotoxic T-cell responses against any of the vaccine antigens, which has been observed in studies where two or more antigens have been combined (8, 25). Here, we aimed to augment the efficacy of known vivax preerythrocytic vaccines by combining CSP and TRAP using two different vaccine platforms for each antigen and two adjuvants suitable for human use: (i) AddaVax, a squalene-based oil-in-water nanoemulsion with a formulation similar to that of MF59 (26), and (ii) Matrix-M, a nanoparticle saponin-based adjuvant (27, 28).
RESULTS
In order to assess in vivo protective efficacy of the different P. vivax vaccine platforms, we developed a rodent challenge model consisting of a double-chimeric P. berghei parasite line that was generated by replacing the Pbtrap with the Pvtrap gene in a single chimeric line in which the Pbcsp gene had initially been replaced by the VK210 allele of Pvcsp. The single chimeric line, PbANKA-PvCSP-VK210(r)PbCSP (line 2196cl1), was developed through the use of gene insertion/marker out (GIMO) transfection technology as described previously (15) and is free of a drug-selectable marker. In addition, the resulting parasite contains the green fluorescent protein (GFP)-luciferase fusion gene as a reporter. The absence of a drug-selectable marker permitted the replacement of the Pbtrap gene with the Pvtrap gene in parasites of the PbANKA-PvCSP-VK210(r)PbCSP line in a single transfection experiment using a trap replacement construct described previously (16). This construct contains a synthetic trap allele composed of the protein-coding sequence of P. vivax TRAP (NCBI accession number XM_001614097) from the Salvador I strain that was codon optimized for expression in P. berghei using GeneOptimizer software. Transfection of PbANKA-PvCSP-VK210(r)PbCSP parasites with the trap replacement construct and positive selection with pyrimethamine resulted in selection of double-chimeric parasites [PbANKA-PvCSP-VK210(r)PbCSP-PvTRAP(r)PbTRAP], referred to as PvCSP-VK210/PvTRAP parasites (see Fig. S1 in the supplemental material). Integration of the Pvcsp and Pvtrap genes in PvCSP/PvTRAP was confirmed by diagnostic Southern analysis of chromosomes separated by pulsed-field gel electrophoresis and diagnostic PCR on genomic DNA (gDNA) (Fig. S2A and B). PvCSP-VK210/PvTRAP parasites are double-chimeric P. berghei parasites that do not contain the P. berghei csp or trap gene coding sequence (CDS) but express both P. vivax csp-VK210 and the P. vivax trap CDS under the control of the P. berghei csp and trap equivalent regulatory sequences and is drug-selectable marker free.
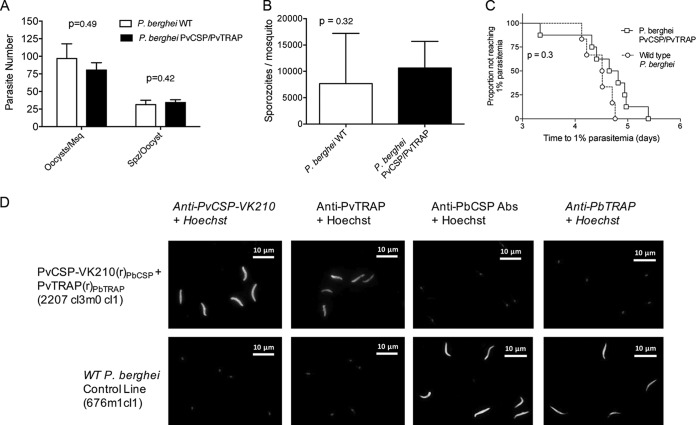
We next assessed the ability of the double-replacement chimeric parasites to produce oocysts and sporozoites since replacing the endogenous csp and trap genes with the P. vivax orthologs could alter these characteristics. Oocyst and sporozoite production in Anopheles stephensi of PvCSP-VK210/PvTRAP was similar to oocyst and sporozoite production of wild-type (WT) P. berghei (Fig. 1A and B). The infectivity of sporozoites of both transgenic parasites was assessed by determination of the prepatent period (i.e., the time to 1% parasitemia) after intravenous injection of 2,000 sporozoites in the tail vein of BALB/c mice. All mice developed blood parasitemia, and the times to reach 1% parasitemia were not different between WT and PvCSP-VK210/PvTRAP parasites (Fig. 1C). Expression of the Pvcsp-VK210 and Pvtrap proteins in PvCSP-VKCSP/PvTRAP sporozoites was confirmed by immunofluorescence microscopy of sporozoites using anti-PvCSP monoclonal antibodies to the repeat region of the PvCSP-VK210 and polyclonal anti-PvTRAP antibodies. PbTRAP was present only in wild-type sporozoites and not in PvCSP-VK210/PvTRAP sporozoites (Fig. 1D). These results demonstrate that the double-chimeric PvCSP-VK210/PvTRAP parasites produce infectious sporozoites, which are able to complete full liver stage development in mice.
FIG 1.
Parasite fitness and phenotype characterization of a double-transgenic P. berghei parasite expressing Plasmodium vivax CSP-VK210 and TRAP. The ability of the PvCSP-VK210/PvTRAP double-transgenic parasite to develop in the invertebrate host was evaluated. (A) Ten days after feeding on infected mice, mosquito midguts were obtained, and oocysts were enumerated for both wild-type and PvCSP-VK210/PvTRAP 2207 cl3m0cl1 parasites. (B) Twenty-one days after feeding, salivary gland sporozoites were extracted and enumerated, and the numbers of sporozoites per mosquito was compared between wild-type and PvCSP-VK210/PvTRAP 2207 cl3m0cl1-infected Anopheles stephensi. Data are shown as means with standard deviations, and P values from t tests. (C) The ability of 2207 cl3m0cl1 to infect vertebrate hosts was evaluated by injecting salivary gland sporozoites into naive BALB/c mice (n = 6) and monitoring for blood stage infection. Outcome is shown as the time to reach 1% blood stage parasitemia. A Mantel-Cox test was performed between groups. (D) Transgenic salivary gland sporozoites were stained with anti-PvCSP-VK210, polyclonal anti-PvTRAP, or with serum from vaccinated mice with ChAd63-MVA expressing PvTRAP or PbTRAP. Alexa Fluor 488-labeled anti-mouse IgG and Hoechst-33342 (nuclear staining) were also included for the assay. As a control, wild-type (WT) P. berghei sporozoites were stained with the same antibodies. Merged images of the different channels are shown for both transgenic and WT P. berghei stained images.
Next, we used these chimeric PvCSP-VK210/PvTRAP sporozoites as a challenge model by infecting immunized mice and assessing protective efficacy of various combinations of PvCSP-VK210 and PvTRAP vaccines.
Combining vvTRAP with vivax CSP enhances protection compared to that of either component alone.
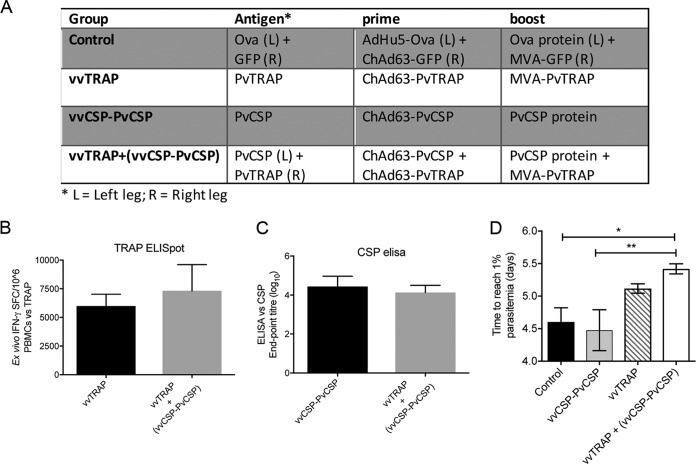
The prime-boost using the malaria vaccine candidate TRAP (thrombospondin-related adhesion protein), expressed by adenoviral or modified vaccinia virus Ankara viral vectors (viral-vectored TRAP, or vvTRAP), combined with a regime consisting of adenovirus expressing CSP followed by a protein boost (Ad-P) CSP has previously been tested, showing enhancement in protection against a challenge with wild-type P. berghei compared to use of either antigen alone (8). Here, we applied a similar vaccination strategy using P. vivax antigens to assess protective efficacy against a challenge with the double-transgenic PvCSP-VK210/PvTRAP P. berghei described above; BALB/c mice were vaccinated as shown in Fig. 2A. High frequencies of ex vivo gamma interferon (IFN-γ) enzyme-linked immunosorbent spot (ELISpot) responses were induced against an immunodominant PvTRAP CD8+ epitope (Fig. 2B). Similarly, high titers of anti-CSP antibodies (Fig. 2C) were obtained, and no significant differences in either antibody titers or T-cell frequencies were observed between a single- and a double-component vaccine. No vaccine approach conferred sterile protection to any mouse following a parasite challenge (Fig. 2D). However, the combination of vvTRAP and Ad-P CSP, vvTRAP plus (vvCSP-PvCSP), significantly enhanced protective efficacy, measured as an increase in the time taken to reach 1% blood stage parasitemia compared to results with either component vaccine alone, confirming that, as with wild-type P. berghei, the combination of vivax vvTRAP and CSP antigens enhances protection against a sporozoite challenge even when equal magnitudes of adaptive humoral and cellular immune responses were induced, indicating the advantage of using two different vaccine candidates simultaneously for vaccination. To determine if higher levels of protection could be obtained, we next substituted vvCSP and PvCSP protein for Rv21, a P. vivax CSP preerythrocytic vaccine based on a hepatitis B virus-like particle (VLP), which has recently been shown to induce high levels of protective efficacy against a challenge with transgenic sporozoites expressing vivax CSP (15).
