Abstract
Over the past decade, a large body of literature has demonstrated that disruptions of the endogenous circadian clock, whether environmental or genetic, lead to metabolic dysfunctions that are associated with obesity, diabetes, and other metabolic disorders. The phrase, “It is not only what you eat and how much you eat, but also when you eat” sends a simple message about circadian timing and body weight regulation. Communicating this message to clinicians and patients, while also elucidating the neuroendocrine, molecular, and genetic mechanisms underlying this phrase is essential to embrace the growing knowledge of the circadian impact on metabolism as a part of healthy life style as well as to incorporate it into clinical practice for improvement of overall human health. In this review, we discuss findings from animal models, as well as epidemiological and clinical studies in humans, which collectively promote the awareness of the role of circadian clock in metabolic functions and dysfunctions.
Keywords: circadian clock, metabolism, obesity
the earth’s rotation imposes 24-h cycles of light and dark, as well as other associated environmental changes to all living organisms. Thus, it is intuitive that a time-keeping system, or circadian (“about-a-day”) clock, which prepares the organism to anticipate these daily rhythmic changes, can be evolutionally advantageous. Indeed, endogenous circadian clocks have been found in organisms ranging from bacteria to humans. Although the exact molecular clock machinery is often distinct across different life domains and kingdoms, suggesting independent evolutionary origins and constant selective pressure during evolutionary history, these clocks are self-sustained and are responsive to light in order to be synchronized to the external light-dark cycle. Competitive advantages of having an accurate or intact circadian clock, including increased growth, longevity, and reproductive success, have been reported in plants, fruit flies, and mammals (2, 11, 12, 21, 82, 121). As a proof of concept, selection against inaccurate clocks has been observed first in mixed cultures of cyanobacteria strains with different circadian periods (74) and, more recently, in mouse populations released into outdoor experimental enclosures (102). It is also evident in humans that natural selection has been fine-tuning our circadian clock, as we moved out of Africa and adapted to a wide range of latitudes and photoperiods (29).
Many clock-regulated biological functions may contribute to adaptive fitness. In particular, energy metabolism, a fundamental necessity of all cellular life forms, has been increasingly appreciated as being tightly linked to the circadian clocks in all organisms that have been studied so far. In mammals, including humans, energy intake and expenditure are regulated by the clock through daily cycles of feed-fast, sleep-wake (or rest-activity), and body temperature cycles, involving many neuroendocrine pathways. Furthermore, in the past decade, we have begun to understand at the cellular and molecular levels how the clock and metabolic machinery is interrelated. Such knowledge has profound implications for human metabolic disorders, especially obesity and diabetes.
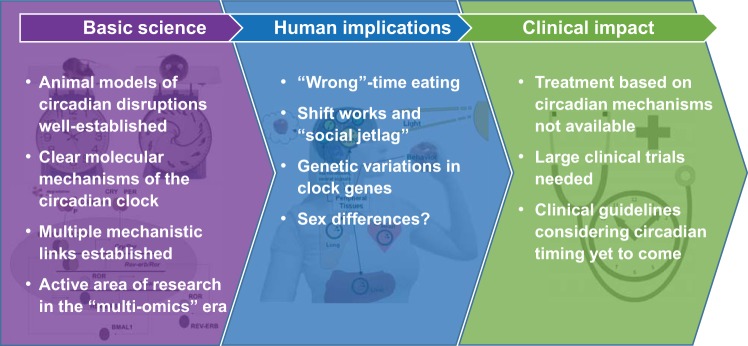
In humans, the circadian system is often challenged by the working and social demands of our modern industrialized society. The availability of artificial light has greatly diminished the limits imposed by the day-night cycle, and exposure to artificial light at night is associated with modulations of circadian behavior in humans, as well as in wild organisms living in urban areas (21). Such changes in humans include shift work and an increasingly common late lifestyle, which are associated with disruptions of the clock and/or dyssynchrony between the clock and metabolic need. As our work and social schedules defy our endogenous timing system that has exquisitely adapted to the 24-h day over millions of years of evolution, adverse physiological consequences of such defiance are to be expected. Numerous studies have now established that disruptions of the circadian clock are linked to metabolic dysfunctions, including obesity and diabetes, along with many other disorders and health problems. In this review, we summarize the current status of circadian-metabolic research and discuss the applications and implications to obesity, diabetes, and metabolic disorders (Fig. 1). We start by discussing key experimental findings in animal models linking the circadian clock to energy metabolism and weight control. We then summarize the current frontier of genetic and molecular studies to understand the mechanisms underlying these interactions. We also present clinical and epidemiological evidence that the lessons learned in animal models are translatable to humans. At the end of this review, we discuss future studies that are needed to embrace these exciting findings and make a clinical impact on the treatment of metabolic disorders, particularly obesity and diabetes.
Fig. 1.
A summary of the current status of circadian-metabolic research.
Wrong-Time Feeding: Lessons from Animal Models
In mammals, the circadian timing system is organized in a hierarchy. The master circadian clock is located in the suprachiasmatic nucleus (SCN) of the brain, which generates self-sustained molecular and electrophysiological rhythms. The SCN clock receives resetting signals via direct synaptic input from the retina and sends neural/humoral output signals to synchronize clocks in other brain regions and peripheral tissues, which, in turn, govern rhythmicity in a wide range of physiological and neurobehavioral functions, including the feeding-fasting cycle, in a coordinated fashion. The coordination between the feeding-fasting cycle and other physiological rhythms is thought to contribute to a tightly regulated metabolic homeostasis and maximizing metabolic efficiency. However, only relatively recently have studies begun to probe the impact of feeding times with regard to the master circadian clock and other clock-controlled rhythms.
