B cell antigen receptor (BCR) diversity increases by several orders of magnitude due to the action of terminal deoxynucleotidyl transferase (TdT) during V(D)J recombination. Unlike adults, infants have limited BCR diversity, in part due to reduced expression of TdT.
KEYWORDS: TdT, Salmonella, Pneumococcus, antibodies, polysaccharide vaccine, antibody repertoire, polysaccharides, vaccines
ABSTRACT
B cell antigen receptor (BCR) diversity increases by several orders of magnitude due to the action of terminal deoxynucleotidyl transferase (TdT) during V(D)J recombination. Unlike adults, infants have limited BCR diversity, in part due to reduced expression of TdT. Since human infants and young mice respond poorly to polysaccharide vaccines, such as the pneumococcal polysaccharide vaccine Pneumovax23 and Vi polysaccharide (ViPS) of Salmonella enterica serovar Typhi, we tested the contribution of TdT-mediated BCR diversity in response to these vaccines. We found that TdT+/− and TdT−/− mice generated comparable antibody responses to Pneumovax23 and survived Streptococcus pneumoniae challenge. Moreover, passive immunization of B cell-deficient mice with serum from Pneumovax23-immunized TdT+/− or TdT−/− mice conferred protection. TdT+/− and TdT−/− mice generated comparable levels of anti-ViPS antibodies and antibody-dependent, complement-mediated bactericidal activity against S. Typhi in vitro. To test the protective immunity conferred by ViPS immunization in vivo, TdT+/− and TdT−/− mice were challenged with a chimeric Salmonella enterica serovar Typhimurium strain expressing ViPS, since mice are nonpermissive hosts for S. Typhi infection. Compared to their unimmunized counterparts, immunized TdT+/− and TdT−/− mice challenged with ViPS-expressing S. Typhimurium exhibited a significant reduction in the bacterial burden and liver pathology. These data suggest that the impaired antibody response to the Pneumovax23 and ViPS vaccines in the young is not due to limited TdT-mediated BCR diversification.
INTRODUCTION
Infants and very young children do not respond efficiently to bacterial polysaccharide (PS) antigens and as a consequence suffer severe infections from PS-encapsulated bacterial pathogens, such as Streptococcus pneumoniae and Salmonella enterica serovar Typhi. Reports from the CDC indicate that 21.6 million cases of typhoid fever due to S. Typhi infection occur each year, resulting in 226,000 deaths, especially in children (1). Invasive pneumococcal diseases kill over 1.5 million people each year globally, and the majority of those deaths occur in young children (2). S. pneumoniae has more than 94 serotypes (based on antigenically distinct PSs), but most pneumococcal diseases in humans are associated with 23 of these serotypes. The single-dose, nonconjugated Pneumovax23 vaccine confers protection against the 23 most prevalent pneumococcal serotypes in adults, but it does not induce an optimal antibody response in young children or young mice (3). Like pneumococcal diseases, typhoid is a vaccine-preventable disease (4, 5). Two types of licensed vaccines are currently available: a live attenuated S. Typhi vaccine and a subunit vaccine composed of Vi polysaccharide (ViPS), e.g., Typhim Vi. The live attenuated vaccine is not recommended for children less than 6 years of age due to safety concerns. Although subunit vaccines are known to be very safe in children, the ViPS vaccine does not induce an adequate antibody response in young children (5).
Impaired responses to PS antigens in the very young have been attributed to several factors, such as delayed marginal zone B cell development, low expression of complement receptor CR1/2 on marginal zone B cells (6, 7), Bruton's tyrosine kinase-mediated B cell antigen receptor (BCR) signaling (8, 9), and a restricted BCR repertoire (10, 11). The ability of B cells to recognize a broad range of antigens is attributed to the diversity of the preimmune BCR repertoire. This diversity is generated by a complete usage of all variable (V), diversity (D), and joining (J) gene segments as well as by the addition of nontemplate (N) nucleotides at the junctions of the V-D, D-J, and V-J gene segments by terminal deoxynucleotidyl transferase (TdT) during the V(D)J recombination process (12).
T cell-independent (TI) type 1 antigens, such as bacterial lipopolysaccharide (LPS), at high concentrations activate B cells primarily by stimulating mitogenic receptors, e.g., Toll-like receptors (TLRs), rather than BCRs. Therefore, the antibodies generated by such stimuli are not necessarily antigen specific. On the other hand, TI type 2 (TI-2) antigens are capsular PSs, including those present on a variety of clinically important human pathogens, such as Streptococcus pneumoniae (13, 14). Unlike protein antigens, polysaccharide (PS) antigens are generally not processed and presented in the context of major histocompatibility complex class II on antigen-presenting cells (13, 14), and as a result, germinal center reactions are not developed upon PS immunization (15, 16). Nevertheless, PS antigens can induce a fairly rapid and antigen-specific antibody response in adults primarily by extensively cross-linking the BCR of antigen-specific B cells (13, 14). Therefore, PS-specific antibody responses are largely dependent on the breadth of the preimmune BCR repertoire.
The human B cell repertoire is developmentally regulated. Notably, fetal B immunoglobulin gene rearrangements use third-heavy-chain complementarity-determining regions (HCDR3s) shorter than those from adults (17, 18). The developing B cells of human infants also express significantly reduced levels of TdT compared to B cells of older children (19). Consistent with this, the B cells of infants but not older children express antibody V genes with reduced numbers of N nucleotides at the junctions of the V-D, D-J, and V-J gene segments (19). Therefore, it was previously hypothesized that infants and young children respond poorly to PS antigens due to reduced BCR junctional diversity (10, 11). In support of this hypothesis, adult mice deficient in TdT do not generate an efficient antibody response to α-1,3 dextran, a PS antigen expressed on several microorganisms, including Enterobacter cloacae (11). To gain a better understanding of why human infants and young mice respond poorly to bacterial PS antigens, we sought to evaluate the contribution of TdT-mediated BCR diversification to antibody responses.
RESULTS AND DISCUSSION
Antibody responses to α-1,3 dextran are impaired in TdT−/− mice.
