Plasmodium falciparum infections are serious in pregnant women, because VAR2CSA allows parasitized erythrocytes to sequester in the placenta, causing placental malaria (PM). In areas of endemicity, women have substantial malarial immunity prior to pregnancy, including antibodies to merozoite antigens, but produce antibodies to VAR2CSA only during pregnancy.
KEYWORDS: Cameroon, IgG, Plasmodium falciparum, VAR2CSA, antibody, immunity, low transmission, malaria, merozoite, pregnancy
ABSTRACT
Plasmodium falciparum infections are serious in pregnant women, because VAR2CSA allows parasitized erythrocytes to sequester in the placenta, causing placental malaria (PM). In areas of endemicity, women have substantial malarial immunity prior to pregnancy, including antibodies to merozoite antigens, but produce antibodies to VAR2CSA only during pregnancy. The current study sought to determine the importance of antibodies to VAR2CSA and merozoite antigens in pregnant women in Yaoundé, Cameroon, where malaria transmission was relatively low. A total of 1,377 archival plasma samples collected at delivery were selected (at a 1:3 ratio of PM-positive [PM+] to PM-negative [PM−] women) and screened for antibodies to full-length VAR2CSA and 7 merozoite antigens. Results showed that many PM+ women and most PM− women lacked antibodies to VAR2CSA at delivery. Among PM+ women, antibodies to VAR2CSA were associated with a reduced risk of having high placental parasitemia (odds ratio [OR], 0.432; confidence interval [CI], 0.272, 0.687; P = 0.0004) and low-birth-weight (LBW) babies (OR = 0.444; CI, 0.247, 0.799; P = 0.0068), even during first pregnancies. Among antibodies to the 7 merozoite antigens, i.e., AMA1, EBA-175, MSP142, MSP2, MSP3, MSP11, and Pf41, only antibodies to MSP3, EBA-175, and Pf41 were associated with reduced risk for high placental parasitemias (P = 0.0389, 0.0291, and 0.0211, respectively) and antibodies to EBA-175 were associated with reduced risk of premature deliveries (P = 0.0211). However, after adjusting for multiple comparisons significance declined. Thus, in PM+ women, antibodies to VAR2CSA were associated with lower placental parasitemias and reduced prevalence of LBW babies in this low-transmission setting.
INTRODUCTION
In areas where malaria is endemic, pregnant women often become infected with Plasmodium falciparum parasites that express the adhesive ligand VAR2CSA (1). As a result, infected erythrocytes (IE) accumulate in the placenta, causing placental malaria (PM), a condition that increases the risk of maternal anemia, low-birth-weight (LBW) babies, and preterm deliveries (2, 3). Women in areas of endemicity have substantial immunity to malaria prior to pregnancy and often produce antibodies (Ab) to VAR2CSA (1, 4–8) that reduce the severity of PM.
The frequency of P. falciparum infection affects Ab acquisition to VAR2CSA. For example, studies in a high-transmission area detected Ab to VAR2CSA in primigravidae at ∼10 gestational weeks, whereas these Ab were not detected until ∼20 weeks in a lower-transmission area (7, 9). Women living in villages with high transmission usually become infected many times during pregnancy and are exposed to multiple parasite genotypes (10). As a result, they rapidly acquire Ab to all six Duffy-like domains (11) of VAR2CSA, whereas the Ab repertoire develops more slowly in women with fewer infections (12). In contrast to high-transmission areas, pregnant women in low-transmission areas may not become infected or may be infected only a few times during a single pregnancy, and it is unclear if infection always results in PM. A study conducted in Yaoundé, Cameroon, found that among 48 women who were PCR positive for P. falciparum before 6 months of pregnancy, 47.9% were PM negative at delivery without having developed Ab to VAR2CSA (12, 13). These data suggest either that the women cleared their infections before PM became established or that Ab to malarial antigens other than VAR2CSA were responsible for eliminating the infection. Thus, the possibility arises that Ab to malarial antigens other than VAR2CSA, e.g., antigens on the surface of merozoites, might be sufficient to protect women in low-transmission areas.
Malaria transmission is declining worldwide due to active efforts for malaria elimination, including implementing intermittent preventive treatment for pregnant women (IPTp), children, and infants, using insecticide-treated bed nets (ITN), residual spraying, and larvicides, and awareness campaigns. In addition, many rural areas are becoming urbanized. As a result, intermediate- and high-transmission areas are transitioning into low-transmission areas. Most comprehensive studies on the role of Ab to VAR2CSA in pregnant women were conducted in areas of relatively high transmission (4, 6). Therefore, it is necessary to determine the relative importance of naturally acquired Ab to VAR2CSA and merozoite antigens in lower-transmission settings.
Accordingly, this study was conducted using archival samples collected between 1995 and 2001 from 1,377 pregnant women residing in Yaoundé, Cameroon. During this period, individuals received ∼1 infectious mosquito bite per month and natural immunity helped control PM infections, since the initial study was conducted before IPTp and ITN implementation. Multivariable analysis of the original data demonstrated that the biggest risk factor for PM was age, not gravidity (14), suggesting that the situation in Yaoundé was different from higher-transmission areas (2, 10, 15–17). Thus, the study sought to determine if at delivery Ab to full-length VAR2CSA (FV2) and seven individual merozoite antigens (MSP142, MSP2, MSP3, MSP11, AMA1, EBA-175, Pf41) were associated with reduced maternal anemia, placental parasitemias, preterm deliveries, and LBW babies.
RESULTS
Characteristics of PM+ and PM− women in the study.
The characteristics of the pregnant women and their neonates are described in Table 1. Placental malaria had a significant impact on maternal health and pregnancy outcomes in Yaoundé, Cameroon. Compared to PM-negative (PM−) women (n = 1,036), PM-positive (PM+) women (n = 341) were younger (P < 0.0001) and had fewer pregnancies (P < 0.0001), lower hemoglobin levels (P < 0.0001), higher prevalence of maternal anemia (P < 0.0001), more preterm deliveries (P = 0.014), and more LBW babies (P = 0.0011), as well as lower birth weight of full-term babies (P < 0.0001).
TABLE 1.
