Abstract
Mucormycosis caused by Apophysomyces variabilis is rarely reported in humans. A case of A. variabilis infection in an immunocompetent men after friction burns in a car accident is described. The infection presented as a rapidly progressive necrotizing infection of the skin and soft tissue, which required extensive surgical debridement and total colonic defunctioning colostomy associated with prolonged antifungal therapy. A. variabilis infection should be considered as a differential diagnosis of rapidly progressive necrotizing skin and soft tissue infections in immunocompetent individuals.
Keywords: Mucormycosis, Zygomycetes, Apophysomyces variabilis, Amphotericin B
1. Introduction
Mucormycosis are uncommon infections that usually affect patients with comorbidities such as diabetes mellitus, immunosuppression, neutropenia and metabolic acidosis, with high morbidity and mortality rates. Species from the genera Rhizopus, Mucor and Lichtheimia are responsible for 80% of the mucormycosis in humans, while Apophysomyces spp. is responsible for less than 3% of these infections [1]. The genus Apophysomyces (phylum Mucoromycota, Subphylum Mucoromycotina) was described by Misra et.al in 1979, isolated from soil samples in northern India [2]. A. elegans was considered the only member of this genus until 2010, when Alvarez et al., described three new species based on morphological and physiological characteristics, and genetic differences (A. variabilis, A. trapeziformis and A. ossiformis) [3]. In 2014 Bonifaz et al. described a fifth new species (A. mexicanus) [4]. We present here a case of a patient with necrotizing fasciitis caused by A. variabilis after a motor vehicle collision in the northern region of Colombia (Valledupar, Cesar).
2. Case
A previously healthy 35-year-old man suffered from polytrauma with blunt chest trauma, multiple open fractures in the left tibia and femur associated with complex wounds in the left hand, and 10 × 10 cm friction burns in the right lumbosacral region. Upon admission (day 0), resuscitation and antibiotic therapy with cefazolin 1 g i.v. every 8 h plus gentamicin 160 mg i.v. daily was initiated, the patient was taken to surgery for femur and tibia osteosynthesis, and a skin flap in the left hand, requiring intensive care unit (ICU) management after the intervention due to the need of non-invasive ventilatory support and multiple transfusions. The patient had an adequate clinical evolution with weaning off ventilatory support and stabilization of hemodynamic parameters and was transferred into the general ward. However, on day 10, the patient reported intense and progressive pain in the right gluteal region with a 15 × 15 cm hardening area, with heat and redness. Soft tissue ultrasonography ruled out collection, and the blood count showed high levels of leukocytes and neutrophilia (leukocytes 25,000 cells/mm3, neutrophils 85%) with elevated C-reactive protein (CRP) (120 mg/L). The antibiotics were changed to piperacillin tazobactam and the patient was assessed by plastic surgery department who discarded surgical treatment. 72 h later (day 13) the patient had a poor evolution with increased pain in the gluteal region, skin sloughing and the appearance of a central necrotic area. The patient was taken to surgical drainage where a sample of purulent secretion (5 ml) was collected. This sample was sent together with tissue samples for histopathology staining and microbiological cultures.
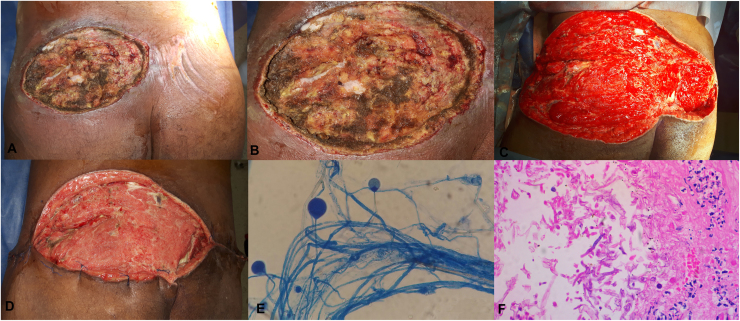
On day 16, due to the appearance of surgical wound necrosis with persistent pain, hardening area, heat and redness in the right gluteus, the patient was taken to surgery again, where skin necrosis, subcutaneous cellular tissue and partial necrosis of the gluteus maximus muscle were observed (Fig. 1A and B). Extensive debridement of necrotic tissues was performed and a support of negative pressure system was implemented (Negative Pressure Wound Therapy (NPWT) systems, Genadyne's Multi-Canister System from New York, USA). During the next 48 h (day 18), there was an increase in the hardening area and necrosis with involvement of the entire gluteus and back right thigh, persistent elevation of CRP levels and fever. The patient was taken to surgery again for total colonic defunctioning colostomy and lumbosacral lesion debridement (Fig. 1C), and transferred to the ICU again due to the need of ventilatory and hemodynamic support.