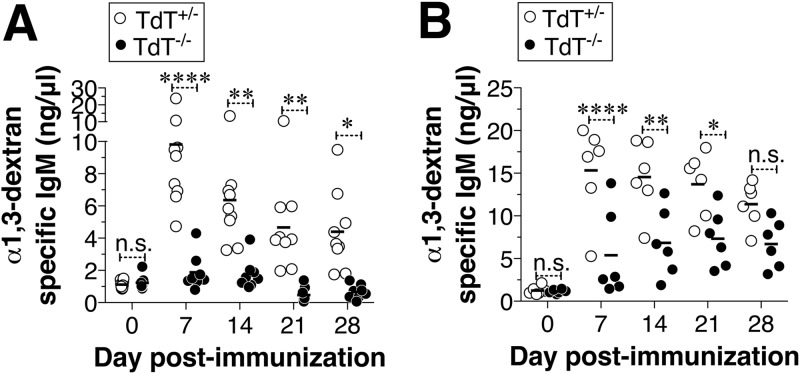
Antibody responses to PS antigens are T cell and germinal center independent (15, 16). PS-specific antibody responses are largely dependent on the breadth of the preimmune BCR repertoire rather than the postimmune repertoire, which is typically shaped by somatic hypermutation in the germinal centers. Therefore, TdT-mediated junctional diversity could be a contributing factor in recognizing PS antigens but not protein antigens, which can induce a germinal center response. This is supported by the efficient antibody response of TdT−/− mice to keyhole limpet hemocyanin, a very immunogenic protein antigen (20). In contrast, the antibody response to α-1,3 dextran is significantly reduced in TdT−/− mice (11). To confirm this finding, we immunized TdT+/− and TdT−/− mice intravenously (i.v.) with E. cloacae, which expresses α-1,3 dextran. We found that TdT-deficient mice, as previously shown (11), are significantly impaired in the α-1,3 dextran antibody response compared to TdT-sufficient mice (Fig. 1A). To examine whether antibody responses to isolated PS antigens, which are commonly used in vaccines, are also impaired in TdT-deficient mice, we immunized mice with B1355 dextran, a homopolymer of d-glucose predominantly with α-1,3 linkages (21, 22). Compared to TdT-sufficient mice, the TdT-deficient mice were significantly impaired in responding to α-1,3 dextran in the context of isolated PS immunization (Fig. 1B).
FIG 1.
Antibody responses to α-1,3 dextran require TdT. TdT+/− or TdT−/− mice were immunized i.v. with E. cloacae strain MK7 (3 × 108 bacterial cells) (A) or i.p. with B1355 dextran (B), and the levels of α-1,3 dextran-specific IgM were measured by ELISA as described previously (11). Each dot represents an individual mouse, and the bars represent the mean antibody level. Statistically significant differences were determined using two-way analysis of variance with the Bonferroni posttest. *, P < 0.05; **, P < 0.01; ****, P < 0.0001; n.s., not statistically significant.
Efficient antibody responses to Pneumovax23 in mice deficient in TdT.
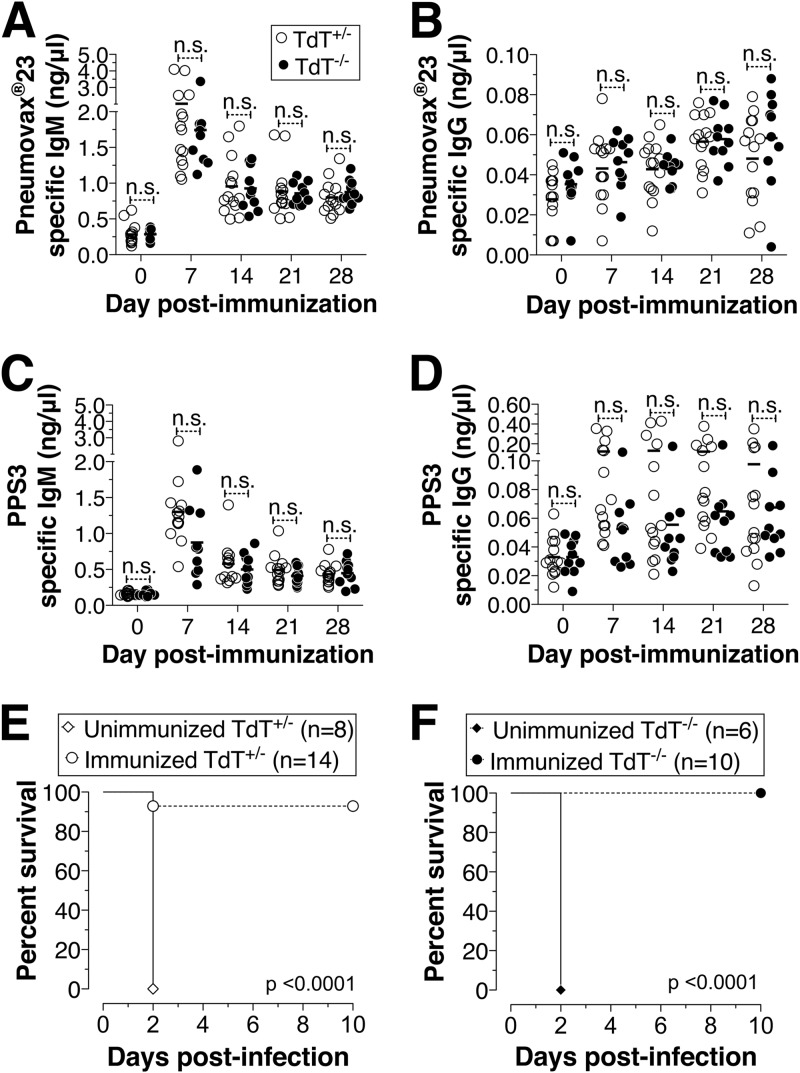
To test whether the lack of TdT-mediated junctional diversity is also the basis for the inefficient antibody responses to pneumococcal PS (PPS) antigens, we immunized adult TdT+/− or TdT−/− mice with Pneumovax23. We found that the levels of Pneumovax23-specific IgM or IgG in immunized TdT+/− and TdT−/− mice were comparable at all time points tested (Fig. 2A and B). We also found that both TdT+/− and TdT−/− mice generated indistinguishable IgM and IgG responses to pneumococcal PS serotype 3 (PPS3) (Fig. 2C and D). To test whether those anti-Pneumovax23 responses confer similar levels of protection, we challenged TdT+/− and TdT−/− mice with serotype 3 (i.e., PPS3) pneumococcus strain WU2. Compared to their unimmunized counterparts, both immunized TdT+/− and immunized TdT−/− mice survived the pneumococcal challenge (Fig. 2E and F). The differences in the survival of immunized TdT+/− and TdT−/− mice were not statistically significant (Fig. 2E versus F). These findings indicate that TdT is not required for generating an efficient and functional antibody response to pneumococcal PS antigens.
FIG 2.
Pneumovax23 immunization of mice sufficient or deficient in TdT results in a comparable antibody response and confers protection against S. pneumoniae. Adult TdT+/− and TdT−/− mice were immunized i.p. with 10 μg of Pneumovax23. Blood samples were obtained at 0, 7, 14, 21, and 28 days postimmunization. (A to D) Pneumovax23-specific (A and B) or PPS3-specific (C and D) IgM and IgG levels were determined by ELISA. Each dot represents an individual mouse, and the bars represent the mean antibody level. The data represent pools from two independent experiments. Statistically significant differences were determined using two-way analysis of variance with the Bonferroni posttest. n.s., not statistically significant. (E and F) At 4 weeks following Pneumovax23 immunization, mice were infected i.p. with 5,000 CFU of S. pneumoniae strain WU2 and survival was monitored. Survival statistics were performed using the log-rank (Mantel-Cox) test, and P values are indicated within the plots.
