Mycobacterium tuberculosis remains a threat to global health, and a more efficacious vaccine is needed to prevent disease caused by M. tuberculosis. We previously reported that the mycobacterial ribosome is a major target of CD4+ T cells in mice immunized with a genetically modified Mycobacterium smegmatis strain (IKEPLUS) but not in mice immunized with Mycobacterium bovis BCG.
KEYWORDS: antigen, cryptic, mycobacteriology, ribosome, T cells, tuberculosis, tuberculosis vaccines
ABSTRACT
Mycobacterium tuberculosis remains a threat to global health, and a more efficacious vaccine is needed to prevent disease caused by M. tuberculosis. We previously reported that the mycobacterial ribosome is a major target of CD4+ T cells in mice immunized with a genetically modified Mycobacterium smegmatis strain (IKEPLUS) but not in mice immunized with Mycobacterium bovis BCG. Two specific ribosomal proteins, RplJ and RpsA, were identified as cross-reactive targets of M. tuberculosis, but the breadth of the CD4+ T cell response to M. tuberculosis ribosomes was not determined. In the present study, a library of M. tuberculosis ribosomal proteins and in silico-predicted peptide libraries were used to screen CD4+ T cell responses in IKEPLUS-immunized mice. This identified 24 out of 57 M. tuberculosis ribosomal proteins distributed over both large and small ribosome subunits as specific CD4+ T cell targets. Although BCG did not induce detectable responses against ribosomal proteins or peptide epitopes, the M. tuberculosis ribosomal protein RplJ produced a robust and multifunctional Th1-like CD4+ T cell population when administered as a booster vaccine to previously BCG-primed mice. Boosting of BCG-primed immunity with the M. tuberculosis RplJ protein led to significantly reduced lung pathology compared to that in BCG-immunized animals and reductions in the bacterial burdens in the mediastinal lymph node compared to those in naive and standard BCG-vaccinated mice. These results identify the mycobacterial ribosome as a potential source of cryptic or subdominant antigenic targets of protective CD4+ T cell responses and suggest that supplementing BCG with ribosomal antigens may enhance protective vaccination against M. tuberculosis.
INTRODUCTION
Tuberculosis is an enormous burden on global health systems. The World Health Organization has concluded that nearly one-fourth of the global population is latently infected with Mycobacterium tuberculosis (http://www.who.int/news-room/fact-sheets/detail/tuberculosis). With 10.4 million new cases and 1.5 million deaths annually, M. tuberculosis remains one of the most serious threats to global public health, and new research is desperately needed to combat its spread (http://www.who.int/tb/publications/global_report/en/). The only currently available vaccine for the prevention and control of M. tuberculosis infection, the attenuated live Mycobacterium bovis bacillus Calmette-Guérin (BCG) strain, has limited and variable efficacy in children and generally fails to prevent pulmonary tuberculosis in adults (1, 2). Lengthy antibiotic treatments that are required for the cure of M. tuberculosis infection are costly and plagued by low compliance, which leads to the emergence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) M. tuberculosis strains (3–5). The HIV epidemic has also led to unforeseen treatment complications for those coinfected with M. tuberculosis (6–8). These issues highlight the necessity of identifying new candidates for vaccination against M. tuberculosis.
M. tuberculosis vaccine candidates that have shown potential for protection greater than that provided by BCG in animal models are currently in every stage of the vaccine development pipeline (9–12). Candidates in clinical trials can be divided into three broad categories, as live mycobacterium vaccines, subunit recombinant protein vaccines, and subunit vaccines delivered by viral vectors (9). The majority of vaccine candidates have focused on immunodominant secreted antigens of M. tuberculosis, such as members of the antigen 85 complex (Ag85a and Ag85b) and proteins secreted by the virulence-related type VII secretion systems of the bacteria (13–22). However, it remains unclear whether any of these antigens will provide useful targets for protective vaccines, and in at least one case, the results from extensive phase II clinical testing have been disappointing (23, 24). Thus, the discovery and analysis of additional antigens for incorporation into new M. tuberculosis vaccines remain areas of high priority in the ongoing effort to develop better strategies for the control and eradication of M. tuberculosis (11, 12, 25).
We previously reported on a genetically modified strain of Mycobacterium smegmatis, which contains the ESX-3 type VII secretion system of M. tuberculosis, designated IKEPLUS, that stimulates strong bactericidal CD4+ T cell responses against subsequent M. tuberculosis challenge in mice (26). Our detailed analysis of the specificity of the CD4+ T cells evoked by IKEPLUS and cross-reactive with M. tuberculosis showed that a majority of this response is specific for structural proteins of the mycobacterial ribosome (27). Using CD4+ T cells from IKEPLUS-immunized mice and epitope mapping with synthetic peptide libraries, we identified conserved epitopes within the ribosomal RplJ/L10 and RpsA/S1 proteins as targets of the immune response. Responses to these antigens were not detected following BCG immunization or aerosol infection with M. tuberculosis, suggesting that they are cryptic antigens during virulent mycobacterial infection and may be a previously untapped source of immunogens for the development of new vaccine candidates.
In the present study, we have expanded on those previous results by broadly screening all structural proteins of the M. tuberculosis ribosome for their ability to be targeted by the CD4+ T cell responses of appropriately immunized mice. We used IKEPLUS immunization along with a recombinant mycobacterial ribosomal protein library to probe for the immune response to the 57 proteins that make up the mycobacterial ribosome. Synthetic peptide libraries were then used to identify specific epitopes within ribosomal proteins that were immunogenic after IKEPLUS immunization. This study also used recombinant RplJ protein to assess the ability of ribosomal proteins to complement BCG immunization. Our findings showed that the mycobacterial ribosome was highly immunogenic and contained many epitopes for the stimulation of T cell responses. Our results also showed that BCG did not inhibit CD4+ T cell responses to ribosomes and that BCG vaccination could be potentially augmented with mycobacterial ribosomal epitopes to enhance protection against M. tuberculosis.
RESULTS
Identification of mycobacterial ribosomal proteins recognized by CD4+ T cells.
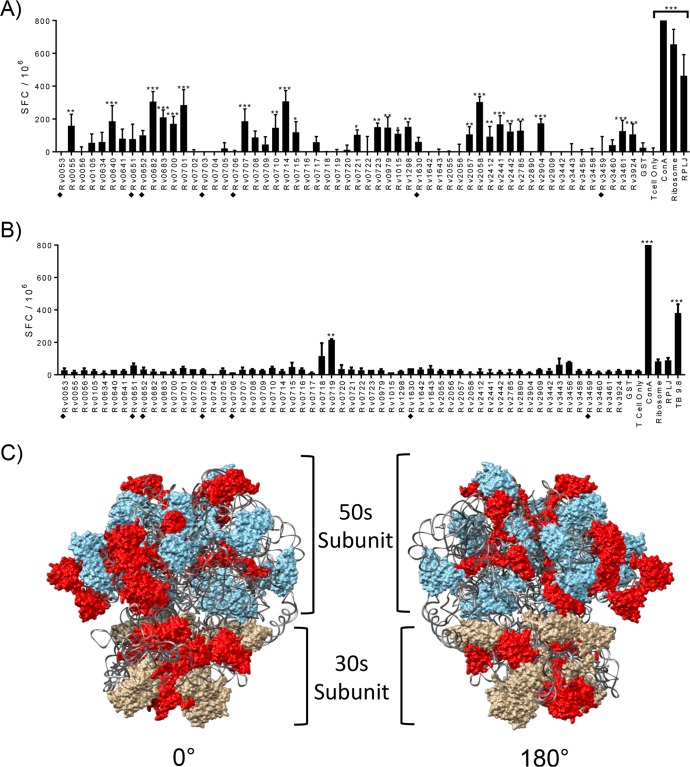
To broadly survey which proteins of the mycobacterial ribosome could induce an immune response in mice immunized with live mycobacterial vaccine strains, we generated all 57 ribosomal proteins of M. tuberculosis by expressing them individually in Escherichia coli and isolating them via affinity tag purification (see Fig. S1 and Table S1 in the supplemental material). CD4+ T cell responses from mice immunized with IKEPLUS or BCG were analyzed for responses to the individual recombinant mycobacterial ribosomal proteins by a gamma interferon (IFN-γ) enzyme-linked immunosorbent spot (ELISPOT) assay of splenic CD4+ T cells. Among the 57 purified recombinant ribosomal proteins, 24 elicited significant numbers of IFN-γ-producing CD4+ T cells in IKEPLUS-immunized mice (Fig. 1A). In contrast, only one ribosomal protein antigen elicited a response that achieved statistical significance with BCG-immunized CD4+ T cells (Fig. 1B). Based on the reported three-dimensional structure of the M. smegmatis ribosome as resolved by cryoelectron microscopy (28, 29), we observed a random distribution in the locations of proteins that stimulated CD4+ T cell responses (Fig. 1C), without obvious clusters in specific areas of either the large or small subunit. Overall, these results indicated that IKEPLUS primed a broad immune response to proteins distributed throughout the structure of the mycobacterial ribosome, while standard BCG vaccination was mostly ineffective at inducing detectable levels of such T cell responses.
FIG 1.
Identification of M. tuberculosis ribosomal proteins recognized by CD4+ T cells from IKEPLUS- and BCG-immunized animals. (A and B) Mice (C57BL/6) (n = 3) were immunized with 5 × 107 CFU IKEPLUS i.v. (A) or 5 × 106 CFU BCG-Danish s.c. (B). Two weeks later, CD4+ T cells were purified from splenocytes and tested by an ELISPOT assay for IFN-γ production in response to ex vivo stimulation with purified recombinant preparations of each of the 57 M. tuberculosis ribosomal proteins (10 μg/ml). Responses that are significantly different from those with the negative-control stimulation with purified glutathione S-transferase (GST) by two-way ANOVA with FDR correction and a 1% cutoff are indicated (*, q < 0.01; **, q < 0.001; ***, q < 0.0001). Positive-control wells were stimulated with concanavalin A (ConA) (5 μg/ml). Additional positive controls were purified M. smegmatis ribosomes (Ribosome) (5 nM) or RplJTB146–160 peptide (RPLJ) (10 μg/ml) (A) and TB9.8 (10 μg/ml) (B). Proteins that were poorly expressed and of lower purity in our system (i.e., potential false negatives) are marked with filled diamonds. Data shown are representative of results from two independent experiments and are displayed as mean numbers of spot-forming cells (SFC) per well, with error bars representing standard errors for quadruplicate (A) and duplicate (B) samples. (C) Proteins that achieved statistical significance for stimulation of CD4+ T cell responses in IKEPLUS-immunized mice in panel A were mapped in red onto the reported structure of the mycobacterial ribosome (PDB accession number 5O61) (28). The 50S subunit is shown in blue, the 30S subunit is in tan, and the rRNA is in gray.