Characteristics of women in the study and the influence of placental malaria on pregnancy outcomes
| Characteristic | PM+ (n = 341) | PM− (n = 1,036) | P valueb |
|---|---|---|---|
| Age, yr (mean ± SD) | 24.3 ± 5.4 | 26.3 ± 5.8 | <0.0001 |
| Gravidity (mean ± SD) | 2.7 ± 1.9 | 3.2 ± 2.1 | <0.0001 |
| Placental parasitemia, % median (25th, 75th) | 0.8 (0.2, 3.1) | 0 | NA |
| Maternal hematocrit, % (mean ± SD) | 31.3 ± 6.2 | 34.9 ± 5.9 | <0.0001 |
| Women with anemia, %a | 26.4 | 15.3 | <0.0001 |
| Preterm deliveries, %a | 24.2 | 17.8 | 0.014 |
| Infant birth weight, g (mean ± SD) | 2,922 ± 656 | 3,134 ± 635 | <0.0001 |
| Infant birth weight from full-term deliveries, g (mean ± SD) | 3,128 ± 513 | 3,300 ± 488 | <0.0001 |
| Low-birth-weight infants, %a | 21.6 | 14.0 | 0.0011 |
These percentages were calculated for samples with available data.
Twins and stillbirths were excluded from the comparisons for birth weight and preterm deliveries. Significant values (i.e., P < 0.05) are highlighted in bold. NA, not applicable.
Percentages of women with Ab to FV2 and merozoite antigens.
The percentages of women who had Ab to FV2 and seven merozoite antigens are shown in Table 2. Overall, more women in the PM+ group had Ab to FV2 than women in the PM− group (P < 0.0001). The percentage of women with Ab to FV2 at delivery increased with gravidity (P < 0.0001 for both PM+ and PM− groups). Interestingly, many women did not have Ab to FV2 even after multiple pregnancies. For example, over one-half of primi- and secundigravidae who were PM+ at delivery were Ab negative to FV2, showing that infection did not always result in Ab production. Even among women who had ≥4 pregnancies and were PM+ at delivery, 26.9% remained Ab negative. Furthermore, among women in the PM− group, only 17.6% of primigravidae (G1), 31.9% of secundigravidae (G2), 32.0% of G3, and 43.8% of G4+ had Ab to FV2. Thus, the proportion of women with Ab to FV2 increased with gravidity, but many women lacked Ab even after multiple pregnancies.
TABLE 2.
Percentages of women who have antibodies to the antigens (seropositivity)a
| Antigen | % of all women (n = 1,342) | % of PM+ women |
% of PM− women |
CMH P valuec | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 328) | G1 (n = 117) | G2 (n = 73) | G3 (n = 45) | G4+ (n = 93) | P valueb | All (n = 1,014) | G1 (n = 244) | G2 (n = 216) | G3 (n = 175) | G4+ (n = 379) | P valueb | |||
| FV2 | 38.6 | 56.1 | 47.0 | 43.8 | 64.4 | 73.1 | <0.0001 | 32.9 | 17.6 | 31.9 | 32.0 | 43.8 | <0.0001 | <0.0001 |
| MSP142 | 62.3 | 73.8 | 69.2 | 68.5 | 75.6 | 82.8 | 0.020 | 58.8 | 52.5 | 56.5 | 60.6 | 62.8 | 0.0076 | 0.0075 |
| MSP2 | 74.7 | 87.5 | 88.0 | 80.3 | 84.4 | 93.4 | 0.21 | 70.5 | 68.9 | 68.5 | 66.3 | 74.7 | 0.11 | 0.033 |
| MSP3 | 19.7 | 27.7 | 22.2 | 24.7 | 24.4 | 38.7 | 0.011 | 17.1 | 11.9 | 19.0 | 12.6 | 21.4 | 0.0093 | 0.0003 |
| MSP11 | 11.1 | 18.9 | 12.0 | 16.4 | 26.7 | 25.8 | 0.0050 | 8.6 | 7.0 | 11.1 | 8.0 | 8.4 | 0.87 | 0.14 |
| AMA1 | 88.4 | 96.6 | 95.7 | 95.9 | 97.8 | 97.9 | 0.34 | 85.7 | 88.1 | 82.4 | 84.0 | 86.8 | 0.98 | 0.32 |
| EBA-175 | 78.2 | 88.7 | 88.0 | 83.6 | 95.6 | 90.3 | 0.33 | 74.8 | 74.6 | 71.8 | 70.9 | 78.4 | 0.21 | 0.15 |
| Pf41 | 48.0 | 61.0 | 59.8 | 60.3 | 64.4 | 61.3 | 0.75 | 43.8 | 41.8 | 44.9 | 40.0 | 46.2 | 0.40 | 0.64 |
Information of the cutoff for positivity for each antigen is provided in Fig. S1 in the supplemental material. Significant P values (i.e., P < 0.05) are highlighted in bold. G, gravidity.
Cochran-Armitage test: trend in binomial proportion of Ab+ cross level of gravidity.
CMH, Cochran-Mantel-Haenszel test for the relationship between Ab+ and gravidity after controlling for malaria status.
Overall, more than 88% of all the women had Ab to at least one merozoite antigen (Table 2). Although fewer than 20% of the women had Ab to MSP3 and MSP11, more than 48% of all the women had Ab to the other merozoite antigens. The proportion of women with Ab to the merozoite antigens significantly increased with gravidity in MSP142 (P = 0.0075), MSP2 (P = 0.033), and MSP3 (P = 0.0003), adjusting for the placental malaria status (Table 2).
Effects of gravidity on antibody levels.