To test whether antibodies from TdT-deficient mice are sufficient to confer protection, serum from Pneumovax23-immunized TdT+/− or TdT−/− mice was transferred to B cell-deficient mice. These mice were challenged with serotype 3 pneumococcus strain WU2 as described previously (23). B cell-deficient mice that did not receive serum succumbed to infection, whereas mice that received immune serum from TdT−/− mice survived (Fig. 3). Although TdT+/− mouse serum recipients did not display 100% survival, there was no significant difference between the survival of TdT−/− and TdT+/− mouse serum recipients (Fig. 3). These data indicate that polysaccharide antibodies generated without junctional diversity can confer protection against S. pneumoniae.
FIG 3.
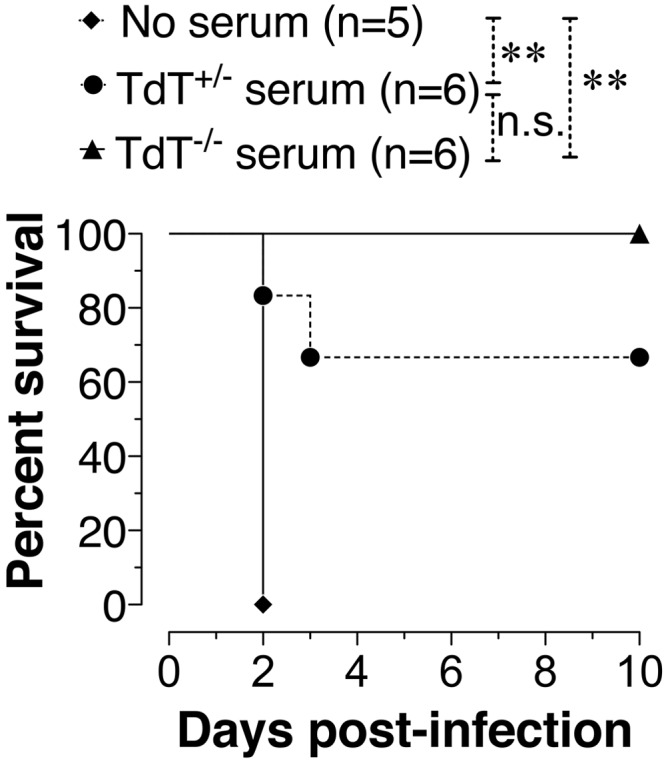
Immune serum from TdT-deficient mice confers protection against S. pneumoniae. B cell-deficient mice were injected with 0.25 ml of serum from either TdT+/− or TdT−/− mice immunized with Pneumovax23. Mice were infected i.p. with 500 CFU of S. pneumoniae strain WU2, and survival was monitored. Survival statistics were performed using the log-rank (Mantel-Cox) test. **, P < 0.01; n.s., not statistically significant.
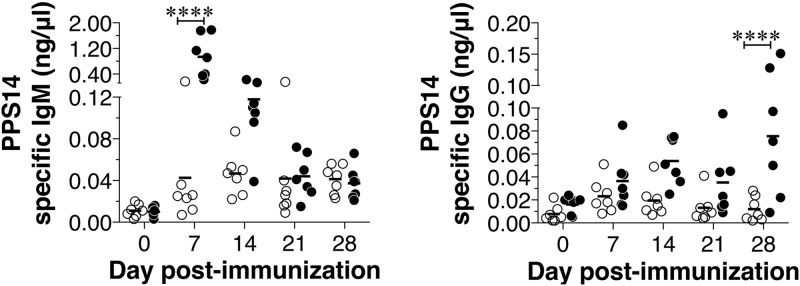
PPS14 is less immunogenic than PPS3 in mice (24). To test whether TdT-mediated junctional diversity plays a role in the antibody response to a less immunogenic PPS serotype, we immunized TdT+/− or TdT−/− mice with PPS14. Remarkably, we found that PPS14-specific IgM and IgG responses were significantly higher in TdT-deficient mice than in TdT-sufficient mice (Fig. 4). These data suggest that impaired responses to certain PPS serotypes in the young are not due to a lack of junctional diversity. There is mounting evidence that the effect of the diversifying activity of TdT is subtle and is better measured at the level of clonal populations. It was shown previously that the presence or absence of TdT had contrasting impacts on B cell repertoire development, depending on the response studied. For example, the generation of the mouse germ line stereotyped response to phosphorylcholine is favored by the absence of TdT expression in neonatal life (23). This window of limited diversity is ablated in TdT short isoform-transgenic mice, where enforced expression of TdT exerts its diversifying activity in fetal and neonatal repertoires and prevents production of the dominant T15 idiotype antiphosphorylcholine antibodies that are protective against S. pneumoniae infection (23). In contrast, TdT−/− mice, which have the opportunity to produce N-less CDR3s even in adult bone marrow, were shown to generate a more robust T15 idiotype antibody response than wild-type mice (25). However, the dominant clones that respond to α-1,3 dextran were highly dependent on TdT activity to add critical residues in the CDR3 responsible for the generation of clones, giving rise to higher-affinity antibodies (26). Thus, TdT expression may enhance (11, 26) or impair (23) antibody responses to certain antigens.
FIG 4.
Increased anti-PSS14 antibody responses in TdT-deficient mice. TdT+/− or TdT−/− mice were immunized i.p. with 1 μg of PPS14, and the levels of PPS14-specific IgM and IgG were measured by ELISA. Each dot represents an individual mouse, and the bars represent the mean antibody level. Statistically significant differences were determined using two-way analysis of variance with the Bonferroni posttest. ****, P < 0.0001.
TdT is not required for antibody responses to ViPS of S. Typhi.