Mapping minimal epitopes of immunogenic mycobacterial ribosomal proteins.
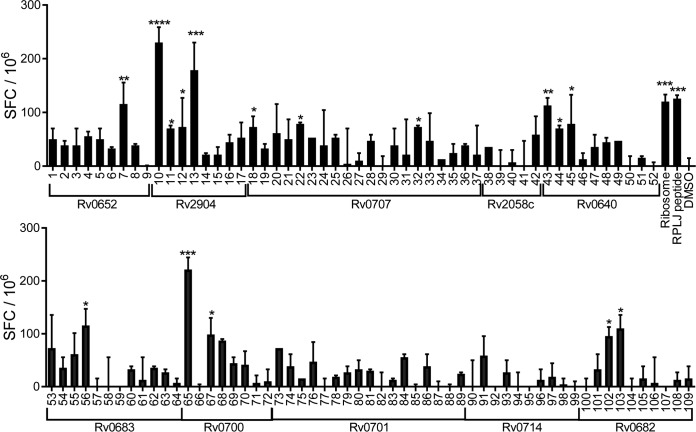
Ten ribosomal proteins that consistently stimulated significant numbers of IFN-γ-secreting CD4+ T cells in the ribosomal protein library screen (Rv0640, Rv0652, Rv0682, Rv0683, Rv0700, Rv0701, Rv0707, Rv0714, Rv2058c, and Rv2904) (Fig. 1 and additional data not shown) were analyzed in silico for candidate major histocompatibility complex (MHC) class II-presented epitopes within all potential 15-amino-acid stretches using the consensus method, as previously described (30). After ranking by predicted binding affinity for MHC class II I-Ab, the results were trimmed by selecting the top 33% strongest predicted binders for each ribosomal protein. Within this group, peptides with overlaps of >3 amino acids were removed, yielding a set of 109 peptides, which were then synthesized (see Table S2 in the supplemental material). To test the recognition of these candidate epitopes, splenic CD4+ T cells were isolated from IKEPLUS-immunized mice and examined for responses to each of these peptides by an IFN-γ ELISPOT assay. Using this approach, we detected 16 stimulatory peptides corresponding to CD4+ T cell epitopes (Fig. 2), 11 of which were nonoverlapping sequences from 7 different ribosomal proteins (Table 1).
FIG 2.
Identification of 15-mer epitopes from immunogenic M. tuberculosis ribosomal proteins. Mice (C57BL/6) (n = 3) were immunized with 5 × 107 CFU IKEPLUS i.v. Two weeks later, CD4+ T cells were purified from splenocytes and tested for IFN-γ production by an ELISPOT assay in response to ex vivo stimulation with 109 mycobacterial ribosomal peptides (10 μg/ml) derived from 10 M. tuberculosis ribosomal proteins, as indicated. Responses that are significantly different from those under the control DMSO conditions by ANOVA are indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001). Positive-control wells were stimulated with purified M. smegmatis ribosomes (Ribosome) (5 nM) or RplJTB146–160 peptide (RPLJ) (10 μg/ml). Data shown represent mean values and standard errors for duplicate samples and are representative of results from two independent experiments.
TABLE 1.
Immunogenic epitopes identified by peptide screens of mycobacterial ribosomal proteinsa
| Peptide | Residues | Sequence | P value |
|---|---|---|---|
| Rv0652 (RplL) | |||
| 7 | 93–107 | AKDLVDGAPKPLLEK | 0.0022 |
| 127 | 91–105 | KEAKDLVDSAPKPLL | 0.0001 |
| Rv2904 (RplS) | |||
| 10 | 69–83 | VERTFPVHSPNIDHI | 0.0001 |
| 11 | 15–29 | DIPAFNPGDTINVHV | 0.0290 |
| 12 | 65–79 | YGVGVERTFPVHSPN | 0.0240 |
| 13 | 73–87 | FPVHSPNIDHIEVVT | 0.0002 |
| Rv0707 (RpsC) | |||
| 16 | 210–224 | GKRELAAAAPAGADR | 0.0240 |
| 20 | 206–220 | DIVGGKRELAAAAPA | 0.0167 |
| 32 | 182–196 | IDYGLYEAKTTFGRI | 0.0240 |
| Rv0640 (RpIK) | |||
| 43 | 12–26 | KLQIVAGQANPAPPV | 0.0025 |
| 44 | 67–81 | SFTFTLKTPPAAKLL | 0.0290 |
| 45 | 16–30 | VAGQANPAPPVGPAL | 0.0167 |
| Rv0651 (RpIJ) | |||
| 123 | 146–160 | KAAGLFNAPASQVAR | 0.0001 |
| 124 | 151–165 | FNAPASQVARLAAAL | 0.0001 |
| Rv0683 (RpsG) | |||
| 56 | 16–30 | PVYGSQLVTQLVNKV | 0.0188 |
| Rv0700 (RpsJ) | |||
| 65 | 32–46 | TGASVVGPVPLPTEK | 0.0006 |
| 67 | 28–42 | TVVRTGASVVGPVPL | 0.0401 |
| 172 | 31–45 | RTGASVVGPVPLPTE | 0.0001 |
| Rv0682 (RpsL) | |||
| 102 | 18–32 | KVKTAALKGSPQRRG | 0.0458 |
| 103 | 30–44 | RRGVCTRVYTTTPKK | 0.0240 |
| Rv1630 (RpsA) | |||
| 154 | 431–445 | QMEKFAAAEAEAANA | 0.0001 |
| Rv3458c (RpsD) | |||
| 162 | 1–15 | MARYTGPATRKSRRL | 0.0018 |
Peptides identified in screens for immunogenic epitopes (Fig. 2 and 3) are listed with their locus tag (name), library identification, peptide number, residue location in the intact protein, amino acid sequence, and statistical significance in the screen conducted. Proteins identified as being immunogenic using the approach illustrated in Fig. 1 are highlighted in boldface type.
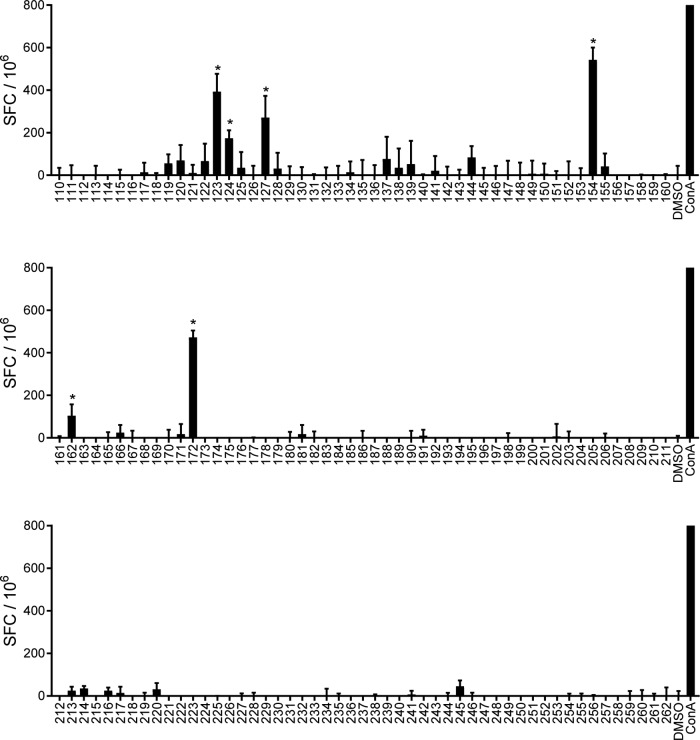
In a complementary but broader approach to epitope identification, we synthesized a second peptide library consisting of predicted I-Ab binding peptides among all 15-mers with fewer than three overlapping residues contained within the entire set of 57 mycobacterial ribosomal proteins. This prediction was done as described above, using the consensus method (30). Peptides were ranked based on hypothetical binding affinity for I-Ab, and the top 10% (153 peptides) were synthesized (Table S3). This library was screened by using an IFN-γ ELISPOT assay as described above, which identified six additional stimulatory peptides from five ribosomal proteins. Two of these overlapped peptides from the proteins RplL and RpsJ identified in the initial, more directed screen. Peptides from the previously reported RplJ and RpsA proteins were also identified, as was one peptide derived from a protein that was not found to be immunogenic in the initial protein screen (RpsD) (Fig. 3 and Table 1). In all, our combined peptide screening identified at least 13 epitopes derived from 10 different M. tuberculosis ribosomal proteins.
FIG 3.
Identification of epitopes predicted from the total M. tuberculosis ribosomal proteome. Mice (C57BL/6) (n = 3) were immunized with 5 × 107 CFU IKEPLUS i.v. Two weeks later, CD4+ T cells were purified from splenocytes and tested for IFN-γ production by an EPLISPOT assay in response to ex vivo stimulation with 153 mycobacterial ribosomal peptides, as indicated (10 μg/ml). Responses that are significantly different from those under the control DMSO conditions are indicated (*, P < 0.05). Positive-control wells were stimulated with concanavalin A (ConA) (5 μg/ml). Data shown represent mean values and standard errors for quadruplicate samples and are representative of results from two independent experiments.
Lack of inhibitory effects of BCG on responses to mycobacterial ribosomal proteins.
