Abstract
We report a rare case of dermatophyte infection of the glabrous skin (Tinea corporis) caused by Nannizzia gypsea (formerly Microsporum gypseum). A 22-year-old Malagasy female who reported close contact reportedly with cats, presented a single round lesion with a peripheral, active, squamous and pruriginous inflammatory bead. Morphologic species identification was confirmed by sequencing the internal transcribed spacer (ITS) region of the genome. Specific treatment with oral loratadine and topical miconazole cream was effective.
Keywords: Nannizzia gypsea, Dermatophyte infection, ITS region, Madagascar
1. Introduction
Nannizzia gypsea, formerly known as Microsporum gypseum [1], is a cosmopolitan geophilic fungus rarely responsible for human infections. It has a particular affinity for keratin (hair, feathers and horns) and might be transmitted to humans and animals through contact with soil [2]. Despite its rarity, the benign nature of its clinical manifestations and its low infectivity [3], this aetiology is important to characterize in order to avoid prescription of erroneous medications and adapt patient management. Here, we report a rare case of N. gypsea infection in whom the initial use of topical corticosteroid led to the worsening of the lesion in this immunocompetent subject living in Antananarivo, Madagascar.
2. Case
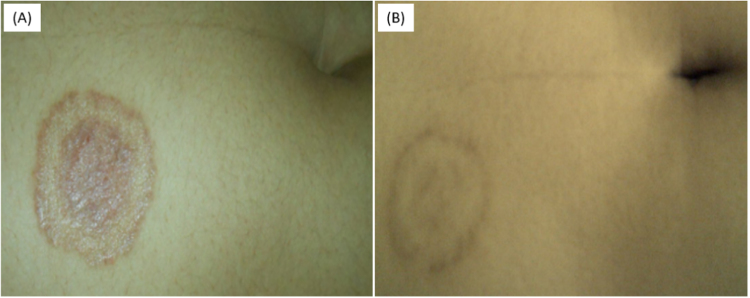
On December 2017 (day 0), a 22-year-old Malagasy female student presented with a 1-month history of moderate inflammation of discreetly squamous lesion located in the glabrous skin of the right upper quadrant. She went to visit a general practitioner and had been receiving topical corticosteroid (betamethasone cream) for 1 month (day +30). This treatment led to the worsening of the lesion that enlarged and get more inflammatory and pruriginous. The patient was referred on February 2017 (day +58) to the Dermatology Department at the University Hospital in Antananarivo. She presented a single rounded lesion of 3 cm on the right upper quadrant with a peripheral, active, squamous and pruriginous inflammatory bead – covered with small vesicles and a central erythematous and hyperchromic zone (Fig. 1A). No other lesion was detected. The patient had no significant medical conditions and no similar cases were found in her relatives. Detailed medical history revealed a permanent habit to be sleeping with cats, yet no history of repeated nor close contact with soils (e.g. gardening, agriculture, barefoot walking). She achieved overall recovery (day +88) after a complete course of oral loratadine (10 mg per day for 7 days) and topical miconazole cream during 4 weeks (Fig. 1B).
Fig. 1.
Clinical appearance of the lesion one month after topical corticosteroids cream (day +58) showing a single rounded lesion of 3 cm in diameter, including a peripheral, active, squamous and pruriginous inflammatory bead covered with small vesicles and a central erythematous hyperchromic zone (A); and after 4 weeks of antifungal treatment (day +88) showing regression of the inflammatory signs and progressive cutaneous healing (B).
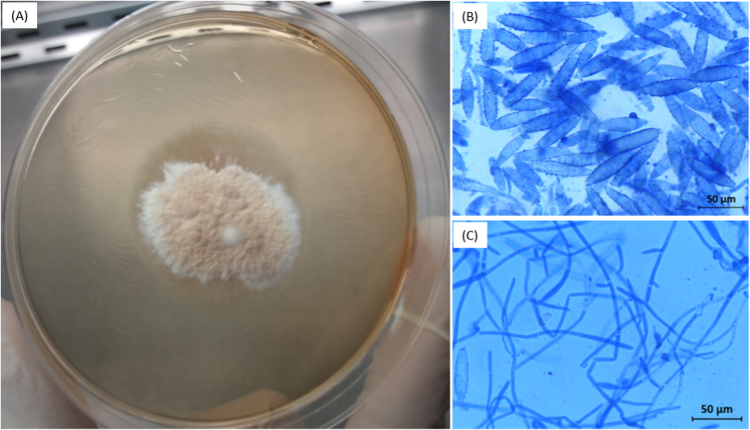
Scales from the peripheral zone of the lesion were sampled on day +58 with a Vidal scraper and collected in a sterile watch glass. After 6 days of incubation at 27 °C on Sabouraud-chloramphenicol medium without cycloheximide, macroscopic examination of the primary culture showed flat, powdery, buff colored colonies with a fringed white outline. A second culture was made on a Petri dish (Fig. 2A). Microscopic examination of the culture colonies after lactophenol blue staining showed a large number of large, thin-walled cocoon-shaped macroconidia with rounded ends and 4–6 compartments (Fig. 2B and C) suggestive of N. gypsea.