Fig. 1.
A: Surgical wound in the right lumbosacral region with skin necrosis, subcutaneous cellular tissue necrosis and partial gluteus major necrosis. B: Previous picture zoom. C: Surgical wound after extensive debridement initiating in the upper and lateral region of thigh and right gluteus to the upper region of the left gluteus. D: 40× lactophenol blue. E: Surgical wound after 12 weeks of evolution, multiple surgical washes and antibiotic and antimitotic treatment. F: Histopathological study with hematoilin eosin staining at 40×.
Testing results showed persistently negative blood cultures, microbiological examination of the tissue sample did not indicate bacterial growth, Gram and Ziehl-Neelsen staining of the secretion were negative. After 96 h of incubation, white and wooly colonies and hyaline aseptate hyphae were observed on agar-blood culture. Suspecting mucormycosis infection, tissue samples were cultured on malt extract agar, Sabouraud agar and water agar to induce the formation of sporangiospores. After 96 h, an overgrowing on all fungi cultures media was observed (white and wooly colonies), but only straight sporangiospores, prominent apophysis and piriform sporangium with evidence of abundant oblong sporangiospores were observed on water agar (Fig. 1D). Taken together, all these characteristics allowed the identification of Apophysomyces sp., and a sample was used for additional molecular identification. The determination of sensitivity by E-test was not possible due to poor growth.
On day 18, with the diagnosis of necrotizing fasciitis secondary to cutaneous mucormycosis a combined treatment with amphotericin B 50 mg i.v. daily, plus posaconazole 300 mg i.v daily, plus tigecycline 50 mg i.v. every 12 h, plus cefepime 2 g i.v. every 8 h, and surgical washings every 72 h was initiated. On day 39, after 21 days of treatment with amphotericin B + posaconazole and 6 surgical washings, reconstruction by plastic surgery with skin grafts was performed (Fig. 1E). On day 84, the patient was discharged with posaconazole 300 mg daily for 3 months. At 6 months follow-up post hospital discharge, 100% healing of the wound was evident, and the procedures for colostomy closure were initiated (day 264).
The histopathological study showed abundant granulation tissue with foreign body reaction, multiple aseptate hyphae in intravascular space with right angle branching (Fig. 1F).
Fungal DNA was isolated using the MoBio PowerSoil® DNA isolation kit (MoBio Laboratories, Carlsbad, CA, US) following manufacturer's protocols. Amplification of the Internal transcribed spacer (ITS) gene region by PCR was performed using universal primers ITS4 and ITS5 as previously described [5]. The PCR fragments were purified by alcohol precipitation and sequenced (Sanger method) using the universal primers ITS1, ITS4 and ITS5. The assembled 774 bp sequence was compared against the NCBI (National Center for Biotechnology Information), UNITE and Warcup Fungal ITS RDP databases, showing a 99% identity percentage in 99% length with sequences reported for Apophysomyces variabilis. The corresponding bioinformatic analysis made it possible to assign this sample to species level. The nucleotide sequence obtained in this study has been submitted to GenBank under the accession number KY996810.