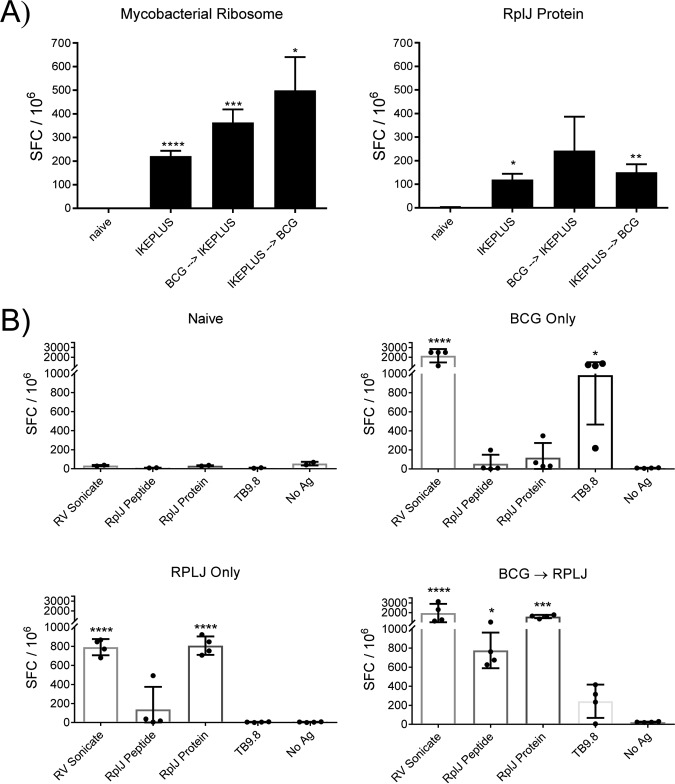
To determine if BCG immunization could inhibit T cell priming against mycobacterial ribosomal proteins or suppress previously primed responses to these antigens, mice were immunized with either IKEPLUS alone, IKEPLUS followed by BCG 2 weeks later, or BCG followed by IKEPLUS 4 weeks later. Splenic CD4+ T cells were isolated and incubated with recombinant RplJ protein or intact 70S mycobacterial ribosomes and naive antigen-presenting cells (APCs). Analysis by an IFN-γ ELISPOT assay showed that mice responded to intact mycobacterial ribosomes when immunized with IKEPLUS, or with IKEPLUS and BCG in either combination, and also responded to recombinant RplJ protein when immunized with IKEPLUS or IKEPLUS followed by BCG (Fig. 4A). Although mice immunized with BCG followed by IKEPLUS did not show responses that reached significance when stimulated with RplJ protein (Fig. 4A), the overall trend revealed that IKEPLUS immunization in any combination elicited a response to mycobacterial ribosomes by CD4+ T cells. These results indicated that prior exposure to BCG did not preclude the stimulation of subsequent priming of responses against ribosomal proteins, suggesting the possibility of using such proteins as immunogens to broaden antimycobacterial immunity induced by BCG vaccination.
FIG 4.
BCG immunization does not impede ribosome-specific priming of CD4+ T cells. (A) Mice (C57BL/6) (n = 3) were immunized with 5 × 107 CFU IKEPLUS i.v., 5 × 106 CFU BCG s.c. followed by 5 × 107 CFU IKEPLUS i.v. 4 weeks later, or 5 × 107 CFU IKEPLUS i.v. followed by 5 × 106 CFU BCG s.c. 2 weeks later. Two weeks after the final immunization, CD4+ T cells were purified from splenocytes and tested for IFN-γ production by an ELISPOT assay in response to ex vivo stimulation with recombinant RplJ protein (10 μg/ml) or purified mycobacterial ribosomes (5 nM). Responses that are significantly different from those in control naive mice by ANOVA are indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001). Data shown represent mean values and standard errors for quadruplicate samples and are representative of results from three independent experiments. (B) Mice (C57BL/6) (n = 4) were immunized with 5 × 106 CFU BCG s.c., 5 × 106 CFU BCG s.c. followed 4 weeks later by two administrations of RplJ protein (25 μg) and CpG-ODN 1826 (20 μg) s.c. 2 weeks apart, or RplJ protein (25 μg) and CpG-ODN 1826 (20 μg) twice 2 weeks apart s.c. Two weeks after the final immunization, CD4+ T cells were purified from splenocytes and tested for IFN-γ production by an ELISPOT assay in response to ex vivo stimulation with recombinant RplJ protein (10 μg/ml), RplJTB146–160 peptide (10 μg/ml), or TB9.8 peptide (10 μg/ml). Positive-control wells were stimulated with the H37Rv sonicate (10 μg/ml). Responses that are significantly different from those of the no-antigen (No Ag) control by ANOVA are indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001). Data shown represent mean values and standard errors for groups of four mice and are representative of results from two independent experiments.
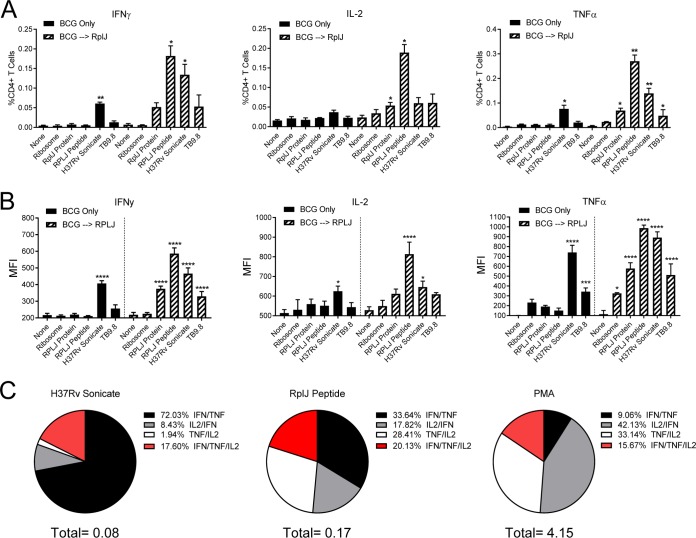
To test the ability of an individual ribosomal antigen to supplement BCG immunization, mice were immunized subcutaneously (s.c.) with either BCG or recombinant RplJ protein or primed with BCG followed by subsequent administrations of RplJ protein. At 56 days postimmunization, mice were sacrificed, and splenic CD4+ T cells were isolated and incubated with naive APCs in the presence of the M. tuberculosis sonicate from H37Rv, RplJTB146–160 peptide, recombinant RplJ protein, or TB9.8 peptide (all at 10 μg/ml). Responses were quantitated by an IFN-γ ELISPOT assay (Fig. 4B). Mice immunized with BCG alone produced a significant CD4+ T cell response to the M. tuberculosis sonicate as well as the TB9.8 peptide, while mice immunized with recombinant RplJ protein alone produced CD4+ T cell responses to the M. tuberculosis sonicate and RplJ protein, as expected. Most notably, mice immunized sequentially with BCG boosted by RplJ protein elicited significant CD4+ T cell responses to the M. tuberculosis sonicate, RplJ protein, and RplJTB146–160 peptide (Fig. 4B). The magnitude of the responses to RplJ protein and peptide in the BCG-primed and RplJ protein-boosted group suggested that BCG may have had some ability to weakly stimulate CD4+ T cell responses to the ribosomal antigen, although these responses rose to a measurable level only after boosting with the purified protein. These results also suggested that the immunodominant antigen TB9.8 may be inhibited, or at least less dominant, in the context of boosting with ribosomal antigen.
Induction of strong multifunctional Th1 CD4+ T cell responses in RplJ-boosted mice.
To determine the strength and multifunctionality of mycobacterial ribosome-specific CD4+ T cell responses, mice were immunized with BCG or with BCG followed by recombinant RplJ protein. On day 70 after the initial BCG administration, mice were sacrificed, and spleens were harvested for intracellular cytokine staining and flow cytometry. Mice immunized with BCG alone showed significant numbers of IFN-γ- and tumor necrosis factor alpha (TNF-α)-producing CD4+ T cells when restimulated with the M. tuberculosis sonicate but not with the other antigens tested. In marked contrast, BCG-immunized mice boosted with RplJ protein showed significant responses to RplJ protein and peptide for each of the three cytokines tested as well as responses to the M. tuberculosis sonicate and TB9.8 (Fig. 5A; see also Fig. S2 in the supplemental material). Whereas BCG-immunized RplJ-boosted mice responded to RplJ-specific stimuli with significant TNF-α, IFN-γ, and interleukin-2 (IL-2) responses, the BCG-specific stimuli (M. tuberculosis sonicate and TB9.8) seemed to stimulate only TNF-α- and IFN-γ-producing cells (Fig. 5A). These results suggest that 70 days after BCG immunization, the mice had a waning CD4+ T cell response to antigens delivered only by the initial priming, while BCG-immunized mice boosted with RplJ protein created strong CD4+ T cell memory populations that maintained responses to mycobacterial ribosomal antigens and to antigens originally primed by BCG.
FIG 5.
Induction of Th1 multifunctional CD4+ T cells by boosting with RplJ protein. Mice (C57BL/6) (n = 4) were immunized with 5 × 106 CFU BCG s.c. or 5 × 106 CFU BCG s.c. followed 4 weeks later by two administrations of RplJ protein (25 μg) and CpG-ODN 1826 (20 μg) s.c. 2 weeks apart. Four weeks after the final immunization, single-cell suspensions of splenocytes were restimulated with recombinant RplJ protein (10 μg/ml), RplJTB146–160 peptide (10 μg/ml), TB9.8 peptide (10 μg/ml), H37Rv sonicate (10 μg/ml), or purified M. smegmatis ribosomes (5 nM). After 6 h of restimulation, cells were fixed and stained as described in Materials and Methods and analyzed by FACS analysis. Positive-control cells were stimulated with PMA plus ionomycin (10 μg/ml) (not shown). (A) Frequencies of CD4+ T cells producing each cytokine in immunized mice in response to antigen restimulation. (B) Mean fluorescence intensity (MFI) as an indication of the average level of cytokine production by CD4+ T cells in response to antigen restimulation. (C) Pie charts showing the relative fractions of dual- and triple-cytokine-producing CD4+ T cells following restimulation with the H37Rv sonicate, RplJ peptide, or PMA-ionomycin. The total below the pie charts is the fraction of total CD4+ T cells producing two cytokines. Percent values in the key represent the percentages of the total dual- and triple-cytokine-producing CD4+ T cells in the sample. Data shown represent mean values and standard errors for groups of four mice; responses that are significantly different from those with the no-antigen control by ANOVA are indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
The immunized CD4+ T cells were also examined for their average levels of cytokine production based on mean fluorescence intensity (MFI) values for intracellular cytokine staining. Although the populations were small, BCG-immunized mice that responded to antigen restimulation produced a large amount of TNF-α and a moderate amount of IFN-γ (Fig. 5B and Fig. S2). This was in contrast to the BCG-immunized RplJ-boosted mice, in which the CD4+ T cells produced large amounts of all three cytokines analyzed in response to mycobacterial ribosomal antigens. Interestingly, the BCG-specific antigens in BCG-immunized RplJ-boosted animals produced a dominant TNF-α CD4+ T cell response with moderate levels of IFN-γ and almost no IL-2 (Fig. 5B). These results suggested that ribosomal antigens may prime a qualitatively different CD4+ T cell response than those produced by BCG immunization.
