Abstract
This case reports cryptococcal meningitis in an HIV positive woman on antiretroviral therapy, presenting with left middle cerebral artery stroke at 30 weeks gestation. The patient had well-controlled HIV (CD4 count over 200 cells/mL). The immunosuppressive effects of the pregnancy likely contributed to the development of cryptococcal disease. The patient was successfully treated with two weeks of amphotericin B followed by fluconazole, delivered a healthy baby, but remained with a permanent severe neurological deficit.
Keywords: Cryptococcal meningitis, Pregnancy, Treatment, HIV, Malawi
1. Introduction
This case reports the difficulties in treating pregnant patients diagnosed with cryptococcal meningitis. Cryptococcal meningitis is a common disease in sub-Saharan Africa and is estimated to cause more than 100,000 deaths annually in the developing world [1], [2], with mortality rates up to 68% [3]. It usually presents with a gradually progressive headache, and commonly results in cranial nerve defects and seizures. Worldwide cryptococcal meningitis is a disease occurring predominantly in those with T-cell immunosuppression secondary to HIV infection, and is an AIDS defining illness. We present a case here of a pregnant patient with an unusual presentation of cryptococcal meningitis.
2. Case
A 34-year-old female HIV-positive pregnant patient presented at 30 weeks gestation with collapse after feeling dizzy, with subsequent aphasia. There were no witnessed seizures, and she had not reported headache prior to collapse. The patient's HIV had been diagnosed 3 years prior, and she had been started at this time on antiretroviral therapy consisting of tenofovir, lamivudine and efavirenz, and co-trimoxazole prophylaxis, with excellent adherence evidenced by family report and medical records. There was no history of hypertension, diabetes, epilepsy, tuberculosis, or previous cryptococcal infection.
On examination, she had a fever of 38.2 °C, reduced movement of the right arm and leg, and a right-sided 7th cranial nerve palsy. Modified Rankin score 5/6. Glasgow coma score 10/15. Cardiovascular, respiratory, abdominal and fundoscopic examinations were unremarkable.
The patient was therefore diagnosed clinically with a left middle cerebral artery stroke. Differential diagnoses included infections such as toxoplasmosis, tuberculosis, cryptococcal meningitis, cerebral bacterial abscess, neurosyphilis, viral encephalitis, HIV encephalopathy, progressive multifocal leukoencephalopathy and cerebral malaria. Non-infective differentials included thromboembolic stroke, primary cerebral lymphoma, primary brain cancer or metastatic brain cancer.
Cerebrospinal fluid (CSF) from day 0 was positive for cryptococcus on India ink microscopy and cryptococcal antigen test (CrAg). Lumbar puncture was repeated on day +2 when the CrAg and India ink remained positive. The opening pressure on day +2 was 25 cm (normal range < 20 cm), white cell count 2 cells/µL (normal range 0–5 cells/µL), glucose 1.1 mmol/L (normal range 2.22–3.88), protein 1.09 g/L (normal range 0.15–0.40), and cryptococcal culture quantitative count was 45 colony forming units (cfu)/mL.
CD4 count on day +1 was 243 cells/mm3, HIV viral load on day +2 was undetectable, syphilis RPR was negative, hepatitis B surface antigen was negative, malaria rapid diagnostic test was negative, and creatinine/potassium levels were normal.
Echocardiogram on day +1 revealed no clots or other abnormalities. Chest X-ray was normal. Computerised tomography and magnetic resonance brain imaging were not available on site.