Fig. 2.
Macroscopic appearance of a secondary cultured colony of Nanizzia gypsea on Sabouraud agar supplemented with chloramphenicol without cycloheximide showing flat, powdery, buff colored colonies with a fringed white outline (A). Photomicrographs (×400) obtained after staining of cultured colonies with lactophenol blue showing cocoon-shape and echinulate macroconidia with rounded ends, thin-walled and thin-partitions delimiting 4–6 compartments (B), and propagules (C).
Fungal genomic DNA was extracted from cultures colonies using the GenElute™ Mammalian Genomic DNA Miniprep Kit (Sigma-Aldrich). The internal transcribed spacer (ITS) encompassing the ITS1, the subunit 5.8 S ribosomal DNA, the ITS2 regions ( ∼ 700 bp) and the D1/D2 domains of large subunit 26 S rDNA ( ∼ 600 bp) were amplified using the universal primers ITS5/ITS4 [4] and NL1/NL4 [5], respectively. The PCR products were sequenced and the sequences were assembled and compared using Basic Local Alignment Search Tool (BLAST, https://blast.ncbi.nlm.nih.gov/) and the International Society for Human and Animal Mycology (ISHAM) ITS Database [6]. Using the BLAST similarity search, the ITS sequences displayed 100% nucleotide identity with the reference strain N. gypsea BCRC 30,542 along the ITS1 (accession number DQ860725.1, 226 nucleotides) and ITS2 (accession number DQ860790.1, 195 nucleotides) regions with a coverage of 100%. Nannizzia is a new genus that include 9 dermatophyte species, among which the former M. gypseum. This result was confirmed by the close relationship with N. gypsea clinical isolates (100% nucleotide identity) using the ISHAM ITS Database. In the D1/D2 region, the analysed sequence was very close (100%, 605/605 nucleotides) to N. gypsea CBS 118,893 (accession number XR_001951142.1). The current taxonomy establishes N. gypsea as the new name of M. gypseum.
The sequences were submitted to the GenBank database under the accession numbers MG437268 to MG437270.
3. Discussion
In this study, we report the isolation of N. gypsea from the glabrous skin lesion of an immunocompetent young female. Tinea gathered a wide range of clinical syndromes caused by various genera of dermatophytes: Epidermophyton, Microsporum, Trichophyton Arthroderma, Lophophyton, Nannizzia and Guarromyces. Previous studies conducted in the central highlands of Madagascar have reported identification of anthropophilic dermatophytes Nannizzia audouinii [7], Trichophyton tonsurans, Nannizzia boullardii, and Trichophyton terrestre [8] during investigation of tinea capitis and tinea corporis outbreaks in primary schools. In our study we isolated N. gypsea, a geophilic dermatophyte involved occasionally in human and animal infections. Based on the detailed medical history, the pathogen has likely been transmitted through direct contact with cats. Age, climatic and socioeconomic status have also been described as the major risk factors of dermatophytoses in the eastern and southern parts of Africa including Madagascar [9]. To our knowledge, this study is the first to report N. gypsea associated-disease in human in Madagascar.
Common clinical manifestations of glabrous skin infections caused by N. gypsea are the classic ringworm, such as those shown in Fig. 1. However, this clinical aspect is not specific to a given dermatophyte species [2]. A single species may be involved in the development of lesions in multiple anatomic sites; and conversely, different species may cause the same lesion. This suggests that laboratory diagnostic is pivotal in understanding the high risk population, the epidemiology and the species concerned. In this study, the diagnostic was made by means of traditional culture followed by morphologic study of growth colonies and microscopic examination but also using molecular method. Our findings showed that sequencing of ITS region is a useful tool to differentiate the dermatophytes species, since there may be a discrepancy between the results obtained from the classical and molecular methods [10].