Identification of polyfunctional CD4+ T cell populations from the same group of mice was also performed. The CD4+ T cell populations from mice immunized with BCG only were too small to permit reliable identification of polyfunctional cells with confidence. However, BCG-immunized mice boosted by RplJ protein had large enough CD4+ T cell populations producing two and three cytokines to allow analysis of their profiles in response to restimulation conditions (Fig. 5C). Stimulation with phorbol myristate acetate (PMA)-ionomycin was used to activate the total CD4+ T cell population in these mice and identify the range of possible polyfunctional CD4+ T cells. This showed that cells with the potential to produce both IFN-γ and IL-2 made up half of the total dual-cytokine-producing CD4+ T cells, followed by 40% of CD4+ T cells producing TNF-α and IL-2 and 10% producing IFN-γ and TNF-α. When stimulated with BCG-specific antigens using a crude M. tuberculosis (H37Rv) sonicate, the CD4+ T cell population was skewed heavily toward dual IFN-γ- and TNF-α-producing cells, with fewer IL-2-producing cells being present overall. In contrast, specific restimulation with RplJ peptide revealed CD4+ T cell populations that were much more diverse in their dual-cytokine-producing profile. Overall, the numbers of triple-cytokine-producing cells were approximately the same among the three stimulations. These results suggested that boosting BCG-primed animals with RplJ protein and adjuvant established a strong multifunctional Th1-like CD4+ T cell population that was not produced by BCG priming alone.
Reduction of lung pathology and bacterial burden following M. tuberculosis challenge in RplJ-boosted mice.
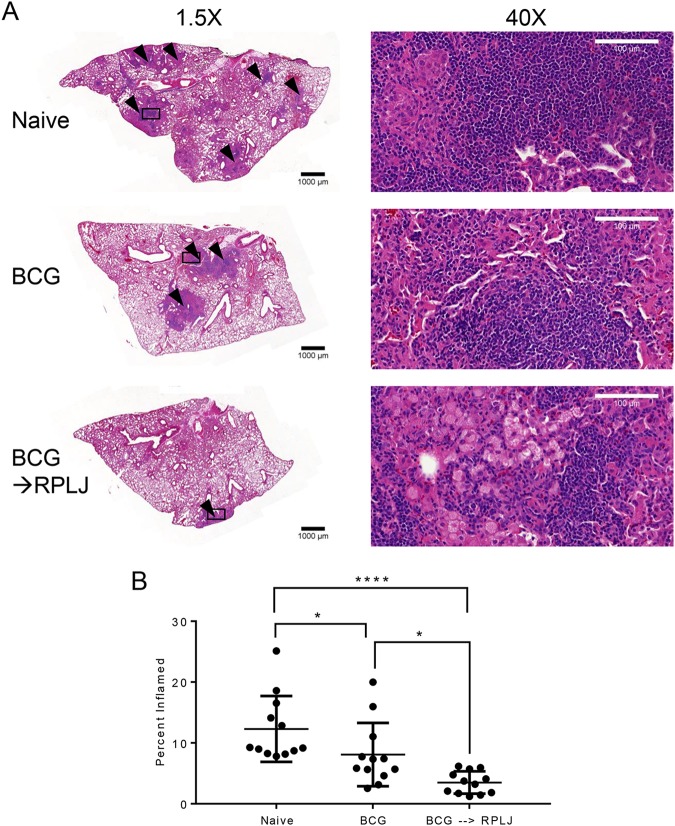
The effects of RplJ protein boosting of BCG-primed mice during challenge with M. tuberculosis were examined by immunizing mice as described above and challenging them with low-dose aerosol delivery of virulent M. tuberculosis (H37Rv) 4 weeks later. Ten weeks after challenge, mice were sacrificed, and lungs were fixed and evaluated for granulomatous inflammation. Naive mice receiving no vaccination had the most severe disease, with extensive coalescing granulomatous inflammation, and this was also the case in BCG-immunized mice, although it was less severe than in naive control mice (Fig. 6). BCG-immunized mice that were boosted with RplJ protein exhibited the least severe disease, with moderate granulomatous inflammation. Mice boosted with RplJ protein showed significantly reduced lung pathology compared to naive mice or BCG-immunized animals. These results suggested that supplementing BCG with ribosomal antigens may improve disease pathology and outcomes.
FIG 6.
RplJ boosting reduces lung pathology in M. tuberculosis-challenged mice. Mice (C57BL/6) (n = 7) were immunized with 5 × 106 CFU BCG s.c. or with 5 × 106 CFU BCG s.c. followed 4 weeks later by two administrations of RplJ protein (25 μg) and CpG-ODN 1826 (20 μg) s.c. 2 weeks apart. Four weeks after the final immunization, mice were exposed to low-dose aerosolized M. tuberculosis for 20 min, resulting in approximately 100 CFU being deposited in the lungs of the mice. Ten weeks after challenge, mice were sacrificed, and lungs were harvested and fixed in 10% neutral buffered formalin. Tissues were embedded in paraffin and sectioned at a 5-μm thickness. Sections were stained with hematoxylin and eosin. (A) Lung sections imaged at the indicated magnifications. Black triangles indicate granulomatous inflammation in the pictured section. Squares indicate the area enlarged to a ×40 magnification. Images are representative of data from groups consisting of four mice each. (B) Sections were evaluated for granulomatous inflammation by using ImageJ software to identify the percent area of inflammation. Data shown represent mean values and standard errors for groups of four mice; responses that are significantly different from those for naive control mice by ANOVA are indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
The effects of RplJ boosting of BCG-primed animals on the bacterial burden in the lungs and mediastinal lymph nodes was also assessed 10 weeks following low-dose aerosol infection. In the lungs of both BCG-primed and BCG-primed RplJ-boosted mice, there were significant reductions in bacterial burdens compared to naive control mice. In the mediastinal lymph node, BCG immunization did not significantly reduce the M. tuberculosis burden, but BCG-primed and RplJ-boosted mice significantly reduced the M. tuberculosis burden compared to both naive and BCG-immunized mice. These results indicated that RplJ boosting had benefits in controlling M. tuberculosis in the lymph nodes but had less ability to augment the effects of BCG in reducing the bacterial burdens in the lungs of challenged mice (Fig. 7).
FIG 7.
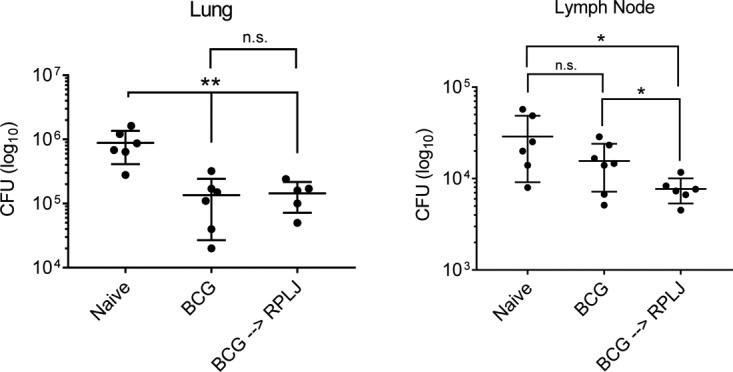
Effect of RplJ boosting on tissue bacterial burden in M. tuberculosis-challenged mice. Mice (C57BL/6) (n = 7) were immunized with 5 × 106 CFU BCG s.c. or with 5 × 106 CFU BCG s.c. followed 4 weeks later by two administrations of RplJ protein (25 μg) and CpG-ODN 1826 (20 μg) s.c. 2 weeks apart. Four weeks after the final immunization, mice were exposed to low-dose aerosolized M. tuberculosis for 20 min, resulting in approximately 100 CFU being deposited in the lungs. Ten weeks after challenge, mice were sacrificed, and single-cell suspensions prepared from lungs and mediastinal lymph nodes were plated onto 7H10 agar. Each symbol is representative of data for one mouse. Data shown represent mean values and standard errors. Responses that are significantly different from another group by a t test are indicated (*, P < 0.05; **, P < 0.01; n.s., not significant).
DISCUSSION
The discovery of new antigens for effective immunization against M. tuberculosis is a daunting task due to multiple factors, such as the complex life cycle of the bacterium, its relatively large genome, multiple immune evasion mechanisms, and the prevalence of previous exposure to mycobacteria prior to immunization (31–34). Although there is relatively limited knowledge of the correlates of protection for an M. tuberculosis vaccine, it is generally accepted that CD4+ T cell responses are critical for controlling M. tuberculosis infections. Recent studies have highlighted that many of the known T cell epitopes in M. tuberculosis and BCG are hyperconserved (35), suggesting a unique survival strategy that overactivates the host immune system and leads to the exhaustion of CD4+ T cells (36). Priming of CD4+ T cell responses to cryptic or subdominant antigens not recognized during natural infection has been shown to promote a less differentiated CD4+ T cell memory phenotype marked by IL-2 secretion and the absence of the marker KLRG1 (37, 38), and there are data that suggest that these T cells may express functions that can reprogram the T cell response to be more protective (39). Taken together, these findings suggest that the incorporation of cryptic antigens into vaccination strategies may be a useful strategy to promote CD4+ T cells that enhance protection against M. tuberculosis and to imprint the protective function of these cells on T cells previously primed by BCG immunization (36).