3. Discussion
Although A. variabilis is found in soil of tropical and subtropical areas with low nitrogen content [6], hereby we describe the first case of mucormycosis caused by A. variabilis in tropical areas of Latin America. Unlike other mucorales, this fungus affects mainly immunocompetent hosts. Infection usually occurs after traumatic inoculation of the microorganism, but can also occur after spore inhalation. The most common clinical presentation is skin and soft tissue infection (52.7%), followed by rhino-orbito-cerebral infection (25.7%), disseminated infection (10.8%) and isolated renal involvement (6.8%) [1].
There are few cases of A. variabilis infection reported in the literature, mostly form India [7], [8], [9], [10], [11], [13], [15], [16] (Table 1). The number of cases is likely to be underestimated because A. variabilis does not produce spores readily in traditional mycological culture media used in clinical laboratories, has special nutritional requirements, grows at high temperatures (37–42 °C) and after prolonged periods of incubation (7–10 days) [9].
Table 1.
17 report of cases of A. variabilis infection.
| aCases | Age (Y) | Type of infection | Area involved | Risk Factor | Primary disease | Outcome |
|---|---|---|---|---|---|---|
| 1 | 9 | Skin | Lower limb | Trauma | Without | Limb amputation [7] |
| 6 | 39–50 | Skin | 4 Gluteal. | 4 IM IY. | 1 DM. | 4 Deaths [8] |
| 1 Upper limb. | 1 Trauma. | 5 Without. | ||||
| 1 Scapular. | 1 Without. | |||||
| 1 | ND | Rhino-orbito-cerebral. | Rhino-orbito-cerebral. | ND | DN | ND [9] |
| 1 | 21 | Skin | Dorsal | Burn 90% TBS | Without. | Death [10] |
| 5 | 29–45 | Skin | 1 Thoracic wall. | 2 Trauma. | 2 DM. | 4 Deaths [13] |
| 3 Abdominal wall. | 2 IM IY. | 3 Without. | ||||
| 1 Upper limb. | 1 Without. | |||||
| 1 | 45 | Skin | Torso and limbs | Toxic epidermic necrosis. | Without | Death [11] |
| 1 | 64 | Skin | Abdominal wall. | Cutaneous zoster. | Chronic use of steroids. | Death [16] |
| 1 | 74 | Rhino-orbito-cerebral. | Rino orbital cerebral. | Without. | Without. | Death [15] |
Cases: number of cases, Y: years, IM IY: intramuscular inyections, ND: No data, TBS: Total Body Surface, DM: diabetes mellitus.
Treatment of mucormycosis caused by A. variabilis requires an early diagnosis, together with an adequate control of predisposing factors and aggressive surgical debridement, associated with the administration of amphotericin B alone or combined with posaconazole. Minimal inhibitory concentration (MIC) of A. variabilis to amphotericin B ranged from 0.03 to 4.0 mg/l, and usually present low MIC to posaconazole (0.0625–2 mg/L). Infections with MIC to amphotericin B > 2 mg/L are associated with worse outcomes [6], [8], [10], [12], [13], [14], [15]. Although in the presented case antifungal sensitivity could not be realized, the combined treatment with amphotericin B and posaconazole was successful.
In conclusion, A. variabilis infection should be considered as a differential diagnosis of rapidly progressive necrotizing skin and soft tissue infections in immunocompetent individuals.
Conflict of interest
The authors have no conflicts of interest to declare and confirm that each one has made substantial contributions to the information or materials submitted for publication.
