Coccidiosis is one of the most serious diseases of livestock and birds in the world. Vaccination with live-parasite anticoccidial vaccines with genetic manipulation improving the immunogenicity of vaccine strains would be the best means for controlling coccidiosis in breeder and layer stocks, even in fast-growing broilers.
KEYWORDS: transgenic Eimeria, immunogenicity, profilin, protective immunity, microbial community
ABSTRACT
Coccidiosis is one of the most serious diseases of livestock and birds in the world. Vaccination with live-parasite anticoccidial vaccines with genetic manipulation improving the immunogenicity of vaccine strains would be the best means for controlling coccidiosis in breeder and layer stocks, even in fast-growing broilers. Profilin from apicomplexan parasites is the first molecularly defined ligand for Toll-like receptor 11 (TLR11) and TLR12 in mice and is a potential molecular adjuvant. Here, we constructed a transgenic Eimeria tenella line (Et-EmPro) expressing the profilin of Eimeria maxima, the most immunogenic species of chicken coccidia, and evaluated the adjuvant effects of EmPro on the immunogenicity of E. tenella. We found that immunization with the transgenic Eimeria parasites, compared with the wild type, elicited greater parasite antigen-specific cell-mediated immunity, characterized by increased numbers of interferon gamma (IFN-γ)-secreting lymphocytes. The transgenic parasite also induced better protective immunity against E. tenella challenge than the wild type. In addition, the diversity of the fecal microbiome of the birds immunized with the transgenic parasite differed from that of the microbiome of the wild-type-immunized birds, indicating interactions of Eimeria with the gut microbiome of chickens. Our results showing enhanced immunogenicity of E. tenella by use of EmPro as a molecular adjuvant derived from the most immunogenic affinis species represent a large step forward in the development of the next generation of coccidiosis vaccines using Eimeria as a vaccine platform expressing molecular adjuvants and potentially other pathogen antigens against not only coccidiosis but also other infectious diseases.
INTRODUCTION
The world chicken flock is estimated at approximately 21 billion, producing 1.1 trillion eggs and approximately 90 billion kilograms of meat each year worldwide (www.fao.org/faostat/en). Coccidiosis, which is caused by parasites in the genus Eimeria, is a major disease in chickens in almost all poultry farms. The disease decreases egg and meat production and causes losses of approximately £2 billion per year worldwide (1–3). Large-scale and long-term use of anticoccidial drugs has been effective in the control of coccidiosis for decades, but this has led to the unavoidable development of parasite resistance to almost all anticoccidial drugs in addition to public health concerns over drug residues in poultry meat when a drug withdrawal period is not strictly observed (4, 5). Instead of control by drugs, vaccination with virulent (e.g., Coccivac and Immucox) or attenuated (e.g., Paracox and Livacox) live parasites is the most efficient method for the protection of breeder and layer flocks from Eimeria infection (6, 7), and vaccination is of low public health risk. Effective immune protection relies on self-boosting immunization with offspring oocysts excreted in the litter through the fecal-oral route (2). For Eimeria parasites with high pathogenicity but low or intermediate immunogenicity, such as E. necatrix and E. tenella, a large quantity of newly invaded offspring parasites of the vaccine strain would damage the intestine and negatively influence intestinal absorption of nutrients, resulting in poor feed conversion (3, 6). Even worse, deaths may occur in circumstances of low vaccination coverage and/or inappropriate animal husbandry (3, 6). The cost of vaccines and, for some vaccines, side effects of vaccination limit the broad use of anticoccidial vaccines in broilers (8, 9). Improving the immunogenicity of pathogenic species would reduce the vaccine dosage and thus potential side effects.
Profilin is an actin-binding protein and is found in most cells of all eukaryotic organisms (10). Profilin of the apicomplexan parasite Toxoplasma gondii (TgPro) is the first molecularly defined ligand for Toll-like receptor 11 (TLR11) and TLR12 and boosts host immune responses through the activation of interleukin 12 (IL-12) and interferon alpha (IFN-α) in mice (11–13). Subsequent findings showed that flagellin and/or profilin present in Salmonella species (14) and uropathogenic Escherichia coli (15), which are also recognized by TLR11, mediate the activation of tumor necrosis factor alpha (15) and IFN-γ secretion (12). Studies with these molecules as adjuvants have showed promising results (16, 17). Eimeria spp. are closely related to T. gondii, and both parasites belong to the apicomplexan phylum. Parasites in the genus Eimeria infect a range of livestock and birds with absolute host specificity. Seven species of Eimeria infect chickens. E. maxima is the most immunogenic species of chicken coccidia, and immunization with as few as five oocysts can induce complete protective immunity against subsequent homologous challenges (3, 18). Here, we hypothesized that profilin, also named 3-1E, of E. maxima (EmPro) applied as an adjuvant would improve the immunogenicity of the intermediate-immunogenicity species E. tenella and enhance immune protection.
The host-pathogen interactions influence the complex body system of the host, including physiology, immunology, nutrition, and the gut microbiome. With advances in next-generation sequencing (NGS), some gastrointestinal tract-associated microorganisms and their potential influence on human and animal health have been identified (19–21). Eimeria parasites are one of the most common intestinal pathogens in chickens, but little is known about their interactions with the gut microbiome.
The objective of this study was to investigate the adjuvant effect of EmPro by constructing a transgenic E. tenella line overexpressing EmPro (Et-EmPro) and comparing the immune protection provided by Et-EmPro and its wild-type strain. In addition, the fecal microbiota was analyzed to reveal and understand interactions of Eimeria with the gut microbiome and the effect of the exogenous profilin on Eimeria-microbiome interactions.
RESULTS
Transgenic Eimeria tenella lines expressing E. maxima and Toxoplasma gondii profilin.