Studies in animal models provided the first direct evidence for a role of circadian timing of food intake in body weight regulation. As a nocturnal species, mice typically consume ~70–80% of the food during the dark phase when they are most active. When food availability is restricted to the light phase (i.e., the “wrong” time of the day), mice fed on a high-fat diet gain more weight in as short as 2 wk, compared with those fed on the same diet but during the dark phase (4). This increased weight gain is not associated with any significant increase in the total amount of daily food intake or any significant decrease in the daily activity levels (4), suggesting that the timing of food intake, like diet and excise, is important for body weight regulation. In addition to restricting food intake to a certain circadian phase, mice kept under constant bright light or a bright-dim light cycle show reduced amplitudes in the activity-rest and feeding-fasting rhythms, as they consume more food during the circadian daytime compared with those housed under the normal light-dark cycle (28). This light-at-night model produces a pronounced increase in body weight and a decrease in glucose tolerance without changes in the total amount of daily activity or food intake (28).
These findings resonate with earlier epidemiology studies in humans suggesting that shift workers are at a higher risk for metabolic disorders, obesity, and cardiovascular diseases (3, 48). It is typical for shift workers to have a significant amount of food intake during the wake-work phase when normally a person would be at sleep-rest (48, 53). Thus, it is possible that wrong-time eating plays a role in shift work-associated health problems, although other confounding factors commonly observed in shift workers, such as unhealthy diet, lack of physical activity, and insufficient sleep, may also contribute to the adverse health outcomes. In a rat model of shift work, rats were kept awake for 8 h during the light phase each day of the five “work days” in the week, resulting in weight gain and metabolic disruptions (90). Interestingly, these changes were, indeed, largely attributable to feeding at the wrong circadian phase, as the adverse effects of being kept awake during the light phase were prevented by restricting feeding to the dark phase (93). Similar to shift work, jet lag creates dyssynchrony between the environmental light-dark cycle and the circadian clock, as well as internally among multiple oscillators of the circadian system. Mouse and rat models of chronic frequent jet lag have demonstrated disruptions in the adipose clock, accompanied by rapid weight gain, elevated plasma leptin levels, as well as central leptin resistance (8, 45, 109).
Importantly, restricting feeding to the right circadian phase can be protective against high-fat diets and excessive caloric intake, which are known to associate with a range of metabolic disorders, such as glucose intolerance, lipid imbalance, insulin resistance, and hormonal dysregulations, in addition to obesity. Compared with ad libitum feeding, restricting caloric intake to the dark phase enhances circadian metabolic rhythms and confers a range of protections against high-fat diet-induced adverse effects in mice, including obesity, glucose intolerance, leptin resistance, and liver damage (39). The beneficial effects of dark-phase restricted feeding are not limited to mice fed on a high-fat diet, and can also be observed in mice fed a high-sucrose or high-fructose diets, and even in mice with preexisting obesity and metabolic disorders (15). Interestingly, similar protective effects have been observed in genetically induced obesity as well. A number of genetic models of obesity in rodents, especially those with defective leptin or leptin receptor, also show blunted circadian activity and/or feeding rhythms (34, 41, 64), and restricting food intake to the dark phase led to a reduced weight gain in rats with mutated leptin receptor (64). Together, these findings suggest time-restricted feeding may be utilized as a therapeutic intervention for treating obesity and metabolic disorders.
It is worth mentioning, however, that some of these studies compare time-restricted feeding with ad libitum feeding. Such comparison may be confounded by an “unnatural” fluctuation of energy flow when the feeding window is exceptionally short, as long fasting (e.g., 20 h a day) can be very challenging to small animals, like mice. In one study, feeding was restricted to a 4-h window during the middle of the light phase, which led to reduced body weight and altered metabolic parameters compared with ad libitum feeding (98), similar to those reported in studies using 4 h of restricted feeding during dark. However, when food availability was restricted to the entire 12 h of the light phase, progressive deterioration in metabolism and eventually metabolic pathologies were observed (65). These findings, thus, suggest severe daily fasting alone can have a major impact on metabolic regulation, at least in mice. Nevertheless, the above-mentioned studies directly comparing daytime vs. nighttime feeding (4, 93) have provided compelling evidence that the particular circadian time of food intake, in addition to time restriction itself, has a major impact on metabolism and body weight regulation.
In summary, convincing data in animal models suggest that circadian timing of food intake affects metabolic outcomes, often without altering the total caloric intake, sending a clear message that body weight regulation involves not only the amount but also the timing of calorie intake. It is also important to note that interactions between the circadian clock and metabolism are reciprocal. Food intake is known to reset clocks in peripheral organs, such as the liver, and recent evidence suggests hormonal pathways, including insulin and oxyntomodulin, are involved in this process (16, 52, 95). Perhaps more strikingly, feeding mice a high-fat diet leads to a slowing down of the central circadian clock, as shown by locomotor activity rhythms (49). These findings clearly suggest that circadian clocks are likely to be tightly coupled with metabolic pathways, calling for a transformation in our thinking of a healthy lifestyle that requires not only healthy diet and exercise but also circadian timing.