Immunization of mice with the genetically modified M. smegmatis strain IKEPLUS was previously shown to significantly enhance the survival of mice when challenged with M. tuberculosis, compared to standard BCG immunization (26). IKEPLUS immunization led to the development of a Th1-like immune response that conferred protection from M. tuberculosis and was transferable to naive animals primarily by CD4+ T cells (26). A recent follow-up study identified the mycobacterial ribosome as a prominent target of CD4+ T cells during IKEPLUS immunization (27) and specifically defined epitopes within the RplJ and RpsA proteins. The present study extended these findings by more definitively surveying all 57 mycobacterial ribosomal proteins to identify those harboring epitopes for CD4+ T cell responses in appropriately immunized mice. As expected, mice immunized with the rapidly growing M. smegmatis IKEPLUS strain formed strong CD4+ T cell responses to ribosomal proteins, recognizing 24 out of the total of 57 proteins, while BCG-immunized CD4+ T cells responded to only one ribosomal protein (Fig. 1). The mechanism for this difference in the priming of antiribosome responses between these mycobacterial species is unknown, although one possibility is that it is related to the larger number of ribosomes in fast-growing mycobacteria like M. smegmatis, versus slow-growing mycobacteria like BCG or M. tuberculosis (40, 41). Thus, the relatively large number of bacterial ribosomes present during a transient M. smegmatis infection may skew the CD4+ T cell response toward the mycobacterial ribosome.
To our knowledge, this is the first study to examine the entire complement of mycobacterial ribosomal proteins for their ability to stimulate CD4+ T cell responses. Early studies suggested that mycobacterial ribosomes were immunogenic in mice and guinea pigs, although the precise identities of the immunogenic components were not definitively established (42–48). In the present study, we identified multiple immunogenic protein antigens present in the mycobacterial ribosome, and for 10 of the 24 proteins identified as being immunogenic, we defined CD4+ T cell epitopes at the 15-mer peptide level (Table 1). Our findings confirmed the immunogenicity of the M. tuberculosis RplJ146–160 peptide identified in our previous work (27) and extended this by showing that immunization with a rapidly growing M. smegmatis strain stimulated a broad response against multiple cross-reactive M. tuberculosis ribosomal proteins. In addition, mycobacterial homologues of two ribosomal proteins identified in previous studies as being protective antigens of other microbial pathogens were identified in our comprehensive survey of mycobacterial ribosomal proteins. These proteins were RplL (L7/12), which is a target for protective immune responses against Brucella abortus, and RplC (L3), which is a target of protective immunity against Leishmania major in animal models (49–53). Overall, our findings provide a rich source of new antigens that potentially can be incorporated into various vaccine delivery systems already in clinical trials or advanced stages of development (11, 35, 54).
We previously reported that BCG was unable to stimulate a significant CD4+ T cell response to mycobacterial ribosomes in mice, even when injected at high doses by the subcutaneous or intravenous (i.v.) route (27). This result raised the possibility that BCG immunization may actively inhibit the presentation of mycobacterial ribosomes and block the formation of ribosome-specific CD4+ T cells. However, our results in the present study suggested that this is not the case, since BCG vaccination actually primed mice to generate stronger ribosome-specific CD4+ T cell responses when followed by immunization with an M. smegmatis strain or with purified ribosomal protein (Fig. 4). Mice that were immunized with BCG and boosted with RplJ protein primed a strong Th1-like CD4+ T cell response to the ribosomal protein, and these responses were dominated by cells with substantial polyfunctionality in terms of the production of IL-2, IFN-γ, and TNF-α (Fig. 5). Analysis of the dual-cytokine-producing cells of mice immunized with BCG and boosted with RplJ protein indicated a preponderance of dual TNF-α- and IFN-γ-producing cells, whereas recall with an epitope of RplJ (RplJTB146–160) revealed a multifunctional CD4+ T cell response that greatly increased the amount of IL-2-producing cells and reduced the number of dual IFN-γ and TNF-α producers. The differences in these responses may be relevant to the protective effects afforded by CD4+ T cells reactive with different antigens, as it has been reported that CD4+ T cells that coproduce IFN-γ and TNF-α are more prevalent in patients with active M. tuberculosis infections and not present in patients who successfully control M. tuberculosis and have latent infection (55, 56). Recent studies have indicated that cryptic antigens are able to prime a CD4+ T cell response with greater TNF-α and IL-2 coproduction and with a higher proliferative capacity, whereas immunodominant antigens were found to prime mainly IFN-γ/TNF-α-coproducing CD4+ T cell responses (57). Our results suggest that boosting BCG-vaccinated animals with RplJ protein may prime CD4+ T cell responses that resemble those associated with cryptic antigen recognition and, possibly, with better control of infection (57, 58).
This study also examined the ability of boosting BCG immunization with RplJ protein to protect mice against M. tuberculosis challenge. Most notably, mice primed with BCG and boosted with RplJ protein also showed significantly reduced pulmonary pathology and lower bacterial burdens in their mediastinal lymph nodes following challenge (Fig. 6 and 7). However, our findings so far have been restricted to proof-of-concept studies using only a single ribosomal protein to boost BCG-induced immunity, and future studies are planned to assess the effects of other immunogenic ribosomal proteins identified in this study as potential vaccines for boosting protective immunity against M. tuberculosis challenge. While we observed no general preference for components of the large or small ribosomal subunits in the CD4+ T cell responses in our studies, understanding the kinetics of expression of these proteins during M. tuberculosis infection, and variations in their posttranslational modifications and associations with cytosolic proteins (59–61), might be critically important in designing future vaccination studies. During normal growth of the bacteria and establishment of infection, ribosomes are relatively abundant (62), and all 57 ribosomal proteins should be available during this stage of infection, with one protein, RplL (L7/L12), present at a higher level than all others due to its formation of multimers (49–51). In contrast, during the latent phase of infection, it is believed that ribosomal silencing factors act on the ribosome, preventing the association of the small and large ribosomal subunits (52, 60). The large ribosomal subunits may be kept stable and present during this phase, while small ribosomal subunits will be subject to degradation and turnover. Thus, we hypothesize that the proteins of the large ribosomal subunit would be more abundant during the latent phase of M. tuberculosis infection. Many vaccine formulations currently in clinical trials are formulated with both active- and latent-phase antigens (11, 53). This raises the likelihood that the proteins of the large ribosomal subunit would act as both active and latent antigens during immunization and that the 15 antigenic proteins of the M. tuberculosis large ribosomal subunit identified in our study are strong candidates for incorporation into future experimental vaccines against M. tuberculosis.
MATERIALS AND METHODS
Mice.
Six- to eight-week-old female C57BL/6J (B6) mice were purchased from The Jackson Laboratories (Bar Harbor, ME). Mice were housed in pathogen-free facilities according to procedures and regulations established by the Albert Einstein College of Medicine Institute for Animal Studies and Biosafety Committees. Mice were allowed to acclimate to their housing for 1 week before experiments. All procedures performed were in accordance with protocols approved by the Albert Einstein College of Medicine Institutional Animal Care and Use Committee (IACUC).
Cell culture.
Mammalian cells were cultured in RPMI 1640 medium or Dulbecco's modified Eagle's medium (DMEM) (ThermoFisher Scientific, Grand Island, NY) containing 0.5% 2-mercaptoethanol (ThermoFisher Scientific, Grand Island, NY), 1% penicillin-streptomycin (ThermoFisher Scientific, Grand Island, NY), 1% HEPES (ThermoFisher Scientific, Grand Island, NY), 1% minimal essential medium (MEM) nonessential amino acids, and 10% fetal calf serum (FCS) (Atlanta Biologicals). Cultures were maintained in a humidified 37°C incubator with 5% CO2 (for RPMI) or 10% CO2 (for DMEM) in air.
Mycobacterial cultures and immunizations.
M. smegmatis strains mc2155 and IKEPLUS were described previously (26) and were grown in liquid Sauton medium (63). Bacillus Calmette-Guérin (BCG)-Danish was obtained from the Statens Serum Institute (Copenhagen, Denmark) and was grown in Middlebrook 7H9 medium (Difco Laboratories, BD Diagnostic Systems, Sparks, MD) with oleic acid-albumin-dextrose-catalase (OADC) enrichment (Difco Laboratories, BD Diagnostic Systems, Sparks, MD) and 0.05% tyloxapol (Sigma-Aldrich, St. Louis, MO). Bacteria were grown to mid-log phase from low-passage-number frozen stocks and then frozen in medium containing 5% glycerol at −80°C. Prior to immunization, bacterial stocks were thawed, washed twice with phosphate-buffered saline (PBS), and resuspended in PBS containing 0.05% Tween 80. The bacteria were then sonicated in a bath sonicator for 5 min to achieve single-cell suspensions. Mice were immunized with 5 × 107 CFU IKEPLUS via the tail vein or 5 × 106 CFU BCG-Danish subcutaneously (s.c.) in the scruff of the neck. For immunization with purified ribosomal proteins, aliquots of the proteins were thawed, diluted with adjuvant and double-distilled water (ddH2O) to a final concentration of 25 μg of protein per 200 μl, and sterilized by passage through a 0.22-μm filter. The adjuvant CPG-ODN 1826 (InvivoGen, San Diego, CA) was added to ribosomal proteins at a concentration of 20 μg per 200 μl. Protein-plus-adjuvant mixtures were injected s.c. in the scruff of the neck with a volume of 200 μl per injection.
Quantification of granulomatous inflammation in lung histology sections.
Lung sections were stained with hematoxylin and eosin, and images were acquired by using the Panoramic 250 Flash II slide scanner (3DHistech, Budapest, Hungary). Images were analyzed by color threshold to determine the total area of lung sections at a ×1.5 magnification. The color threshold was adjusted to isolate areas containing a high density of nuclei indicative of granulomatous inflammation, and the area was recorded. The inflamed area measured was divided by the total area measured and expressed as a percentage to represent the extent of granulomatous inflammation.