FIG 2.
Immunogenicity and protective efficacy of a combination P. vivax CSP-VK210 with adenoviral or MVA viral vectors expressing P. vivax TRAP (vvTRAP). (A) Inbred female BALB/c mice (n = 7 to 8/group) were primed with PvCSP-VK210 viral vector (vv) followed by the PvCSP protein (vvCSP-PvCSP), ChAd63-MVA viral vector expressing PvTRAP (vvTRAP), or their combination (vvTRAP+vvCSP-PvCSP). (B) Two weeks postboost ex vivo IFN-γ ELISpot responses were measured upon restimulation of whole-blood PBMCs with three subpools of peptides representing the full-length PvTRAP. Values are means and standard errors of the means. (C) An endpoint titer ELISA was done to quantify anti-PvCSP-VK210 antibodies. Bars indicate median responses. (D) Two weeks after the last vaccination, animals were challenged by intravenous injection of 2,000 double-transgenic P. berghei PvCSP-VK210 and PvTRAP sporozoites. Blood stage infection was recorded over three consecutive days starting at day 5 postchallenge, and a linear regression model predicting time to 1% blood stage parasitemia for individual animals was applied, showing a significant delay in the vvTRAP+vvCSP-PvCSP group that received a combination vaccine compared to time for the other treatment groups. Values are means and standard errors of the means. *, P < 0.05; **, P < 0.01 (Mann-Whitney test comparing two groups).
Effect on immunogenicity of a combination of vvTRAP, Rv21, and two adjuvants.
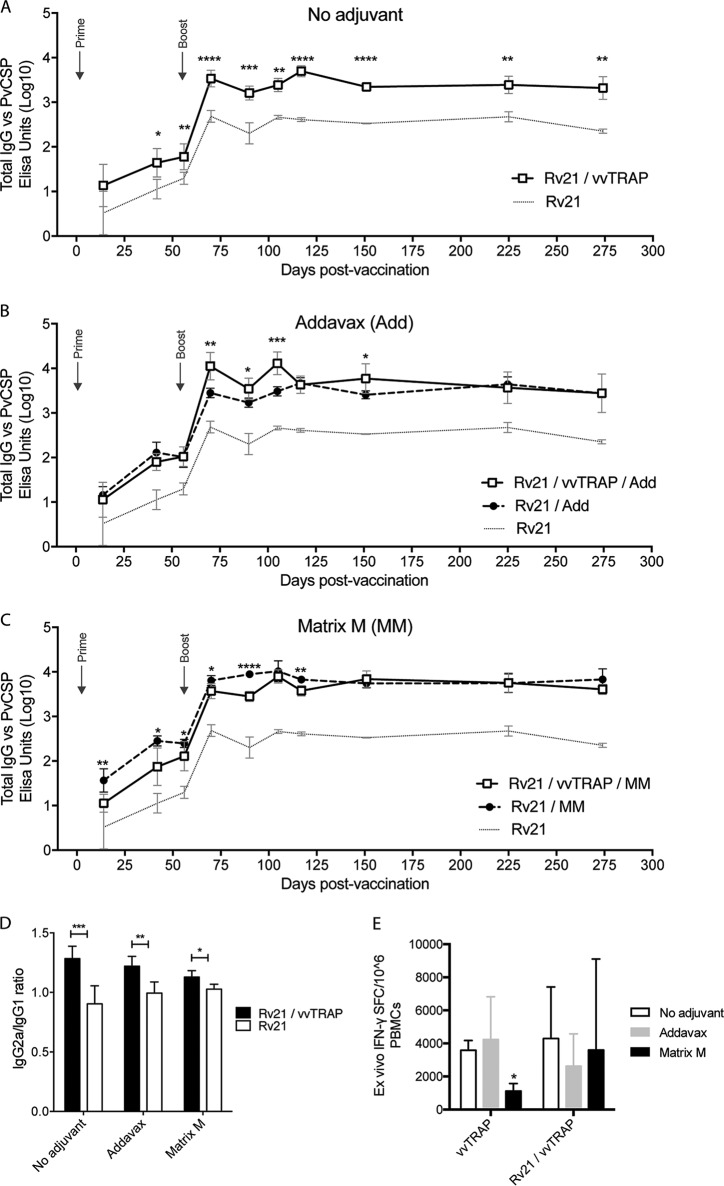
We investigated various approaches to adjuvant immune responses to the P. vivax vaccine candidates CSP and TRAP. To this end, BALB/c mice were vaccinated with combinations of vvTRAP and Rv21 in the presence or absence of two adjuvants suitable for human use (Table 1). The combination consisted of a mixture of TRAP viral vectors (vvTRAP) delivered as an adenovirus expressing vivax TRAP with Rv21 presenting CSP used for priming and followed by a boost 8 weeks later using a combination of MVA-TRAP mixed with Rv21. Of interest, we observed a significant adjuvanting effect on the vivax CSP antibodies just by mixing vvTRAP with Rv21 at the doses indicated in Table 1 in the absence of any chemical adjuvant. This was evident both after a prime with adenovirus plus Rv21 and after a boost with MVA plus Rv21 and for a follow-up period of 275 days (Fig. 3A). Next, we studied the effect of adding two clinically relevant adjuvants to Rv21 or to the mixture of Rv21 plus vvTRAP (Rv21+vvTRAP). We first tested AddaVax, a squalene-based oil-in-water nanoemulsion with a formulation similar to that of MF59 (26). AddaVax-adjuvanted Rv21 increased significantly the anti-PvCSP titers after both a prime and homologous Rv21 prime/boost regimens compared to results with Rv21 alone. When AddaVax was added to the mixture of Rv21+vvTRAP, a significant increase in titers was seen after an MVA boost compared to results using Rv21 with AddaVax alone (Fig. 3B). This trend continued for up to 150 days, a time when responses became similar in both groups and remained comparable until the end of the study. Additionally, we tested Matrix-M, a saponin-based adjuvant (27, 28) that has been shown to enhance Rv21 immunity against a sporozoite challenge (15). As expected, Matrix-M significantly enhanced the anti-PvCSP responses elicited by Rv21 compared to those of the group with no adjuvant. However, the inclusion of viral vectors to the mixture resulted in a decrease in PvCSP-specific antibodies, which was evident at various time points after the prime or after the boost. The antigenic interference effect of viral vectors with Matrix-M appeared to be temporary as beyond 150 days postprime, anti-CSP titers in both groups receiving Matrix-M adjuvants were similar (Fig. 3C). Mixing vvTRAP with Rv21 significantly increased the IgG2a/IgG1 ratio, and the use of adjuvants also contributed to enhance this ratio in the absence of adjuvants (Fig. 3D). TRAP-specific CD8+ responses induced by viral vectors did not show major differences in the presence or absence of adjuvants, with the exception of a decrease in IFN-γ released by CD8+ T cells in the Matrix-M group, measured in an ex vivo IFN-γ ELISpot assay after a boost with a mixture (Fig. 3E). This confirms recent observations made in our laboratory (29).
TABLE 1.
Vaccination regimes using viral vectors expressing TRAP (vvTRAP) and Rv21 in BALB/c mice
| Vaccine regimen (adjuvant)a | Prime |
Boost |
||
|---|---|---|---|---|
| Rv21-CSP (μg) | Ad-PvTRAP (IU) | Rv21-CSP (μg) | MVA-PvTRAP (PFU) | |
| Rv21 + vvTRAP | 1.5 | 1 × 108 | 1.5 | 1 × 107 |
| Rv21 | 1.5 | 1.5 | ||
| vvTRAP | 1 × 108 | 1 × 107 | ||
| Rv21 + vvTRAP (AddaVax) | 1.5 | 1 × 108 | 1.5 | 1 × 107 |
| Rv21 (AddaVax) | 1.5 | 1.5 | ||
| vvTRAP (AddaVax) | 1 × 108 | 1 × 107 | ||
| Rv21 + vvTRAP (Matrix-M) | 1.5 | 1 × 108 | 1.5 | 1 × 107 |
| Rv21 (Matrix-M) | 1.5 | 1.5 | ||
| vvTRAP (Matrix-M) | 1 × 108 | 1 × 107 | ||
Adjuvant, where present, was used in both prime and boost. For all regimens, the prime-boost interval was 8 weeks.