Clock and Metabolism: Intertwined Molecular Machinery
At the core of the molecular clock machinery, a transcriptional and translational feedback loop generates self-sustained ~24-h rhythms. Transcriptional activators CLOCK and BMAL1 dimerize and activate expression of clock-controlled genes, including their repressors Per1/2/3 and Cry1/2. After translation, the PER and CRY proteins dimerize and inhibit the transcriptional activity of the CLOCK-BMAL1 heterodimers, thus shutting down their own transcription. This core clock machinery is cell-autonomous and can be found in virtually every cell throughout the body, providing a basis for the interactions between circadian rhythms and tissue-specific functions. The transcriptional output of the clock machinery drives waves of oscillatory patterns in the transcriptome with tissue specificity. These clock-driven rhythms in the transcriptome have been shown to represent tissue-specific metabolic needs (50, 62, 76, 104, 125) and are highly plastic to nutritional input (24). Feeding time drives rhythmic clock gene expression in the fat body (a major metabolic organ) in flies (121), and time-restricted feeding in rodents uncouples clock oscillations in peripheral tissues, particularly, the liver, from the SCN (20, 81, 103). Thus, by modulating the clock, as well as direct control of metabolic and/or stress regulators, time-restricted feeding reprograms transcriptomic oscillations and the metabolic organization in peripheral tissues (117, 121). It is conceivable that disruptions of the clock will lead to dysregulation and discoordination of clock-controlled transcriptomic oscillations, which, in turn, is likely to cause impaired cellular functions, including metabolic state and overall metabolic outcome. Elucidation of the molecular mechanisms of the circadian clock has, therefore, provided a blueprint allowing for a fast growing body of mechanistic studies linking the clock machinery to metabolic pathways in the past decade (6, 9, 44, 75). These efforts have led to a revolutionized understanding of the interactions between circadian rhythms and metabolic functions at genetic, molecular, and cellular levels, with important implications for human health and metabolic disorders (10, 111).
Genetic disruptions in core clock genes are directly linked to metabolic dysfunctions and obesity and have been associated with disturbed feeding rhythms and/or excessive daytime feeding. The first direct evidence of this comes from a study of mice bearing the ClockΔ19 mutation (112), the same mutation identified in a mutagenesis screen that led to the discovery of the Clock gene and ultimately the transcriptional-translational feedback loop. Homozygous ClockΔ19 mutant animals exhibit severely blunted feeding rhythms, with nearly 50% of the food intake during the daytime. These mutant animals fed either on a regular chow or high-fat diet develop obesity as early as 5–6 wk of age, accompanied by an overall decreased energy expenditure, as well as a range of metabolic disorders, including hyperglycemia, hyperlipidemia, hyperleptinemia, and liver damage (112). Similarly, ablation of BMAL1, the binding partner of CLOCK, also leads to an obese phenotype and increased adipose mass, as the heterodimer regulates leptin expression in adipose cells (45, 51). In addition, Bmal1-null mice also exhibit insulin hypersensitivity, decreased insulin levels, and glucose imbalance (51, 91). These profound metabolic dysfunctions can be dissected in a tissue-specific manner. Deletion of Bmal1 specifically in the adipose tissue alone is sufficient to cause obesity and reduced feeding rhythms, which may be due to altered fatty acid feedback signals from adipocytes to hypothalamic neurons regulating food intake (77). In contrast, obesity phenotypes were not observed in mice with tissue-specific knockout of Bmal1 in the pancreatic islets or the liver (51, 60). Instead, loss of Bmal1 specifically in the pancreatic islets leads to diabetes due to insufficient insulin secretion (60), an effect that has been linked to disrupted coordination in the transcriptional activity of the CLOCK-BMAL1 heterodimers and the pancreatic transcription factor PDX1 (80). Liver-specific Bmal1 deficiency causes hypoglycemia during the resting/fasting phase of the day and faster glucose clearance (51). Interestingly, in contrast to the loss of Bmal1 in the liver, Bmal1 ablation in the skeletal muscle, the major glucose “sink” in the body, causes nonfasting hyperglycemia and glucose intolerance, which are associated with impaired glucose uptake by the muscle in response to insulin but not significant changes in food intake or activity levels (38). Last but not least, deletion of Bmal1 specifically in the ventromedial hypothalamus of the brain has recently been shown to alter cyclic thermogenesis and energy expenditure in brown adipose tissue, revealing an intriguing pathway by which a core circadian clock gene is involved in the control of peripheral metabolic outcomes in brain regions outside of the SCN (73).
In addition, genetic disruptions in the negative limb of the transcriptional-translational feedback loop are also associated with metabolic phenotypes. Higher body weight and disrupted energy homeostasis have been found in Per1/Per2 double-knockout mice in which circadian rhythms are abated (45) as well as in a Per1 mutant line that shows accelerated circadian rhythms and an advanced phase in the feeding behavior (58). Interestingly, Cry1/Cry2 double-knockout animals exhibit significantly lower body weight, despite similar circadian dysfunctions as observed in Per1/Per2 double-knockout mice (45). A similar phenotype of lower body weight has also been observed in mice carrying mutant or null alleles of the Csnk1e gene (127), which encodes a casein kinase that phosphorylates PER proteins and regulates circadian period, suggesting the downstream effector pathways linking these closely related clock components to metabolic outcomes are likely to be diverse and complex.