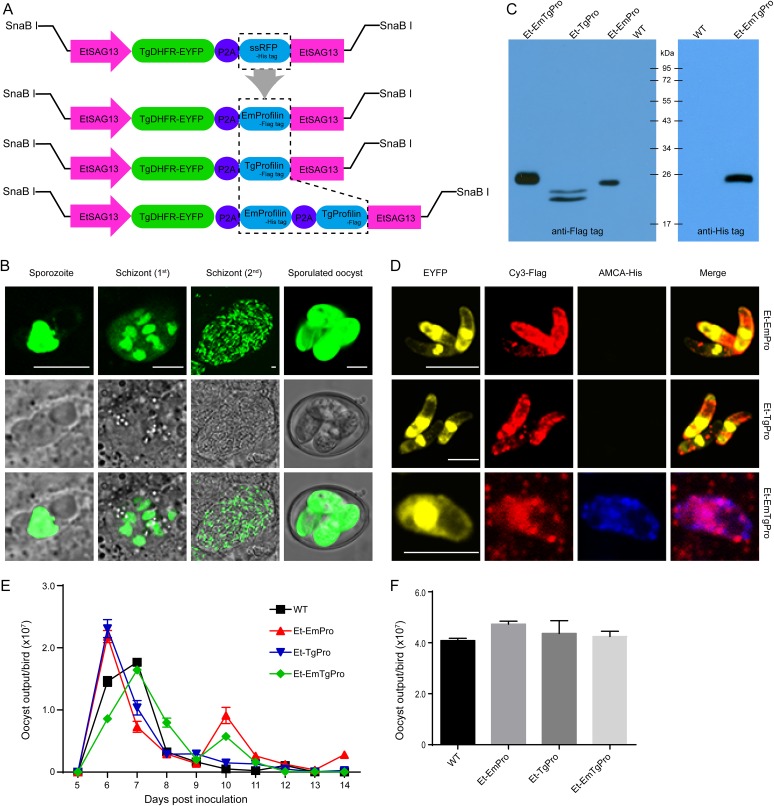
We previously demonstrated that the EtSAG13 promoter was a powerful promoter and drove high-level expression of fluorescent proteins (22). Here, using the EtSAG13 regulatory elements to overexpress E. maxima profilin (EmPro), we constructed a recombinant vector that coexpresses reporter and EmPro genes linked by the P2A sequence to generate E. tenella (Et-EmPro), which expressed both the reporter and EmPro proteins (Fig. 1A). Similarly, we also generated the Et-TgPro parasite, in which EmPro was replaced by TgPro (Toxoplasma gondii profilin), and Et-EmTgPro parasites carrying both EmPro and TgPro (EmTgPro) linked by the P2A sequence (Fig. 1A).
FIG 1.
Construction of transgenic Eimeria lines expressing EmPro and/or TgPro. (A) Schematic and design of recombinant vectors. Exogenous profilins and reporter EYFP were coexpressed in a single expression cassette linked by P2A. (B) Stably transfected Et-EmPro expressing the reporter EYFP in its sporozoite, schizont, and sporulated oocyst stages. Bar, 5 μm. (C) Validation of the expression of exogenous profilins by Western blotting. Parasites from sporozoite stages were immunoblotted with mouse anti-Flag tag or mouse anti-His tag monoclonal antibody. The molecular weight of EmPro with Flag tag was 20.5 kDa, that of TgPro with Flag tag was 18.9 kDa, and that of EmPro with His tag and P2A peptide was 22.6 kDa. (D) Distribution of exogenous profilins in transgenic sporozoites analyzed by IFA with mouse anti-Flag tag and mouse anti-His tag monoclonal antibody. Bar, 5 μm. (E and F) Comparison of oocyst shedding patterns (E) and reproduction (F) of the transgenic Eimeria lines and the wild type. Each value represents the mean for three birds.
After continuous in vivo selection, we obtained stably transfected Et-EmPro, Et-TgPro, and Et-EmTgPro parasites expressing the reporter gene in all stages of the endogenously complex life cycle: sporozoites, first- and second-generation schizonts, and sporulated oocysts (Fig. 1B). The expression pattern of the reporter gene of Et-TgPro and Et-EmTgPro was consistent with that for Et-EmPro (data not shown). These results suggest that EmPro, TgPro, and EmTgPro were expressed in the whole life cycle of the transgenic parasites, as both enhanced yellow fluorescent protein (EYFP) and exogenous profilins were controlled by one set of regulatory elements, EtSAG13, that regulated exogenous genes with high expression levels during the whole life cycle of the transgenic Eimeria parasites.
To further confirm the expression of profilin by the transgenic parasites, we conducted an immunoblot assay and showed that EmPro, TgPro, and EmTgPro were expressed in the sporozoite stage of each transgenic parasite line, consistent with EYFP expression (Fig. 1C). The exogenous profilins were distributed mainly in the cytoplasm and cell surface of sporozoites, not colocalizing with EYFP, which was expressed in the nucleus (Fig. 1D). Exogenous profilins were expressed without interference by EYFP, although the reporter and profilin genes were constructed in one cassette.
We further studied the biological characteristics (oocyst shedding dynamics and fecundity) of each transgenic E. tenella line. Oocyst shedding patterns and the total oocysts shed by the three transgenic lines were comparable with those for the wild type (Fig. 1F) except for a small increase in oocyst shedding of Et-EmPro and Et-EmTgPro at 10 days postinfection (dpi) (Fig. 1E). Thus, we obtained three transgenic E. tenella lines expressing exogenous profilin for the investigation of the adjuvant effects of E. maxima profilin.
Et-EmPro enhances parasite-specific cell-mediated immunity.
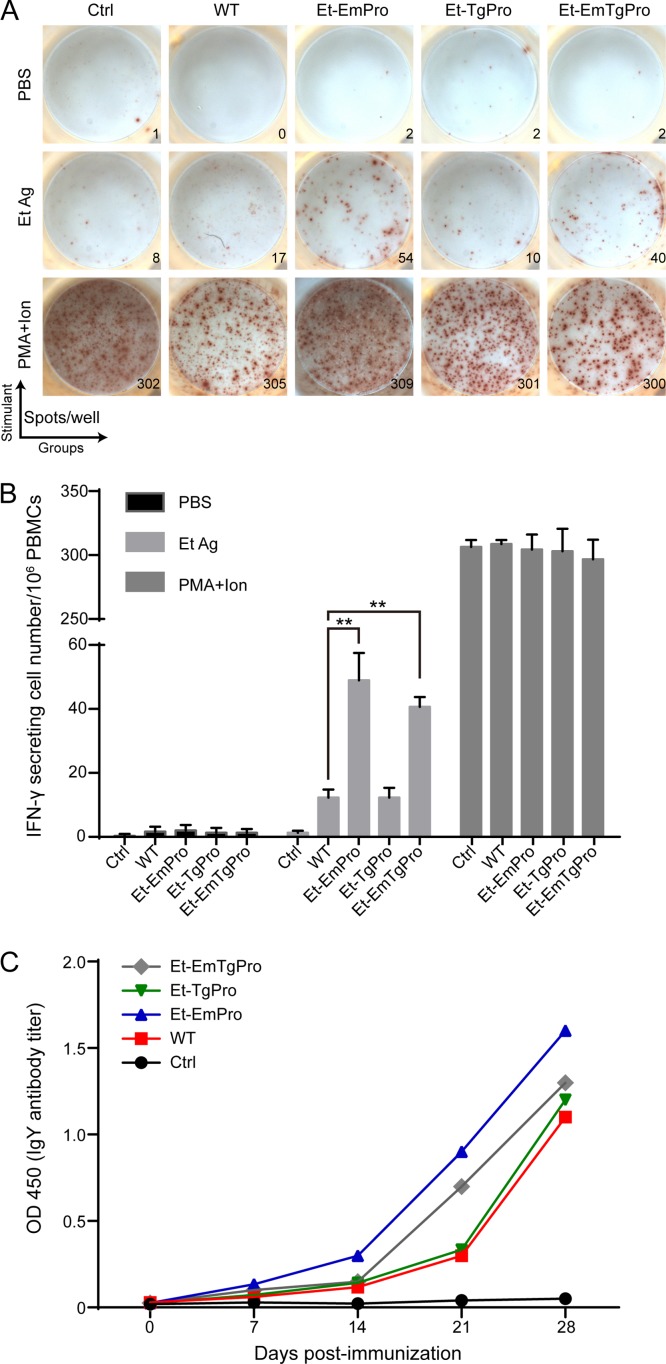
TgPro recognized by Toll-like receptor 11 activates the IL-12 response and IFN-γ secretion, which trigger Th1-dominant immune responses in mice (13). We first investigated the cell-mediated immunity, which is critical in the host defense against Eimeria infection (23). IFN-γ-secreting lymphocytes at 4 weeks after immunization were detected by enzyme-linked immunosorbent spot assay (ELISPOT assay). The proportion of E. tenella antigen-specific IFN-γ-secreting lymphocytes in peripheral blood mononuclear cells (PBMCs) in the birds immunized with Et-EmPro or Et-EmTgPro was significantly higher than that in the wild-type-immunized group, while the proportion of parasite antigen-specific IFN-γ-secreting lymphocytes in the Et-TgPro-immunized birds was comparable with that in the wild-type-parasite-immunized birds (Fig. 2A and B). These results indicate that EmPro enhanced the immunogenicity of E. tenella but that TgPro, which is a more phylogenetically related species to E. tenella than E. maxima, did not increase the immunogenicity of E. tenella.