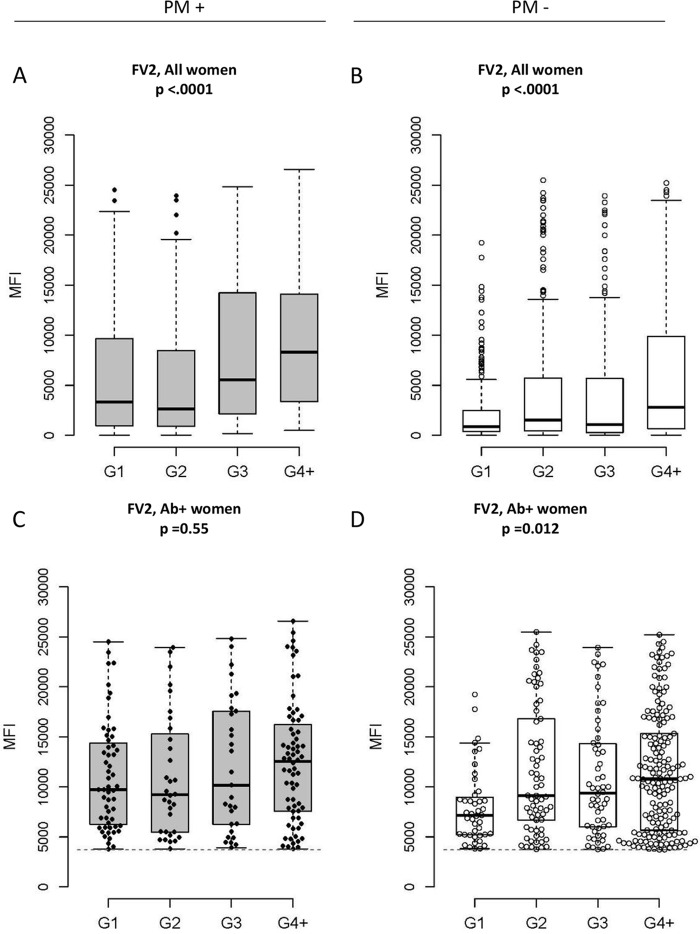
Figure 1 illustrates the Ab levels to FV2 across gravidity by PM status. Ab levels to FV2 increase with gravidity (P < 0.0001 for PM+ and PM−) (Fig. 1A and B) in part because the number of Ab-positive women increased in each gravidity group. On the other hand, when Ab levels were compared among women who had Ab to FV2 (i.e., FV2 Ab-positive women), the amount of Ab increased with gravidity in PM− women (P = 0.012) (Fig. 1D), but not in PM+ women (P = 0.55) (Fig. 1C). Although most primigravidae remained Ab negative (Table 2), those who produced Ab had levels of Ab to FV2 similar to those of the multigravidae (Fig. 1C). That is, Ab levels to FV2 in PM+ women were similar at delivery in primigravid and multigravid women.
FIG 1.
Antibody levels to FV2 in PM+ (n = 328) and PM− (n = 1,014) women stratified by gravidity (G). P values were based on the Cochran-Armitage trend tests. Boxes illustrate the median and IQR of the median fluorescence intensity (MFI). Note that equivalent data for the subset of women who were Ab positive for FV2 are shown in the second row; other antigens are shown in Fig. S2 in the supplemental material.
Overall, Ab levels to the seven merozoite antigens were also higher in PM+ than PM− women (all P values were <0.0001; see Fig. S2 in the supplemental material). Levels of Ab to five of the seven merozoite antigens increased with gravidity, primarily in PM+ women; these five included MSP142 (P = 0.041 in PM+ women), MSP2 (P = 0.002 in PM− women), MSP3 (P = 0.027 in PM+ and P = 0.007 in PM− women), MSP11 (P = 0.0085 in PM+ women), and EBA-175 (P = 0.031 in PM+ women). The age range for women in the different gravidity groups is provided in Table S1 in the supplemental material. Ab levels to MSP142 had a significant but small correlation with age (r = 0.19 and P = 0.0007 for PM+ women; r = 0.09 and P = 0.0039 for PM− women), but this was not the case for other merozoite antigens. Thus, levels of Ab to merozoite antigens at delivery increased with infection, age, and gravidity.
Influence of Ab in PM+ and PM− women on pregnancy outcomes.
Table 3 shows that Ab to FV2 were associated with a protective effect in PM+ but not PM− women. PM+ women who had Ab to FV2 had lower median placental parasitemia (P < 0.0001), fewer preterm deliveries (P = 0.022), higher-birth-weight babies (P = 0.0005), and fewer LBW babies (P = 0.0014) than PM+ mothers without Ab to FV2. Having Ab to FV2 reduced the odds of having high placental parasitemia (odds ratio [OR] = 0.432; 95% confidence interval [CI], 0.272 to 0.687) and LBW babies (OR = 0.444; 95% CI, 0.247 to 0.799) after adjusting for age and gravidity (Table 4). On the other hand, among PM− women, Ab to FV2 were associated with negative outcomes, including slightly lower hematocrits (P = 0.022), increased prevalence of anemia (P = 0.021), and more preterm deliveries (P = 0.014).
TABLE 3.
Comparison of pregnancy outcomes in women with and without antibodies to FV2a
| Clinical outcome, unit | PM+ (n = 328) |
PM− (n = 1,014) |
||||
|---|---|---|---|---|---|---|
| FV2 Ab+ (n = 184) | FV2 Ab− (n = 144) | P value | FV2 Ab+ (n = 334) | FV2 Ab− (n = 680) | P value | |
| Placental parasitemia, % median (25th, 75th) | 0.5 (0.1, 1.9) | 1.7 (0.3, 9.9) | <0.0001 | 0 | 0 | NA |
| Maternal hematocrit, % (mean ± SD) | 31.4 ± 5.9 | 31.3 ± 6.3 | 0.87 | 34.2 ± 5.5 | 35.1 ± 6.0 | 0.022 |
| Women with anemia, %b | 24.5 | 29.2 | 0.18 | 15.8 | 12.1 | 0.021 |
| Preterm deliveries, %b | 16.9 | 27.1 | 0.022 | 20.4 | 14.2 | 0.014 |
| Infant birth weight, g (mean ± SD) | 3,059 ± 578 | 2,807 ± 661 | 0.0005 | 3,147 ± 624 | 3,148 ± 624 | 0.99 |
| Infant birth weight from full-term deliveries, g (mean ± SD) | 3,194 ± 484 | 3,067 ± 484 | 0.052 | 3,319 ± 477 | 3,305 ± 480 | 0.72 |
| Infant birth weight from preterm deliveries, g (mean ± SD) | 2,493 ± 632 | 2,233 ± 651 | 0.097 | 2,587 ± 713 | 2,432 ± 665 | 0.17 |
| Low-birth-weight infants, %b | 13.0 | 27.1 | 0.0014 | 13.5 | 12.5 | 0.64 |
Significant values (i.e., P < 0.05) are highlighted in bold. NA, not applicable.
These percentages were calculated for samples with available data.
TABLE 4.