The patient received Ceftriaxone 2 g intravenously once daily to treat bacterial meningitis. This was stopped on day +1 following positive CSF tests for cryptococcal meningitis. She was started on aspirin 75 mg orally once/day. Anti-retroviral treatment and co-trimoxazole prophylaxis were continued. Two weeks of intravenous amphotericin B (AmB) 1 mg/kg once/day was started on day +2. Two litres of intravenous normal saline with 20 mmol potassium chloride, 1200 mg twice daily of oral potassium chloride, and three tablets once daily of magnesium glycerophosphate were given on each day of AmB treatment. Dexamethasone treatment (12 mg intravenously twice daily for 2 days) was given on days +3 and +4 to mature the foetal lungs, in view of the chance of premature delivery. The patient received a unit of blood on day +8 following a drop in haemoglobin from 9.1 g/dL on day +1 to 7.0 g/dL on day +6 (haemoglobin normal range 11.8–14.8 g/dL).
Lumbar puncture was repeated on day +7, when the opening pressure was 45 cm water, and the cryptococcal quantitative count was 30 cfu/mL. A final lumbar puncture was performed on day +14 when the opening pressure was 22, and the cryptococcal quantitative count was 0 cfu/mL.
The patient improved with the two weeks of AmB treatment, and oral fluconazole 400 mg twice per day then started. The fluconazole dose was reduced to 400 mg orally once/day 2 weeks later. The patient spontaneously entered labour 6 weeks following her presentation at 36 weeks gestation and delivered a healthy, but slightly underweight, baby boy (weight = 1.8 kg) without any noticeable congenital abnormalities.
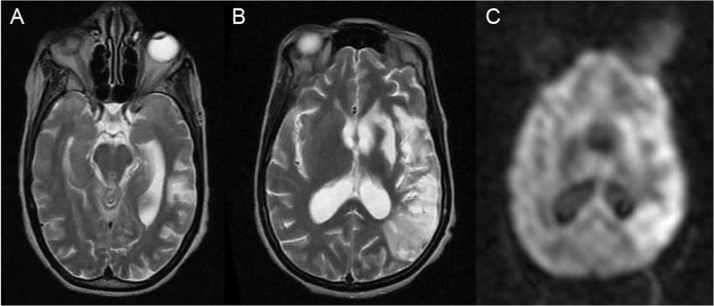
An MRI Brain was performed after discharge (day +59) and is displayed in Fig. 1. Brain imaging was not performed earlier due to the clinical risk to the patient and her baby of problems arising during transport to another hospital 1 hour away. Brain imaging was still thought helpful to exclude differential diagnoses (e.g. brain abscess, tuberculoma, toxoplasmosis, lymphoma etc).
Fig. 1.
MRI Brain Imaging of the patient at day +59. T2-weighted images (A, B) and DWI image (C) demonstrate extensive hyperintensity in the left frontal, parietal and temporal lobes, as well as the left caudate and lentiform nucleus, in keeping with a large left middle cerebral artery territory infarct. There is ventricular dilatation indicating hydrocephalus.
The patient was reviewed in clinic on day +123. She had gradually been improving at home, but severe neurological impairment persisted. At review her right-sided hemiparesis remained evident with contractures of the right foot and hand. She could walk with the assistance of one person, was able to eat and drink herself, and was breastfeeding. At this time she remained on fluconazole 200 mg once daily, tenofovir/lamivudine/efavirenz once daily, co-trimoxazole 960 mg once daily and aspirin 75 mg once daily.
3. Discussion
Experience on the treatment of patients with cryptococcal meningitis, disseminated cryptococcosis and pulmonary cryptococcosis in pregnancy is limited to case reports and small series totaling 50 cases (Table 1). There have been only 3 documented cases of mother-to-child transmission of cryptococcus [4], [5], [6]. Three of the 8 (38%) cases reported of cryptococcal meningitis in confirmed HIV positive patients resulted in maternal death (Table 1).
Table 1.