FIG 2.
Parasite antigen-specific immune responses elicited by the transgenic Eimeria lines. (A) Et-EmPro and Et-EmTgPro stimulate higher Eimeria parasite antigen-specific cellular immune responses than the wild type and Et-TgPro. The numbers of IFN-γ secretion lymphocytes (spots) in PBMCs from naive birds (Ctrl) and birds immunized with wild-type E. tenella (WT) or transgenic Eimeria parasites are shown. (B) Mean numbers of E. tenella antigen-specific IFN-γ secretion lymphocytes in PBMCs in birds immunized with transgenic Eimeria parasites or the wild type (n = 6). (C) The E. tenella antigen-specific antibody titer increases after primary self-boosting immunization with both transgenic Eimeria parasites and the wild type. Each value represents the mean for six birds. Each panel represents three independent experiments. **, P ≤ 0.01.
We also determined adaptive humoral immune responses elicited by the transgenic parasites. IgY antibody specific to E. tenella sporozoite soluble antigen was assessed in the immunized chickens by enzyme-linked immunosorbent assay (ELISA). There was no significant difference in IgY titers in the birds immunized with transgenic lines or the wild type (Fig. 2C). Similar increases of IgY antibody titer in all groups immunized with transgenic or wild-type parasites were observed after the booster immunization on day 14. These results suggest that exogenous profilin plays no role in enhancing humoral immunity.
EmPro enhances immunoprotection by E. tenella vaccine.
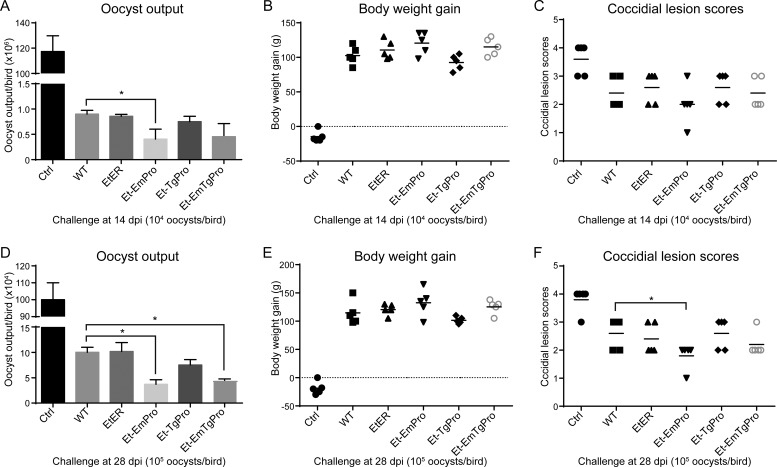
To assess the protective efficacy of transgenic Eimeria lines expressing exogenous profilin as an avian coccidiosis vaccine strain against infection with its parental parasites, we challenged Et-EmPro-, Et-TgPro-, and Et-EmTgPro-immunized birds by oral inoculation with 10,000 wild-type E. tenella parasites at 14 dpi and analyzed the oocyst output, body weight gain, and cecum lesion score as indices of protective efficacy. With significantly reduced oocyst output (4.07 [± 0.72] × 105 versus 9.0 [± 0.54] × 105) (Fig. 3A), Et-EmPro-immunized birds gained slightly more body weight (120.6 ± 3.2 g versus 102.6 ± 3.4 g) (Fig. 3B) and had lower cecum lesion scores (2.0 ± 0.63 versus 2.4 ± 0.49) (Fig. 3C) than the wild-type-immunized group and also the transgenic manipulation control group (EtER, a transgenic E. tenella line stably expressing EYFP and red fluorescent protein [RFP] that has been previously described [22]). Although the Et-EmTgPro-immunized birds showed reduced oocyst output (Fig. 3A), neither Et-TgPro- nor Et-EmTgPro-immunized birds showed enhanced protection (measured by oocyst output and cecal lesions) compared with birds immunized with the wild type (Fig. 3A to C).
FIG 3.
Protection of the birds immunized with transgenic Eimeria parasites after wild-type E. tenella challenge. Oocyst output (A and D), body weight gain (B and E), and cecum lesion scores (C and F) after challenge with E. tenella in the chickens immunized with or without transgenic Eimeria parasites or the wild type at 14 dpi (A to C) or 28 dpi (D to F) are shown. The birds were challenged with wild-type E. tenella at 10,000 oocysts per bird at 14 dpi and with 100,000 oocysts per bird at 28 dpi. Each value represents the mean for five birds. Each panel represents three independent experiments. *, P ≤ 0.05.
In birds challenged with 10,000 E. tenella oocysts at 28 dpi, we found no oocyst output from the birds immunized with the transgenic parasite lines or the wild type (data not shown). The absence of oocysts in feces after challenge was probably due to the solid immunity elicited by self-boosting immunization from parasite offspring. These results indicated that solid protective immunity was established in the birds after one round of self-boosting immunization. To further test whether the protective immunity could protect chickens from a high dose of wild-type E. tenella challenge infection, we challenged the birds at 28 dpi with a 10-fold-higher challenge dosage (100,000 E. tenella oocysts). We found significantly reduced oocyst output in birds immunized with Et-EmPro or Et-EmTgPro, but not Et-TgPro, compared with that in the wild-type-immunized group (Fig. 3D). In addition, the cecum lesion scores of Et-EmPro- and Et-EmTgPro-immunized birds were lower than those of Et-TgPro- and wild-type-immunized birds (Fig. 3F), although the difference was not statistically significant. The trends of body weight gain after the high challenge dose were similar to those for the low challenge dose at 14 dpi, where Et-EmPro-immunized birds showed a slightly higher body weight than the wild-type-immunized group (Fig. 3E). These results demonstrated that enhanced protective immunity was established in the birds immunized with the transgenic parasite lines expressing EmPro after one round of self-boosting immunization.