Preparation of bacterial sonicates and purified ribosomes.
The H37Rv sonicate was produced by sonicating irradiated M. tuberculosis H37Rv (BEI Resources, Manassas, VA) in lysis buffer (30 mM Tris-HCl, pH 8.0) containing 0.05% tyloxapol and protease inhibitors (cOmplete, Mini, EDTA-free protease inhibitor cocktail [Roche], at 1 tablet per 10 ml) for 20 min on ice. The resulting crude sonicate was centrifuged at 1,500 × g for 12 min at 4°C. The supernatant was collected and retained, and the pellet was resuspended and sonicated as described above. This step was repeated three times, and the supernatants were combined. The combined supernatant was centrifuged at 14,000 × g for 30 min at 4°C and filtered through a 0.22-μm filter. The protein concentration was determined by using a bicinchoninic acid (BCA) protein assay kit (ThermoFisher Scientific, Grand Island, NY).
Ribosomes from IKEPLUS were purified and quantitated as described previously (27).
Generation of an M. tuberculosis ribosomal protein library.
H37Rv genomic DNA was obtained from BEI Resources (Manassas, VA). By using the primers indicated in Table S1 in the supplemental material, each of the 57 M. tuberculosis ribosomal protein genes was amplified by PCR using Q5 high-fidelity polymerase 2× master mix (NEB, Ipswich, MA). Cloning of inserts was accomplished via ligation-independent cloning. The resulting inserts were treated with T4 polymerase (NEB, Ipswich, MA) at room temperature for 30 min to create nucleotide overhangs. The vector pmscg9 (64) was linearized with the SspI restriction enzyme (NEB, Ipswich, MA) and similarly treated with T4 polymerase. The digested vector and insert were incubated together at a molar ratio of 1:3 at room temperature for 5 min. The resulting ligation reaction mixtures were used to transform E. coli MAX Efficiency DH5α cells (ThermoFisher Scientific, Grand Island, NY) according to the manufacturer's instructions. Transformed DH5α cells were grown in LB broth (ThermoFisher Scientific, Grand Island, NY), and plasmid DNA was isolated by using the QIAprep spin miniprep kit (Qiagen, Hilden, Germany). Plasmid inserts were validated by sequencing by the Sanger method (Genewiz, South Plainfield, NJ). For the production and purification of recombinant M. tuberculosis ribosomal proteins, 5-ml cultures of E. coli BL21 harboring plasmids encoding ribosomal proteins were grown overnight in 1 liter of autoinduction medium [Terrific broth (Sigma), 1 mM MgSO4, 25 mM (NH4)2SO4, 50 mM KH2PO4, 50 mM Na2HPO4, 0.9% glycerol, 0.05% glucose, 0.2% α-lactose]. Cultures were centrifuged at 3,000 × g for 10 min, and pellets were frozen at −80°C, thawed at room temperature, and resuspended by using 5 ml bacterial protein extraction reagent (BPER) (ThermoFisher Scientific) per g of wet pellet. The mixture was rotated for 15 min at room temperature to lyse the cells and then centrifuged at 16,000 × g for 20 min to separate the insoluble and soluble fractions. Insoluble pellets were resuspended with 5 ml per g of wet pellet of inclusion body solubilization buffer (8 M urea, 1% Triton X-100, 0.6 M NaCl, 20 mM sodium phosphate), rotated for 2 h at room temperature, and then centrifuged at 27,000 × g for 15 min to remove debris and nonsolubilized proteins. Recombinant ribosomal proteins were purified by using a peristaltic pump (GE Healthcare, Marlborough, MA) and HisTrap HP columns (GE Healthcare). Protein was eluted by using 8 column volumes of elution buffer (500 mM imidazole, 0.5 M NaCl, 20 mM sodium phosphate), and 1-ml fractions were collected for analysis. Fractions that contained protein, as determined by the absorption at 280 nm, were pooled and depleted of imidazole by using a 7,000-molecular-weight-cutoff (MWCO) Zeba desalting column (Thermo). The concentrations of the desalted pure protein fractions were determined by using the Bradford assay (Thermo), and purified proteins were run on Any kD precast protein gels (Bio-Rad) to determine the molecular weight and purity. Purified ribosomal proteins were aliquoted in 150-μg stocks and frozen at −80°C.
Peptide libraries.
A peptide library based on the sequences of 10 selected M. tuberculosis ribosomal proteins (RplL, RplS, RpsJ, RpmB2, RplK, RpsG, RpsC, RplC, RplN, and RpsL) was designed based on strain H37Rv sequences collected from Tuberculist (http://genolist.pasteur.fr/TubercuList/). The sequences were ranked by their predicted binding affinity for MHC class II (I-Ab). MHC binding affinity was predicted by using the IEDB (http://www.iedb.org/) analysis resource consensus tool (30). Peptides with a consensus rank of ≤33.33 (i.e., top one-third best binders) were selected, and those with an overlap of 3 amino acids or more with a higher-ranking peptide were removed. This analysis resulted in 109 predicted peptides, which were synthesized and isolated by using a standard commercial service for generating peptide batches with 70 to 80% purity (Mimotopes, Mulgrave, Victoria, Australia). A second peptide library was independently constructed based on areas of homology between M. tuberculosis and M. smegmatis ribosomal proteins. The sequences of all 57 ribosomal proteins from the Mycobacterium smegmatis (strain mc2155) and M. tuberculosis (H37Rv) strains were collected from UniProt (http://www.uniprot.org/). The sequences were then clustered at a 30% amino acid sequence identity level to group similar sequences into separate clusters with the epitope cluster analysis tool available at the IEDB tools website (65). Each cluster of sequences was then aligned separately by using the ClustalW algorithm as implemented in the MEGA tool (66). For these clusters, 15-mer peptides overlapping by 10 amino acids were generated, and duplicates were removed. The remaining peptides were then ranked based on their hypothetical binding affinity for MHC class II (I-Ab), as predicted by using the IEDB analysis resource consensus tool (30). Peptides with an IEDB consensus percentile rank of ≤10.0 were selected, and variants (peptides from same sequence position but having amino acid mismatches) were removed by eliminating the variant with the higher consensus percentile. This resulted in 153 peptides, which were synthesized on a small scale (∼1 mg) and provided at 70 to 80% purity by A&A Labs (San Diego, CA). All peptides were reconstituted in dimethyl sulfoxide (DMSO) and used at a final concentration of 10 μg/ml per peptide.
IFN-γ ELISPOT and flow cytometry analyses.
Measurement of IFN-γ-producing CD4+ T cells purified from the spleens of previously immunized animals was done by an ELISPOT assay as described previously (27). For staining and analysis of surface markers by flow cytometry, CD4+ T cells were restimulated with antigens in culture and collected by centrifugation at 300 × g for 5 min. Cells were then resuspended in 50 μl of Live/Dead fixable blue reagent (Thermo) diluted 1:500 in PBS and incubated at room temperature for 30 min. After washing with 1× fluorescence-activated cell sorter (FACS) buffer (PBS, 2% FCS, 0.05% NaN3), cells were resuspended in FACS buffer containing a 1:200 dilution of CD11b-biotin (Thermo), a 1:200 dilution of CD19-biotin (BioLegend), and 5 μg/ml of 2.4G2 antibody (BioLegend). The cells were incubated for 30 min at room temperature and washed once in FACS buffer. A surface marker staining cocktail (CD4-Alexa 488 clone GK1.5 [BioLegend], CD3-peridinin chlorophyll protein [PerCP]/Cy5.5 clone 145-2C11 [Thermo], and CD8α-allophycocyanin [APC]/Cy7 clone 53-6.7 [BioLegend], all at a 1:200 dilution), also containing 5 μg/ml 2.4G2 antibody and a 1:200 dilution of streptavidin-Pacific Blue, was prepared in FACS buffer, and 50 μl was added to each well. The cells were incubated for 30 min at room temperature and washed once in FACS buffer. The cells were then washed once in PBS, resuspended in 50 μl of 1% paraformaldehyde (Sigma), and incubated at 4°C overnight. The next day, cells were washed once and resuspended in 100 μl FACS buffer. For intracellular cytokine staining, fixed and surface-stained cells were washed once with PBS, resuspended in 50 μl of FoxP3 fixation/permeabilization buffer (eBioscience), and incubated at room temperature. After 1 h, 200 μl of intracellular cytokine staining (ICS) permeabilization buffer (Dulbecco's phospate-buffered saline [DPBS] plus Ca2+ and Mg2+ [Thermo], 1.0% HEPES [Thermo], 2.0% FCS, 0.1% saponin [Sigma], and 0.05% NaN3 [Sigma]) was added to the wells. The plates were washed once with ICS permeabilization buffer. An intracellular cytokine staining cocktail (1:100 dilution of anti-IFN-γ–phycoerythrin [PE] clone XMG1.2 [BioLegend], 1:400 dilution of anti-IL-2–PE/Cy7 clone JES6-5H4 [BioLegend], 1:100 dilution of anti-TNF-α–APC clone MP6-XT22 [BioLegend]) was prepared in ICS permeabilization buffer with 5% normal mouse serum, and 50 μl was added to each well. Cells were incubated at 37°C for 30 min and transferred to 4°C overnight. The next day, cells were washed twice with permeabilization buffer and resuspended in 200 μl FACS buffer. All analyses and data collection were done by using an LSRII benchtop flow cytometer (BD Biosciences) and FlowJo software (BD Biosciences).
Statistical analyses.
The significance of ELISPOT responses to proteins of the total library of 57 mycobacterial ribosomal proteins was determined by using two-way analysis of variance (ANOVA) with a false discovery rate (FDR) correction. The cutoff for significance was a 1% FDR. All data reported are represented as mean values ± 1 standard error of the mean (SEM). Statistical significance was determined by using one-way ANOVA in the case of multiple comparisons, unless stated otherwise in the figure legends. GraphPad Prism (GraphPad, La Jolla, CA) was used for all statistical calculations.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by NIH grant AI063537 to William R. Jacobs, Jr., and Steven A. Porcelli; NIH grant AI093649 to Steven A. Porcelli; contract HHSN272200900044C to Alessandro Sette; and NIH training grant T32 AI07506 support to Alison J. Johnson. Flow cytometry and analytical imaging core resources used in this study were supported by the Albert Einstein Cancer Center support grant from the National Institutes of Health under award number P30CA013330.