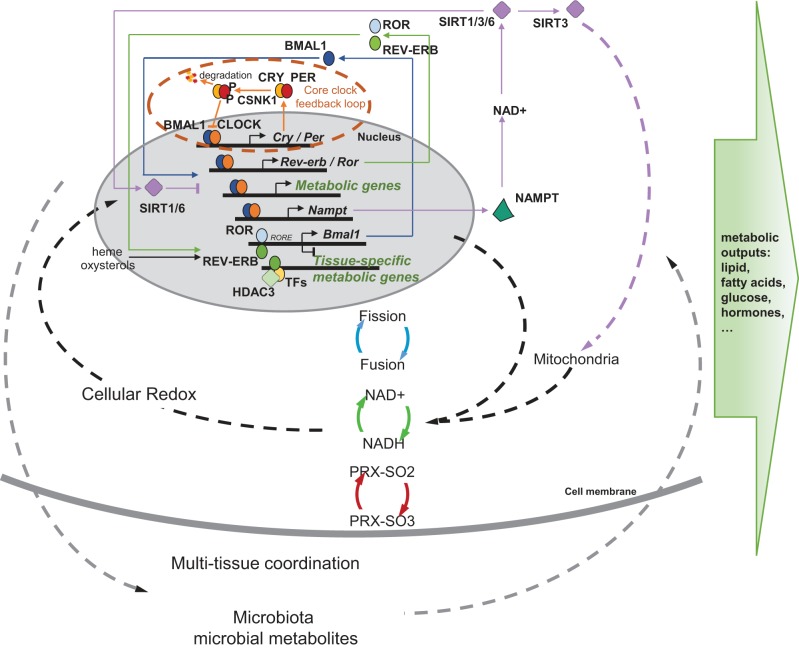
A multilayer molecular network is involved in the coupling between the circadian clock and metabolic pathways (Fig. 2). The core transcriptional/translational feedback loop of the circadian clock is regulated by a “stabilizing” loop involving nuclear receptors REV-ERBα/β and retinoid-related orphan receptors RORα/β/γ. Rev-erbs and Rors are rhythmically expressed under the direct transcriptional control by the CLOCK-BMAL1 heterodimers. REV-ERBs suppress and RORs activate the transcription of Bmal1 by competing to bind the same cis-regulatory element. REV-ERBα and its downstream pathways, in particular, are known to play a critical role in the regulation of lipid metabolism, energy homeostasis, adipogenesis, as well as systemic and vascular inflammatory processes (reviewed in Ref. 22). In addition, REV-ERBs and RORs can function as nuclear receptors for heme and oxysterols, respectively (35, 84, 122), and, thus, may provide a mechanism for sensing metabolic and nutrient flux. Given their intimate transcriptional coupling with the core clock, REV-ERBs and RORs may serve as a critical relay that adjusts the circadian clock for nutrient/metabolic cues and integrates timing and metabolic signals in the regulation of energy homeostasis. In line with this hypothesis, a recent study partitioned circadian and metabolic functions of REV-ERBα. REV-ERBα acts directly on the canonical REV-ERB DNA response elements to regulate the circadian clock in the brain, liver, and adipose tissues; meanwhile, it also forms a complex with histone deacetylase HDAC3 and other tissue-specific transcriptional regulators to regulate the expression of downstream metabolic genes to accommodate the metabolic need of the tissue (126). HDAC3 is recruited to the transcriptional regulatory site in a circadian fashion directed by REV-ERBα, and loss of either HDAC3 or REV-ERBα causes disrupted hepatic lipid homeostasis, as well as steatosis (27).
Fig. 2.
A multilayer molecular network couples the circadian clock to metabolic functions. The core circadian clock machinery consists of a transcriptional and translational feedback loop, in which transcription of the repressors (PER1/2/3 and CRY1/2) is activated by the transcriptional activators (CLOCK and BMAL1) and is turned off as the repressor proteins accumulate and suppress the activity of the CLOCK-BMAL1 heterodimers. CSNK1 phosphorylates the PER proteins to reregulate PER nuclear entry and turnover, controlling the velocity of the clock. This transcriptional/translational clock machinery produces a cyclic transcriptional profile of metabolic genes and is coupled with metabolic functions via multiple mechanisms. The clock is stabilized by a feedback loop involving nuclear receptors RORs and REV-ERBs, which is sensitive to cellular metabolic state and is involved in the transcriptional programming of tissue-specific metabolic output. The clock also directs NAD+ biosynthesis, which, in turn, negatively feeds back on CLOCK-BMAL1 transcriptional activity through the NAD+-dependent deacetylases (SIRTs). SIRT1 and SIRT6 are involved in the epigenetic regulation of metabolic genes, and SIRT3 regulates mitochondrial activity. Through this link and many others, the clock is coupled with daily fluctuations of the cellular redox state, which involves mitochondrial fission and fusion, NAD+/NADH flux, and peroxiredoxin hyperoxidation (PRX-SO2/3). These cellular circadian-metabolic mechanisms are also coordinated at the whole organism level among multiple tissues and interact with the microbiota to shape the overall profile of metabolic health.
In addition to the metabolic coupling via REV-ERBs and RORs, the clock is further coupled with energy homeostasis via a feedback loop involving NAD+ and histone deacetylase sirtuins. NAD+ biosynthesis is controlled by the circadian clock through CLOCK-BMAL1-driven transcription of NAMPT, the rate-limiting enzyme in NAD+ salvage pathway, conferring a circadian rhythm in NAD+ levels (68, 85). Meanwhile, NAD+ activates SIRT1 (NAD+-dependent deacetylase sirtuin 1), which suppresses the transcriptional activity of CLOCK-BMAL1 by counteracting the histone acyltransferase activity of CLOCK and by deacetylating BMAL1 and PER2 to influence their protein stabilities (5, 67), thereby completing a feedback loop. A number of NAD+-dependent sirtuins are regulated by this clock-driven NAD+ oscillation, and each of these sirtuins can be linked to specific downstream circadian- metabolic pathways. For example, as histone deacetylases involved in epigenetic modulation of gene expression, SIRT1 and SIRT6 regulate distinct groups of metabolic genes. Genes involved in processing peptide metabolites and metabolic cofactors are mostly regulated by SIRT1, while those key to lipid and carbohydrate metabolism are regulated by SIRT6 (61). Another sirtuin, SIRT3, has recently been implicated in the circadian regulation of mitochondrial oxidative respiration by altering acetylation and activities of oxidative enzymes (79).