FIG 3.
Adjuvanticity in a combination of Rv21 P. vivax CSP VLP with adenovirus and modified vaccinia Ankara viral vectors expressing P. vivax TRAP (vvTRAP). Inbred BALB/c mice (n = 6 per group) were vaccinated with combinations of CSP and vvTRAP as described in Table 1. Kinetics analysis of anti-CSP antibodies, measured by ELISA at the indicated time points, was determined with or without coadministration of vvTRAP. (A) Effect of the coadministration of Rv21 (PvCSP) with vvTRAP without addition of adjuvants. (B) Effect of AddaVax on anti-PvCSP antibodies induced by Rv21 alone or by Rv21+vvTRAP. (C) Effect of Matrix-M on anti-PvCSP antibodies induced by Rv21 alone or by Rv21+vvTRAP. (D) IgG2a/IgG1 ratios in BALB/c mice using Rv21 with or without vvTRAP and adjuvants. (E) Effect of Rv21, AddaVax, and Matrix-M on ex vivo IFN-γ responses against PvTRAP in mice. t tests were used for statistical analyses. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Efficacy of a preerythrocytic vivax vaccine is increased using a bivalent vaccine consisting of vvTRAP and PvCSP in VLPs.
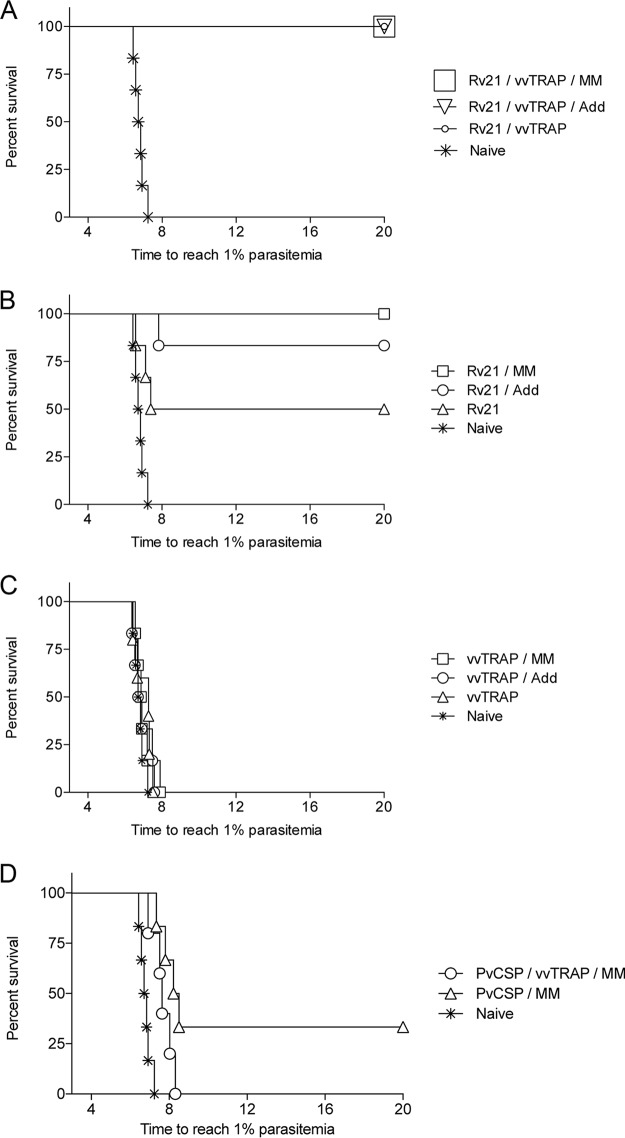
The initial results indicating an adjuvant effect on P. vivax CSP by the use of a combination of viral vectors, virus-like particles (VLPs), Matrix-M, and AddaVax prompted us to investigate if the increased antibody titers resulted in enhanced protective efficacy against malaria infection. To this end, we challenged vaccinated mice with our newly developed chimeric PvCSP-VK210/PvTRAP sporozoites (Fig. 4). Unadjuvanted bivalent vaccines consisting of vvTRAP, mixed with only 1.5 μg of Rv21 VLP presenting PvCSP on its surface, protected 100% of the challenged mice, providing sterile efficacy with no signs of parasitemia for up to 20 days postchallenge. Inclusion of Matrix-M and AddaVax in the formulation retained the high protective levels of combined vaccines (Fig. 4A). Importantly, removing vvTRAP from the vaccine composition still yielded 100% sterile efficacy in Rv21 plus Matrix-M, while there was a trend toward reduction of efficacy in Rv21 plus AddaVax on a challenge (83.3% protection with AddaVax versus 100% with Matrix-M; P = 0.36) under conditions where only 50% of the mice were sterilely protected after vaccination with unadjuvanted Rv21 (Fig. 4B). In contrast, prime/boost vaccination with only vvTRAP did not protect mice against a sporozoite challenge, regardless of the presence or absence of adjuvants (Fig. 4C). Our results indicate a dose-sparing effect of the vaccines by administering Rv21 combined with either vvTRAP or adjuvants to enhance immunity against malaria without the need of increasing vaccine doses and open a possibility for the use of the combination as a fractional dose while retaining protection against malaria. Finally, we investigated if a protein in adjuvant had similar effects on protection and injected vivax CSP protein (pPvCS) in Matrix-M. Sterile protective efficacy was achieved in only 33% of the vaccinated mice (Fig. 4D) in contrast to 100% of protection elicited by Rv21 in Matrix-M, stressing the advantage of using a VLP over a soluble antigen. A summary of the survival efficacy for all groups is presented in Table 2.
FIG 4.
Protective efficacy against a transgenic sporozoite challenge using a combination of the Rv21 and vvTRAP, in the presence of Matrix-M or AddaVax adjuvants. Inbred BALB/c mice (n = 6 per group) vaccinated with Rv21 and/or vvTRAP as described in Table 1 were challenged by intravenous administration of transgenic P. berghei PvCSP-VK210/PvTRAP double-replacement parasites using a stringent challenge with 2,000 sporozoites. (A) Efficacy of a vaccine consisting of Rv21 combined with either vvTRAP or of vvTRAP plus the adjuvant Matrix-M (MM) or AddaVax (Add). (B) Efficacy of Rv21 in the presence of chemical adjuvants. (C) Efficacy of a prime-boost vaccination regimen using vvTRAP. (D) Efficacy of a P. vivax CSP protein in combination with vvTRAP and Matrix-M adjuvants.
TABLE 2.
Summary of the survival efficacy induced by P. vivax CSP plus TRAP with vaccination regimens using vvTRAP, protein, or Rv21 in mice challenged by P. berghei sporozoitesa
| Vaccine regimenb | No. of protected mice/total no. of mice | Sterile protection (%) | Median survival (days) | Survival limit (days) | Mean survival ± SD (days) | SE |
|---|---|---|---|---|---|---|
| Naive | 0/6 | 0 | 6.78 | 6.49–7.08 | 6.78 ± 0.28 | 0.12 |
| vvTRAP | 0/6 | 0 | 7.28 | 6.46–7.62 | 7.12 ± 2.5 | 0.21 |
| Rv21 | 3/6 | 50 | 13.69 | 6.06–20.98 | 13.52 ± 7.1 | 2.9 |
| Rv21+vvTRAP | 6/6 | 100 | 20 | 20.0–20.0 | 20.0 ± 0 | 0 |
| vvTRAP+MM | 0/6 | 0 | 6.89 | 6.52–7.53 | 7.03 ± 0.48 | 0.20 |
| Rv21+MM | 6/6 | 100 | 20 | 20.0–20.0 | 20.0 ± 0 | 0 |
| Rv21+vvTRAP+MM | 6/6 | 100 | 20 | 20.0–20.0 | 20.0 ± 0 | 0 |
| pCS+MM | 3/6 | 33 | 8.36 | 5.44–18.51 | 11.98 ± 6.23 | 2.54 |
| vvTRAP+pCS+MM | 0/6 | 0 | 7.64 | 7.02–8.35 | 7.68 ± 0.53 | 0.24 |
| vvTRAP+Add | 0/6 | 0 | 6.81 | 6.41–7.46 | 6.9 ± 0.5 | 0.20 |
| Rv21+Add | 5/6 | 83.3 | 20 | 12.7–23.2 | 20.0 ± 0 | 2.03 |
| Rv21+vvTRAP+Add | 6/6 | 100 | 20 | 20.0–20.0 | 20.0 ± 0 | 0 |
Vaccinated BALB/c mice were challenged with 2,000 sporozoites of the PvCSP-PvTRAP 2207 cl1 strain, as described in the legend of Fig. 4.
Adjuvants used were Matrix-M (MM) and AddaVax (Add).
A combination of Rv21 VLP and viral vectors requires vivax antigens in both platforms to enhance protective efficacy against a sporozoite challenge.