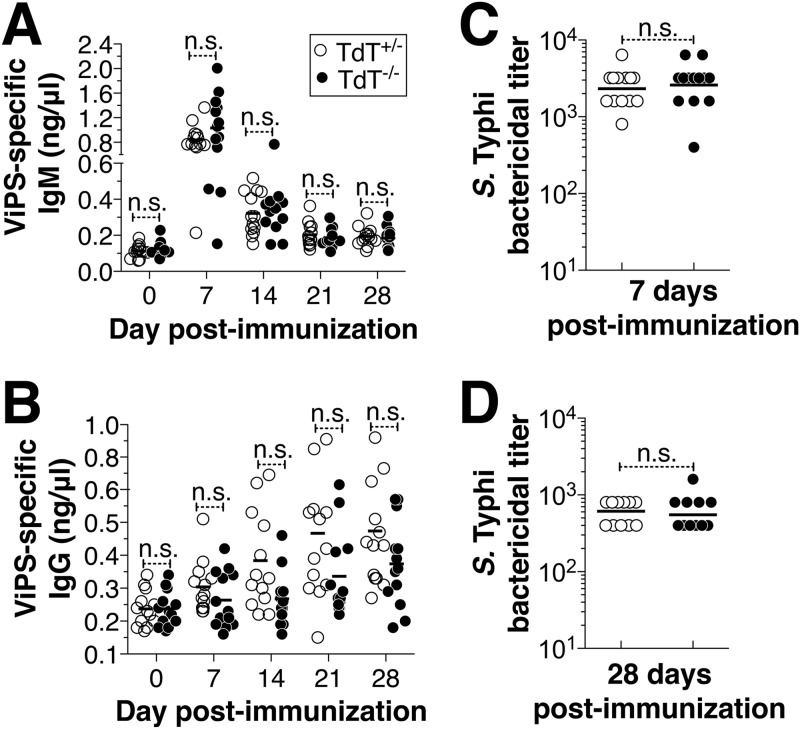
To test whether TdT is also dispensable for antibody responses to other bacterial PS vaccines, we immunized adult TdT+/− and TdT−/− mice with ViPS, the critical component in the current typhoid subunit vaccine, Typhim Vi. In adult mice, administration of a single dose of ViPS induces a robust anti-ViPS IgM and IgG response (27). We found that ViPS immunization results in comparable ViPS-specific IgM and IgG responses in both TdT+/− and TdT−/− mice (Fig. 5A and B). Since the IgM and IgG responses peaked at 7 and 28 days postimmunization, respectively, we chose these time points for evaluating complement-dependent serum bactericidal activity against S. Typhi. We found that the immune sera from both TdT+/− and TdT−/− mice exerted similar bactericidal activity against S. Typhi, and the differences in the serum bactericidal titers were not statistically significant at either time point (Fig. 5C and D).
FIG 5.
Immunization with Vi polysaccharide results in comparable antibody and bactericidal responses in TdT-sufficient and -deficient mice. (A and B) TdT+/− and TdT−/− mice were immunized i.p. with 2.5 μg of ViPS, and the levels of ViPS-specific IgM (A) and IgG (B) were measured by ELISA. Each dot represents an individual mouse, and the bars represent the mean antibody level. The data represent pools from two independent experiments. Statistically significant differences were determined using two-way analysis of variance with the Bonferroni posttest. n.s., not statistically significant. (C and D) Serum bactericidal antibody titers against S. Typhi strain Ty2 were determined at 7 (C) and 28 (D) days postimmunization. Each dot represents an individual mouse, and the bars represent the geometric mean. Statistically significant differences were determined by the Mann-Whitney test.
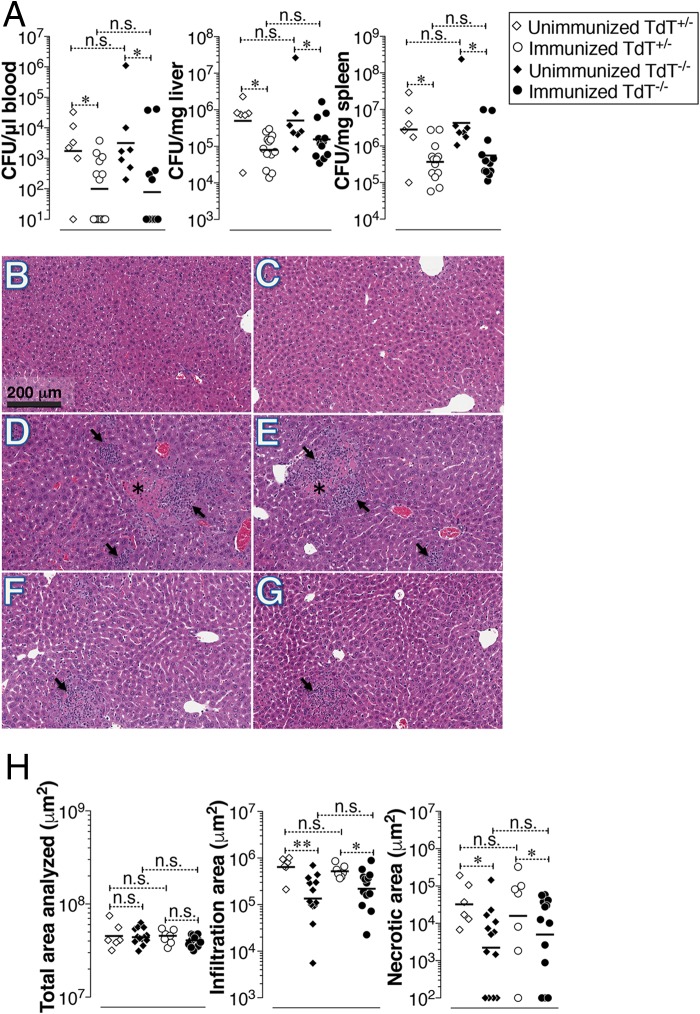
S. Typhi is a human-restricted pathogen, and experimental models to test the efficacy of typhoid vaccines are currently limited (28). S. Typhimurium causes a typhoid-like systemic disease in mice. Therefore, S. Typhimurium infection in mice is widely used as an experimental model to understand certain aspects of human typhoid (29). Investigating the role of ViPS-specific antibody responses using S. Typhimurium is limited by the fact that, unlike S. Typhi, S. Typhimurium does not express ViPS. A chimeric strain of S. Typhimurium (RC60) was previously engineered to express all genes that are necessary for ViPS synthesis, export, and regulation in S. Typhi and was shown to exhibit cell surface and other characteristics of S. Typhi (30). To test the ViPS-mediated protective immunity, unimmunized or immunized TdT+/− and TdT−/− mice were challenged with RC60 on day 28 postimmunization, and the bacterial burden in the blood, liver, and spleen was measured 3 days later. Compared to unimmunized mice, we found a significant decrease in the bacterial burden in immunized TdT+/− and TdT−/− mice (Fig. 6A); however, no significant differences in bacterial burden between immunized TdT+/− and TdT−/− mice were observed. It was previously shown that mouse complement is not effective in activating the classical complement pathway on certain nontyphoidal Salmonella serovars, including S. Typhimurium (31). Therefore, it is important to note that in the in vitro serum bactericidal assay (SBA) we used baby rabbit serum as a source of complement. Thus, the decrease in bacterial burden in ViPS-immunized mice could be due to other defense mechanisms, such as antibody-mediated opsonophagocytosis, as previously suggested (31, 32).
FIG 6.
Comparable reductions in bacterial burdens and liver damage in TdT+/− and TdT−/− mice immunized with Vi polysaccharide. TdT+/− and TdT−/− mice were immunized with ViPS as described in the legend to Fig. 5. (A) At 4 weeks after immunization, mice were infected i.p. with 3 × 104 CFU of ViPS-expressing S. Typhimurium strain RC60, and at 3 days postchallenge, the bacterial burden was determined as described in Materials and Methods. (B to G) Livers from naive uninfected mice (B and C), naive infected mice (D and E), and ViPS-immunized and infected mice (F and G) were analyzed for histopathology. Asterisks, necrotic lesions; arrows, areas of cellular infiltration. (H) Quantification of liver damage in multiple mice. Each dot represents an individual mouse, and the bars represent the geometric mean. The data represent pools from two independent experiments. Statistically significant differences were determined by the Mann-Whitney test. **, P < 0.01; *, P < 0.05; n.s., not statistically significant.