Profilin has been considered an immunoprotective antigen of Eimeria parasites. Immunization with recombinant profilin partly protects chickens from subsequent Eimeria parasite infection (24, 25). Transgenic E. tenella as a vaccine delivery vector expressing heterogeneous antigens such as TgSAG1 partly protects hosts from T. gondii infection (26). Here we challenged the Et-EmPro-immunized birds with 50 E. maxima oocysts to evaluate the protective immunity against heterogeneous parasite infection. There was no significant reduction in oocyst output of Et-EmPro-immunized birds compared with the wild-type-immunized birds (data not shown). These results indicated that EmPro enhanced E. tenella-specific immunity as a molecular adjuvant but that EmPro as an exogenous antigen did not elicit protective immunity against E. maxima.
Impact of transgenic parasites on diversity of the bird gut microbiome.
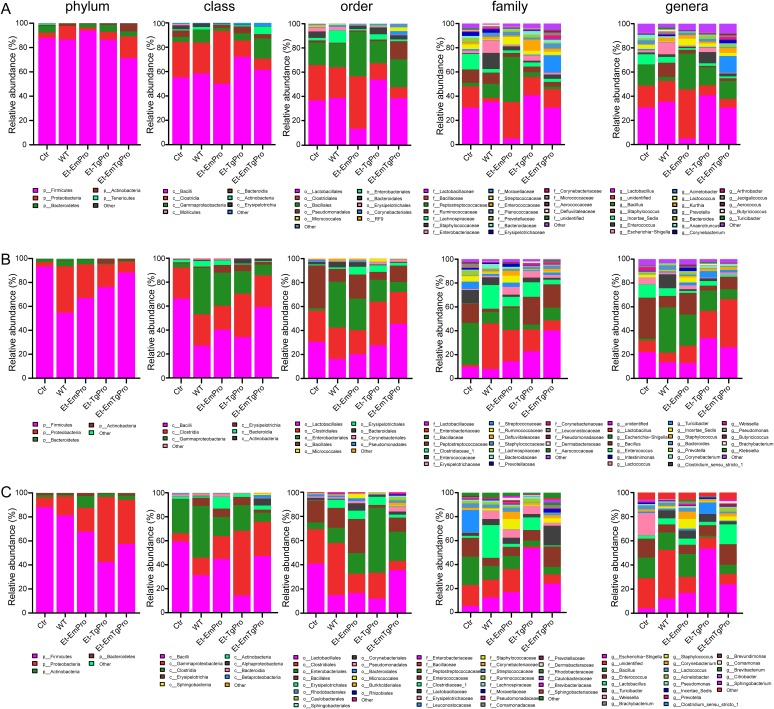
The gut microbiota is a complex ecosystem and has profound effects on the health of the host, including physiology, immune system, and nutrition, and also pathogens in the gut (27). We analyzed the core gut flora of fecal samples from individual birds after inoculation with transgenic parasites and the wild type. Fragments encompassing V3 and V4 16S rRNA hypervariable regions were PCR amplified from each of those fecal microbiota DNA samples and were sequenced by Illumina MiSeq. The taxon abundance of each sample was classified into phylum, class, order, family, and genus levels using the RDP database (rdp.cme.msu.edu), aided by the Greengene (www.greengene.com) and SSU (www.arb-silva.de) databases. Total fecal microbiota in both transgenic-parasite- and wild-type-immunized birds and naive birds were classified into two major phyla, namely, Firmicutes and Proteobacteria, at 7 dpi (Fig. 4A), 14 dpi (Fig. 4B), and 21 dpi (Fig. 4C). The percentage of Proteobacteria was increased after one round of self-boosting immunization from the offspring of both the transgenic-parasite- and wild-type immunized birds at 14 dpi and 21 dpi (Fig. 4B and C). The phylum Actinobacteria was increased as the third major phylum in the Et-EmPro-immunized birds at 21 dpi (Fig. 4C). We found significantly different compositions of microbiota from class to family levels in Et-EmPro-immunized birds compared with the other two transgenic lines and wild-type-immunized or naive birds at 7 dpi (Fig. 4A), 14 dpi (Fig. 4B), and 21 dpi (Fig. 4C).
FIG 4.
Dynamic distribution for the fecal microbiome composite for birds immunized with transgenic Eimeria parasites and the wild-type from the phylum to genus level. The distribution of the fecal microbiome composite for the naive and immunized birds at the phylum, class, order, family, and genus levels at 7 dpi (A), 14 dpi (B), and 21 dpi (C) is shown.
A more detailed analysis on the genus level was performed to learn about interactions between microbiota and parasites. The genus Bacillus was the most prominent member of the microbiota in Et-EmPro-immunized birds, but in other groups the major member was Lactobacillus, except for the unidentified microbiota at 7 dpi (Fig. 4A). At 14 dpi, major members of the microbiota in transgenic-parasite- and wild-type-immunized birds were the genera Lactobacillus and Escherichia-Shigella, while Bacillus was the major member in naive birds (Fig. 4B). The microbial community was more abundant at 21 dpi than at both 7 dpi and 14 dpi in all chicken fecal samples from phylum to genus levels (Fig. 4). In particular, more than 50% of the microbiota belonged to the genus Escherichia-Shigella in Et-TgPro-immunized birds, and the genus Weissella was dominant in the naive birds at 21 dpi (Fig. 4C). Consistently, the genus Bacillus was the dominant member of the microbiota in all fecal samples of the birds at various times in parasite-infected and naive birds.
To further study the microbial community of the birds with different treatments, we analyzed unique operational taxonomical units (OTUs) of each group. Unique OTUs in the Et-TgPro- and Et-EmTgPro-immunized birds were considerably higher than in the wild-type- and Et-EmPro-immunized birds at 7 dpi, suggesting that transgenic Eimeria parasites expressing exogenous profilin of a closely related strain (E. maxima) influenced the microbial community less than those expressing profilin from a distantly related one (T. gondii) during primary infection at 7 dpi (Fig. 5A). The number of differentially abundant OTUs between the transgenic and wild-type groups at 14 dpi was reduced after one round of self-boosting immunization from the parasite offspring (Fig. 5B). The phenomenon of the birds “eating” too much parasite offspring, which severely damages the cecum epithelium and dilutes the difference caused by exogenous profilin, may contribute to the reduced difference in unique OTUs among the immunized birds but not naive birds. Interestingly, the unique microbiota in the Et-EmPro- and Et-EmTgPro-immunized birds rapidly recovered by 21 dpi (Fig. 5C).
FIG 5.
The unique OTUs of the fecal microbiome. The unique OTUs for the fecal microbiome for birds immunized with transgenic Eimeria parasites and the wild-type were analyzed at 7 dpi (A), 14 dpi (B), and 21 dpi (C).