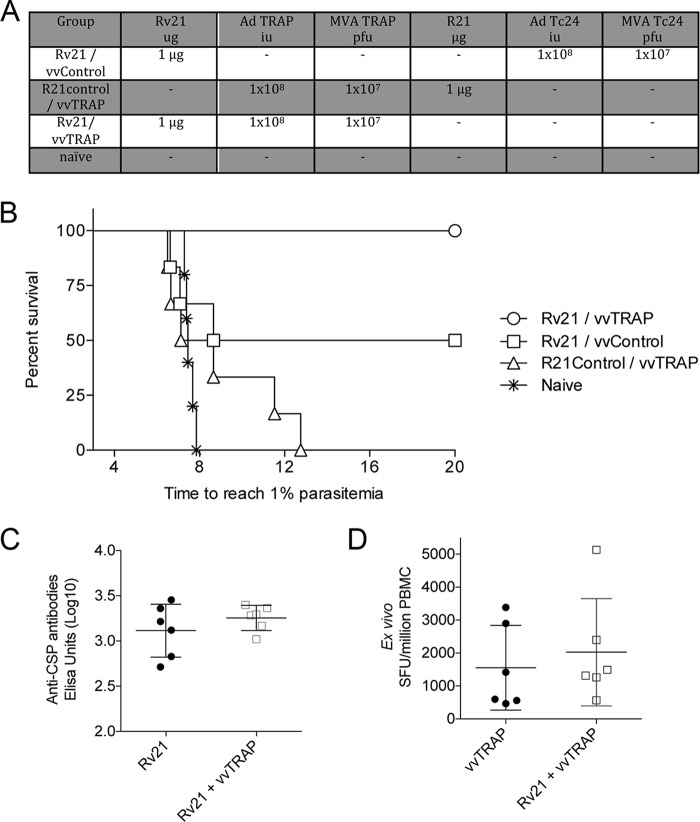
In the experiments described in the sections above, anti-PvCSP antibody responses induced by injection of 1.5 μg of Rv21 were adjuvanted by coimmunization with vvTRAP. The adjuvanting effect, unless properly controlled for, would make the interpretation of enhancement of protection from Rv21 and vvTRAP coadministration difficult as it would not be clear if any enhancement in protection derived from the increased breadth of response to multiple antigens or merely from an improved immunogenicity to PvCSP titers resulting from inclusion of vvTRAP in the vaccine formulation. To this end, lower doses of 1 μg of Rv21 were mixed with viral vectors expressing an unrelated Trypanosoma cruzi antigen, Tc24 (30), while a control group received vvTRAP mixed with R21 presenting P. falciparum CSP (Fig. 5A) (31). This resulted, as desired, in identical anti-CSP titers in the Rv21 and Rv21+vvTRAP groups and identical TRAP-specific ELISpot assay responses in the vvTRAP and Rv21+vvTRAP groups, thus eliminating the possibility that an enhancement of protection in the Rv21+vvTRAP group compared to protection of the single-component groups was due to vvTRAP enhancing antibody responses to PvCSP (Fig. 5C and D). Complete protection was achieved only in the group in which Rv21 and vvTRAP were combined (Fig. 5B). In contrast, when Rv21 was mixed with viral vectors expressing an unrelated Chagas Tc24 antigen (vvControl), only 50% of the mice were completely protected, and no sterile protection was afforded upon vaccination with vvTRAP mixed with R21 presenting P. falciparum CSP.
FIG 5.
Assessment of vaccine efficacy using a combination of Rv21 with viral-vectored vaccines expressing P. vivax TRAP (vvTRAP) or an unrelated transgene (vvControl). (A) Table indicating the vaccination components in an 8-week prime-boost regimen, utilized to compare the efficacy against a sporozoite challenge with a combination of a P. vivax VLP (Rv21), a P. falciparum VLP (R21) with vvTRAP, or an unrelated nonmalaria antigen vvControl. (B) Survival of mice (n = 6) after a sporozoite challenge with the vaccination regimens described in panel A. (C) Titer of anti-PvCSP antibodies by Rv21 or a combination of Rv21 plus vvTRAP. (D) Ex vivo IFN-γ responses against TRAP measured by ELISpot assay. SFU, spot-forming units.
DISCUSSION
Here, it is demonstrated that immunity against malaria using parasites expressing P. vivax antigens is enhanced by a combination of two preerythrocytic vaccine candidates, PvCSP and PvTRAP. This is achieved in mice using a newly developed double-transgenic P. berghei parasite in which the endogenous PbCSP and PbTRAP were replaced by their P. vivax homologues. Our initial observations using a combination of a P. vivax CSP protein with vvTRAP resulted in suboptimal protection against a sporozoite challenge with the double-transgenic parasite. Importantly, when the CSP protein was replaced with the virus-like particle (VLP) Rv21, 100% protection was obtained even in the absence of any adjuvant. Our results open an opportunity to replace an adjuvant by viral vectors expressing other malaria antigens to enhance protective immunity through the improvement of immune responses or by targeting additional malaria antigens. This is important due to the limited number of adjuvants that are currently available for human use (32). Greater availability of immunostimulatory alternatives could contribute to reducing reactogenicity as this is the inevitable price for improving immunogenicity in adjuvanted vaccines (33). Moreover, our results support the use of fractional doses of a VLP due to a dose-sparing effect when Rv21 and viral vectors were combined, and while an adjuvant affects the cost of a vaccine, replacing it with a viral vector may not significantly alter the total cost of a vaccination approach and gives an opportunity to assess alternative methods to limit reactogenicity while improving the breadth of responses, thus enhancing immunity against malaria.
Our results support the notion that protective immunity against malaria may require harnessing humoral and cellular responses against multiple antigens (7), which has been shown in earlier studies where a combination the two preerythrocytic antigens, CSP and TRAP, has enhanced protection against mouse malaria in a P. berghei mouse sporozoite challenge (8). Such studies stressed the importance of the protective effect of monoclonal antibodies against CSP and how, at suboptimal doses, protection was rescued by viral vector vaccination against P. berghei TRAP. In the current study, we have confirmed this observation using the P. vivax proteins as vaccine targets by maximizing anti-CSP antibody responses through the use of the Rv21 VLP and T-cell responses using viral vectors expressing TRAP. Similar observations have been made for P. falciparum. In a recent phase IIa clinical trial (9), a leading P. falciparum malaria vaccine was assessed for protective efficacy against a sporozoite challenge. This consisted of a CSP-based VLP RTS,S in combination with viral-vectored ME-TRAP. In that study, the combination showed evidence of being more efficacious (82.4%) than RTS,S alone (75%). This demonstrates a difficulty also encountered in the design of the present study: establishing that the combination of TRAP and CSP enhances efficacy requires that both regimens alone elicit suboptimal protection. A mathematical model (34) predicts that combining an RTS,S vaccine of 50% efficacy with a viral-vectored ME-TRAP regime with 21% efficacy would confer protective efficacy of 97%. The murine evidence presented here and elsewhere (16, 23) is consistent with this prediction and suggests that a strategy of combining subunit vaccines could prove crucial in developing a malaria vaccine of the desired 75% efficacy (35) and yet provide a dose-sparing effect where improved efficacy with low doses of vaccines could contribute to a decrease in vaccine costs.
Our results also indicate that an improvement in protective efficacy requires the combination of Rv21 with vivax vvTRAP to act synergistically in order to completely eliminate infection since the use of viral vectors expressing unrelated transgenes does not produce improved immunity against malaria. This indicates that augmenting the breadth of responses to multiple antigens plays an important role in the increase in protective efficacy seen here with Rv21 and vvTRAP coadministration. This supports the observations made previously stressing the major challenge posed by the complexity of the malaria parasite that transitions from extracellular to intracellular stages during the life cycle in the mammalian host, thus requiring harnessing cellular and humoral responses against more than one antigen to induce protective immunity (7).
It is possible that a CSP/TRAP combination regime without adjuvants could be used in future P. vivax clinical trials, given that viral vectors have been shown here to possess as potent an adjuvant effect as the other adjuvants tested. It remains to be seen whether the adjuvanting effects of VLPs and viral vectors on each other in the absence of adjuvant are recapitulated in phase I and II trials.
When considering the implications of these results on the choice of adjuvant for a CSP/TRAP combination vaccine, we must also consider the long-term effects of adjuvants on immune responses. Antigenic interference was not seen in the medium to long term with Matrix-M or AddaVax in this study. Indeed, 150 days after priming, anti-CSP titers were highest in Matrix-M-vaccinated groups. Given that the malaria research community is aiming for a vaccine which remains 75% effective for 2 years (35), longevity of protection may prove more important than short-term antigenic effects on interference in the decision about the most appropriate adjuvant to use. In this regard, Matrix-M and AddaVax are equally good in the induction of protective immunity against malaria, at least in terms of antibody titers.