The liver lesions observed in S. Typhimurium infection in mice (33–37) are very similar to those described for human typhoid patients (38–40). We confirmed that systemic infection of naive mice with live S. Typhimurium strain RC60 resulted in extensive liver damage, as shown by large areas of hepatocyte necrosis and infiltration of mononuclear cells (Fig. 6D and E). In contrast, TdT+/− and TdT−/− mice immunized with ViPS had significantly reduced necrotic regions in the liver (Fig. 6F and G). Moreover, the infiltrated regions in the ViPS-immunized mice were significantly reduced compared to those in unimmunized mice (Fig. 6H). These data suggest that TdT is not essential for generating an efficient and functional antibody response to ViPS.
TdT expression early in life temporally coincides with IL-7-dependent B lymphopoiesis. For example, human fetal B cell precursors have reduced expression of both IL-7 receptor α (IL-7Rα) and TdT (19). Furthermore, humans with IL-7Rα mutations have reduced expression of TdT in their pro-B cells (19). We have previously shown that murine antibody responses to Pneumovax23 require IL-7 (3). Recently, we have found that IL-7-deficient mice are also impaired in responding to ViPS (41). Since the data from the present study obtained using these two bacterial PS vaccines show that TdT is dispensable for the generation of protective antibody responses, it is likely that IL-7 plays a role in generating antibody responses to some PS antigens independently of TdT. Mice deficient in IL-7Rα signaling have a restricted immunoglobulin repertoire, partly because they are inefficient in their usage of distal VH gene segments during V(D)J recombination (42), similar to the inefficiency of the usage of distal VH gene segments seen in newborn mice (43). Human pro-B cells that develop early in life express mainly proximal VH rather than distal VH segments (19). It was previously shown that the majority of adult human B cells that bind to PPS serotypes 14 and 4 utilize distal VH genes VH3-74 and VH3-48 (44), whereas those that bind to PPS serotypes 6b and 23f utilize the VH3-23 located in the center of the human Ig heavy-chain locus (45, 46). We have recently found that the majority of ViPS-binding B cells of adult wild-type mice express immunoglobulin heavy-chain sequences containing distal VH gene segments, the most frequent of which were VH1-53 and VH1-55 (41). Therefore, the reasons for the impaired anti-PS responses in the young could be attributable to a restricted Ig repertoire due to an inefficient expression of distal VH genes. Adjuvants that increase the breadth of preimmune BCR diversification by increasing the usage of distal VH segments could help overcome the impaired responses to certain PS vaccines in infants and young children.
MATERIALS AND METHODS
Mice.
The Thomas Jefferson University Institutional Animal Care and Use Committee approved these studies. Mice were housed in microisolator cages with free access to food and water and were maintained in a specific-pathogen-free facility. TdT−/− mice were generated previously (47) and were backcrossed onto a BALB/c mouse background for more than 10 generations. Two independent studies confirmed that the TdT+/− mice had increased numbers of N nucleotides between their VH-DH and DH-JH junctions and that such nucleotides were absent in TdT−/− mice (47, 48). In the present study, we used TdT+/− and TdT−/− littermates for a controlled comparison, and the disruption of the gene encoding TdT was confirmed by PCR. B cell-deficient mice (mb1-cre) on the C57BL6 mouse background (49) were originally generated by Michael Reth (Max Planck Institute of Immunobiology, Freiburg, Germany) and obtained from Ann Feeney (The Scripps Research Institute, La Jolla, CA). The genotype of the mb1-cre mice was confirmed by PCR. All mice were bred in the Thomas Jefferson University's Laboratory Animal facility, and 8- to 12-week-old mice of both sexes were used.
Immunization.
Mice were immunized intravenously (i.v.) with 108 paraformaldehyde-fixed Enterobacter cloacae strain MK7 bacteria in 100 μl of Dulbecco's phosphate-buffered saline (DPBS; Mediatech, Herndon, VA). Fifty micrograms of α-1,3 dextran (B1355; a gift from A. Jeanes) dissolved in 100 μl DPBS was used to immunize mice intraperitoneally (i.p.). Two and a half micrograms of unconjugated Vi polysaccharide (ViPS; lot 5 PDMI 158299, obtained from the U.S. Food and Drug Administration, Silver Spring, MD) dissolved in 100 μl DPBS was used to immunize mice i.p. The ViPS used for immunization was isolated from Citrobacter freundii strain WR7011 (O29 serotype) (50) and is structurally identical to the ViPS from S. Typhi (51, 52). Previously, immunization with this ViPS in the range of 0.25 to 50 μg in 100 μl phosphate-buffered saline (PBS) was shown to induce an anti-ViPS antibody response comparable to the response induced by ViPS from S. Typhi (52, 53). Ten micrograms of the 23-valent pneumococcal polysaccharide (PPS) vaccine (Pneumovax23; Merck & Co. Inc., Whitehouse Station, NJ) or 1 μg of PPS14 (197-X; American Type Culture Collection, Manassas, VA) was dissolved in 100 μl DPBS and used to immunize mice i.p. as described previously (3). Blood samples were obtained at 0, 7, 14, 21, and 28 days following immunization and stored at −20°C.
ELISA.
For measuring α-1,3 dextran-specific IgM, 96-well enzyme immunoassay/radioimmunoassay plates (Costar 9017; Corning Inc., Corning, NY) were coated with 50 μl of dextran B1355 (1 μg/ml). Pneumovax23-, PPS3-, or PPS14-specific IgM and IgG were measured by coating 96-well plates (Nunc MultiSorp 467340; Nunc A/S, Roskilde, Denmark) with 50 μl of either Pneumovax23 (5 μg/ml), PPS3 (5 μg/ml; 169-X; American Type Culture Collection, Manassas, VA), or PPS14 (5 μg/ml). ViPS-specific IgM and IgG were measured by incubating 96-well microtiter plates (Nunc MultiSorp 467340) with 50 μl of ViPS (2 μg/ml) in DPBS overnight at room temperature. All plates were washed and blocked with 2% bovine serum albumin (BSA) in PBS (pH 7.2) for 2 h at room temperature. For measuring Pneumovax23-, PPS3-, and PPS14-specific antibody responses, serum samples were preincubated with C-polysaccharide (5 μg/ml; Statens Serum Institut Diagnostica A/S, Denmark) to decrease background binding (54). The serum dilutions for measuring the antibody response to each polysaccharide were chosen after evaluating various serum dilutions within the linear range by enzyme-linked immunosorbent assay (ELISA) (data not shown). The chosen serum dilutions were as follows: α-1,3 dextran-specific IgM, 1:250; Pneumovax23-specific IgM, 1:250; Pneumovax23-specific IgG, 1:50; PPS3-specific IgM, 1:100; PPS3-specific IgG, 1:50; PPS14-specific IgM, 1:60; PPS3-specific IgG, 1:30; ViPS-specific IgM, 1:50; and ViPS-specific IgG, 1:25. Blood samples were diluted and centrifuged (16,000 × g for 10 min), and the supernatant was used. Since each of the PS-specific mouse IgM and IgG reference standards is not available and the PS-specific antibodies are likely to be of an oligoclonal nature with various affinities, the antigen-specific antibody levels in the present study were interpreted as nanogram-per-microliter equivalents using normal mouse serum IgM or IgG standards (Bethyl Laboratories, Montgomery, TX), as described previously (3, 55, 56).