Odds ratios for the influence of antibodies to FV2 and merozoite antigens on pregnancy outcome in PM+ womena
| Antigen | OR (95% CI) |
|||
|---|---|---|---|---|
| Anemia | High placental parasitemia | LBW | Preterm delivery | |
| FV2 | 0.726 (0.429, 1.229) | 0.432 (0.272, 0.687)* (P = 0.0004) | 0.444 (0.247, 0.799)* (P = 0.0068) | 0.590 (0.335, 1.040) |
| MSP142 | 1.408 (0.768, 2.583) | 1.367 (0.828, 2.257) | 0.873 (0.468, 1.632) | 1.210 (0.641, 2.283) |
| MSP2 | 0.608 (0.289, 1.278) | 0.538 (0.272, 1.063) | 0.546 (0.256, 1.163) | 0.720 (0.329, 1.572) |
| MSP3 | 0.727 (0.405, 1.304) | 0.593 (0.361, 0.974) (P = 0.0389) | 1.268 (0.680, 2.363) | 0.927 (0.494, 1.737) |
| MSP11 | 0.653 (0.317, 1.346) | 1.197 (0.679, 2.108) | 1.547 (0.767, 3.123) | 2.168 (1.114, 4.222) |
| AMA1 | 0.777 (0.180, 3.354) | 0.216 (0.046, 1.019) | 0.990 (0.197, 4.981) | 0.410 (0.103, 1.636) |
| EBA-175 | 0.690 (0.307, 1.553) | 0.444 (0.214, 0.920) (P = 0.0291) | 0.575 (0.263, 1.257) | 0.406 (0.188, 0.873) (P = 0.0211) |
| Pf41 | 0.685 (0.409, 1.149) | 0.486 (0.309, 0.765)* (P = 0.0018) | 0.832 (0.471, 1.469) | 0.722 (0.414, 1.257) |
ORs with 95% CIs reported were based on logistic models, adjusted for age and gravidity. The 95% CIs of ORs reaching outside 1.0 (highlighted in bold) were statistically significant. *, statistically significant ORs after Bonferroni correction for multiple comparisons among four pregnancy outcomes.
Antibodies to some merozoite antigens were also associated with a protective effect in PM+ but not PM− women (Table 4; see Table S2 in the supplemental material for data for PM− women). A reduced risk of having high placental parasitemia was associated with the presence of Ab to MSP3 (OR = 0.593; 95% CI, 0.361 to 0.974), EBA-175 (OR = 0.444; 95% CI, 0.214 to 0.920), and Pf41 (OR = 0.486; 95% CI, 0.309 to 0.765). Antibodies to EBA-175 were associated with a reduced risk of preterm delivery (OR = 0.406; 95% CI, 0.118 to 0.873) (Table 4). When adjustments were made for multiple comparisons, only Ab to Pf41 remained significantly associated with improved outcomes. No significant association was found between Ab to any of the merozoite antigens and maternal anemia. Thus, Ab to FV2 and possibly MSP3, EBA-175, and Pf41 were associated with lower placental parasitemia in PM+ women but not PM− women.
DISCUSSION
This study sought to determine if Ab to FV2 or merozoite antigens were associated with better pregnancy outcomes, including reductions in maternal anemia, placental parasitemia, and premature deliveries and higher infant birth weight. The initial study was conducted between 1998 and 2001 in Yaoundé, Cameroon, a typical African urban area. At that time, the entomological inoculation rate was relatively low (1 to 2 infectious bites per month) and pregnant women had easy access to health care at government-supported facilities. Results from the prior epidemiological study found that young women had a higher risk of PM (P = 0.008), with adjusted ORs (95% CI) of 2.0 (1.3 to 3.0) for women of <20 years and 1.4 (1.0 to 19.9) for women aged 20 to 25 years (14). Surprisingly, after adjusting for age and other covariates, gravidity was no longer a major risk factor for PM (P = 0.278), indicating that malaria in pregnant women in this urban setting was different from that in rural areas, where malaria transmission was higher (2, 10, 15–17). Today, malaria transmission is declining globally due to malaria elimination efforts, and many rural areas are becoming urbanized and transitioning into low-transmission areas. Thus, it is important to understand what constitutes natural protective immunity to PM in lower-transmission areas like Yaoundé, Cameroon.
The first step was to define the impact of PM in this urban setting (Table 1). Like in higher-transmission rural areas, PM+ women residing in Yaoundé were younger and of lower gravidity and had lower hematocrits, higher prevalence of anemia, more preterm deliveries, and babies with lower mean birth weights than PM− women (Table 1). Clearly, PM had a negative influence on mothers and their newborns in this relatively low transmission area, showing data similar to those reported previously for rural settings. Thus, it was possible to evaluate the influence of Ab to FV2 and merozoite antigens on these PM-associated conditions.
The failure to detect a positive effect of Ab to FV2 (Table 3) and/or the merozoite antigens in PM− women was unexpected. Initially, it was hypothesized that PM− women would have higher Ab levels to FV2 and/or the merozoite antigens that would protect them from infection, but such was not the case. In fact, higher Ab levels were generally found in PM+ women with ongoing infections (Fig. 1), making high Ab levels a marker of infection, not protection, as proposed in other studies (4, 8). It is possible that some of the PM− women had not become infected during pregnancy, since approximately two-thirds (680/1,014) lacked Ab to FV2 at delivery. If they were not infected, then the women had malaria-free pregnancies and would not suffer the consequences of PM, explaining their favorable “normal” pregnancy outcomes. Interestingly, PM− women who had Ab to FV2, most likely resulting from PM during the current pregnancy, had lower hematocrits (P = 0.022), higher prevalence of anemia (P = 0.021), and a higher percent premature delivery (P = 0.014) than PM− women without Ab, suggesting that they had malaria during pregnancy, which affected the health of the mother, but the women cleared their infections prior to delivery. Overall, there was no direct evidence that Ab levels at delivery were associated with improved pregnancy outcomes in PM− women.
On the other hand, Ab to FV2 were associated with better pregnancy outcomes in PM+ women. That is, PM+ women with Ab to these antigens had better outcomes than those who lacked Ab. For example, when PM+ women were stratified into two groups, those above and those below the median placental parasitemia of 0.8%, women who had Ab to FV2 were more likely to be in the low-placental-parasitemia group. Antibodies to FV2 most likely reduced placental parasitemia by preventing cytoadherence of IE to the placental tissue, resulting in IE being eliminated in the spleen. PM+ women who had Ab to FV2 were also less likely to have LBW babies. Antibodies to FV2 may have helped prevent LBW by inhibiting the direct binding of IE to the placenta and/or by reducing inflammation in the intervillous space (IVS) that can result in dysregulation of metabolic pathways, including those involved in nutrient transport, production of factors for placental angiogenesis and vasculogenesis, and growth hormones (18–22).