The latter highlights an intriguing coupling between the clock and cellular redox homeostasis. In addition to the SIRT3 link, other mechanisms contribute to the circadian control of mitochondrial oxidative function. In mouse liver, 38% of mitochondrial proteins, including rate-limiting enzymes for lipid and carbohydrate catabolism, show diurnal rhythms of protein levels that are dependent on an intact circadian clock (70). The clock-driven oscillatory expression of mitochondrial genes in mouse liver and oxidative stress regulation are disrupted during aging, suggesting interesting implications for age-related metabolic disorders (33). In addition, mitochondrial fusion/fission and mitophagy, dynamic energy regulatory processes that involve morphological and functional remodeling of the mitochondria, exhibit circadian rhythms in the liver (43). These rhythms in mitochondrial remodeling are controlled by clock-dependent rhythmic transcription of mitochondrial dynamics genes and are abolished by liver-specific deletion of Bmal1 (43). Other clock genes have also been implicated in mitochondrial function. Expression of a “stabilized” REV-ERBα (a mutant protein with a long half-life due to reduced protein degradation) or REV-ERBα overexpression leads to increased mitochondrial mass and number as well as an enhanced respiratory capacity (97, 119). Together, these studies provide a mechanistic basis for the circadian flux of cellular redox state in the form of NAD+/NADH ratios, which can be observed in cultured cells (42), as well as in vivo at the single-cell level (105). Such diurnal redox oscillations may also feedback to the clock. An earlier study has found DNA binding affinities of CLOCK-BMAL1 are dependent on NAD+/NADH ratios (92). In addition, perturbing the conversion of NADP+ to NADPH by inhibiting the pentose phosphate pathway modulates the circadian clock, as well as its molecular and behavior outputs (87).
Diurnal redox flux can also be observed in the form of oscillatory reactive oxygen species. Hyperoxidation of peroxiredoxin displays robust circadian rhythms, which is highly conserved across different life domains (26, 72). In humans, the peroxiredoxin rhythms even persist in mature red blood cells in the absence of the nucleus and the transactional/translational clock (71). A recent study also reported autonomous redox rhythms in the mitochondria involving peroxiredoxin and its reducing enzyme sulfiredoxin (46). Interestingly, when the nucleus is present, the peroxiredoxin oscillator might be coupled with the transcriptional clock, since the loss of a functional clock caused by ablating Cry1 and Cry2 leads to disrupted peroxiredoxin rhythms in mouse embryonic fibroblasts (71), although the detailed mechanisms of this coupling are still largely unclear. Such organization between self-sustained transcription-dependent and transcription-independent oscillators is similar to the circadian clock system observed in the cyanobacteria, in which the nontranscriptional oscillator is also self-sustained and directly senses redox state (47, 69, 120). Collectively, these findings raise an intriguing hypothesis that the coupling between the circadian clock and cellular redox flux may be deeply rooted in the evolutionary origins of the circadian clock system. Since the exact evolutionary history of the clock is known, it is open to debate whether the transcription-independent cellular redox oscillations may represent a more “fundamental” (or conserved) form of the time-keeping system (26, 87). The emergence of transcriptional-translational clocks in different eukaryotic organisms may have allowed for more robust timekeeping than the cellular redox oscillations, which are presumably more sensitive to noncircadian fluctuations in nutrient and other environmental factors. Nevertheless, the bidirectional coupling between the circadian clock and cellular redox state, which is supported by a large body of evidence, provides a fundamental mechanism linking the circadian clock with cellular energy metabolism with important implications for health and disease in humans.
In summary, studies over the last decade have revealed a highly regulated coupling and functional coordination between the circadian clock machinery and metabolic flux. At the cellular level, such coupling involves multiple layers of molecular networks transducing signals reciprocally between the circadian transcriptional/translational feedback loop and daily nutrient/metabolite/energy fluctuations. Dysregulation of this intertwined clock-metabolic network leads to profound alternations in cellular metabolic outcomes, providing a basis for the interactions between disrupted circadian rhythms and metabolic dysfunctions. As alluded to above, circadian transcriptomic studies have revealed tissue-specific programs of transcriptomic oscillations that are associated with particular metabolic functions of the tissue (62, 76, 104, 125). These tissue-specific circadian metabolic programs are likely to be coordinated to achieve energy balance at the whole organism level. Thus, it is expected that circadian-metabolic defects in specific tissues can lead to and/or be caused by altered coordination among circadian oscillators in multiple organs, resulting in an overall metabolic imbalance, such as seen in circadian misalignment caused by wrong-time feeding, shift work, and jet lag.
Circadian-Metabolic Coordination via Interactions Between the Host and Gut Microbiota
The mechanisms underlying the circadian-metabolic interactions may reside beyond the cells and organs of our body. Microorganisms living in the gastrointestinal track encompass thousands of microbial species and millions of genes, whose physiological importance has only recently been recognized. A large body of data has now linked the gut microbial community (i.e., microbiota), which is highly dynamic and sensitive to dietary modulations, to host digestive, metabolic, immune, and neurobehavioral functions (17). In particular, the findings that fecal microbiota transplantation from obese mice or humans to lean mice leads to adiposity phenotypes in the recipients (88, 113) have demonstrated a pivotal role of gut microbiota in host metabolism and body weight regulation (for a review, see Ref. 99).