SBA.
The serum bactericidal assay (SBA) was performed as previously described (57). In brief, log-phase cultures (optical density at 600 nm [OD600], 0.5 at 37°C) of S. Typhi strain Ty2 were prepared in Luria-Bertani (LB) broth with 10 mM NaCl. Bacterial cells were washed in DPBS, and the bacterial cell density was adjusted to 2.5 × 104 to 5.0 × 104 CFU per ml in DPBS. The expression of ViPS was assessed by a slide agglutination test using a commercial Vi monoclonal antibody reagent (lot 188L-8; Statens Serum Institut Diagnostica A/S, Denmark). Serum samples were heat inactivated by incubation at 56°C for 30 min prior to use in the assay. Ten microliters of S. Typhi strain Ty2 in DPBS (250 to 500 CFU) was added to each well of a round-bottom polypropylene 96-well plate containing 50 μl of heat-inactivated serum in serial dilutions, 12.5 μl baby rabbit complement (Pel-Freeze, Rogers, AR), and 27.5 μl DPBS. Triplicate samples of each dilution were incubated for 120 min at 37°C with gentle rocking, and 10 μl of this mixture was plated on LB agar plates for counting of the number of CFU. Serum bactericidal antibody titers were defined as the reciprocal of the highest dilution that produced 50% killing in relation to that in control wells containing complement but no mouse serum. Naive mouse serum served as a negative control, whereas S. Typhi anti-Vi human IgG (lot R1, 2011; U.S. Food and Drug Administration, Silver Spring, MD) served as a positive control.
Infections.
For pneumococcal infections, 5 × 103 CFU of S. pneumoniae WU2, a serotype 3 strain (58, 59), was injected i.p., and the survival of the immunized mice was monitored. To test the relative protection conferred by ViPS immunization, mice were infected with a chimeric strain of S. Typhimurium (strain RC60) that expresses the genes necessary for ViPS synthesis, export, and regulation in S. Typhi (30). Strain RC60 was grown to an OD600 of ∼1.0 in LB broth containing 10 mM NaCl. The expression of ViPS was assessed by a slide agglutination test using a commercial Vi monoclonal antibody reagent (lot 188L-8; Statens Serum Institut Diagnostica A/S, Denmark). The bacteria were washed twice in DPBS, and 100 μl of DPBS containing 3 × 104 CFU was injected i.p. At 3 days postinfection, the liver and spleen were collected and the tissues were processed using a Minilys tissue homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France). Blood was collected into anticoagulant, and the bacterial burden in the blood and tissue homogenates was measured by counting of the number of CFU on LB agar plates.
Passive immunization.
The protection mediated by passive immunization was determined as described previously (23). Seven TdT+/− or TdT−/− mice were immunized with 10 μg of Pneumovax23 (3). At 40 days postimmunization, mice were sacrificed and serum was collected. Pooled serum was filter sterilized (pore size, 0.22 μm), and 250 μl of this filter-sterilized serum was injected i.p. into B cell-deficient mice (49). Three hours later, mice were infected i.p. with 500 CFU of S. pneumoniae (strain WU2), and the survival of the infected mice was monitored for 10 days.
Histopathology analysis.
Liver tissues obtained on day 3 postinfection were fixed in 10% buffered formalin, and 4-μm paraffin-embedded sections were stained with hematoxylin and eosin. The specimen slides were scanned at a ×20 magnification on an Aperio CS2 ScanScope scanner (Leica Biosystems Inc.); total, necrotic, and infiltration areas composed of lymphocytes and other mononuclear cells in the entire specimen were quantified using Aperio ImageScope software (Leica Biosystems Inc.).
Statistical analysis.
The data presented throughout depict pooled data from at least two independent experiments unless otherwise noted. Statistical analyses were performed using Prism (version 5) software (GraphPad Software, Inc., La Jolla, CA).
ACKNOWLEDGMENTS
We thank David Briles for providing S. pneumoniae strain WU2, Tim Manser for editing the manuscript, and Cynthia Benedict for discussion. We also thank Shousun Szu and John Cipollo for providing Vi polysaccharide and the S. Typhi anti-Vi human IgG standard.
This work was supported by Public Health Service grant AI121270 to K.R.A., by AI14782-39 AI100005-06 to J.F.K., and by R01AI04417 to A.J.B.
REFERENCES
- 1.Crump JA, Mintz ED. 2010. Global trends in typhoid and paratyphoid fever. Clin Infect Dis 50:241–246. doi: 10.1086/649541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T, Hib Pneumococcal Global Burden of Disease Study Team. 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 3.Shriner AK, Liu H, Sun G, Guimond M, Alugupalli KR. 2010. IL-7-dependent B lymphocytes are essential for the anti-polysaccharide response and protective immunity to Streptococcus pneumoniae. J Immunol 185:525–531. doi: 10.4049/jimmunol.0902841. [DOI] [PubMed] [Google Scholar]
- 4.Guzman CA, Borsutzky S, Griot-Wenk M, Metcalfe IC, Pearman J, Collioud A, Favre D, Dietrich G. 2006. Vaccines against typhoid fever. Vaccine 24:3804–3811. doi: 10.1016/j.vaccine.2005.07.111. [DOI] [PubMed] [Google Scholar]
- 5.Levine MM. 2008. Typhoid fever vaccine, 5th ed Saunders Elsevier, Philadelphia, PA. [Google Scholar]
- 6.Zandvoort A, Lodewijk ME, de Boer NK, Dammers PM, Kroese FG, Timens W. 2001. CD27 expression in the human splenic marginal zone: the infant marginal zone is populated by naive B cells. Tissue Antigens 58:234–242. doi: 10.1034/j.1399-0039.2001.580403.x. [DOI] [PubMed] [Google Scholar]
- 7.Zandvoort A, Timens W. 2002. The dual function of the splenic marginal zone: essential for initiation of anti-TI-2 responses but also vital in the general first-line defense against blood-borne antigens. Clin Exp Immunol 130:4–11. doi: 10.1046/j.1365-2249.2002.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chelvarajan RL, Gilbert NL, Bondada S. 1998. Neonatal murine B lymphocytes respond to polysaccharide antigens in the presence of IL-1 and IL-6. J Immunol 161:3315–3324. [PubMed] [Google Scholar]