50 published cases of cryptococcal disease in pregnancy.
| Author | Year | HIV status | Cryptococcal diagnosis | Gestation at disease onset (trimester) | Outcome mother | Outcome baby |
|---|---|---|---|---|---|---|
| Timerman | 1935 | Not specified | CM | 3rd | Died | Died |
| Wager | 1954 | Not specified | CM | 3rd | Died | Survived |
| Gantz | 1958 | Not specified | CM | Postpartum | Died | Survived |
| Feldman | 1959 | Not specified | CM | 2nd | Survived | Survived |
| Littman | 1959 | Not specified | CM | 2nd | Survived | Not specified |
| Aitken | 1962 | Not specified | CM | 1st | Survived | Survived |
| Kuo | 1962 | Not specified | CM | 3rd | Survived | Survived |
| Crotty | 1965 | Not specified | CM | 1st | Died | Not specified |
| Crotty | 1965 | Not specified | CM | 2nd | Died | Not specified |
| Crotty | 1965 | Not specified | CM | 3rd | Died | Not specified |
| Crotty | 1965 | Not specified | CM + PC | Postpartum | Died | Not specified |
| Philpot | 1972 | Not specified | CM | 1st | Died | Died |
| Philpot | 1972 | Not specified | CM + PC | 1st | Survived | Survived |
| Silberfarb | 1972 | Not specified | CM | Postpartum | Survived | Survived |
| Curole | 1981 | Not specified | CM | 1st | Survived | Survived |
| Curole | 1981 | Not specified | CM | 2nd | Survived | Survived |
| Jones | 1983 | Not specified | CM | 3rd | Survived | Survived |
| Stafford | 1983 | Not specified | CM | 2nd | Not specified | Not specified |
| Chotmongkol | 1991 | Not specified | CM | Pre Pregnancy | Survived | Survived |
| Chotmongkol | 1992 | Not specified | CM | 1st | Not specified | Not specified |
| Pereira | 1993 | Not specified | CM | 1st | Survived | Survived |
| Pereira | 1993 | Not specified | CM | 2nd | Survived | Survived |
| Chen | 1996 | Not specified | CM | 3rd | Survived | Survived |
| Ely [7] | 1998 | Not specified | CM | 3rd | Survived | Survived |
| Ely [7] | 1998 | Not specified | PC | Pre-pregnancy | Survived | Survived |
| Ely [7] | 1998 | Not specified | PC | 3rd | Survived | Survived |
| Nakamura | 2009 | Not specified | PC | 3rd | Survived | Survived |
| Nakamura | 2009 | Not specified | CM + PC | 3rd | Survived | Survived |
| Molnar-Nadasdy | 1994 | Negative | CM | 2nd | Survived | Died |
| Ely [7] | 1998 | Negative | PC | 3rd | Survived | Survived |
| Ely [7] | 1998 | Negative | PC | 2nd | Survived | Survived |
| LaGatta | 1998 | Negative | PC | Postpartum | Survived | Survived |
| Nucci | 1999 | Negative | CM | 2nd | Died | Died |
| Vawda | 2008 | Negative | PC | 2nd | Survived | Survived |
| Costa | 2009 | Negative | CM | 1st | Survived | Survived |
| Mudumbi | 2010 | Negative | PC + DC | 2nd | Survived | Died |
| Pastagia | 2010 | Negative | PC + DC | 2nd | Survived | Died |
| Lachhab | 2012 | Negative | CM | 3rd | Survived | Survived |
| Chopra [8] | 2015 | Negative | PC | 2nd | Survived | Died |
| Chopra [8] | 2015 | Negative | PC + CM | 3rd | Survived | Survived |
| Nath | 2016 | Negative | CM | 2nd | Not known | Not known |
| Kida | 1989 | Positive | DC | 3rd | Died | Survived |
| Sirinavin [5] | 2004 | Positive | CM | 3rd | Died | Survived |
| Castro | 2006 | Positive | CM | 2nd | Died | Died |
| Darko | 2011 | Positive | CM | 2nd | Survived | Survived |
| Nayak | 2011 | Positive | CM | 2nd | Survived | Survived |
| Patel | 2012 | Positive | CM | 2nd | Survived | Survived |
| Kiggundu [6] | 2014 | Positive | CM | 3rd | Died | Survived |
| Ngwenya | 2016 | Positive | CM | 3rd | Survived | Survived |
| This report | 2017 | Positive | CM | 3rd | Survived | Survived |
CM = Cryptococcal Meningitis, DC = Disseminated Cryptococcosis, PC = Pulmonary Cryptococcosis. See Supplementary appendix for non-numbered references.