Results for Ab to the merozoite antigens were less clear. Like for FV2, women with higher (above the median) Ab levels to MSP3, EBA-175, and Pf41 were more likely to have lower placental parasitemias, suggesting that Ab to these antigens helped reduce the circulating parasite burden, including within the IVS. In addition, Ab to EBA-175 were associated with a reduced risk of premature deliveries (Table 4). Why Ab to EBA-175 were associated with reduced risk of premature deliveries whereas the other merozoite antigens were not remains unclear. However, Andersen et al. (23) reported that the interdomain 2 region (ID2) of VAR2CSA has homology with EBA-175. Today, it is clear that the major binding site for VAR2CSA is in the DBL1-2 or ID1-ID2a region. Thus, it is tempting to speculate that Ab to EBA-175 might bind to shared epitopes and alter IE binding. However, when the Bonferroni adjustment was applied, some of the P values lost significance. This may not be surprising, as it has been difficult to correlate Ab levels to any single antigen and protection from malaria (24, 25). Clearly, further studies are warranted, but the current data suggest only a possible role of Ab to merozoite antigens in reducing placental parasitemias and better pregnancy outcomes.
Although the epidemiology of PM in Yaoundé appears to differ somewhat from that in higher-transmission areas, it was surprising that many women failed to produce Ab to FV2 even after multiple pregnancies. For example, among women who were infected and had PM at delivery, only 47% of primigravidae and 60% of multigravidae had Ab to FV2 (Table 2). The reason why women failed to produce Ab to FV2 is unclear, but possible explanations include the following: (i) the women did not become infected until close to term, and the time was too short for them to mount a detectable Ab response; (ii) the women had sufficient natural immunity to P. falciparum to rapidly clear the parasite before it switched to the VAR2CSA phenotype; (iii) they had sufficient immunity, e.g., via Ab to merozoites, to maintain VAR2CSA-expressing IE at levels too low to induce a primary, acquired Ab response (i.e., at very low submicroscopic levels); (iv) the women were immunologically tolerant to VAR2CSA; or (v) women had VAR2CSA Ab that did not cross-react with the parasite serotype used in the assay. Data provided by Walker-Abbey et al. support the possibility of the second, third, and fourth explanations, since from 1996 to 1998, 27.5% of pregnant women at delivery were peripheral and placental blood smear positive, but an additional 54.9% had submicroscopic infections (26). Further studies are needed to clarify why many of the women lacked Ab to FV2, in order to help ensure the immunogenicity of future VAR2CSA-based vaccines.
Few prior studies have assessed the importance of Ab to malarial antigens other than to VAR2CSA in pregnant women. Two early studies reported that pregnant women who did not have Ab to RESA were more susceptible to P. falciparum (27, 28), but three other studies did not (29–31). Women who lacked Ab to MSP119 were also reported to be at a higher risk of having PM and reduced placental parasite densities than women who had Ab to MSP119 (29, 32, 33), but there was no evidence that the presence or absence of Ab to MSP119 or MSP142 influenced infant birth weight (33). More recently, a study by Mayor et al. demonstrated that babies born to mothers in Mozambique with high levels of Ab against AMA1 had increased birth weights and gestational ages, but only if the mothers had experienced malaria episodes during pregnancy (34). Recently, Ab to the serine repeat antigen-5 (SE36) were found to be associated with both reduced risk of PM and better infant birth weight in women residing in Kampala, Uganda, a city with low, seasonal malaria transmission (33). Clearly, further efforts to evaluate the role of other malarial antigens as well as VAR2CSA are needed to obtain a clear picture of the natural protective immunity of pregnant women living in urban areas like Yaoundé, Cameroon.
MATERIALS AND METHODS
Ethics statement.
The original study was approved by the National Ethics Committee, Cameroon, and the Institutional Review Board at Georgetown University. Women participating in the study gave written informed consent to use their plasma and clinical data in studies on immunity to malaria. The use of deidentified, archival plasma samples and clinical information was determined to be exempt from human subject research by the Committee on Human Studies, University of Hawai'i, Mānoa (CHS #19912).
Study site.
Clinical specimens had been collected in a study conducted between 1995 and 2001 in Yaoundé, Cameroon (14, 26). Pregnant women were consecutively recruited at two government-supported hospitals: the Biyem-Assi Hospital, a district hospital that provides care for women in the surrounding area, and the Central Maternity Hospital, a referral hospital for a diverse group of women. When the initial study was conducted, P. falciparum transmission in Yaoundé was perennial, with an annual estimated entomological inoculation rate of ∼13 infectious bites per individual per year (35). Women were not tested for HIV; but the average prevalence of HIV among pregnant women visiting antenatal clinics in major urban areas in Cameroon was 7.14% (36). Thus, it is unlikely that HIV had a great effect on the study.
Sample collection and parasitological studies.
Upon obtaining informed consent, information on each woman's age, gravidity, gestational age, and residence was recorded on a standardized questionnaire. Peripheral blood samples and a biopsy specimen of the placenta were collected at delivery, and information on the baby's birth weight was recorded.
Samples were processed at the Biotechnology Center, University of Yaoundé I. Thick and thin blood smears of maternal peripheral and placental blood were stained with Diff-Quick (Polysciences, Warrington, PA) and examined for IE by two microscopists. Heparinized microhematocrit tubes, filled with maternal peripheral blood, were centrifuged, and the packed cell volume was measured. Whole peripheral blood was centrifuged at 400 × g for 10 min, and plasma was stored in −80°C until used. Small pieces of placental biopsy specimens were used to make impression smears that were stained and examined for IE. Placental biopsy specimens were also fixed in buffered formalin for histology. A woman was considered to be PM positive (PM+) if IE were detected in the placental blood smear, impression smear, or histological section. Women whose samples showed absence of parasites in both peripheral blood and placenta were considered PM negative (PM−). Women who were peripheral blood smear positive but placental negative were excluded from the study.
Study design.