The gut microbiota has also been implicated in the interactions between the circadian clock and metabolism. Multiple studies have shown that the gut microbial composition exhibits diurnal variations, which are regulated by the host circadian clock and feeding time (54, 56, 108, 124). Dysbiosis, a deviation from normal microbiota composition that is often associated with adverse health outcomes, can result from disruptions of the circadian clock, including genetic perturbations of core clock genes (56, 108, 116), as well as frequent phase shifts in mice (115) and jet lag in humans (108). Conversely, removing gut microbiota impairs expression of clock genes in the intestinal epithelial cells, liver, and mediobasal hypothalamus (54, 66). Such manipulation also reprograms the transcriptomic rhythms in the intestine and liver (107). Interestingly, jet-lagged human subjects show changes in gut microbiota that are known to be associated with metabolic disorders, and fecal transplantation from these jet-lagged subjects into germ-free mice causes obesity and glucose intolerance in mice (108). Pathways and mechanisms underlying this “trans-kingdom” link are yet to be fully understood, but they are likely to involve multiorgan functions and perhaps coordination of multiple circadian oscillators in different tissues. For example, gut microbiota may program the intestinal and hepatic transcriptomic rhythms by influencing diurnal metabolome fluctuations in the intestinal lumen and serum, and the programed hepatic transcriptome, in turn, determines hepatic metabolic output (107). Future studies are needed to gain a more comprehensive understanding of circadian system organization and its role in metabolic outcomes at the whole organism level that involves the circadian-metabolic rhythms in multiple organs, as well as the gut microbiota.
Circadian Rhythms and Metabolic Disorders: Applications to Humans
Rodent studies linking the clock and metabolism using genetic/molecular and environmental/dietary perturbations have profound implications for human metabolic disorders. The circadian clock machinery and metabolic pathways are highly conserved between rodents and humans. Thus, it is expected that the same circadian-metabolic genes and pathways identified in mice are also involved in the development of metabolic disorders, particularly obesity and diabetes, in humans. Interestingly, genome-wide association studies have identified strong associations between Type 2 diabetes and genetic variants of the clock gene CRY2 (23, 57), as well as MTNR1B (13, 59, 83), which encodes a receptor of melatonin, a well-studied circadian hormone directly controlled by the SCN clock. Genetic variations in these two genes have also been reported to affect energy expenditure responses to weight-loss diets in a two-year clinical trial testing the effectiveness of dietary interventions in overweight or obese adults (63). Genetic associations with metabolic parameters have also been observed in other clock genes, especially polymorphisms in the CLOCK and PER2 genes that are associated with individual “chronotypes” (i.e., preference of morningness and eveningness) and timing of food intake (reviewed in Ref. 31). Together, these studies indicate that the genetic links between clock genes and metabolic outcomes are present in humans as in animal models, suggesting that the circadian-metabolic pathways identified in rodents are likely to be functionally conserved in humans.
In addition, recent studies focusing on the metabolic outcomes of meal times have provided support that findings in animal models of wrong-time feeding are also translatable to humans. A study of 420 subjects in Spain participating in a 20-wk weight-loss treatment found that the timing of food intake affects the effectiveness of the treatment and that late-lunch eaters show a slower weight-loss rate during the treatment as compared with early eaters (32). Similarly, another study compared subjects assigned to early and late meal groups and found that late meal time over the 2 wk of trial led to higher levels of triglycerides, total cholesterol, and LDL cholesterol in the serum (123). In more extreme cases, a large amount of food consumed late at night is a key clinical manifestation of the night-eating syndrome, which has long been recognized as characteristic in a significant proportion of obese patients (especially during stressful periods) and is positively associated with body mass index (BMI) (18, 30, 106). Furthermore, it has been noted that foods with a high fat content are more likely to be consumed when eating later during the day (118), implying potentially exacerbated metabolic dysfunctions caused by the combined effects of unhealthy diet and wrong-time eating. These studies complement animal studies and further stress the importance of the timing of meals for maintaining a healthy lifestyle. In addition, given the protective role of time-restricted feeding as suggested in animal studies, future studies in randomized clinical settings are needed to test whether restricting food intake to desirable circadian times can be used as a therapeutic intervention for obesity and other metabolic disorders in humans.
As alluded to earlier in this review, adverse metabolic outcomes of wrong-time feeding have important implications for health challenges faced by shift workers and individuals exposed to frequent occurrences of jet lag. Shift work and jet lag are associated with higher risks of obesity and diabetes, and eating at a wrong time of the day often occurs as a result of circadian misalignment induced by these conditions. In addition, as a late lifestyle has become increasingly common, large survey studies of self-reported data estimate ~69% of the working population experience at least 1 h of “social jet lag,” a term used to describe weekly shifts between an earlier sleep/wake schedule during workdays and a delayed schedule during weekends (90). The severity of social jet lag is a strong predictor of higher BMI, as found in an online survey of more than 65,000 participants (89).