In summary, the combination of two P. vivax antigens, CSP and TRAP, using appropriate platforms for each candidate (a VLP for CSP and viral vectors for TRAP), improves protective efficacy compared to use of either alone. Improved immunogenicity and protective efficacy was also seen with the use of AddaVax and Matrix-M adjuvants in combination with Rv21. This study demonstrates the potential of combining subunit antigens using suitable platforms as a method of enhancing the protective efficacy of malaria vaccines.
MATERIALS AND METHODS
Generation of DNA constructs and genotyping of the double-chimeric parasite line PvCSP/PvTRAP.
Two reference WT lines of P. berghei ANKA were used to construct a double-chimeric parasite: the wild-type (WT) reference line cl15cy1 of P. berghei ANKA (36) and the reporter PbANKA parasite line PbGFP-Luccon (676m1cl1). The PbGFP-Luccon parasite expresses a fusion protein of GFP (mutant 3) and firefly luciferase (LUC-IAV) under the constitutive eef1a promoter and is selectable marker (SM) free (37). The reporter cassette is integrated into the neutral 230p locus (Eukaryotic Pathogen Database [EuPathDB] accession number PBANKA_030600). For details of PbGFP-Luccon, see the RMgm (Rodent Malaria genetically modified) database (entry RMgm-29 [http://www.pberghei.eu/index.php?rmgm=29]).
For the generation and genotyping of the chimeric parasites, we used Swiss mice (OF1/ico, construct 242; 6 weeks old; 25 to 26 g) (Charles River). All animal experiments performed at the Leiden University Medical Center (LUMC) were approved by the Animal Experiments Committee of the Leiden University Medical Center (DEC 12042). The Dutch Experiments on Animals Act was established under European guidelines (European Union directive no. 86/609/EEC regarding the Protection of Animals used for Experimental and Other Scientific Purposes).
The double-chimeric line PvCSP-VK210/PvTRAP in which both the csp (EuPathDB accession number PBANKA_040320) and the trap (EuPathDB accession number PBANKA_134980) CDSs of P. berghei were replaced with csp (EuPathDB accession number PVX_119355) and trap (NCBI accession number XM_001614097) genes of P. vivax was generated by replacing the Pbtrap gene with Pvtrap in a single chimeric line in which the Pbcsp gene had been previously replaced by the VK210 allele of Pvcsp. The single-chimeric line, PbANKA-PvCSP-VK210(r)PbCSP (line 2196cl1), had been generated using the gene insertion/marker out (GIMO)-based transfection technology as described previously (15) and is free of a drug-selectable marker. In addition, it contains the GFP-luciferase fusion gene as a reporter. The absence of a drug-selectable marker allowed the replacement of the Pbtrap gene with the Pvtrap gene in parasites of the PbANKA-PvCSP-VK210(r)PbCSP line in a single transfection experiment using the trap replacement construct described by Bauza et al. (16). This construct contains a synthetic trap allele composed of the protein coding sequence of P. vivax TRAP (NCBI accession number XM_001614097) from the Salvador I strain, which was codon optimized for expression in P. berghei using GeneOptimizer software. In addition, this construct contains a positive/negative selectable marker (SM) cassette with the human dhfr and yeast fcu (hdhfr::yfcu) fusion gene. Transfection of PbANKA-PvCSP-VK210(r)PbCSP parasites with the Pvtrap replacement construct and positive selection with pyrimethamine were performed using standard methods for transfection of P. berghei (36). This resulted in selection of double-chimeric parasites (line 2207). Selected double-chimeric parasites were cloned by the method of limiting dilution (38), resulting in the PbANKA-PvCSP-VK210(r)PbCSP+PvTRAP(r)PbTRAP+SM line (2207 cl3). Correct integration of the constructs into the genome of the double-replacement gene [DRG] chimeric parasite was analyzed by diagnostic PCR analysis on gDNA and Southern analysis of pulsed-field gel electrophoresis (PFGE)-separated chromosomes as described previously (36). Primers used for PCR genotyping are listed in Table S1 in the supplemental material.
Subsequently, we recycled the positive-negative SM cassette from the parasites of line 2207 cl3 by applying negative selection by providing 5-fluorocytosine (5-FC) in the drinking water of mice (39). Negative selection and removal of the hdhfr::yfcu SM cassette resulted in selection of PbANKA-PvCSP-VK210(r)PbCSP+PvTRAP(r)PbTRAP parasites (2207 cl3m0). Selected parasites were cloned by the method of limiting dilution (38) resulting in parasite line 2207 cl3m0cl1. This double-chimeric line PbANKA-PvCSP-VK210(r)PbCSP+PvTRAP(r)PbTRAP is referred to as PvCSP-VK210/PvTRAP parasites.
Correct integration of the constructs into the genome of double-chimeric parasites was analyzed by diagnostic PCR analysis on gDNA and Southern analysis of pulsed-field gel electrophoresis (PFGE)-separated chromosomes as described previously (37). Primers used for PCR genotyping are listed in Table S1.
Phenotyping of the double-chimeric parasites.
Growth of blood stages of the reporter and chimeric P. berghei parasites was determined during the cloning period, as described previously (36, 40). Feeding of A. stephensi mosquitoes, determination of oocyst production, and sporozoite collection were performed as described previously (40). Expression of PvCSP-VK210 and PvTRAP antigens in sporozoites of the chimeric parasites was analyzed by immunofluorescence staining assay (IFA), using two anti-P. vivax antigen monoclonal antibodies, anti-PvCSP-VK210 (obtained through BEI Resources, NIAID, NIH; hybridoma 2E10.E9 anti-Plasmodium vivax circumsporozoite protein, catalogue no. MRA-185, contributed by Elizabeth Nardin) (diluted 200 times) or anti-PvTRAP polyclonal antibodies elicited by viral vector vaccination. In addition we used anti-PbCSP 3D11 (41) antibodies as a control (diluted 1,000 times) and serum from mice vaccinated with ChAd63-MVA expressing PbTRAP (diluted 50 times). Purified sporozoites were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min on ice, washed three times with PBS, and blocked with 20 μl of 10% fetal calf serum (FCS) plus 1% bovine serum albumin (BSA) in PBS for 30 min at room temperature. The excess blocking medium was removed, followed by the addition of 20 to 25 μl of primary monoclonal antibody in 10% FCS–1% BSA in PBS (blocking medium) for 1 to 2 h at room temperature or overnight at 4°C. After incubation, the primary antibody was removed, and the slides were washed three times with PBS, followed by staining with a secondary antibody, Alexa Fluor 488 goat anti-mouse IgG (catalog number A-11001; Life Technologies), diluted 800 times in 10% FCS–1% BSA in PBS (blocking medium) for 1 h at room temperature. After three washes with PBS, nuclei were stained with 2% Hoechst-33342 (4082S; Cell Signaling Technology) in PBS for 10 min at room temperature, washed twice with PBS, and left to air dry; this was followed by the addition of fluorescence mounting medium (code S3023; Dako) before complete dry out. Coverslips were mounted onto the slides, and the slides were sealed with nail polish and left to dry overnight in the dark. The parasites in both blue and green channels were analyzed using a DMI-300B Leica fluorescence microscope, and images were processed using ImageJ software.
Viral vector vaccines.
Mice were primed with simian adenoviral vector 63 (ChAd63) encoding PvTRAP, as described earlier (16), and PvCSP VK210 (15) and 8 weeks later boosted with modified vaccinia virus strain Ankara (MVA) carrying the same transgene at a dose of 1 × 107 PFU (15, 16). A control viral vector was designed and constructed to express Tc24-IMX (a Trypanosoma cruzi antigen; GenBank accession number U70035.1). Briefly, the Tc24 gene from Trypanosoma cruzi was synthesized by GeneArt (Germany) and cloned into the plasmid pMono2 using the restriction sites Acc651 and BamHI. Tc24 was cloned into ChAd63, and the presence of the Tc24 gene was confirmed by PCR, while the integrity of the antigenic DNA sequence and absence of contaminating adenovirus were confirmed by PCR followed by restriction fragment length polymorphism (RFLP).
The virus was titrated to obtain the number of infectious units (IU) per milliliter and assayed by spectrophotometry to quantify the number of virus particles per milliliter. The sterility of the virus was also confirmed by inoculation of tryptic soy broth (TSB) with 10 μl of purified virus and incubation for 3 days at 35°C. Quality control tests indicated infectious units by titration of 1.0E+11 IU/ml, a virus particle (VP) concentration of 7.2E+12 VP/ml, and a particle/infectious units (P/I) ratio of 70.3 at a dose of 1 × 108 IU. To construct MVA-Tc24, the gene was cloned into a plasmid (p2773) suitable for MVA recombination. The virus and plasmid were recombined, and the cell lysate was harvested and used at 1:10 and 1:50 dilutions to infect chicken embryonic fibroblasts (CEF). CEF cells were MoFlo sorted into 96-well plates. Recombinant virus was purified by selecting individual plaques to be subsequently cultured to eliminate parental virus, and purity was determined through amplification of the DNA sequence by PCR followed by RFLP. The virus was then amplified to confirm the presence of the antigen in question and lack of original, parental virus expressing the red fluorescent protein in the final stock. Sterility of the virus was confirmed by inoculation of TSB with 10 μl of purified virus and incubation for 3 days at 35°C. The numbers of PFU were calculated upon titration, yielding 4.6 × 108 PFU/ml. Additional control viral vectors expressing ovalbumin (Ova) and GFP have been published previously, such as AdHu5 Ova (42), ChAd63 Ova, and MVA GFP (8).