We thank Hillary Guzik for aiding in histology slide image acquisition and analysis and the Albert Einstein College of Medicine Macromolecular Therapeutics Development Facility for aiding in high-throughput ribosomal protein expression and purification.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00009-18.
REFERENCES
- 1.Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698–702. doi: 10.1001/jama.1994.03510330076038. [DOI] [PubMed] [Google Scholar]
- 2.Fine PE. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339–1345. doi: 10.1016/S0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 3.Ellner JJ. 2009. The emergence of extensively drug-resistant tuberculosis: a global health crisis requiring new interventions. Part II: scientific advances that may provide solutions. Clin Transl Sci 2:80–84. doi: 10.1111/j.1752-8062.2008.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellner JJ. 2008. The emergence of extensively drug-resistant tuberculosis: a global health crisis requiring new interventions. Part I: the origins and nature of the problem. Clin Transl Sci 1:249–254. doi: 10.1111/j.1752-8062.2008.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pang Y, Lu J, Huo F, Ma Y, Zhao L, Li Y, Liang Q, Chu N, Gao M, Huang H. 11 August 2017. Prevalence and treatment outcome of extensively drug-resistant tuberculosis plus additional drug resistance from the National Clinical Center for Tuberculosis in China: a five-year review. J Infect doi: 10.1016/j.jinf.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Teshome Kefale A, Anagaw YK. 2017. Outcome of tuberculosis treatment and its predictors among HIV infected patients in southwest Ethiopia. Int J Gen Med 10:161–169. doi: 10.2147/IJGM.S135305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osei E, Der J, Owusu R, Kofie P, Axame WK. 2017. The burden of HIV on tuberculosis patients in the Volta region of Ghana from 2012 to 2015: implication for tuberculosis control. BMC Infect Dis 17:504. doi: 10.1186/s12879-017-2598-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandhi NR, Shah NS, Andrews JR, Vella V, Moll AP, Scott M, Weissman D, Marra C, Lalloo UG, Friedland GH. 2010. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med 181:80–86. doi: 10.1164/rccm.200907-0989OC. [DOI] [PubMed] [Google Scholar]
- 9.Tang J, Yam WC, Chen Z. 2016. Mycobacterium tuberculosis infection and vaccine development. Tuberculosis (Edinb) 98:30–41. doi: 10.1016/j.tube.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Ng TW, Saavedra-Avila NA, Kennedy SC, Carreno LJ, Porcelli SA. 2015. Current efforts and future prospects in the development of live mycobacteria as vaccines. Expert Rev Vaccines 14:1493–1507. doi: 10.1586/14760584.2015.1089175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fletcher HA, Schrager L. 2016. TB vaccine development and the End TB Strategy: importance and current status. Trans R Soc Trop Med Hyg 110:212–218. doi: 10.1093/trstmh/trw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans TG, Schrager L, Thole J. 2016. Status of vaccine research and development of vaccines for tuberculosis. Vaccine 34:2911–2914. doi: 10.1016/j.vaccine.2016.02.079. [DOI] [PubMed] [Google Scholar]
- 13.Abel B, Tameris M, Mansoor N, Gelderbloem S, Hughes J, Abrahams D, Makhethe L, Erasmus M, de Kock M, van der Merwe L, Hawkridge A, Veldsman A, Hatherill M, Schirru G, Pau MG, Hendriks J, Weverling GJ, Goudsmit J, Sizemore D, McClain JB, Goetz M, Gearhart J, Mahomed H, Hussey GD, Sadoff JC, Hanekom WA. 2010. The novel tuberculosis vaccine, AERAS-402, induces robust and polyfunctional CD4+ and CD8+ T cells in adults. Am J Respir Crit Care Med 181:1407–1417. doi: 10.1164/rccm.200910-1484OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beveridge NE, Price DA, Casazza JP, Pathan AA, Sander CR, Asher TE, Ambrozak DR, Precopio ML, Scheinberg P, Alder NC, Roederer M, Koup RA, Douek DC, Hill AV, McShane H. 2007. Immunisation with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis-specific CD4+ memory T lymphocyte populations. Eur J Immunol 37:3089–3100. doi: 10.1002/eji.200737504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowland R, Pathan AA, Satti I, Poulton ID, Matsumiya MM, Whittaker M, Minassian AM, O'Hara GA, Hamill M, Scott JT, Harris SA, Poyntz HC, Bateman C, Meyer J, Williams N, Gilbert SC, Lawrie AM, Hill AV, McShane H. 2013. Safety and immunogenicity of an FP9-vectored candidate tuberculosis vaccine (FP85A), alone and with candidate vaccine MVA85A in BCG-vaccinated healthy adults: a phase I clinical trial. Hum Vaccin Immunother 9:50–62. doi: 10.4161/hv.22464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun R, Skeiky YA, Izzo A, Dheenadhayalan V, Imam Z, Penn E, Stagliano K, Haddock S, Mueller S, Fulkerson J, Scanga C, Grover A, Derrick SC, Morris S, Hone DM, Horwitz MA, Kaufmann SH, Sadoff JC. 2009. Novel recombinant BCG expressing perfringolysin O and the over-expression of key immunodominant antigens; pre-clinical characterization, safety and protection against challenge with Mycobacterium tuberculosis. Vaccine 27:4412–4423. doi: 10.1016/j.vaccine.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 17.Billeskov R, Elvang TT, Andersen PL, Dietrich J. 2012. The HyVac4 subunit vaccine efficiently boosts BCG-primed anti-mycobacterial protective immunity. PLoS One 7:e39909. doi: 10.1371/journal.pone.0039909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic S. 2000. Recombinant bacillus Calmette-Guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc Natl Acad Sci U S A 97:13853–13858. doi: 10.1073/pnas.250480397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luabeya AK, Kagina BM, Tameris MD, Geldenhuys H, Hoff ST, Shi Z, Kromann I, Hatherill M, Mahomed H, Hanekom WA, Andersen P, Scriba TJ, Schoeman E, Krohn C, Day CL, Africa H, Makhethe L, Smit E, Brown Y, Suliman S, Hughes EJ, Bang P, Snowden MA, McClain B, Hussey GD. 2015. First-in-human trial of the post-exposure tuberculosis vaccine H56:IC31 in Mycobacterium tuberculosis infected and non-infected healthy adults. Vaccine 33:4130–4140. doi: 10.1016/j.vaccine.2015.06.051. [DOI] [PubMed] [Google Scholar]
- 20.Ottenhoff TH, Doherty TM, van Dissel JT, Bang P, Lingnau K, Kromann I, Andersen P. 2010. First in humans: a new molecularly defined vaccine shows excellent safety and strong induction of long-lived Mycobacterium tuberculosis-specific Th1-cell like responses. Hum Vaccin 6:1007–1015. doi: 10.4161/hv.6.12.13143. [DOI] [PubMed] [Google Scholar]
- 21.Skeiky YA, Dietrich J, Lasco TM, Stagliano K, Dheenadhayalan V, Goetz MA, Cantarero L, Basaraba RJ, Bang P, Kromann I, McMclain JB, Sadoff JC, Andersen P. 2010. Non-clinical efficacy and safety of HyVac4:IC31 vaccine administered in a BCG prime-boost regimen. Vaccine 28:1084–1093. doi: 10.1016/j.vaccine.2009.10.114. [DOI] [PubMed] [Google Scholar]
- 22.Von Eschen K, Morrison R, Braun M, Ofori-Anyinam O, De Kock E, Pavithran P, Koutsoukos M, Moris P, Cain D, Dubois MC, Cohen J, Ballou WR. 2009. The candidate tuberculosis vaccine Mtb72F/AS02A: tolerability and immunogenicity in humans. Hum Vaccin 5:475–482. doi: 10.4161/hv.8570. [DOI] [PubMed] [Google Scholar]
- 23.Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, Mahomed H, McShane H, MVA85A 020 Trial Study Team . 2013. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 381:1021–1028. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ndiaye BP, Thienemann F, Ota M, Landry BS, Camara M, Dieye S, Dieye TN, Esmail H, Goliath R, Huygen K, January V, Ndiaye I, Oni T, Raine M, Romano M, Satti I, Sutton S, Thiam A, Wilkinson KA, Mboup S, Wilkinson RJ, McShane H, MVA85A 030 Trial Investigators . 2015. Safety, immunogenicity, and efficacy of the candidate tuberculosis vaccine MVA85A in healthy adults infected with HIV-1: a randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 3:190–200. doi: 10.1016/S2213-2600(15)00037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufmann SHE, Evans TG, Hanekom WA. 2015. Tuberculosis vaccines: time for a global strategy. Sci Transl Med 7:276fs8. doi: 10.1126/scitranslmed.aaa4730. [DOI] [PubMed] [Google Scholar]
- 26.Sweeney KA, Dao DN, Goldberg MF, Hsu T, Venkataswamy MM, Henao-Tamayo M, Ordway D, Sellers RS, Jain P, Chen B, Chen M, Kim J, Lukose R, Chan J, Orme IM, Porcelli SA, Jacobs WR Jr. 2011. A recombinant Mycobacterium smegmatis induces potent bactericidal immunity against Mycobacterium tuberculosis. Nat Med 17:1261–1268. doi: 10.1038/nm.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson AJ, Kennedy SC, Lindestam Arlehamn CS, Goldberg MF, Saini NK, Xu J, Paul S, Hegde SS, Blanchard JS, Chan J, Jacobs WR Jr, Sette A, Porcelli SA. 2017. Identification of mycobacterial RplJ/L10 and RpsA/S1 proteins as novel targets for CD4+ T cells. Infect Immun 85:e01023-16. doi: 10.1128/IAI.01023-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hentschel J, Burnside C, Mignot I, Leibundgut M, Boehringer D, Ban N. 2017. The complete structure of the Mycobacterium smegmatis 70S ribosome. Cell Rep 20:149–160. doi: 10.1016/j.celrep.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 29.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. 2004. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 30.Wang P, Sidney J, Dow C, Mothé B, Sette A, Peters B. 2008. A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput Biol 4:e1000048. doi: 10.1371/journal.pcbi.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ernst JD. 2012. The immunological life cycle of tuberculosis. Nat Rev Immunol 12:581–591. doi: 10.1038/nri3259. [DOI] [PubMed] [Google Scholar]