These results demonstrated that Eimeria parasite infection influenced the fecal microbial community, and transgenic Eimeria parasites with expression of only one exogenous protein influenced the community of microbiota.
DISCUSSION
In this study, we demonstrated that profilin of E. maxima expressed by E. tenella enhanced the immunogenicity of E. tenella, eliciting higher protective immunity than the wild-type strain against E. tenella infection, without affecting the fecundity of the transgenic parasite. In addition, the diverse community of the fecal microbiota was altered by Eimeria infection and the expression of exogenous profilin.
Both Et-EmPro and Et-EmTgPro stimulated enhanced protective immunity against subsequent parasite infection, while Et-TgPro elicited protective immunity similar to that elicited by wild-type E. tenella. Toxoplasma gondii profilin is recognized by TLR11 in mice (13). Our findings suggest that TgPro might not be recognized by chicken TLR and that profilin itself is not a protective immunogen. It probably acts as an adjuvant and enhances the immunogenicity of antigens of the host parasites. EmPro could be used as an effective molecular adjuvant in Eimeria vaccines.
Genetic manipulation to improve the safety and efficacy of anticoccidiosis vaccines have been extensively studied in recent years (28–31). Enhancing the immunogenicity of live oocyst formulations via expressing molecular adjuvants is one of the most active and promising attempts (29, 30). Li and colleagues used interleukin 2 (IL-2), a critical cytokine for T cell activation and proliferation, to enhance the immunogenicity of E. mitis and demonstrated that the protection provided by transgenic E. mitis expressing IL-2 is nearly 3-fold better than that provided by the wild type in experimental settings (29). Parallel experiments showed that transgenic Eimeria expressing the Fc fragment of immunoglobulin stimulated better protective immunity than its wild type (30). Compared with the adjuvants derived from the host immune-related molecules, cytokines or ligands of immune receptors, adjuvants derived from closely related parasites like EmPro should be more readily expressed by Eimeria parasites with fewer side effects. Our study is the first to show that an affinis molecular adjuvant, EmPro, expressed by E. tenella enhanced the immunogenicity of the transgenic parasite without deleterious effects on reproductivity. However, Et-EmPro could not protect chickens against E. maxima infection, although profilin has been tested as an immunoprotective antigen in other species such as E. acervulina and E. tenella (32, 33). We speculated that EmPro helps E. tenella propagate an effective E. tenella-specific immune response but probably is not an immunodominant antigen of E. maxima.
Transgenic E. tenella with enhanced immunogenicity stimulates vaccinated chickens to develop solid immunity rapidly, reduces vaccination side effects through preventing reinfection by offspring oocysts, and is a promising anticoccidial vaccine strain (9). Our results suggested that genetic manipulation with molecule adjuvants could also be applied to other Eimeria species such as E. necatrix, which is highly pathogenic but has intermediate immunogenicity, for developing attenuated anticoccidial vaccine strains for their safe application in breeder and layer flocks and even in broilers.
The gastrointestinal tract represents one of the primary sites of exposure to pathogens (34) and the regulation of immunity. The gut microbiota is a microbial organ with characteristic stages of development, and disruption of this developmental process is related to the pathogenesis of undernutrition in humans and animals (35). Immunization with an attenuated Salmonella enterica serovar Typhimurium strain or prebiotic galacto-oligosaccharides changed the intestinal microbiome structure and resulted in a faster clearance of virulent Salmonella after challenge infection in chickens (36). E. tenella infection changes the abundance of some microbial taxa, although the diversity of the cecal microbiome remained relatively stable (37). Consistent with the fecal microbial taxa in this study, the cecal microflora of Firmicutes and Proteobacteria were significantly increased after E. tenella infection (38). Our results demonstrated that Eimeria parasite infection influenced the fecal microbial community and that exogenous profilin has an impact on the diversity of microbiota in chickens. The gut microbiome could be utilized as an indicator for the evaluation of interactions between the immune response and parasite infection (39).
Our results are encouraging in the development of next-generation anticoccidial vaccines using genetic manipulation and have huge implications for the development of transgenic Eimeria parasites as vaccine vectors and beyond. Immune-related molecules derived from affinis organisms with high immunogenicity could be excellent adjuvant candidates for developing effective vaccines against pathogens in animals and even in humans.
MATERIALS AND METHODS
Ethics statement.
All animal experiments were performed in strict accordance with the China Agricultural University Institutional Animal Care and Use Committee guidelines (CAU20160812-1) and followed the International Guiding Principles for Biomedical Research Involving Animals. Experiments were approved by the Beijing Administration Committee of Laboratory Animals.
Parasites and animals.
Eimeria tenella (XJ strain) and E. maxima (BJ strain) were maintained by passaging in coccidian-free, 2- to 5-week-old Arbor Acres (AA) broilers. The procedures for collection, purification, and sporulation were carried out as previously described (40).
Toxoplasma gondii (RH strain) was maintained by serial passages in African green monkey kidney (Vero) cells (Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences) in Dulbecco modified Eagle medium (DMEM) supplemented with fetal bovine serum (FBS) (10%, vol/vol), penicillin (200 U ml−1), and streptomycin (20 mg ml−1) in a humidified atmosphere of 5% CO2 at 37°C.
One-week-old specific-pathogen-free (SPF) chickens were purchased from Merial Animal Health Co., Ltd. (Beijing, China) and were fed a pathogen-free diet and water ad libitum.
Plasmid construction.
Total RNAs were isolated from T. gondii tachyzoites and E. maxima sporozoites using the TRIzol reagent (Invitrogen, USA). cDNA was synthesized utilizing random primers and a high-capacity cDNA reverse transcription kit (Applied Biosystems, USA). According to the T. gondii profilin (TgPro) and E. maxima profilin (EmPro) sequences (GenBank Accession number AY937257 and XM_013481936), the open reading frames (ORFs) of profilins were amplified by PCR using TgPro-F (5′-CATATGATGTCCGACTGGGACCCTGTT-3′)/TgPro-R (5′-GTACCCAGACTGGTGAAGATACT-3′) and EmPro-F (5′-CATATGATGGGAGAAGAAGCAGCAGATAC-3′)/EmPro-R (5′-GAATCCTCCTTGATATAGGTATTC-3′) primers, respectively. The ORF of EmPro and TgPro was fused with the Flag tag at each 3′ end by a 2nd round PCR using EmPro-F/EmPro-R2 (5′-CCGCGGTCACTTATCGTCGTCATCCTTGTAATCGAATCCTCCTTGATATAGGTATTC-3′) and TgPro-F/TgPro-R2 (5′-CCGCGGTCACTTATCGTCGTCATCCTTGTAATCGTACCCAGACTGGTGAAGATACT-3′) primers, respectively. To improve the transfection efficacy, the complementary control plasmid coexpressing EmPro and TgPro was constructed in a single expression cassette linked by P2A. EmPro was similarly fused with a His tag by PCR. Fused EmPro-His and TgPro-Flag were linked by P2A using overlapping PCR to produce the dual-profilin fragment, EmPro-His-P2A-TgPro-Flag. The three fragments (EmPro-Flag, TgPro-Flag, and EmPro-His-P2A-TgPro-Flag) replacing the RFP gene of the plasmid pSDEP2ARS (22) generated the recombinant vectors pSDEP2AEmProS, pSDEP2ATgProS, and pSDEP2AEmTgProS, respectively (Fig. 1A).