Initially, 1,649 blood samples were available to study. Because the goal of the study was to determine if Ab to FV2 and merozoite antigens had a role in influencing pregnancy outcomes, all PM-positive women (n = 341) were included in the study to create a group representative of women with PM in Yaoundé. To enable comparisons, approximately three times the number of PM− women (n = 1,036) were randomly selected to provide the control group of women who were PM− at delivery.
Measuring Ab to FV2 and merozoite antigens.
A multiplex bead-based assay was used to measure IgG to the malarial antigens. Characteristics of the antigens (37, 38), methods for coupling and optimization of the Luminex-based multianalyte platform assay (12, 39), and reproducibility (38) have been reported previously. Antigens used in the current study included recombinant FV2 and 7 merozoite antigens (AMA1, EBA-175, MSP142, MSP2, MSP3, MSP11, and Pf41). Briefly, the optimal amount of each recombinant protein was covalently coupled to seroMAP beads with different spectral addresses using a carbodiimide cross-linking reaction with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and sulfo-N-hydroxysulfosuccinimide. Beads were blocked and stored at 4°C in phosphate-buffered saline (PBS) containing 0.05% sodium azide and pooled immediately prior to use. During the optimization process, beads were assayed as a monoplex and multiplex to ensure that inhibition or competition among the antigens did not occur.
Plasma samples were diluted to 1:200 in PBS–1% bovine serum albumin (BSA), and 50 μl was incubated with 50 μl of pooled beads (i.e., 1:400 dilution was combined with 2,000 beads of each antigen) in microtiter wells for 1 h at room temperature on a shaker. Four positive and two negative controls were included on each plate to control for plate-to-plate and day-to-day variation (38), including 4 pools of plasma from women with high levels of Ab to FV2 (positive controls), from Cameroonian males who had high levels of Ab to merozoite antigens but lacked Ab to FV2, and from pregnant U.S. women who lacked Ab to malarial antigens. After being washed three times, beads were incubated with 50 μl R-phycoerythrin-conjugated, affinity purified F(ab′)2, heavy-chain-specific goat anti-human IgG (2 μg/ml; Jackson ImmunoResearch) in PBS–1% BSA for 1 h. After three washes, beads were analyzed using a LiquiChip 100 reader (Luminex Corp., Austin, TX), and results are reported as median fluorescence intensity (MFI). The cutoff for Ab positivity for Ab to FV2 was the mean plus 2 standard deviations (SD) for 30 Cameroonian male adults who lacked Ab to FV2, and the cutoff for the merozoite antigens was the mean plus 2 SD for 30 plasma samples collected at delivery from U.S. women who lacked Ab to malaria. Data for establishing the cutoffs for each antigen are provided in Fig. S1 in the supplemental material.
Statistical analysis.
Demographic, clinical variables, and assay results were summarized with means and SD for continuous variables and with frequencies and percentages for categorical variables. Women with <30% packed cell volume (PCV) were classified as having anemia (WHO definition), singletons weighing <2,500 g were considered LBW, and newborns delivered between 28 and 37 weeks of gestation were considered preterm. Since the measurements for parasitemia were not normally distributed, median and interquartile ranges (IQR) were reported. In the analyses related to Ab, women whose pregnancies ended with stillbirth (at >28 gestational weeks) were excluded, because there might not have been sufficient time for Ab production. Differences between the outcome groups (PM+ versus PM−, FV2 Ab+ versus Ab−) were compared using two-sample t tests or the Mann-Whitney U test for continuous variables and chi-square or Fisher's exact tests for categorical variables. The Cochran-Armitage test was performed to evaluate the trends in the proportion of seropositive women across gravidity in PM+ and PM− women separately. Further, the Cochran-Mantel-Haenszel test was performed to test if the association between seropositivity and gravidity changed after controlling for the placental malaria status. Logistic regression models were developed to assess the association between seropositivity and frequency of (i) low-birth-weight babies, (ii) high parasitemia (defined as samples above the median parasitemia, which was ≥0.8%), (iii) maternal anemia, and (iv) preterm deliveries, with or without adjustment for demographic and/or clinical variables. The odds ratios (40) with 95% confidence intervals (41) were reported, stratified by placental malaria status. Ab to each antigen were investigated individually. To adjust for multiple testing among the four clinical outcomes, the Bonferroni correction procedure was applied to each antigen. All statistical analyses were performed using SAS 9.4 software (2013; SAS Institute Inc., Cary, NC), and P values of less than 0.05 were regarded as statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank researchers and the laboratory staff at the Biotechnology Centre, University of Yaoundé I, Cameroon, for collecting, processing, and archiving the samples.
This study was supported by NIH grants R21AI 105286 to D.W.T. and J.J.C.; U54MD007584 and U54MD007601 supported R.F. and J.J.C.; FIC training grant 5D43TW009074 to D.W.T. supported E.N. and K.V.; NIAID R21AI09449 supported Y.M.L.
Author contributions: D.W.T. conceived the project and designed the experiments. N.B., M.J.S.-Q., E.N., and K.V. carried out the experiments. Y.M.L., R.F., and J.J.C. analyzed the data. A.S. provided the recombinant proteins. R.G.F.L. supervised the collection of samples. Y.M.L. and D.W.T. wrote the manuscript.
We declare that we have no competing financial interests.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00166-18.