Pregnancy may well be a predisposing factor for the development of cryptococcal disease [8]. Protection from cryptococcal disease is dependent on a good Th1 weighted cell-mediated immune response. The predisposition to cryptococcal disease in pregnancy likely relates to the necessity for tolerance by the mother's adaptive immune system to paternal antigens present in the foetus. CD4+ T-helper responses and number are reduced during pregnancy, with the greatest suppression in responses in the third trimester [9]. Additionally, in the context of patients with HIV infection off antiretroviral treatment, pregnancy reversibly reduces CD4 counts [10]. It is also possible to demonstrate a reduction in delayed hypersensitivity skin responses in pregnancy [7]. The weighting of T-cell responses in pregnancy changes from a Th1 cell-mediated (IFN-γ, IL-12) to a Th2 (IL-4, IL-10) antibody-mediated and T-regulatory (TGF-β) response [11]. The number of T-regulatory cells in peripheral blood actually increases during early pregnancy, peaks during the second trimester, and then declines post-partum [11]. Oestrogen reduces the activation of T-cells [12] and T-regulatory cell numbers correlate with oestrogen levels [13]. Progesterone is also considered to be immunosuppressive [14]. Progesterone has a positive role in the production of the T-regulatory cytokine IL-10, can induce Th2 weighted immune responses, reduce inflammatory cytokine production, reduce Th1 weighted immune responses and suppress allogeneic responses [11]. Therefore pregnancy-related hormonal increases in both progesterone and oestrogen appear immunosuppressive.
Table 1 demonstrates that symptoms of cryptococcosis began pre-pregnancy in 2 cases (4%), during the first trimester in 8 cases (16%), the second trimester in 18 cases (36%), the third trimester in 18 cases (36%) and post-partum in 4 cases (8%). Symptomatic presentation therefore appears to be most common in the latter stages of pregnancy in keeping with the increased immunosuppression at this time.
This patient presented with a left middle cerebral artery stroke. Seventeen of 87 (20%) HIV positive cryptococcal meningitis patients had cortical or lacunar infarction [2], and 7 of 37 (19%) of immunocompetent patients with cryptococcal meningitis had cerebral infarction [15]. Therefore stroke is not an uncommon component of cryptococcal meningitis presentation.
Options for the treatment of cryptococcal disease include Amphotericin B based intravenous therapy, oral flucytosine and oral fluconazole. The Infectious Diseases Society of America (IDSA) guidelines on the treatment of disseminated cryptococcal disease in pregnancy recommends AmB based therapy with or without flucytosine [16]. AmB is a category B pregnancy drug indicating that there is no evidence of pregnancy risk in humans from its use following extensive clinical use for both cryptococcosis and other infections [17]. Flucytosine is a category C pregnancy drug indicating that not enough research has been done to determine if it is safe. Flucytosine crosses the placenta and animal studies have shown the potential for teratogenicity at doses lower than the human dose [17]. However, flucytosine has been used in three cases of cryptococcal disease in the later stages of pregnancy without adverse effects on the fetus [17]. There is no information to guide the safety of flucytosine whilst breastfeeding although the manufacturer advises avoidance.