- 9.Khan AQ, Sen G, Guo S, Witte ON, Snapper CM. 2006. Induction of in vivo antipolysaccharide immunoglobulin responses to intact Streptococcus pneumoniae is more heavily dependent on Btk-mediated B-cell receptor signaling than antiprotein responses. Infect Immun 74:1419–1424. doi: 10.1128/IAI.74.2.1419-1424.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez C, Moller G. 1978. Immunological unresponsiveness to native dextran B512 in young animals of dextran high responder strains is due to lack of Ig receptors expression. Evidence for a nonrandom expression of V-genes. J Exp Med 147:645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahmoud TI, Kearney JF. 2010. Terminal deoxynucleotidyl transferase is required for an optimal response to the polysaccharide alpha-1,3 dextran. J Immunol 184:851–858. doi: 10.4049/jimmunol.0902791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benedict CL, Gilfillan S, Thai TH, Kearney JF. 2000. Terminal deoxynucleotidyl transferase and repertoire development. Immunol Rev 175:150–157. doi: 10.1111/j.1600-065X.2000.imr017518.x. [DOI] [PubMed] [Google Scholar]
- 13.Vos Q, Lees A, Wu Z, Snapper CM, Mond JJ. 2000. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev 176:154–170. doi: 10.1034/j.1600-065X.2000.00607.x. [DOI] [PubMed] [Google Scholar]
- 14.Lesinski GB, Westerink MA. 2001. Novel vaccine strategies to T-independent antigens. J Microbiol Methods 47:135–149. doi: 10.1016/S0167-7012(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 15.de Vinuesa CG, Cook MC, Ball J, Drew M, Sunners Y, Cascalho M, Wabl M, Klaus GG, MacLennan IC. 2000. Germinal centers without T cells. J Exp Med 191:485–494. doi: 10.1084/jem.191.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manser T. 2004. Textbook germinal centers? J Immunol 172:3369–3375. doi: 10.4049/jimmunol.172.6.3369. [DOI] [PubMed] [Google Scholar]
- 17.Schroeder HW Jr, Mortari F, Shiokawa S, Kirkham PM, Elgavish RA, Bertrand FE III. 1995. Developmental regulation of the human antibody repertoire. Ann N Y Acad Sci 764:242–260. doi: 10.1111/j.1749-6632.1995.tb55834.x. [DOI] [PubMed] [Google Scholar]
- 18.Schroeder HW Jr, Zhang L, Philips JB III. 2001. Slow, programmed maturation of the immunoglobulin HCDR3 repertoire during the third trimester of fetal life. Blood 98:2745–2751. doi: 10.1182/blood.V98.9.2745. [DOI] [PubMed] [Google Scholar]
- 19.Rother MB, Jensen K, van der Burg M, van de Bovenkamp FS, Kroek R, van IJcken WF, van der Velden VH, Cupedo T, Olstad OK, van Dongen JJ, van Zelm MC. 2016. Decreased IL7Ralpha and TdT expression underlie the skewed immunoglobulin repertoire of human B-cell precursors from fetal origin. Sci Rep 6:33924. doi: 10.1038/srep33924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilfillan S, Bachmann M, Trembleau S, Adorini L, Kalinke U, Zinkernagel R, Benoist C, Mathis D. 1995. Efficient immune responses in mice lacking N-region diversity. Eur J Immunol 25:3115–3122. doi: 10.1002/eji.1830251119. [DOI] [PubMed] [Google Scholar]
- 21.Newman B, Sugii S, Kabat EA, Torii M, Clevinger BL, Schilling J, Bond M, Davie JM, Hood L. 1983. Combining site specificities of mouse hybridoma antibodies to dextran B1355S. J Exp Med 157:130–140. doi: 10.1084/jem.157.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foote JB, Kearney JF. 2009. Generation of B cell memory to the bacterial polysaccharide alpha-1,3 dextran. J Immunol 183:6359–6368. doi: 10.4049/jimmunol.0902473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benedict CL, Kearney JF. 1999. Increased junctional diversity in fetal B cells results in a loss of protective anti-phosphorylcholine antibodies in adult mice. Immunity 10:607–617. doi: 10.1016/S1074-7613(00)80060-6. [DOI] [PubMed] [Google Scholar]
- 24.Moens L, Jeurissen A, Wuyts G, Fallon PG, Louis B, Ceuppens JL, Bossuyt X. 2007. Specific intracellular adhesion molecule-grabbing nonintegrin R1 is not involved in the murine antibody response to pneumococcal polysaccharides. Infect Immun 75:5748–5752. doi: 10.1128/IAI.00574-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benedict CL, Gilfillan S, Kearney JF. 2001. The long isoform of terminal deoxynucleotidyl transferase enters the nucleus and, rather than catalyzing nontemplated nucleotide addition, modulates the catalytic activity of the short isoform. J Exp Med 193:89–99. doi: 10.1084/jem.193.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahmoud TI, Schroeder HW Jr, Kearney JF. 2011. Limiting CDR-H3 diversity abrogates the antibody response to the bacterial polysaccharide alpha 1→3 dextran. J Immunol 187:879–886. doi: 10.4049/jimmunol.1100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandya KD, Palomo-Caturla I, Walker JA, Sandilya VK, Zhong Z, Alugupalli KR. 2018. An unmutated IgM response to the Vi polysaccharide of Salmonella typhi contributes to protective immunity in a murine model of typhoid. J Immunol 200:4078–4084. doi: 10.4049/jimmunol.1701348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darton TC, Blohmke CJ, Pollard AJ. 2014. Typhoid epidemiology, diagnostics and the human challenge model. Curr Opin Gastroenterol 30:7–17. doi: 10.1097/MOG.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 29.McSorley SJ. 2014. Immunity to intestinal pathogens: lessons learned from Salmonella. Immunol Rev 260:168–182. doi: 10.1111/imr.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crawford RW, Wangdi T, Spees AM, Xavier MN, Tsolis RM, Baumler AJ. 2013. Loss of very-long O-antigen chains optimizes capsule-mediated immune evasion by Salmonella enterica serovar Typhi. mBio 4:e00232-. doi: 10.1128/mBio.00232-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siggins MK, Cunningham AF, Marshall JL, Chamberlain JL, Henderson IR, MacLennan CA. 2011. Absent bactericidal activity of mouse serum against invasive African nontyphoidal Salmonella results from impaired complement function but not a lack of antibody. J Immunol 186:2365–2371. doi: 10.4049/jimmunol.1000284. [DOI] [PubMed] [Google Scholar]
- 32.Hart PJ, O'Shaughnessy CM, Siggins MK, Bobat S, Kingsley RA, Goulding DA, Crump JA, Reyburn H, Micoli F, Dougan G, Cunningham AF, MacLennan CA. 2016. Differential killing of Salmonella enterica serovar Typhi by antibodies targeting Vi and lipopolysaccharide O:9 antigen. PLoS One 11:e0145945. doi: 10.1371/journal.pone.0145945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakoneczna I, Hsu HS. 1980. The comparative histopathology of primary and secondary lesions in murine salmonellosis. Br J Exp Pathol 61:76–84. [PMC free article] [PubMed] [Google Scholar]