Rv21 virus-like particle vaccine.
Rv21 is a chimeric fusion of the central repeat regions of PvCSP-VK210 (NCBI accession no: P08677) and VK247 (NCBI accession no: M69059) and the C terminus of PvCSP-VK210, fused to the N terminus of hepatitis B surface antigen (HepBsAg) and expressed in Pichia pastoris (15). A gene containing a chimeric CSP sequence comprising the repeat regions VK210 and VK247, followed by the C-terminal sequence (210/247C), was designed to be fused to HepBsAg. This VLP was described previously (15). Characterization of the Rv21 VLP was made by Western blotting using anti-PvCSP-VK210 (obtained through BEI Resources, NIAID, NIH; hybridoma 2E10.E9 anti-Plasmodium vivax circumsporozoite protein, catalogue mo. MRA-185, contributed by Elizabeth Nardin) and anti-PvCSP-VK247 (obtained through BEI Resources, NIAID, NIH; hybridoma 2F2 anti-Plasmodium vivax circumsporozoite protein, catalogue no. MRA-184, contributed by Elizabeth Nardin) primary mouse antibodies, diluted in 3% BSA-PBS to 1:200, 1:20,000, and 1:20,000. A secondary donkey anti-mouse alkaline phosphatase (AP) conjugate in 3% BSA-PBS and Sigmafast BCIP/NBT (5-bromo-4-chloro-3-indolylphosphate/nitroblue tetrazolium) tablets (Sigma-Aldrich) were used for development.
Vaccination.
All viral vector, VLP, and protein vaccines were administered intramuscularly in endotoxin-free PBS. Combinations of vaccines were mixed prior to administration (called coadministration in this paper) unless otherwise stated.
Animals.
Female inbred BALB/c (H-2d) mice were used for the assessment of immunogenicity and protection after challenge. Six to 8 mice per group were used for the studies. Tuck-ordinary (TO) outbred mice were used for parasite production and transmission. The mice were purchased from Harlan (United Kingdom).
Ethics statement.
All animals and procedures were used in accordance with the terms of the United Kingdom Home Office Animals Act Project License. The procedures were approved by the University of Oxford Animal Care and Ethical Review Committee (PPL 30/2414).
Protein production.
PvTRAP protein used for enzyme-linked immunosorbent assays (ELISAs) was prepared using HEK293 cells as described earlier (16, 43). PvCSP was produced as described earlier (15). Use of Ova protein was described earlier (44).
Ex vivo IFN-γ ELISpot assay.
Ex vivo IFN-γ ELISpot assays were carried out using peripheral blood mononuclear cells (PBMCs) isolated from blood as previously described (8, 16). Twenty-amino-acid peptides from PvTRAP previously shown to be immunodominant in BALB/c mice (ITKVIPMLNGLINSLSLSRD) (16) (ThinkPeptides) or peptide pools of 20 amino acids representing the full-length protein and overlapping by 10 amino acids (Mimotopes) were used to stimulate PBMCs at a final concentration of 5 μg/ml. MAIP ELISpot plates (Millipore) were used to plate cells. Anti-mouse IFN-γ monoclonal antibody and development reagents were used according to the manufacturer's specifications (Mabtech).
Whole IgG ELISA, avidity ELISA, and IgG subclass ELISA.
Enzyme-linked immunosorbent assays (ELISAs) measuring total IgG were carried out as described previously (45), and serum antibody endpoint titers were taken as the x axis intercept of the dilution curve at an absorbance value 3 standard deviations greater than the optical density at 405 nm (OD405) for serum from a naive mouse.
For standard-curve ELISAs, plates were prepared as for endpoint ELISAs, and then serum was diluted between 1:500 and 1:20,000 in PBS-Tween before application in triplicate at 50 μl/well. A standard curve was produced in duplicate on each plate by 3-fold serial dilution from 1:750 (for PvCSP) or 2-fold serial dilution from 1:100 (for PvTRAP) through 10 wells using stock sera from Rv21 (15) and Ad-M PvTRAP-vaccinated mice (16). Following 2 h of incubation at room temperature, plates were treated as for endpoint ELISAs, with plates scanned at 405 nm after 14 min for PvCSP-coated plates and 18 min for PvTRAP-coated plates. MARS (Multivariate Adaptive Regression Splines) software was used to obtain four-parameter logistic values of the standard curve on each plate for analysis and expression of serum OD values in terms of arbitrary ELISA units (EU) relative to the standard curve.
For avidity ELISAs, standard-curve ELISAs were performed as described above except that after 2 h of incubation of the serum at room temperature, plates were washed six times in PBS-Tween, and 100 μl of 7 M urea was added to serum wells for 10 min. Plates were then washed six times in PBS-Tween, and the standard-curve ELISA was carried out as before. The avidity index was calculated as the percentage of log10 EU of urea-treated compared to untreated serum samples, following the procedure described in (46).
For IgG subclass ELISAs, standard-curve ELISAs were performed at the end of the study, day 275, using a Bio-Rad Mouse Typer Sub-Isotyping kit according to the manufacturer's protocol (Bio-Rad) with the modification that after serum incubation, subclass-specific rabbit anti-mouse IgG was applied at 50 μl per well in triplicate. Serum for a standard curve was incubated with kappa-chain-specific IgG. After 1 h of incubation, plates were washed six times in PBS-Tween, and goat anti-rabbit horseradish peroxidase conjugate was applied (50 μl/well; 1:3,000). After 1 h plates were washed six times in PBS-Tween and developed for 14 min (PvCSP-coated plates) or 18 min (PvTRAP-coated plates) using 100 μl/well peroxidase substrate solution (Bio-Rad). Reactions were stopped by the addition of 100 μl/well of 2% oxalic acid.
Parasite production and challenge.
Wild-type and transgenic parasites used to challenge mice were produced at the Jenner Institute insectary. Female Anopheles stephensi mosquitoes were fed on infected TO mice. Exflagellation was first confirmed, and mosquitoes were exposed to anesthetized infected mice for 15 min. The mosquitoes were then maintained for 21 days in a humidified incubator at a temperature of 19 to 21°C on a 12-h night-day cycle and fed with a fructose–p-aminobenzoic acid (PABA) solution. At 21 days salivary glands were dissected from mosquitoes into Schneider's medium (Pan Biotech), and sporozoites were gently liberated using a glass homogenizer. Sporozoites were diluted to the required concentration for 100 μl of intravenous injection into the tail vein of the mouse.
Adjuvants.
When used, adjuvants were mixed and coinjected with vaccines. AddaVax was used at 25 μl per dose; Matrix-M was used at 5 μg per dose. AddaVax (InvivoGen) was kindly provided by the Jenner Institute adjuvant bank by Anita Milicic, and Matrix-M was obtained from Novavax AB.
Statistical model for parasitemia prediction.
To extrapolate the liver-to-blood parasite load or predict the time to 1% blood stage infection, a linear regression model was used as described previously (47). Briefly, blood parasite counts were obtained for 3 to 5 consecutive days starting on day 4 after challenge. Blood smears were stained with Giemsa stain, and percentages of parasitemia were calculated in all animals. The log10 value of the calculated percentage of parasitemia was plotted against time after challenge, and Prism, version 6 (GraphPad Software), for Mac OS X was used for generating a linear regression model on the linear part of the blood stage growth curve.
Statistical analysis.
For all statistical analyses, GraphPad Prism, version 6.0, for Mac OS X was used unless otherwise indicated. Prior to statistical analysis to compare two or more populations, the Kolmogorov-Smirnov test for normality was used to determine whether the values followed a Gaussian distribution. An unpaired t test was employed to compare two normally distributed groups, whereas a Mann-Whitney rank test was used to compare two nonparametric groups. If more than two groups were present, nonparametric data were compared using a Kruskal-Wallis test with Dunn's multiple-comparison posttest, whereas normally distributed data were analyzed by one-way analysis of variance (ANOVA) with Tukey's multiple-comparison posttest. Correlation strength was assessed using either Pearson's or Spearman's test, as indicated in Results. Kaplan-Meier survival curves were used to represent protective efficacy against challenge with P. berghei parasite lines. All ELISA titers were log10 transformed before analysis.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Jenner Insectary for supplying infected mosquitoes for the studies. We are also grateful to Alex Fyfe for assisting with the production of transgenic P. berghei parasites in the insectary of the University of Oxford, Isconova and Anita Milicic from the adjuvant core facility for supplying the Matrix-M adjuvant, and The Jenner Vector Core Facility for producing the recombinant viral vectors.