It is noteworthy that circadian misalignment and late sleep timing are often accompanied by insufficient sleep (1, 7), which can also contribute to metabolic dysfunctions. The vast majority of epidemiological studies, particularly large studies and meta-analyses that involve tens of thousands of subjects, have found that short sleep time is associated with higher BMI and an increased risk of diabetes in children, adults, and the elderly (for reviews, see Refs. 14, 37, 78, 114). Under controlled laboratory conditions, sleep restriction of 4 h (1 AM–5 AM) daily for 6 days leads to reduced insulin sensitivity in healthy young men (100). Other metabolic alterations, such as reduced leptin, elevated ghrelin, and increased hunger and appetite, have also been reported to result from acute sleep restrictions (101). These metabolic changes are likely to contribute to the observed associations between short sleep and obesity/diabetes in epidemiological studies. Interestingly, a recent study found a phase-delayed plasma melatonin profile in relation to the wake time when sleep was restricted to 5 h centered at the middle of habitual sleep time, suggesting a misalignment between activities (including feeding) during this earlier awake period and the central clock (25). A significant correlation was observed between the circadian phase of awakening and insulin sensitivity, arguing that circadian misalignment may contribute to the sleep-restriction-induced decline of insulin sensitivity (25). Other studies argue further for a sleep-independent contribution of circadian misalignment to adverse metabolic outcomes. When subjects are exposed to a 28-h sleep/wake cycle, the scheduled sleep time occurs at different phases of the endogenous circadian timing. Under this “forced dyssynchrony” protocol, decreased leptin, increased glucose, and increased insulin levels are observed when eating and sleeping occurs at the opposite circadian phase of habitual times, and this effect of circadian misalignment is still prominent after the contribution from lowered sleep efficiency has been statistically removed (96). Furthermore, one study directly compared 8 days of 5-h sleep restriction that was given with or without circadian misalignment and found that circadian misalignment led to a decreased insulin sensitivity and elevated inflammation markers without changing the amount of sleep (55). Finally, an epidemiology study following patients with Type 2 diabetes found that poor glycemic control is associated with a late chronotype and a large-size dinner after statistically controlling for sleep variables (86). Regardless the exact magnitude of contributions, circadian misalignment, wrong-time eating, as well as insufficient sleep, are likely to lead to worsened metabolic outcomes associated with obesity and diabetes, either as individual factors or in combination with one another leading to exacerbated effects. Together, studies in humans, as well as in animal models urge for a healthy lifestyle that respects our endogenous circadian clock and the development of intervention strategies against circadian-metabolic dysfunctions, while still enjoying all the social and economic benefits of the modern 24/7 society.
Concluding Remarks
The discovery of the molecular clock machinery in the 1990s has led to an explosive growth of our knowledge in how the self-sustained and cell-autonomous circadian clockwork is intertwined with a multilayer molecular network to convey time to metabolic functions. Future studies are expected to continue to elucidate mechanisms underlying the interactions between the circadian clock and metabolism, particularly the mechanisms of how the circadian-metabolic networks in different organ/tissues are synchronized and functionally coordinated. Progressing toward this goal, there has been a fast accumulation of circadian omics data (e.g., transcriptomics, proteomics, metabolomics, as well as the microbiome) collected from studies of multiple tissues under various experimental conditions. Integrated analyses of these rich data sets will add to our understanding of how genetic and environmental (e.g., wrong-time feeding, shift work, social jet lag) disruptions of the clock lead to internal dyssynchrony within and among tissues and how this may contribute to metabolic dysfunction, obesity, diabetes, as well as other diseases such as cardiovascular disease, neurological disease, and cancer.
A potential shortcoming of current circadian-metabolic studies in both animals and humans is the lack of direct comparisons between males and females. Since sex differences have been observed in the circadian control of sleep-wake cycle, hormone secretion, and a range of other physiological processes (36, 94, 110), it is reasonable to expect that clock-regulated metabolic processes and overall metabolic outcome also exhibit sex differences, which is an important aspect of biomedical research for future studies to pursue. Nevertheless, an enormous amount of data has now established clear links between the circadian clock and metabolic processes at the hormonal, cellular, and molecular levels. In humans, results from epidemiological, laboratory/clinical, and genetic studies have supported findings from animal studies, providing evidence that circadian misalignment and wrong-time eating contribute to obesity and diabetes, especially in industrialized societies. Collectively, the current status of knowledge strongly argues for appropriate circadian timing as a pivotal component of a healthy lifestyle in addition to healthy diet and exercise. Such implications are particularly important given the high rate of obesity and diabetes that are associated with the increasing challenges to the “circadian hygiene” in our industrialized 24/7 society.
In addition to further mechanistic studies, as well as studies that include both sexes, a major task of future studies is to demonstrate whether and/or how metabolic disorders can be treated according to circadian principles and mechanisms. The success in understanding the basic science that has established a role of circadian clock in energy metabolism has yet to make an impact on the clinical practice of treating obesity, diabetes, and other metabolic disorders. Thus, large studies in humans and clinical trials are needed to demonstrate the benefit of applying circadian principles in clinical practice, in order to engage the medical professionals, the pharmaceutical industry, and, importantly, the regulatory bodies to fully integrate the circadian organization into the prevention, treatment, and patient care of metabolic disorders.
The applications of circadian principles in the treatment of metabolic disorders consist of two main aspects. First, since a wide range of physiological and metabolic processes are under circadian regulation, it can be hypothesized that a particular treatment may be most effective or least toxic when given at a particular time of day. Indeed, circadian transcriptomic studies have found over 50% of the top 100 best-selling medicines directly target the products of rhythmic genes (125). Since many of these drugs have short half-lives, this finding strongly argues for a time-of-day-dependent therapy (i.e., chronotherapy) when using these drugs. Studies in the rapidly growing research area known as chronopharmacology have now identified many drugs for a range of diseases with dosing-time-dependent efficacy or toxicity in animal and clinical studies (for a review, see Ref. 19), including an antidiabetic medicine that targets the GLUT4 (glucose transporter 4) gene. Large clinical trials are needed to validate these findings and allow them to be adopted into standard clinical care. A potential challenge of such clinical trials is that the heterogeneity in human chronotypes may complicate the true circadian time of dosing and thus, the trial outcome. There is, therefore, a critical need for the development of circadian biomarkers that can be easily assessed with minimal invasion and that can differentiate circadian timing in disease-relevant tissues against other tissues. As such, circadian timing and chronotype of individual subjects at the system level, as well as at the level of disease-relevant tissues can be screened in a cost-efficient manner to identify subpopulations of obese and diabetic patients, in which a certain treatment may be most effective when given at a particular time of the day. In addition, real-time monitoring of such circadian biomarkers, which may be enabled by future advances in the wearable device technologies, will truly empower medical professionals and patients to embrace the concept of chronotherapy.