This study also raises the issue of improper use of corticosteroid – especially in developing countries. Indications of corticosteroid rely essentially on its anti-inflammatory, immunosuppressive and anti-allergic properties and should be targeted and monitored strictly to avoid adverse effects as observed in our case. In fact, misuse of topical [11], [12] or injectable [13] corticosteroid is common in developing countries and presents a major threat to public health.
In conclusion, this study sheds more light in understanding the epidemiology of tinea in Madagascar. Further studies should be confirmed by molecular method and include an etiological research of the role of domestic pets in the transmission of the pathogen.
Acknowledgements
This study was supported partly by grants from Fondation Mérieux, Lyon, France, Lyon, France.
Acknowledgments
Conflict of interest
No conflict of interests is declared.
References
- 1.de Hoog G.S., Dukik K., Monod M., Packeu A., Stubbe D., Hendrickx M. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia. 2017;182:5–31. doi: 10.1007/s11046-016-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weitzman I., Summerbell R.C. The dermatophytes. Clin. Microbiol. Rev. 1995;8:240–259. doi: 10.1128/cmr.8.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chmel L., Buchvald J. Ecology and transmission of Microsporum gypseum from soil to man. Sabouraudia. 1970;8:149–156. [PubMed] [Google Scholar]
- 4.White T.J., Bruns T.D., Lee S.B., Taylor J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, part three. Genet. Evol. 1990;38 [Google Scholar]
- 5.Abliz P., Fukushima K., Takizawa K., Nishimura K. Identification of pathogenic dematiaceous fungi and related taxa based on large subunit ribosomal DNA D1/D2 domain sequence analysis. FEMS Immunol. Med. Microbiol. 2004;40:41–49. doi: 10.1016/S0928-8244(03)00275-X. [DOI] [PubMed] [Google Scholar]
- 6.Irinyi L., Serena C., Garcia-Hermoso D., Arabatzis M., Desnos-Ollivier M., Vu D. International society of human and animal mycology (ISHAM)-ITS reference DNA barcoding database – the quality controlled standard tool for routine identification of human and animal pathogenic fungi. Med. Mycol. 2015;53:313–337. doi: 10.1093/mmy/myv008. [DOI] [PubMed] [Google Scholar]
- 7.Carod J.F., Ratsitorahina M., Raherimandimby H., Hincky Vitrat V., Ravaolimalala Andrianaja V., Contet-Audonneau N. Outbreak of tinea capitis and corporis in a primary school in Antananarivo, Madagascar. J. Infect. Dev. Ctries. 2011;5:732–736. doi: 10.3855/jidc.1944. [DOI] [PubMed] [Google Scholar]
- 8.Contet-Audonneau N., Grosjean P., Razanakolona L.R., Andriantsinjovina T., Rapelanoro R. Tinea capitis in Madagascar: a survey in a primary school in Antsirabe. Ann. Dermatol. Venereol. 2006;133:22–25. doi: 10.1016/s0151-9638(06)70837-7. [DOI] [PubMed] [Google Scholar]
- 9.Nweze E.I., Eke I.E. Dermatophytes and dermatophytosis in the eastern and southern parts of Africa. Med. Mycol. 2017 doi: 10.1093/mmy/myx025. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 10.Li H.C., Bouchara J.P., Hsu M.M., Barton R., Su S., Chang T.C. Identification of dermatophytes by sequence analysis of the rRNA gene internal transcribed spacer regions. J. Med. Microbiol. 2008;57:592–600. doi: 10.1099/jmm.0.47607-0. [DOI] [PubMed] [Google Scholar]
- 11.Sendrasoa F.A., Ranaivo I.M., Andrianarison M., Raharolahy O., Razanakoto N.H., Ramarozatovo L.S. Misuse of topical corticosteroids for cosmetic purpose in Antananarivo, Madagascar. Biomed. Res. Int. 2017:4. doi: 10.1155/2017/9637083. 9637083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma S.B. Topical corticosteroid misuse in India is harmful and out of control. Br. Med. J. 2015;351:h6079. doi: 10.1136/bmj.h6079. [DOI] [PubMed] [Google Scholar]
- 13.Raharolahy O., Ramarozatovo L.S., Ranaivo I.M., Sendrasoa Fandresena A., Andrianarison M., Rakoto Andrianarivelo M. A case of fluoroquinolone-resistant leprosy discovered after 9 years of misdiagnosis. Case Rep. Infect. Dis. 2016 doi: 10.1155/2016/4632369. [DOI] [PMC free article] [PubMed] [Google Scholar]