Construction of transgenic E. tenella lines.
Restriction enzyme-mediated integration (REMI) was adapted for the transfection of E. tenella sporozoites (41). Briefly, 1 × 107 freshly isolated E. tenella sporozoites were transfected with 10 μg linear plasmid and 5 μl SnaBI restriction enzyme. Transfected sporozoites were inoculated into chicks via the cloacal route for the selection of stably transfected oocysts with pyrimethamine stress. Stably transfected E. tenella lines, Et-EmPro, Et-TgPro, and Et-EmTgPro, were selected using pyrimethamine combined with fluorescence-activated cell sorting.
To observe the expression patterns of reporting genes in the endogenous development of the stably transfected parasites, 6 groups of two inbred SPF chickens were inoculated with 5 × 107, 1 × 107, 1 × 106, 1 × 105, 1 × 104, and 1 × 103 sporulated oocysts, respectively, at 1 week of age. The chickens were given limited feed to reduce the cecal content and were euthanized every 24 h. Fresh smears of the mucous membrane of the cecum were visualized using a confocal laser scanning microscope (SP5; Leica, Germany).
Western blotting and indirect immunofluorescence assay (IFA) were conducted to confirm exogenous profilin expression and distribution in transgenic parasites using previously described protocols (42, 43). Soluble antigens of sporozoites were resolved by SDS-PAGE and immunoblot analysis following standard protocols with mouse anti-Flag tag or anti-His tag monoclonal antibodies and horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Proteintech, USA) as primary and secondary antibodies, respectively.
Indirect immunofluorescence assays were conducted to detect the distribution and relative expression level of exogenous profilin using mouse anti-Flag monoclonal antibody and rabbit anti-His monoclonal antibody as the primary antibodies and Cy3-conjugated goat anti-mouse IgG or aminomethylcoumarin (AMCA)-conjugated goat anti-rabbit IgG (Proteintech, USA) as the secondary antibody.
To quantify and compare the replication of different transgenic parasite lines with that of the wild type, four groups of three inbred SPF chickens were inoculated with 500 sporulated wild-type E. tenella, Et-EmPro, Et-TgPro, and Et-EmTgPro oocysts at 1 week of age. The output of oocysts of the transgenic lines and wild-type parasites was measured every 24 h using a McMaster egg counting chamber between 5 and 14 days postinoculation (44).
Vaccination, ELISA, and ELISPOT assay.
Five groups of 6 inbred SPF chickens were immunized by inoculation with 200 sporulated wild-type E. tenella, Et-EmPro, Et-TgPro, and Et-EmTgPro oocysts at 1 week of age. Serum was collected at 1-week intervals and stored at −20°C until analysis.
ELISA for detecting E. tenella-specific IgY was conducted as previously described (26, 42). Briefly, 5 μg/ml E. tenella oocyst antigens was applied to individual wells of microplates and then incubated with chicken serum (1:100). The secondary antibody used in this experiment was the HRP-conjugated goat anti-chicken IgY Fc fragment (Bethyl Laboratories, Inc.).
Parasite-specific cellular immune responses were evaluated by ELISPOT assay detecting IFN-γ-secreting peripheral blood mononuclear cells (PBMCs) following established protocols in our laboratory (45). Briefly, 1 × 106 PBMCs from naive birds and birds immunized with wild-type E. tenella and transgenic Eimeria lines were stimulated with 10 μl phosphate-buffered saline (PBS), 10 μl E. tenella oocyst antigen (10 μg/ml), or 10 μl phorbol myristate acetate (PMA) (10 ng/ml) plus ionomycin (5 μg/ml). Spots indicating IFN-γ-secreting lymphocytes were detected after 24 h of stimulation.
Challenge infection.
Inbred SPF chickens (10/group) were immunized by infection with 200 sporulated wild-type E. tenella, Et-EmPro, Et-TgPro, or Et-EmTgPro oocysts at 1 week of age. One naive control group was not immunized. Five chickens of each group were removed to new cages and challenged with wild-type E. tenella at 14 dpi (10,000 oocysts/bird), and the other 5 birds of each group were challenged at 28 dpi (100,000 oocysts/bird). Oocyst output was determined at 5 to 10 days, body weight gain between 0 and 10 days, and cecum lesions 7 days after each challenge infection (46).
Fecal microbiome analysis.
Inbred SPF chickens (n = 3/group) were immunized by infection with 200 sporulated wild-type E. tenella, Et-EmPro, Et-TgPro, and Et-EmTgPro oocysts at 1 week of age. One naive control group was not immunized. Individual fecal samples were collected at 1, 2, and 3 weeks after immunization. Samples were homogenized and stored in sterile 1.5-ml centrifuge tubes at −80°C.
Microbial genome DNA was extracted from fecal samples using the QIAamp DNA stool minikit (Qiagen) following the manufacturer's recommendation. The V3-V4 hypervariable regions of 16S rRNA were PCR amplified from microbial genome DNA using barcoded fusion primers (338-F, 5′-GTACTCCTACGGGAGGCAGCA-3′; 806-R, 5′-GTGGACTACHVGGGTWTCTAAT-3′). The PCR products were subjected to high-throughput sequencing using the Illumina MiSeq PE300 sequencing platform (Illumina, Inc., CA, USA) by Allwegene Technology Inc. (Beijing, China). Sequences with an average Phred score lower than 25, containing ambiguous bases, with homopolymer runs exceeding 6, having mismatches in primers, or with a sequence length of less than 100 bp were removed. For V3 and V4 pair-end reads, only sequences with overlap longer than 10 bp and without any mismatch were assembled according to their overlap sequence. Reads which could not be assembled were discarded. Each sample's trimmed sequence was compared to the RDP, Greengene, and SSU databases using the best-hit classification option to classify the abundance count of each taxon and analyzed by Allwegene Technology Inc. (Beijing, China).
Statistical analysis.
GraphPad Prism 6.01 (GraphPad Software) was used for statistical analysis. Differences in experimental treatments were tested using Duncan's multiple-range test following analysis of variance (ANOVA), with significance reported at a P value of ≤0.05.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (31572507) and the National Key Research and Development Program of China (2017YFD0501200).
X.T. and X.S. conceived and designed this study and analyzed the data. X.T. and J.S. carried out the experiments and drafted the manuscript. C.L. and M.D. contributed to the microbiota data analysis. C.Y., D.H., C.D., and Y.L. assisted in conducting the animal experiments and helped draft the manuscript. X.L. and X.S. supervised the study implementation and revised the manuscript. All authors read and approved the final version of the manuscript.