- 34.Moncure CW, Guo YN, Xu HR, Hsu HS. 1998. Comparative histopathology in mouse typhoid among genetically diverse mice. Int J Exp Pathol 79:183–192. doi: 10.1046/j.1365-2613.1998.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alam MS, Akaike T, Okamoto S, Kubota T, Yoshitake J, Sawa T, Miyamoto Y, Tamura F, Maeda H. 2002. Role of nitric oxide in host defense in murine salmonellosis as a function of its antibacterial and antiapoptotic activities. Infect Immun 70:3130–3142. doi: 10.1128/IAI.70.6.3130-3142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sebastiani G, Blais V, Sancho V, Vogel SN, Stevenson MM, Gros P, Lapointe JM, Rivest S, Malo D. 2002. Host immune response to Salmonella enterica serovar Typhimurium infection in mice derived from wild strains. Infect Immun 70:1997–2009. doi: 10.1128/IAI.70.4.1997-2009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez-Escobedo G, La Perle KM, Gunn JS. 2013. Histopathological analysis of Salmonella chronic carriage in the mouse hepatopancreatobiliary system. PLoS One 8:e84058. doi: 10.1371/journal.pone.0084058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mert A, Tabak F, Ozaras R, Ozturk R, Aki H, Aktuglu Y. 2004. Typhoid fever as a rare cause of hepatic, splenic, and bone marrow granulomas. Intern Med 43:436–439. doi: 10.2169/internalmedicine.43.436. [DOI] [PubMed] [Google Scholar]
- 39.Narechania S, Duran M, Karivedu V, Gopalakrishna KV. 2015. A case of typhoid fever with hepatic granulomas and enteritis. Case Rep Pathol 2015:745461. doi: 10.1155/2015/745461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramachandran S, Godfrey JJ, Perera MV. 1974. Typhoid hepatitis. JAMA 230:236–240. [PubMed] [Google Scholar]
- 41.Dickinson GS, Levenson EA, Walker JA, Kearney JF, Alugupalli KR. 2018. Interleukin-7 enables antibody responses to bacterial polysaccharides by promoting B cell antigen receptor diversity. J Immunol 201:1229–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corcoran AE, Riddell A, Krooshoop D, Venkitaraman AR. 1998. Impaired immunoglobulin gene rearrangement in mice lacking the IL-7 receptor. 2018. Nature 391:904–907. doi: 10.1038/36122. [DOI] [PubMed] [Google Scholar]
- 43.Malynn BA, Yancopoulos GD, Barth JE, Bona CA, Alt FW. 1990. Biased expression of JH-proximal VH genes occurs in the newly generated repertoire of neonatal and adult mice. J Exp Med 171:843–859. doi: 10.1084/jem.171.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolibab K, Smithson SL, Rabquer B, Khuder S, Westerink MA. 2005. Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults: analysis of the variable heavy chain repertoire. Infect Immun 73:7465–7476. doi: 10.1128/IAI.73.11.7465-7476.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou J, Lottenbach KR, Barenkamp SJ, Lucas AH, Reason DC. 2002. Recurrent variable region gene usage and somatic mutation in the human antibody response to the capsular polysaccharide of Streptococcus pneumoniae type 23F. Infect Immun 70:4083–4091. doi: 10.1128/IAI.70.8.4083-4091.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou J, Lottenbach KR, Barenkamp SJ, Reason DC. 2004. Somatic hypermutation and diverse immunoglobulin gene usage in the human antibody response to the capsular polysaccharide of Streptococcus pneumoniae type 6B. Infect Immun 72:3505–3514. doi: 10.1128/IAI.72.6.3505-3514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gilfillan S, Dierich A, Lemeur M, Benoist C, Mathis D. 1993. Mice lacking TdT: mature animals with an immature lymphocyte repertoire. Science 261:1175–1178. doi: 10.1126/science.8356452. [DOI] [PubMed] [Google Scholar]
- 48.Komori T, Okada A, Stewart V, Alt FW. 1993. Lack of N regions in antigen receptor variable region genes of TdT-deficient lymphocytes. Science 261:1171–1175. doi: 10.1126/science.8356451. [DOI] [PubMed] [Google Scholar]
- 49.Hobeika E, Thiemann S, Storch B, Jumaa H, Nielsen PJ, Pelanda R, Reth M. 2006. Testing gene function early in the B cell lineage in mb1-cre mice. Proc Natl Acad Sci U S A 103:13789–13794. doi: 10.1073/pnas.0605944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snellings NJ, Johnson EM, Kopecko DJ, Collins HH, Baron LS. 1981. Genetic regulation of variable Vi antigen expression in a strain of Citrobacter freundii. J Bacteriol 145:1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szu SC, Li XR, Schneerson R, Vickers JH, Bryla D, Robbins JB. 1989. Comparative immunogenicities of Vi polysaccharide-protein conjugates composed of cholera toxin or its B subunit as a carrier bound to high- or lower-molecular-weight Vi. Infect Immun 57:3823–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szu SC, Li XR, Stone AL, Robbins JB. 1991. Relation between structure and immunologic properties of the Vi capsular polysaccharide. Infect Immun 59:4555–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szu SC, Stone AL, Robbins JD, Schneerson R, Robbins JB. 1987. Vi capsular polysaccharide-protein conjugates for prevention of typhoid fever. Preparation, characterization, and immunogenicity in laboratory animals. J Exp Med 166:1510–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Konradsen HB, Sorensen UB, Henrichsen J. 1993. A modified enzyme-linked immunosorbent assay for measuring type-specific anti-pneumococcal capsular polysaccharide antibodies. J Immunol Methods 164:13–20. doi: 10.1016/0022-1759(93)90270-H. [DOI] [PubMed] [Google Scholar]
- 55.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. 2004. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 56.Dickinson GS, Sun G, Bram RJ, Alugupalli KR. 2014. Efficient B cell responses to Borrelia hermsii infection depend on BAFF and BAFFR but not TACI. Infect Immun 82:453–459. doi: 10.1128/IAI.01147-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boyd MA, Tennant SM, Saague VA, Simon R, Muhsen K, Ramachandran G, Cross AS, Galen JE, Pasetti MF, Levine MM. 2014. Serum bactericidal assays to evaluate typhoidal and nontyphoidal Salmonella vaccines. Clin Vaccine Immunol 21:712–721. doi: 10.1128/CVI.00115-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Briles DE, Nahm M, Schroer K, Davie J, Baker P, Kearney J, Barletta R. 1981. Anti-phosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J Exp Med 153:694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swiatlo E, King J, Nabors GS, Mathews B, Briles DE. 2003. Pneumococcal surface protein A is expressed in vivo, and antibodies to PspA are effective for therapy in a murine model of pneumococcal sepsis. Infect Immun 71:7149–7153. doi: 10.1128/IAI.71.12.7149-7153.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]