References
- 1.Gomes M.Z.R., Lewis R.E., Kontoyiannis D.P. Mucormycosis caused by unusual mucormycetes, non-Rhizopus, -Mucor, and -Lichtheimia species. Clin. Microbiol. Rev. 2011;24(2):411–445. doi: 10.1128/CMR.00056-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Misra P., Srivastava K., Lata K. Apophysomyces, a new genus of the mucorales. Mycotaxon. 1979;8(2):377–382. [Google Scholar]
- 3.Alvarez E., Stchigel A.M., Cano J., Sutton D.A., Fothergill A.W., Chander J. Molecular phylogenetic diversity of the emerging mucoralean fungus apophysomyces: proposal of three new species. Rev. Iberoam. Micol. 2010;27(2):80–89. doi: 10.1016/j.riam.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Bonifaz A., Stchigel A.M., Guarro J., Guevara E., Pintos L., Sanchis M. Primary cutaneous mucormycosis produced by the new species apophysomyces mexicanus. J. Clin. Microbiol. 2014;52(12):4428–4431. doi: 10.1128/JCM.02138-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White T.J., Bruns T., Lee S., Taylor J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR Protocols: A Guide to Methods and Applications. Academic Press, Inc; New York, N.Y: 1990. pp. 315–322. [Google Scholar]
- 6.Prakash H., Ghosh A.K., Rudramurthy S.M., Paul R.A., Gupta S., Negi V. The environmental source of emerging apophysomyces variabilis infection in India. Med. Mycol. 2016;54(6):567–575. doi: 10.1093/mmy/myw014. [DOI] [PubMed] [Google Scholar]
- 7.Al-Zaydani I.A., Al-Hakami A.M., Joseph M.R.P., Kassem W.M., Almaghrabi M.K., Nageeb A. Aggressive cutaneous zygomycosis caused by apophysomyces variabilis in an immunocompetent child. Med. Mycol. Case Rep. 2015;10:11–13. doi: 10.1016/j.mmcr.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chander J., Stchigel A.M., Alastruey-Izquierdo A., Jayant M., Bala K., Rani H. Fungal necrotizing fasciitis, an emerging infectious disease caused by apophysomyces (Mucorales) Rev. Iberoam. Micol. 2015;32(2):93–98. doi: 10.1016/j.riam.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Bala K., Chander J., Handa U., Punia R.S., Attri A.K. A prospective study of mucormycosis in north India: experience from a tertiary care hospital. Med. Mycol. 2015;53(3):248–257. doi: 10.1093/mmy/myu086. [DOI] [PubMed] [Google Scholar]
- 10.dela Cruz W.P., Calvano T.P., Griffith M.E., White C.E., Kim S.H., Sutton D.A. Invasive apophysomyces variabilis infection in a burn patient. J. Clin. Microbiol. 2012;50(8):2814–2817. doi: 10.1128/JCM.00671-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Razmi T.M., Shivaprakash M.R., Saikia U.N., De D., Handa S. All that necroses is not toxic epidermal necrolysis. J. Cutan. Med. Surg. 2017;21(2):172–173. doi: 10.1177/1203475416679827. [DOI] [PubMed] [Google Scholar]
- 12.Espinel-Ingroff A., Chakrabarti A., Chowdhary A., Cordoba S., Dannaoui E., Dufresne P. MulticenteR evaluation of mic distributions for epidemiologic cutoff value definition to detect amphotericin B, posaconazole, and itraconazole resistance among the most clinically relevant species of mucorales. Antimicrob. Agents Chemother. 2015;59(3):1745–1750. doi: 10.1128/AAC.04435-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guarro J., Chander J., Alvarez E., Stchigel A.M., Robin K., Dalal U. Apophysomyces variabilis infections in humans. Emerg. Infect. Dis. 2011;17(1):134–135. doi: 10.3201/eid1701.101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salas V., Pastor F.J., Calvo E., Sutton D.A., Chander J., Mayayo E. Efficacy of posaconazole in a murine model of disseminated infection caused by apophysomyces variabilis. J. Antimicrob. Chemother. 2012;67(7):1712–1715. doi: 10.1093/jac/dks090. [DOI] [PubMed] [Google Scholar]
- 15.Wolkow N., Jakobiec F.A., Stagner A.M., Cunnane M.E., Piantadosi A.L., Basgoz N. Chronic orbital and calvarial fungal infection with apophysomyces variabilis in an immunocompetent patient. Surv. Ophthalmol. 2017;62(1):70–82. doi: 10.1016/j.survophthal.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Gowdara V.K., Shivappa S.G., Chandgal M., Ramakrishna R., Ramalingaiah R., Puranik V. Apophysomyces variabilis a flesh eating fungus – a case report. IOSR J. Dent. Med. Sci. 2014;13(3Ver. II):86–90. [Google Scholar]