We declare that no competing interests exist.
REFERENCES
- 1.Blake DP, Clark EL, Macdonald SE, Thenmozhi V, Kundu K, Garg R, Jatau ID, Ayoade S, Kawahara F, Moftah A, Reid AJ, Adebambo AO, Zapatai RA, Rao ASRS, Thangaraj K, Banerjee PS, Dhinakar-Raj G, Raman M, Tomley FM. 2015. Population, genetic, and antigenic diversity of the apicomplexan Eimeria tenella and their relevance to vaccine development. Proc Natl Acad Sci U S A 112:E5343–E5350. doi: 10.1073/pnas.1506468112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalloul RA, Lillehoj HS. 2006. Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev Vaccines 5:143–163. doi: 10.1586/14760584.5.1.143. [DOI] [PubMed] [Google Scholar]
- 3.Shirley MW, Smith AL, Tomley FM. 2005. The biology of avian Eimeria with an emphasis on their control by vaccination. Adv Parasitol 60:285–330. doi: 10.1016/S0065-308X(05)60005-X. [DOI] [PubMed] [Google Scholar]
- 4.Chapman HD. 1997. Biochemical, genetic and applied aspects of drug resistance in Eimeria parasites of the fowl. Avian Pathol 26:221–244. doi: 10.1080/03079459708419208. [DOI] [PubMed] [Google Scholar]
- 5.Peek HW, Landman WJM. 2011. Coccidiosis in poultry: anticoccidial products, vaccines and other prevention strategies. Vet Q 31:143–161. doi: 10.1080/01652176.2011.605247. [DOI] [PubMed] [Google Scholar]
- 6.Chapman HD, Barta JR, Blake D, Gruber A, Jenkins M, Smith NC, Suo X, Tomley FM. 2013. A selective review of advances in coccidiosis research. Adv Parasitol 83:93–171. doi: 10.1016/B978-0-12-407705-8.00002-1. [DOI] [PubMed] [Google Scholar]
- 7.Shirley MW, Lillehoj HS. 2012. The long view: a selective review of 40 years of coccidiosis research. Avian Pathol 41:111–121. doi: 10.1080/03079457.2012.666338. [DOI] [PubMed] [Google Scholar]
- 8.Williams RB. 1999. A compartmentalised model for the estimation of the cost of coccidiosis to the world's chicken production industry. Int J Parasitol 29:1209–1229. doi: 10.1016/S0020-7519(99)00086-7. [DOI] [PubMed] [Google Scholar]
- 9.Williams RB. 2002. Anticoccidial vaccines for broiler chickens: pathways to success. Avian Pathol 31:317–353. doi: 10.1080/03079450220148988. [DOI] [PubMed] [Google Scholar]
- 10.Gunning PW, Ghoshdastider U, Whitaker S, Popp D, Robinson RC. 2015. The evolution of compositionally and functionally distinct actin filaments. J Cell Sci 128:2009–2019. doi: 10.1242/jcs.165563. [DOI] [PubMed] [Google Scholar]
- 11.Koblansky AA, Jankovic D, Oh H, Hieny S, Sungnak W, Mathur R, Hayden MS, Akira S, Sher A, Ghosh S. 2013. Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii. Immunity 38:119–130. doi: 10.1016/j.immuni.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yarovinsky F, Hieny S, Sher A. 2008. Recognition of Toxoplasma gondii by TLR11 prevents parasite-induced immunopathology. J Immunol 181:8478–8484. doi: 10.4049/jimmunol.181.12.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yarovinsky F, Zhang DK, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, Sher A. 2005. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 14.Mathur R, Oh H, Zhang DK, Park SG, Seo J, Koblansky A, Hayden MS, Ghosh S. 2012. A mouse model of Salmonella typhi infection. Cell 151:590–602. doi: 10.1016/j.cell.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang DK, Zhang GL, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. 2004. A Toll-like receptor that prevents infection by uropathogenic bacteria. Science 303:1522–1526. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 16.Applequist SE, Rollman E, Wareing MA, Liden M, Rozell B, Hinkula J, Ljunggren HG. 2005. Activation of innate immunity, inflammation, and potentiation of DNA vaccination through mammalian expression of the TLR5 agonist flagellin. J Immunol 175:3882–3891. doi: 10.4049/jimmunol.175.6.3882. [DOI] [PubMed] [Google Scholar]
- 17.Ha H, Lee JH, Kim HN, Kwak HB, Kim HM, Lee SE, Rhee JH, Kim HH, Lee ZH. 2008. Stimulation by TLR5 modulates osteoclast differentiation through STAT1/IFN-beta. J Immunol 180:1382–1389. doi: 10.4049/jimmunol.180.3.1382. [DOI] [PubMed] [Google Scholar]
- 18.Blake DP, Billington KJ, Copestake SL, Oakes RD, Quail MA, Wan KL, Shirley MW, Smith AL. 2011. Genetic mapping identifies novel highly protective antigens for an apicomplexan parasite. PLoS Pathog 7:e1001279. doi: 10.1371/journal.ppat.1001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. 2013. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benson A, Pifer R, Behrendt CL, Hooper LV, Yarovinsky F. 2009. Gut commensal bacteria direct a protective immune response against Toxoplasma gondii. Cell Host Microbe 6:187–196. doi: 10.1016/j.chom.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hess M, Sczyrba A, Egan R, Kim TW, Chokhawala H, Schroth G, Luo SJ, Clark DS, Chen F, Zhang T, Mackie RI, Pennacchio LA, Tringe SG, Visel A, Woyke T, Wang Z, Rubin EM. 2011. metagenomic discovery of biomass-degrading genes and genomes from cow rumen. Science 331:463–467. doi: 10.1126/science.1200387. [DOI] [PubMed] [Google Scholar]
- 22.Tang XM, Liu XY, Tao GR, Qin M, Yin GW, Suo JX, Suo X. 2016. “Self-cleaving” 2A peptide from porcine teschovirus-1 mediates cleavage of dual fluorescent proteins in transgenic Eimeria tenella. Vet Res 47:68. doi: 10.1186/s13567-016-0351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prowse SJ. 1991. Cell-mediated immunity to Eimeria in the fowl: the absence of cross-species protection is not due to the lack of cross-reactive T cells. Int J Parasitol 21:133–135. doi: 10.1016/0020-7519(91)90134-S. [DOI] [PubMed] [Google Scholar]
- 24.Ding XC, Lillehoj HS, Quiroz MA, Bevensee E, Lillehoj EP. 2004. Protective immunity against Eimeria acervulina following in ovo immunization with a recombinant subunit vaccine and cytokine genes. Infect Immun 72:6939–6944. doi: 10.1128/IAI.72.12.6939-6944.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Min WG, Lillehoj HS, Burnside J, Weining KC, Staeheli P, Zhu JJ. 2001. Adjuvant effects of IL-1 beta, IL-2, IL-8, IL-15, IFN-alpha, IFN-gamma TGF-beta 4 and lymphotactin on DNA vaccination against Eimeria acervulina. Vaccine 20:267–274. doi: 10.1016/S0264-410X(01)00270-5. [DOI] [PubMed] [Google Scholar]