REFERENCES
- 1.Salanti A, Dahlback M, Turner L, Nielsen MA, Barfod L, Magistrado P, Jensen AT, Lavstsen T, Ofori MF, Marsh K, Hviid L, Theander TG. 2004. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J Exp Med 200:1197–1203. doi: 10.1084/jem.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD. 2007. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 3.Huynh BT, Fievet N, Gbaguidi G, Dechavanne S, Borgella S, Guezo-Mevo B, Massougbodji A, Ndam NT, Deloron P, Cot M. 2011. Influence of the timing of malaria infection during pregnancy on birth weight and on maternal anemia in Benin. Am J Trop Med Hyg 85:214–220. doi: 10.4269/ajtmh.2011.11-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ndam NT, Denoeud-Ndam L, Doritchamou J, Viwami F, Salanti A, Nielsen MA, Fievet N, Massougbodji A, Luty AJ, Deloron P. 2015. Protective antibodies against placental malaria and poor outcomes during pregnancy, Benin. Emerg Infect Dis 21:813–823. doi: 10.3201/eid2105.141626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng G, Aitken E, Yosaatmadja F, Kalilani L, Meshnick SR, Jaworowski A, Simpson JA, Rogerson SJ. 2009. Antibodies to variant surface antigens of Plasmodium falciparum-infected erythrocytes are associated with protection from treatment failure and the development of anemia in pregnancy. J Infect Dis 200:299–306. doi: 10.1086/599841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duffy PE, Fried M. 2003. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect Immun 71:6620–6623. doi: 10.1128/IAI.71.11.6620-6623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Neil-Dunne I, Achur RN, Agbor-Enoh ST, Valiyaveettil M, Naik RS, Ockenhouse CF, Zhou A, Megnekou R, Leke R, Taylor DW, Gowda DC. 2001. Gravidity-dependent production of antibodies that inhibit binding of Plasmodium falciparum-infected erythrocytes to placental chondroitin sulfate proteoglycan during pregnancy. Infect Immun 69:7487–7492. doi: 10.1128/IAI.69.12.7487-7492.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staalsoe T, Megnekou R, Fievet N, Ricke CH, Zornig HD, Leke R, Taylor DW, Deloron P, Hviid L. 2001. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum-infected erythrocytes that protect against placental parasitemia. J Infect Dis 184:618–626. doi: 10.1086/322809. [DOI] [PubMed] [Google Scholar]
- 9.Cox SE, Staalsoe T, Arthur P, Bulmer JN, Hviid L, Yeboah-Antwi K, Kirkwood BR, Riley EM. 2005. Rapid acquisition of isolate-specific antibodies to chondroitin sulfate A-adherent Plasmodium falciparum isolates in Ghanaian primigravidae. Infect Immun 73:2841–2847. doi: 10.1128/IAI.73.5.2841-2847.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leke RF, Bioga JD, Zhou J, Fouda GG, Leke RJ, Tchinda V, Megnekou R, Fogako J, Sama G, Gwanmesia P, Bomback G, Nama C, Diouf A, Bobbili N, Taylor DW. 2010. Longitudinal studies of Plasmodium falciparum malaria in pregnant women living in a rural Cameroonian village with high perennial transmission. Am J Trop Med Hyg 83:996–1004. doi: 10.4269/ajtmh.2010.10-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiwuwa MS, Ribacke U, Moll K, Byarugaba J, Lundblom K, Farnert A, Fred K, Wahlgren M. 2013. Genetic diversity of Plasmodium falciparum infections in mild and severe malaria of children from Kampala, Uganda. Parasitol Res 112:1691–1700. doi: 10.1007/s00436-013-3325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tutterrow YL, Avril M, Singh K, Long CA, Leke RJ, Sama G, Salanti A, Smith JD, Leke RG, Taylor DW. 2012. High levels of antibodies to multiple domains and strains of VAR2CSA correlate with the absence of placental malaria in Cameroonian women living in an area of high Plasmodium falciparum transmission. Infect Immun 80:1479–1490. doi: 10.1128/IAI.00071-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tutterrow YL, Salanti A, Avril M, Smith JD, Pagano IS, Ako S, Fogako J, Leke RG, Taylor DW. 2012. High avidity antibodies to full-length VAR2CSA correlate with absence of placental malaria. PLoS One 7:e40049. doi: 10.1371/journal.pone.0040049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tako EA, Zhou A, Lohoue J, Leke R, Taylor DW, Leke RF. 2005. Risk factors for placental malaria and its effect on pregnancy outcome in Yaounde, Cameroon. Am J Trop Med Hyg 72:236–242. [PubMed] [Google Scholar]
- 15.Brabin BJ. 1983. An analysis of malaria in pregnancy in Africa. Bull World Health Organ 61:1005–1016. [PMC free article] [PubMed] [Google Scholar]
- 16.Menendez C. 1995. Malaria during pregnancy: a priority area of malaria research and control. Parasitol Today 11:178–183. doi: 10.1016/0169-4758(95)80151-0. [DOI] [PubMed] [Google Scholar]
- 17.Guyatt HL, Snow RW. 2001. The epidemiology and burden of Plasmodium falciparum-related anemia among pregnant women in sub-Saharan Africa. Am J Trop Med Hyg 64:36–44. doi: 10.4269/ajtmh.2001.64.36. [DOI] [PubMed] [Google Scholar]
- 18.Rogerson SJ, Brown HC, Pollina E, Abrams ET, Tadesse E, Lema VM, Molyneux ME. 2003. Placental tumor necrosis factor alpha but not gamma interferon is associated with placental malaria and low birth weight in Malawian women. Infect Immun 71:267–270. doi: 10.1128/IAI.71.1.267-270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moormann AM, Sullivan AD, Rochford RA, Chensue SW, Bock PJ, Nyirenda T, Meshnick SR. 1999. Malaria and pregnancy: placental cytokine expression and its relationship to intrauterine growth retardation. J Infect Dis 180:1987–1993. doi: 10.1086/315135. [DOI] [PubMed] [Google Scholar]