The work was funded by a Wellcome Trust Career Development Fellowship award (grant 097395/Z/11/Z) to A.R.-S., who is also a Jenner Investigator and an Oxford Martin Fellow and is supported by MRC-DPFS (grant MR/N019008/1). A.M.S. was funded by EVIMalaR's program funding (FP7/2007-2013) under grant agreement number 242095. E.A. was funded by CAPES from Science without Border program. A.V.S.H. is supported by a Wellcome Trust grant (number 095540/Z/11/Z) and is a Jenner Investigator and an Oxford Martin Fellow.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00114-18.
REFERENCES
- 1.Galinski MR, Barnwell JW. 2008. Plasmodium vivax: who cares? Malar J 7(Suppl 1):S9. doi: 10.1186/1475-2875-7-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reyes-Sandoval A, Bachmann MF. 2013. Plasmodium vivax malaria vaccines: why are we where we are? Hum Vaccin Immunother 9:2558–2565. doi: 10.4161/hv.26157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. 2007. Vivax malaria: neglected and not benign. Am J Trop Med Hyg 77(6 Suppl):79–87. [PMC free article] [PubMed] [Google Scholar]
- 4.Gething PW, Elyazar IR, Moyes CL, Smith DL, Battle KE, Guerra CA, Patil AP, Tatem AJ, Howes RE, Myers MF, George DB, Horby P, Wertheim HF, Price RN, Mueller I, Baird JK, Hay SI. 2012. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl Trop Dis 6:e1814. doi: 10.1371/journal.pntd.0001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maire N, Tediosi F, Ross A, Smith T. 2006. Predictions of the epidemiologic impact of introducing a pre-erythrocytic vaccine into the expanded program on immunization in sub-Saharan Africa. Am J Trop Med Hyg 75:111–118. doi: 10.4269/ajtmh.2006.75.111. [DOI] [PubMed] [Google Scholar]
- 6.Penny MA, Maire N, Studer A, Schapira A, Smith TA. 2008. What should vaccine developers ask? Simulation of the effectiveness of malaria vaccines. PLoS One 3:e3193. doi: 10.1371/journal.pone.0003193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunachie S, Hill AV, Fletcher HA. 2015. Profiling the host response to malaria vaccination and malaria challenge. Vaccine 33:5316–5320. doi: 10.1016/j.vaccine.2015.07.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauza K, Atcheson E, Malinauskas T, Blagborough AM, Reyes-Sandoval A. 2015. Tailoring a combination preerythrocytic malaria vaccine. Infect Immun 84:622–634. doi: 10.1128/IAI.01063-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rampling T, Ewer KJ, Bowyer G, Bliss CM, Edwards NJ, Wright D, Payne RO, Venkatraman N, de Barra E, Snudden CM, Poulton ID, de Graaf H, Sukhtankar P, Roberts R, Ivinson K, Weltzin R, Rajkumar BY, Wille-Reece U, Lee CK, Ockenhouse CF, Sinden RE, Gerry S, Lawrie AM, Vekemans J, Morelle D, Lievens M, Ballou RW, Cooke GS, Faust SN, Gilbert S, Hill AV. 2016. Safety and high level efficacy of the combination malaria vaccine regimen of RTS,S/AS01B with chimpanzee adenovirus 63 and modified vaccinia Ankara vectored vaccines expressing ME-TRAP. J Infect Dis 214:772–781. doi: 10.1093/infdis/jiw244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett JW, Yadava A, Tosh D, Sattabongkot J, Komisar J, Ware LA, McCarthy WF, Cowden JJ, Regules J, Spring MD, Paolino K, Hartzell JD, Cummings JF, Richie TL, Lumsden J, Kamau E, Murphy J, Lee C, Parekh F, Birkett A, Cohen J, Ballou WR, Polhemus ME, Vanloubbeeck YF, Vekemans J, Ockenhouse CF. 2016. Phase 1/2a trial of Plasmodium vivax malaria vaccine candidate VMP001/AS01B in malaria-naive adults: safety, immunogenicity, and efficacy. PLoS Negl Trop Dis 10:e0004423. doi: 10.1371/journal.pntd.0004423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrera S, Fernandez OL, Vera O, Cardenas W, Ramirez O, Palacios R, Chen-Mok M, Corradin G, Arevalo-Herrera M. 2011. Phase I safety and immunogenicity trial of Plasmodium vivax CS derived long synthetic peptides adjuvanted with Montanide ISA 720 or Montanide ISA 51. Am J Trop Med Hyg 84:12–20. doi: 10.4269/ajtmh.2011.09-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimberg BT, Udomsangpetch R, Xainli J, McHenry A, Panichakul T, Sattabongkot J, Cui L, Bockarie M, Chitnis C, Adams J, Zimmerman PA, King CL. 2007. Plasmodium vivax invasion of human erythrocytes inhibited by antibodies directed against the Duffy binding protein. PLoS Med 4:e337. doi: 10.1371/journal.pmed.0040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh AP, Puri SK, Chitnis CE. 2002. Antibodies raised against receptor-binding domain of Plasmodium knowlesi Duffy binding protein inhibit erythrocyte invasion. Mol Biochem Parasitol 121:21–31. doi: 10.1016/S0166-6851(02)00017-8. [DOI] [PubMed] [Google Scholar]
- 14.Vanloubbeeck Y, Pichyangkul S, Bayat B, Yongvanitchit K, Bennett JW, Sattabongkot J, Schaecher K, Ockenhouse CF, Cohen J, Yadava A, P. vivax Vaccine Study Group. 2013. Comparison of the immune responses induced by soluble and particulate Plasmodium vivax circumsporozoite vaccine candidates formulated in AS01 in rhesus macaques. Vaccine 31:6216–6224. doi: 10.1016/j.vaccine.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 15.Salman AM, Montoya-Diaz E, West H, Lall A, Atcheson E, Lopez-Camacho C, Ramesar J, Bauza K, Collins KA, Brod F, Reis F, Pappas L, Gonzalez-Ceron L, Janse CJ, Hill AVS, Khan SM, Reyes-Sandoval A. 2017. Rational development of a protective P. vivax vaccine evaluated with transgenic rodent parasite challenge models. Sci Rep 7:46482. doi: 10.1038/srep46482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauza K, Malinauskas T, Pfander C, Anar B, Jones EY, Billker O, Hill AV, Reyes-Sandoval A. 2014. Efficacy of a Plasmodium vivax malaria vaccine using ChAd63 and modified vaccinia Ankara expressing thrombospondin-related anonymous protein as assessed with transgenic Plasmodium berghei parasites. Infect Immun 82:1277–1286. doi: 10.1128/IAI.01187-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krotoski WA, Collins WE, Bray RS, Garnham PC, Cogswell FB, Gwadz RW, Killick-Kendrick R, Wolf R, Sinden R, Koontz LC, Stanfill PS. 1982. Demonstration of hypnozoites in sporozoite-transmitted Plasmodium vivax infection. Am J Trop Med Hyg 31:1291–1293. doi: 10.4269/ajtmh.1982.31.1291. [DOI] [PubMed] [Google Scholar]
- 18.Hodgson SH, Ewer KJ, Bliss CM, Edwards NJ, Rampling T, Anagnostou NA, de Barra E, Havelock T, Bowyer G, Poulton ID, de Cassan S, Longley R, Illingworth JJ, Douglas AD, Mange PB, Collins KA, Roberts R, Gerry S, Berrie E, Moyle S, Colloca S, Cortese R, Sinden RE, Gilbert SC, Bejon P, Lawrie AM, Nicosia A, Faust SN, Hill AV. 2015. Evaluation of the efficacy of ChAd63-MVA vectored vaccines expressing circumsporozoite protein and ME-TRAP against controlled human malaria infection in malaria-naive individuals. J Infect Dis 211:1076–1086. doi: 10.1093/infdis/jiu579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gosling R, von Seidlein L. 2016. The future of the RTS,S/AS01 malaria vaccine: an alternative development plan. PLoS Med 13:e1001994. doi: 10.1371/journal.pmed.1001994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.RTS,S Clinical Trials Partnership, Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, Adegnika AA, Mordmuller B, Issifou S, Kremsner PG, Sacarlal J, Aide P, Lanaspa M, Aponte JJ, Machevo S, Acacio S, Bulo H, Sigauque B, Macete E, Alonso P, Abdulla S, Salim N, Minja R, Mpina M, Ahmed S, Ali AM, Mtoro AT, Hamad AS, Mutani P, Tanner M, Tinto H, D'Alessandro U, Sorgho H, Valea I, Bihoun B, Guiraud I, Kabore B, Sombie O, Guiguemde RT, Ouedraogo JB, Hamel MJ, Kariuki S, Oneko M, Odero C, Otieno K, Awino N, McMorrow M, Muturi-Kioi V, Laserson KF, et al. . 2012. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med 367:2284–2295. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, Methogo BG, Doucka Y, Flamen A, Mordmuller B, Issifou S, Kremsner PG, Sacarlal J, Aide P, Lanaspa M, Aponte JJ, Nhamuave A, Quelhas D, Bassat Q, Mandjate S, Macete E, Alonso P, Abdulla S, Salim N, Juma O, Shomari M, Shubis K, Machera F, Hamad AS, Minja R, Mtoro A, Sykes A, Ahmed S, Urassa AM, Ali AM, Mwangoka G, Tanner M, Tinto H, D'Alessandro U, Sorgho H, Valea I, Tahita MC, Kabore W, Ouedraogo S, Sandrine Y, Guiguemde RT, Ouedraogo JB, Hamel MJ, Kariuki S, Odero C, et al. . 2011. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 365:1863–1875. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 22.Ewer KJ, O'Hara GA, Duncan CJ, Collins KA, Sheehy SH, Reyes-Sandoval A, Goodman AL, Edwards NJ, Elias SC, Halstead FD, Longley RJ, Rowland R, Poulton ID, Draper SJ, Blagborough AM, Berrie E, Moyle S, Williams N, Siani L, Folgori A, Colloca S, Sinden RE, Lawrie AM, Cortese R, Gilbert SC, Nicosia A, Hill AV. 2013. Protective CD8+ T-cell immunity to human malaria induced by chimpanzee adenovirus-MVA immunisation. Nat Commun 4:2836. doi: 10.1038/ncomms3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khusmith S, Charoenvit Y, Kumar S, Sedegah M, Beaudoin RL, Hoffman SL. 1991. Protection against malaria by vaccination with sporozoite surface protein 2 plus CS protein. Science 252:715–718. doi: 10.1126/science.1827210. [DOI] [PubMed] [Google Scholar]
- 24.O'Hara GA, Duncan CJ, Ewer KJ, Collins KA, Elias SC, Halstead FD, Goodman AL, Edwards NJ, Reyes-Sandoval A, Bird P, Rowland R, Sheehy SH, Poulton ID, Hutchings C, Todryk S, Andrews L, Folgori A, Berrie E, Moyle S, Nicosia A, Colloca S, Cortese R, Siani L, Lawrie AM, Gilbert SC, Hill AV. 2012. Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector. J Infect Dis 205:772–781. doi: 10.1093/infdis/jir850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pichyangkul S, Tongtawe P, Kum-Arb U, Yongvanitchit K, Gettayacamin M, Hollingdale MR, Limsalakpetch A, Stewart VA, Lanar DE, Dutta S, Angov E, Ware LA, Bergmann-Leitner ES, House B, Voss G, Dubois MC, Cohen JD, Fukuda MM, Heppner DG, Miller RS. 2009. Evaluation of the safety and immunogenicity of Plasmodium falciparum apical membrane antigen 1, merozoite surface protein 1 or RTS,S vaccines with adjuvant system AS02A administered alone or concurrently in rhesus monkeys. Vaccine 28:452–462. doi: 10.1016/j.vaccine.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 26.O'Hagan DT, Ott GS, Nest GV, Rappuoli R, Giudice GD. 2013. The history of MF59 adjuvant: a phoenix that arose from the ashes. Expert Rev Vaccines 12:13–30. doi: 10.1586/erv.12.140. [DOI] [PubMed] [Google Scholar]
- 27.Bengtsson KL, Karlsson KH, Magnusson SE, Reimer JM, Stertman L. 2013. Matrix-M adjuvant: enhancing immune responses by “setting the stage” for the antigen. Expert Rev Vaccines 12:821–823. doi: 10.1586/14760584.2013.814822. [DOI] [PubMed] [Google Scholar]
- 28.Reimer JM, Karlsson KH, Lovgren-Bengtsson K, Magnusson SE, Fuentes A, Stertman L. 2012. Matrix-M adjuvant induces local recruitment, activation and maturation of central immune cells in absence of antigen. PLoS One 7:e41451. doi: 10.1371/journal.pone.0041451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milicic A, C SR, Tang CK, Longley R, Hill AVS, Reyes-Sandoval A. 2017. Adjuvanting a viral vectored vaccine against pre-erythrocytic malaria. Sci Rep 7:7284. doi: 10.1038/s41598-017-07246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez-Campos V, Martinez-Vega P, Ramirez-Sierra MJ, Rosado-Vallado M, Seid CA, Hudspeth EM, Wei J, Liu Z, Kwityn C, Hammond M, Ortega-Lopez J, Zhan B, Hotez PJ, Bottazzi ME, Dumonteil E. 2015. Expression, purification, immunogenicity, and protective efficacy of a recombinant Tc24 antigen as a vaccine against Trypanosoma cruzi infection in mice. Vaccine 33:4505–4512. doi: 10.1016/j.vaccine.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Collins KA, Snaith R, Cottingham MG, Gilbert SC, Hill AVS. 2017. Enhancing protective immunity to malaria with a highly immunogenic virus-like particle vaccine. Sci Rep 7:46621. doi: 10.1038/srep46621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S, Nguyen MT. 2015. Recent advances of vaccine adjuvants for infectious diseases. Immune Netw 15:51–57. doi: 10.4110/in.2015.15.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrovsky N. 2015. Comparative safety of vaccine adjuvants: a summary of current evidence and future needs. Drug Saf 38:1059–1074. doi: 10.1007/s40264-015-0350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker AS, Lourenco J, Hill AV, Gupta S. 2015. Modeling combinations of pre-erythrocytic Plasmodium falciparum malaria vaccines. Am J Trop Med Hyg 93:1254–1259. doi: 10.4269/ajtmh.14-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. 2014. World malaria report 2014. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/world_malaria_report_2014/report/en/. [Google Scholar]
- 36.Janse CJ, Ramesar J, Waters AP. 2006. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat Protoc 1:346–356. doi: 10.1038/nprot.2006.53. [DOI] [PubMed] [Google Scholar]
- 37.Janse CJ, Franke-Fayard B, Waters AP. 2006. Selection by flow-sorting of genetically transformed, GFP-expressing blood stages of the rodent malaria parasite, Plasmodium berghei. Nat Protoc 1:614–623. doi: 10.1038/nprot.2006.88. [DOI] [PubMed] [Google Scholar]
- 38.Menard R, Janse C. 1997. Gene targeting in malaria parasites. Methods 13:148–157. doi: 10.1006/meth.1997.0507. [DOI] [PubMed] [Google Scholar]
- 39.Orr RY, Philip N, Waters AP. 2012. Improved negative selection protocol for Plasmodium berghei in the rodent malarial model. Malar J 11:103. doi: 10.1186/1475-2875-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Annoura T, Chevalley S, Janse CJ, Franke-Fayard B, Khan SM. 2013. Quantitative analysis of Plasmodium berghei liver stages by bioluminescence imaging. Methods Mol Biol 923:429–443. doi: 10.1007/978-1-62703-026-7_30. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida N, Nussenzweig RS, Potocnjak P, Nussenzweig V, Aikawa M. 1980. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science 207:71–73. doi: 10.1126/science.6985745. [DOI] [PubMed] [Google Scholar]
- 42.de Cassan SC, Forbes EK, Douglas AD, Milicic A, Singh B, Gupta P, Chauhan VS, Chitnis CE, Gilbert SC, Hill AV, Draper SJ. 2011. The requirement for potent adjuvants to enhance the immunogenicity and protective efficacy of protein vaccines can be overcome by prior immunization with a recombinant adenovirus. J Immunol 187:2602–2616. doi: 10.4049/jimmunol.1101004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aricescu AR, Lu W, Jones EY. 2006. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr D Biol Crystallogr 62:1243–1250. doi: 10.1107/S0907444906029799. [DOI] [PubMed] [Google Scholar]
- 44.Milicic A, Kaur R, Reyes-Sandoval A, Tang CK, Honeycutt J, Perrie Y, Hill AV. 2012. Small cationic DDA:TDB liposomes as protein vaccine adjuvants obviate the need for TLR agonists in inducing cellular and humoral responses. PLoS One 7:e34255. doi: 10.1371/journal.pone.0034255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reyes-Sandoval A, Berthoud T, Alder N, Siani L, Gilbert SC, Nicosia A, Colloca S, Cortese R, Hill AV. 2010. Prime-boost immunization with adenoviral and modified vaccinia virus Ankara vectors enhances the durability and polyfunctionality of protective malaria CD8+ T-cell responses. Infect Immun 78:145–153. doi: 10.1128/IAI.00740-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grangeot-Keros L, Mayaux MJ, Lebon P, Freymuth F, Eugene G, Stricker R, Dussaix E. 1997. Value of cytomegalovirus (CMV) IgG avidity index for the diagnosis of primary CMV infection in pregnant women. J Infect Dis 175: 944–946. doi: 10.1086/513996. [DOI] [PubMed] [Google Scholar]
- 47.Reyes-Sandoval A, Wyllie DH, Bauza K, Milicic A, Forbes EK, Rollier CS, Hill AV. 2011. CD8+ T effector memory cells protect against liver-stage malaria. J Immunol 187:1347–1357. doi: 10.4049/jimmunol.1100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.