- 26.Tang XM, Yin GW, Qin M, Tao GR, Suo JX, Liu XY, Suo X. 2016. Transgenic Eimeria tenella as a vaccine vehicle: expressing TgSAG1 elicits protective immunity against Toxoplasma gondii infections in chickens and mice. Sci Rep 6:29379. doi: 10.1038/srep29379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao LL, Wang G, Siegel P, He C, Wang HZ, Zhao WJ, Zhai ZX, Tian FW, Zhao JX, Zhang H, Sun ZK, Chen W, Zhang Y, Meng H. 2013. Quantitative genetic background of the host influences gut microbiomes in chickens. Sci Rep 3:1163. doi: 10.1038/srep01163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanig S, Entzeroth R, Kurth M. 2012. Chimeric fluorescent reporter as a tool for generation of transgenic Eimeria (Apicomplexa, Coccidia) strains with stage specific reporter gene expression. Parasitol Int 61:391–398. doi: 10.1016/j.parint.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Li ZR, Tang XM, Suo JX, Qin M, Yin GW, Liu XY, Suo X. 2015. Transgenic Eimeria mitis expressing chicken interleukin 2 stimulated higher cellular immune response in chickens compared with the wild-type parasites. Front Microbiol 6:533. doi: 10.3389/fmicb.2015.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin M, Tang XM, Yin GW, Liu XY, Suo JX, Tao GR, El-Ashram S, Li Y, Suo X. 2016. Chicken IgY Fc expressed by Eimeria mitis enhances the immunogenicity of E-mitis. Parasit Vectors 9:164. doi: 10.1186/s13071-016-1451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tao GR, Shi TY, Tang XM, Duszynski DW, Wang YZ, Li C, Suo JX, Tian XL, Liu XY, Suo X. 2017. Transgenic Eimeria magna Perard, 1925 displays similar parasitological properties to the wild-type strain and induces an exogenous protein-specific immune response in rabbits (Oryctolagus cuniculus L.). Front Immunol 8:2. doi: 10.3389/fimmu.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.del Cacho E, Gallego M, Lee SH, Lillehoj HS, Quilez J, Lillehoj EP, Sanchez-Acedo C. 2011. Induction of protective immunity against Eimeria tenella infection using antigen-loaded dendritic cells (DC) and DC-derived exosomes. Vaccine 29:3818–3825. doi: 10.1016/j.vaccine.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 33.Song KD, Lillehoj HS, Choi KD, Yun CH, Parcells MS, Huynh JT, Han JY. 2000. A DNA vaccine encoding a conserved Eimeria protein induces protective immunity against live Eimeria acervulina challenge. Vaccine 19:243–252. doi: 10.1016/S0264-410X(00)00169-9. [DOI] [PubMed] [Google Scholar]
- 34.Molloy MJ, Grainger JR, Bouladoux N, Hand TW, Koo LY, Naik S, Quinones M, Dzutsev AK, Gao JL, Trinchieri G, Murphy PM, Belkaid Y. 2013. Intraluminal containment of commensal outgrowth in the gut during infection-induced dysbiosis. Cell Host Microbe 14:318–328. doi: 10.1016/j.chom.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. 2014. Gut microbiota: the neglected endocrine organ. Mol Endocrinol 28:1221–1238. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azcarate-Peril MA, Butz N, Cadenas MB, Koci M, Ballou A, Mendoza M, Ali R, Hassan H. 2018. An attenuated Salmonella enterica serovar Typhimurium strain and galacto-oligosaccharides accelerate clearance of Salmonella infections in poultry through modifications to the gut microbiome. Appl Environ Microbiol 84:e02526-. doi: 10.1128/AEM.02526-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Macdonald SE, Nolan MJ, Harman K, Boulton K, Hume DA, Tomley FM, Stabler RA, Blake DP. 2017. Effects of Eimeria tenella infection on chicken caecal microbiome diversity, exploring variation associated with severity of pathology. PLoS One 12:e0184890. doi: 10.1371/journal.pone.0184890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Z, Nie K, Huang Q, Li K, Sun Y, Zhou R, Wang Z, Hu S. 2017. Changes of cecal microflora in chickens following Eimeria tenella challenge and regulating effect of coated sodium butyrate. Exp Parasitol 177:73–81. doi: 10.1016/j.exppara.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Young VB, Raffals LH, Huse SM, Vital M, Dai DJ, Schloss PD, Brulc JM, Antonopoulos DA, Arrieta RL, Kwon JH, Reddy KG, Hubert NA, Grim SL, Vineis JH, Dalal S, Morrison HG, Eren AM, Meyer F, Schmidt TM, Tiedje JM, Chang EB, Sogin ML. 2013. Multiphasic analysis of the temporal development of the distal gut microbiota in patients following ileal pouch anal anastomosis. Microbiome 1:9. doi: 10.1186/2049-2618-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Long PL, Millard BJ, Joyner LP, Norton CC. 1976. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet Lat 6:201–217. [PubMed] [Google Scholar]
- 41.Liu XY, Shi TY, Ren HB, Su HL, Yan WC, Suo X. 2008. Restriction enzyme-mediated transfection improved transfection efficiency in vitro in Apicomplexan parasite Eimeria tenella. Mol Biochem Parasitol 161:72–75. doi: 10.1016/j.molbiopara.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Huang XX, Zou J, Xu HQ, Ding Y, Yin GW, Liu XY, Suo X. 2011. Transgenic Eimeria tenella expressing enhanced yellow fluorescent protein targeted to different cellular compartments stimulated dichotomic immune responses in chickens. J Immunol 187:3595–3602. doi: 10.4049/jimmunol.1100043. [DOI] [PubMed] [Google Scholar]
- 43.Liu XY, Zou J, Yin GW, Su HL, Huang XX, Li JN, Xie L, Cao YQ, Cui YJ, Suo X. 2013. Development of transgenic lines of Eimeria tenella expressing M2e-enhanced yellow fluorescent protein (M2e-EYFP). Vet Parasitol 193:1–7. doi: 10.1016/j.vetpar.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 44.Haug A, Williams RB, Larsen S. 2006. Counting coccidial oocysts in chicken faeces: a comparative study of a standard McMaster technique and a new rapid method. Vet Parasitol 136:233–242. doi: 10.1016/j.vetpar.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 45.Yin GW, Qin M, Liu XY, Suo JX, Suo X. 2013. Interferon-gamma enzyme-linked immunosorbent spot assay as a tool to study T cell responses to Eimeria tenella infection in chickens. Poult Sci 92:1758–1763. doi: 10.3382/ps.2012-02998. [DOI] [PubMed] [Google Scholar]
- 46.Johnson J, Reid WM. 1970. Anticoccidial drugs—lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol 28:30. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]