- 20.Muehlenbachs A, Mutabingwa TK, Edmonds S, Fried M, Duffy PE. 2006. Hypertension and maternal-fetal conflict during placental malaria. PLoS Med 3:e446. doi: 10.1371/journal.pmed.0030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silver KL, Zhong K, Leke RG, Taylor DW, Kain KC. 2010. Dysregulation of angiopoietins is associated with placental malaria and low birth weight. PLoS One 5:e9481. doi: 10.1371/journal.pone.0009481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silver KL, Conroy AL, Leke RG, Leke RJ, Gwanmesia P, Molyneux ME, Taylor DW, Rogerson SJ, Kain KC. 2011. Circulating soluble endoglin levels in pregnant women in Cameroon and Malawi—associations with placental malaria and fetal growth restriction. PLoS One 6:e24985. doi: 10.1371/journal.pone.0024985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersen P, Nielsen MA, Resende M, Rask TS, Dahlback M, Theander T, Lund O, Salanti A. 2008. Structural insight into epitopes in the pregnancy-associated malaria protein VAR2CSA. PLoS Pathog 4:e42. doi: 10.1371/journal.ppat.0040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osier FH, Fegan G, Polley SD, Murungi L, Verra F, Tetteh KK, Lowe B, Mwangi T, Bull PC, Thomas AW, Cavanagh DR, McBride JS, Lanar DE, Mackinnon MJ, Conway DJ, Marsh K. 2008. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun 76:2240–2248. doi: 10.1128/IAI.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osier FH, Mackinnon MJ, Crosnier C, Fegan G, Kamuyu G, Wanaguru M, Ogada E, McDade B, Rayner JC, Wright GJ, Marsh K. 2014. New antigens for a multicomponent blood-stage malaria vaccine. Sci Transl Med 6:247ra102. doi: 10.1126/scitranslmed.3008705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker-Abbey A, Djokam RR, Eno A, Leke RF, Titanji VP, Fogako J, Sama G, Thuita LH, Beardslee E, Snounou G, Zhou A, Taylor DW. 2005. Malaria in pregnant Cameroonian women: the effect of age and gravidity on submicroscopic and mixed-species infections and multiple parasite genotypes. Am J Trop Med Hyg 72:229–235. [PubMed] [Google Scholar]
- 27.Astagneau P, Steketee RW, Wirima JJ, Khoromana CO, Millet P. 1994. Antibodies to ring-infected erythrocyte surface antigen (Pf155/RESA) protect against P. falciparum parasitemia in highly exposed multigravidas women in Malawi. Acta Trop 57:317–325. doi: 10.1016/0001-706X(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 28.Mvondo JL, James MA, Sulzer AJ, Campbell CC. 1992. Malaria and pregnancy in Cameroonian women. Naturally acquired antibody responses to asexual blood-stage antigens and the circumsporozoite protein of Plasmodium falciparum. Trans R Soc Trop Med Hyg 86:486–490. [DOI] [PubMed] [Google Scholar]
- 29.Taylor DW, Zhou A, Marsillio LE, Thuita LW, Leke EB, Branch O, Gowda DC, Long C, Leke RF. 2004. Antibodies that inhibit binding of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A and to the C terminus of merozoite surface protein 1 correlate with reduced placental malaria in Cameroonian women. Infect Immun 72:1603–1607. doi: 10.1128/IAI.72.3.1603-1607.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deloron P, Steketee RW, Campbell GH, Peyron F, Kaseje DC, Brandling-Bennett AD. 1989. Serological reactivity to the ring-infected erythrocyte surface antigen and circumsporozoite protein in gravid and nulligravid women infected with Plasmodium falciparum. Trans R Soc Trop Med Hyg 83:58–62. doi: 10.1016/0035-9203(89)90705-0. [DOI] [PubMed] [Google Scholar]
- 31.Fievet N, Cot M, Chougnet C, Maubert B, Bickii J, Dubois B, Le Hesran JY, Frobert Y, Migot F, Romain F, Verhave JP, Louis F, Deloron P. 1995. Malaria and pregnancy in Cameroonian primigravidae: humoral and cellular immune responses to Plasmodium falciparum blood-stage antigens. Am J Trop Med Hyg 53:612–617. doi: 10.4269/ajtmh.1995.53.612. [DOI] [PubMed] [Google Scholar]
- 32.Branch OH, Udhayakumar V, Hightower AW, Oloo AJ, Hawley WA, Nahlen BL, Bloland PB, Kaslow DC, Lal AA. 1998. A longitudinal investigation of IgG and IgM antibody responses to the merozoite surface protein-1 19-kiloDalton domain of Plasmodium falciparum in pregnant women and infants: associations with febrile illness, parasitemia, and anemia. Am J Trop Med Hyg 58:211–219. doi: 10.4269/ajtmh.1998.58.211. [DOI] [PubMed] [Google Scholar]
- 33.Owalla TJ, Palacpac NM, Shirai H, Horii T, Egwang TG. 2013. Association of naturally acquired IgG antibodies against Plasmodium falciparum serine repeat antigen-5 with reduced placental parasitemia and normal birth weight in pregnant Ugandan women: a pilot study. Parasitol Int 62:237–239. doi: 10.1016/j.parint.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Mayor A, Kumar U, Bardaji A, Gupta P, Jimenez A, Hamad A, Sigauque B, Singh B, Quinto L, Kumar S, Gupta PK, Chauhan VS, Dobano C, Alonso PL, Menendez C, Chitnis CE. 2013. Improved pregnancy outcomes in women exposed to malaria with high antibody levels against Plasmodium falciparum. J Infect Dis 207:1664–1674. doi: 10.1093/infdis/jit083. [DOI] [PubMed] [Google Scholar]
- 35.Manga L, Traore O, Cot M, Mooh E, Carnevale P. 1993. Malaria in the village of Yaounde (Cameroon). 3. Parasitological study in 2 central districts. Bull Soc Pathol Exot 86:56–61. (In French.) [PubMed] [Google Scholar]
- 36.UNAIDS/WHO. 2004. Cameroon: epidemiological fact sheets on HIV/AIDS and sexually transmitted infections—2004 update. WHO, Geneva, Switzerland. [Google Scholar]
- 37.Siriwardhana C, Fang R, Salanti A, Leke RGF, Bobbili N, Taylor DW, Chen JJ. 2017. Statistical prediction of immunity to placental malaria based on multi-assay antibody data for malarial antigens. Malar J 16:391. doi: 10.1186/s12936-017-2041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang R, Wey A, Bobbili NK, Leke RFG, Taylor DW, Chen JJ. 2017. An analytical approach to reduce between-plate variation in multiplex assays that measure antibodies to Plasmodium falciparum antigens. Malar J 16:287. doi: 10.1186/s12936-017-1933-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fouda GG, Leke RF, Long C, Druilhe P, Zhou A, Taylor DW, Johnson AH. 2006. Multiplex assay for simultaneous measurement of antibodies to multiple Plasmodium falciparum antigens. Clin Vaccine Immunol 13:1307–1313. doi: 10.1128/CVI.00183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bockhorst J, Lu F, Janes JH, Keebler J, Gamain B, Awadalla P, Su XZ, Samudrala R, Jojic N, Smith JD. 2007. Structural polymorphism and diversifying selection on the pregnancy malaria vaccine candidate VAR2CSA. Mol Biochem Parasitol 155:103–112. doi: 10.1016/j.molbiopara.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Oleinikov AV, Rossnagle E, Francis S, Mutabingwa TK, Fried M, Duffy PE. 2007. Effects of sex, parity, and sequence variation on seroreactivity to candidate pregnancy malaria vaccine antigens. J Infect Dis 196:155–164. doi: 10.1086/518513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.