Fluconazole treatment of more than a single 150 mg dose is a category D pregnancy drug indicating that there is positive evidence of human fetal risk. Fluconazole is a triazole which selectively inhibits fungal cytochrome P-450 preventing the fungus from building its cell wall [18]. It is potentially teratogenic, and results in a characteristic pattern of malformations [18]. It seems that a relatively high dose (over 400 mg) is required, and that the critical period of exposure is likely to be the first half of pregnancy [18]. Fluconazole is secreted in breast milk but is considered safe in children [19]. The IDSA guidelines [16] recommend avoiding fluconazole in the first trimester, judging the use of fluconazole in the second and third trimester according to need, and starting fluconazole after delivery. Ely and colleagues suggest 4–6 weeks of treatment with intravenous AmB with co-administration of oral flucytosine, followed by oral fluconazole after delivery [7].
This case highlights the difficulties in diagnosing and treating cryptococcal disease in pregnancy. The presentation of cryptococcal meningitis in pregnancy with a stroke in the absence of headache, in the context of well-controlled HIV, an unexpectedly high CD4 count (243 cells/mm3) and low cryptococcal CSF count (45 cfu/mL) is unusual. It is likely that the immunosuppression of pregnancy contributed to the development of this opportunistic infection. We would advocate the initial use of AmB in the treatment of similar patients, with or without a 1–2 week course of flucytosine. Results from the recent ACTA trial suggest one week of AmB plus flucytosine provides effective induction with fewer side effects than 2 weeks [20], but special circumstances such as this case, duration of induction could also be guided by culture results. If it is decided to avoid fluconazole, especially early in pregnancy, further alternate day and/or intermittent dosing of AmB could provide consolidation and maintenance while minimising side effects. However, in the developing world setting of this case AmB, flucytosine, and CSF culture are often unavailable. The use of maintenance fluconazole may be reasonable in the second half of pregnancy, and fluconazole treatment should be instituted following delivery of the baby. We would also advocate a low threshold for as required therapeutic lumbar punctures as part of the treatment for cryptococcal meningitis.
Acknowledgements
The authors would like to thank the ACTA study for donating the treatment medications, and for providing advice and support in managing this patient. No funding for this case.
Acknowledgments
Conflict of interest statement
PDB: No conflicts of interest.
DL: No conflicts of interest.
JJvO: No conflicts of interest.
AChen: No conflicts of interest.
TSH: No conflicts of interest.
AChan: No conflicts of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.mmcr.2017.10.002.
Appendix A. Supplementary material
Supplementary material
References
- 1.Park B.J., Wannemuehler K.A., Marston B.J., Govender N., Pappas P.G., Chiller T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23(4):525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Loyse A., Moodley A., Rich P., Molloy S.F., Bicanic T., Bishop L. Neurological, visual, and MRI brain scan findings in 87 South African patients with HIV-associated cryptococcal meningoencephalitis. J. Infect. 2015;70(6):668–675. doi: 10.1016/j.jinf.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Veltman J.A., Bristow C.C., Klausner J.D. Meningitis in HIV-positive patients in sub-Saharan Africa: a review. J. Int. AIDS Soc. 2014;17:19184. doi: 10.7448/IAS.17.1.19184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neuhauser E.B.D., Tucker A. The roentgen changes produced by diffuse torulosis in the newborn. Am. J. Roentgenol. Radium Ther. 1948;59(6):805–815. [PubMed] [Google Scholar]
- 5.Sirinavin S., Intusoma U., Tuntirungsee S. Mother-to-child transmission of cryptococcus neoformans. Pediatr. Infect. Dis. J. 2004;23(3):278–279. doi: 10.1097/01.inf.0000115639.43305.9b. [DOI] [PubMed] [Google Scholar]