- 32.Kato-Maeda M, Rhee JT, Gingeras TR, Salamon H, Drenkow J, Smittipat N, Small PM. 2001. Comparing genomes within the species Mycobacterium tuberculosis. Genome Res 11:547–554. doi: 10.1101/gr.166401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poyntz HC, Stylianou E, Griffiths KL, Marsay L, Checkley AM, McShane H. 2014. Non-tuberculous mycobacteria have diverse effects on BCG efficacy against Mycobacterium tuberculosis. Tuberculosis (Edinb) 94:226–237. doi: 10.1016/j.tube.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baena A, Porcelli SA. 2009. Evasion and subversion of antigen presentation by Mycobacterium tuberculosis. Tissue Antigens 74:189–204. doi: 10.1111/j.1399-0039.2009.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coscolla M, Copin R, Sutherland J, Gehre F, de Jong B, Owolabi O, Mbayo G, Giardina F, Ernst JD, Gagneux S. 2015. M. tuberculosis T cell epitope analysis reveals paucity of antigenic variation and identifies rare variable TB antigens. Cell Host Microbe 18:538–548. doi: 10.1016/j.chom.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woodworth JS, Andersen P. 2016. Reprogramming the T cell response to tuberculosis. Trends Immunol 37:81–83. doi: 10.1016/j.it.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orme IM, Robinson RT, Cooper AM. 2015. The balance between protective and pathogenic immune responses in the TB-infected lung. Nat Immunol 16:57–63. doi: 10.1038/ni.3048. [DOI] [PubMed] [Google Scholar]
- 38.Lindenstrom T, Knudsen NP, Agger EM, Andersen P. 2013. Control of chronic Mycobacterium tuberculosis infection by CD4 KLRG1-IL-2-secreting central memory cells. J Immunol 190:6311–6319. doi: 10.4049/jimmunol.1300248. [DOI] [PubMed] [Google Scholar]
- 39.Hoang T, Aagaard C, Dietrich J, Cassidy JP, Dolganov G, Schoolnik GK, Lundberg CV, Agger EM, Andersen P. 2013. ESAT-6 (EsxA) and TB10.4 (EsxH) based vaccines for pre- and post-exposure tuberculosis vaccination. PLoS One 8:e80579. doi: 10.1371/journal.pone.0080579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia MJ, Nuñez MC, Cox RA. 2010. Measurement of the rates of synthesis of three components of ribosomes of Mycobacterium fortuitum: a theoretical approach to qRT-PCR experimentation. PLoS One 5:e11575. doi: 10.1371/journal.pone.0011575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamada H, Yamaguchi M, Chikamatsu K, Aono A, Mitarai S. 2015. Structome analysis of virulent Mycobacterium tuberculosis, which survives with only 700 ribosomes per 0.1 fl of cytoplasm. PLoS One 10:e0117109. doi: 10.1371/journal.pone.0117109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyazaki C, Ohara N, Yukitake H, Kinomoto M, Matsushita K, Matsumoto S, Mizuno A, Yamada T. 1999. Host immune responses to ribosome, ribosomal proteins, and RNA from Mycobacterium bovis bacille de Calmette-Guerin. Vaccine 17:245–251. doi: 10.1016/S0264-410X(98)00191-1. [DOI] [PubMed] [Google Scholar]
- 43.Patterson RJ, Youmans GP. 1970. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun 1:600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sinha S, Kosalai K, Arora S, Namane A, Sharma P, Gaikwad AN, Brodin P, Cole ST. 2005. Immunogenic membrane-associated proteins of Mycobacterium tuberculosis revealed by proteomics. Microbiology 151:2411–2419. doi: 10.1099/mic.0.27799-0. [DOI] [PubMed] [Google Scholar]
- 45.Youmans AS, Youmans GP. 1965. Immunogenic activity of a ribosomal fraction obtained from Mycobacterium tuberculosis. J Bacteriol 89:1291–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Youmans AS, Youmans GP. 1966. Effect of trypsin and ribonuclease on the immunogenic activity of ribosomes and ribonucleic acid isolated from Mycobacterium tuberculosis. J Bacteriol 91:2146–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Youmans AS, Youmans GP. 1973. The relationship between sedimentation value and immunogenic activity of mycobacterial ribonucleic acid. J Immunol 110:581–586. [PubMed] [Google Scholar]
- 48.Youmans GP, Youmans AS. 1969. Allergenicity of mycobacterial ribosomal and ribonucleic acid preparations in mice and guinea pigs. J Bacteriol 97:134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deroo S, Hyung S-J, Marcoux J, Gordiyenko Y, Koripella RK, Sanyal S, Robinson CV. 2012. Mechanism and rates of exchange of L7/L12 between ribosomes and the effects of binding EF-G. ACS Chem Biol 7:1120–1127. doi: 10.1021/cb300081s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin Y, Li Y, Zhu N, Han Y, Jiang W, Wang Y, Si S, Jiang J. 2014. The antituberculosis antibiotic capreomycin inhibits protein synthesis by disrupting interaction between ribosomal proteins L12 and L10. Antimicrob Agents Chemother 58:2038–2044. doi: 10.1128/AAC.02394-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin Y, Li Y, Zhu Y, Zhang J, Li Y, Liu X, Jiang W, Yu S, You X-F, Xiao C, Hong B, Wang Y, Jiang J-D, Si S. 2012. Identification of antituberculosis agents that target ribosomal protein interactions using a yeast two-hybrid system. Proc Natl Acad Sci U S A 109:17412–17417. doi: 10.1073/pnas.1110271109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKay SL, Portnoy DA. 2015. Ribosome hibernation facilitates tolerance of stationary-phase bacteria to aminoglycosides. Antimicrob Agents Chemother 59:6992–6999. doi: 10.1128/AAC.01532-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahsan MJ. 2015. Recent advances in the development of vaccines for tuberculosis. Ther Adv Vaccines 3:66–75. doi: 10.1177/2051013615593891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orr MT, Ireton GC, Beebe EA, Huang PW, Reese VA, Argilla D, Coler RN, Reed SG. 2014. Immune subdominant antigens as vaccine candidates against Mycobacterium tuberculosis. J Immunol 193:2911–2918. doi: 10.4049/jimmunol.1401103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harari A, Rozot V, Bellutti Enders F, Perreau M, Stalder JM, Nicod LP, Cavassini M, Calandra T, Blanchet CL, Jaton K, Faouzi M, Day CL, Hanekom WA, Bart PA, Pantaleo G. 2011. Dominant TNF-alpha+ Mycobacterium tuberculosis-specific CD4+ T cell responses discriminate between latent infection and active disease. Nat Med 17:372–376. doi: 10.1038/nm.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petruccioli E, Petrone L, Vanini V, Sampaolesi A, Gualano G, Girardi E, Palmieri F, Goletti D. 2013. IFNγ/TNFα specific-cells and effector memory phenotype associate with active tuberculosis. J Infect 66:475–486. doi: 10.1016/j.jinf.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 57.Woodworth JS, Aagaard CS, Hansen PR, Cassidy JP, Agger EM, Andersen P. 2014. Protective CD4 T cells targeting cryptic epitopes of Mycobacterium tuberculosis resist infection-driven terminal differentiation. J Immunol 192:3247–3258. doi: 10.4049/jimmunol.1300283. [DOI] [PubMed] [Google Scholar]
- 58.Bertholet S, Ireton GC, Ordway DJ, Windish HP, Pine SO, Kahn M, Phan T, Orme IM, Vedvick TS, Baldwin SL, Coler RN, Reed SG. 2010. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug resistant Mycobacterium tuberculosis. Sci Transl Med 2:53ra74. doi: 10.1126/scitranslmed.3001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prisic S, Hwang H, Dow A, Barnaby O, Pan TS, Lonzanida JA, Chazin WJ, Steen H, Husson RN. 2015. Zinc regulates a switch between primary and alternative S18 ribosomal proteins in Mycobacterium tuberculosis. Mol Microbiol 97:263–280. doi: 10.1111/mmi.13022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li X, Sun Q, Jiang C, Yang K, Hung L-W, Zhang J, Sacchettini JC. 2015. Structure of ribosomal silencing factor bound to Mycobacterium tuberculosis ribosome. Structure 23:1858–1865. doi: 10.1016/j.str.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trauner A, Lougheed KEA, Bennett MH, Hingley-Wilson SM, Williams HD. 2012. The dormancy regulator DosR controls ribosome stability in hypoxic mycobacteria. J Biol Chem 287:24053–24063. doi: 10.1074/jbc.M112.364851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Piir K, Paier A, Liiv A, Tenson T, Maiväli Ü. 2011. Ribosome degradation in growing bacteria. EMBO Rep 12:458–462. doi: 10.1038/embor.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stager CE, Gangadharam PRJ. 1978. Comparison of several culture media used for studies on mycobacteriophages (plates XII–XIV). J Med Microbiol 11:187–191. doi: 10.1099/00222615-11-2-187. [DOI] [PubMed] [Google Scholar]
- 64.Donnelly MI, Zhou M, Millard CS, Clancy S, Stols L, Eschenfeldt WH, Collart FR, Joachimiak A. 2006. An expression vector tailored for large-scale, high-throughput purification of recombinant proteins. Protein Expr Purif 47:446–454. doi: 10.1016/j.pep.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim Y, Ponomarenko J, Zhu Z, Tamang D, Wang P, Greenbaum J, Lundegaard C, Sette A, Lund O, Bourne PE, Nielsen M, Peters B. 2012. Immune epitope database analysis resource. Nucleic Acids Res 40:W525–W530. doi: 10.1093/nar/gks438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.