- 6.Kiggundu R., Rhein J., Meya D.B., Boulware D.R., Bahr N.C. Unmasking cryptococcal meningitis immune reconstitution inflammatory syndrome in pregnancy induced by HIV antiretroviral therapy with postpartum paradoxical exacerbation. Med. Mycol. Case Rep. 2014;5:16–19. doi: 10.1016/j.mmcr.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ely E.W., Peacock J.E., Haponik E.F., Washburn R.G. Cryptococcal pneumonia complicating pregnancy. Medicine. 1998;77(3):153–167. doi: 10.1097/00005792-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Chopra S., Capoor M.R., Mallik R., Gupta S., Ray A., Khanna G. Pulmonary Cryptococcosis in HIV sero-negative patients: case series from India. Mycoses. 2015;58(5):288–293. doi: 10.1111/myc.12313. [DOI] [PubMed] [Google Scholar]
- 9.Sabahi F., Rola-Plesczcynski M., O’Connell S., Frenkel L.D. Qualitative and quantitative analysis of T lymphocytes during normal human pregnancy. Am. J. Reprod. Immunol. 1995;33(5):381–393. doi: 10.1111/j.1600-0897.1995.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 10.Heffron R., Donnell D., Kiarie J., Rees H., Ngure K., Mugo N. A prospective study of the effect of pregnancy on CD4 counts and plasma HIV-1 RNA concentrations of antiretroviral-naive HIV-1–infected women. J. Acquir. Immune Defic. Syndr. 2014;65(2):231–236. doi: 10.1097/QAI.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song D., Shi Y. Immune system modifications and feto-maternal immune tolerance. Chin. Med. J. 2014;127(17):3171–3180. [PubMed] [Google Scholar]
- 12.Segerer S.E., Müller N., van den Brandt J., Kapp M., Dietl J., Reichardt H.M. Impact of female sex hormones on the maturation and function of human dendritic cells. Am. J. Reprod. Immunol. 2009;62(3):165–173. doi: 10.1111/j.1600-0897.2009.00726.x. [DOI] [PubMed] [Google Scholar]
- 13.Arruvito L., Sanz M., Banham A.H., Fainboim L. Expansion of CD4+CD25+ and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J. Immunol. 2007;178(4):2572–2578. doi: 10.4049/jimmunol.178.4.2572. [DOI] [PubMed] [Google Scholar]
- 14.Adar T., Grisaru-Granovsky S., Ben Ya’acov A., Goldin E., Bar-Gil Shitrit A. Pregnancy and the immune system: general overview and the gastroenterological perspective. Dig. Dis. Sci. 2015;60(9):2581–2589. doi: 10.1007/s10620-015-3683-z. [DOI] [PubMed] [Google Scholar]
- 15.Chen S.-F., Lu C.-H., Lui C.-C., Huang C.-R., Chuang Y.-C., Tan T.-Y. Acute/subacute cerebral infarction (ASCI) in HIV-negative adults with cryptococcal meningoencephalitis (CM): a MRI-based follow-up study and a clinical comparison to HIV-negative CM adults without ASCI. BMC Neurol. BioMed. Cent. 2011;11:12. doi: 10.1186/1471-2377-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perfect J.R., Dismukes W.E., Dromer F., Goldman D.L., Graybill J.R., Hamill R.J. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin. Infect. Dis. 2010;50(3):291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King C.T., Rogers P.D., Cleary J.D., Chapman S.W. Antifungal therapy during pregnancy. Clin. Infect. Dis. 1998;27(5):1151–1160. doi: 10.1086/514977. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Rangel E., Van Allen M.I. Prenatal exposure to fluconazole: an identifiable dysmorphic phenotype. Birth Defects Res. A Clin. Mol. Teratol. 2005;73(11):919–923. doi: 10.1002/bdra.20189. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan Y.C., Koren G., Ito S., Bozzo P. Fluconazole use during breastfeeding. Can. Fam. Physician. 2015;61(10):875–876. [PMC free article] [PubMed] [Google Scholar]
- 20.S. Molloy, C. Kanyama, R. Heyderman, A. Loyse, C. Kouanfack, D. Chanda, et al., A randomized controlled trial for the treatment of HIV-associated cryptococcal meningitis in Africa: oral fluconazole plus flucytosine or one week amphotericin-based therapy vs two weeks amphotericin-based therapy, The ACTA Trial, Oral Presentation. International AIDS Society, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material