The other important aspect of clock-based therapy for metabolic disorders is to treat the disorders by targeting or modulating the circadian-metabolic machinery itself. Animal studies have, indeed, suggested that circadian-based interventions can be used to treat preexisting obesity, as exemplified by the protective effects of time-restricted feeding against obesity induced by high-fat diet or leptin receptor deficiency (15, 64). Similar studies in humans are needed to test the therapeutic value of time-restricted feeding. Most recently, a study in mice found that a drug targeting the clock component RORs protects against diet- or genetically induced obesity and metabolic disorders in a clock-dependent manner (40). This exciting finding in animal models provides proof-of-concept evidence that clock genes and its coupling with metabolic genes can be used as therapeutic targets for the treatment of metabolic disorders. Successes like these call for additional studies to test therapeutic effects of bioactive compounds that target key converging nodes of circadian and metabolic networks, which will enable future interventional studies in humans and large clinical trials to test clock-targeting drugs and/or chronotherapy as new therapeutic options for metabolic disorders. Perhaps then, the exciting area of circadian-metabolic research can make an impact on the clinical guidelines and practice for the treatment of obesity, diabetes, and other metabolic disorders.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.J. prepared figures; P.J. drafted manuscript; P.J. and F.W.T. approved final version of manuscript; F.W.T. edited and revised manuscript.
ACKNOWLEDGMENTS
This work is supported by National Institute on Aging Grant NIH P01 AG011412.
REFERENCES
- 1.Åkerstedt T, Wright KP Jr. Sleep loss and fatigue in shift work and shift work disorder. Sleep Med Clin : 257–271, 2009. doi: 10.1016/j.jsmc.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez JD, Hansen A, Ord T, Bebas P, Chappell PE, Giebultowicz JM, Williams C, Moss S, Sehgal A. The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J Biol Rhythms : 26–36, 2008. doi: 10.1177/0748730407311254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antunes LC, Levandovski R, Dantas G, Caumo W, Hidalgo MP. Obesity and shift work: chronobiological aspects. Nutr Res Rev : 155–168, 2010. doi: 10.1017/S0954422410000016. [DOI] [PubMed] [Google Scholar]
- 4.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) : 2100–2102, 2009. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell : 317–328, 2008. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 6.Asher G, Sassone-Corsi P. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell : 84–92, 2015. doi: 10.1016/j.cell.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) : 1374–1381, 2011. doi: 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- 8.Bartol-Munier I, Gourmelen S, Pevet P, Challet E. Combined effects of high-fat feeding and circadian desynchronization. Int J Obes : 60–67, 2006. doi: 10.1038/sj.ijo.0803048. [DOI] [PubMed] [Google Scholar]
- 9.Bass J. Circadian topology of metabolism. Nature : 348–356, 2012. doi: 10.1038/nature11704. [DOI] [PubMed] [Google Scholar]
- 10.Bass J, Lazar MA. Circadian time signatures of fitness and disease. Science : 994–999, 2016. doi: 10.1126/science.aah4965. [DOI] [PubMed] [Google Scholar]
- 11.Beaver LM, Gvakharia BO, Vollintine TS, Hege DM, Stanewsky R, Giebultowicz JM. Loss of circadian clock function decreases reproductive fitness in males of Drosophila melanogaster. Proc Natl Acad Sci USA : 2134–2139, 2002. doi: 10.1073/pnas.032426699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boden MJ, Kennaway DJ. Circadian rhythms and reproduction. Reproduction : 379–392, 2006. doi: 10.1530/rep.1.00614. [DOI] [PubMed] [Google Scholar]
- 13.Bouatia-Naji N, Bonnefond A, Cavalcanti-Proença C, Sparsø T, Holmkvist J, Marchand M, Delplanque J, Lobbens S, Rocheleau G, Durand E, De Graeve F, Chèvre JC, Borch-Johnsen K, Hartikainen AL, Ruokonen A, Tichet J, Marre M, Weill J, Heude B, Tauber M, Lemaire K, Schuit F, Elliott P, Jørgensen T, Charpentier G, Hadjadj S, Cauchi S, Vaxillaire M, Sladek R, Visvikis-Siest S, Balkau B, Lévy-Marchal C, Pattou F, Meyre D, Blakemore AI, Jarvelin MR, Walley AJ, Hansen T, Dina C, Pedersen O, Froguel P. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet : 89–94, 2009. doi: 10.1038/ng.277. [DOI] [PubMed] [Google Scholar]
- 14.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care : 414–420, 2010. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab : 991–1005, 2014. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaves I, van der Horst GT, Schellevis R, Nijman RM, Koerkamp MG, Holstege FC, Smidt MP, Hoekman MF. Insulin-FOXO3 signaling modulates circadian rhythms via regulation of clock transcription. Curr Biol : 1248–1255, 2014. doi: 10.1016/j.cub.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 17.Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell : 1258–1270, 2012. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colles SL, Dixon JB, O’Brien PE. Night eating syndrome and nocturnal snacking: association with obesity, binge eating and psychological distress. Int J Obes : 1722–1730, 2007. doi: 10.1038/sj.ijo.0803664. [DOI] [PubMed] [Google Scholar]
- 19.Dallmann R, Okyar A, Lévi F. Dosing-time makes the poison: circadian regulation and pharmacotherapy. Trends Mol Med : 430–445, 2016. doi: 10.1016/j.molmed.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev : 2950–2961, 2000. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dodd AN, Salathia N, Hall A, Kévei E, Tóth R, Nagy F, Hibberd JM, Millar AJ, Webb AA. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science : 630–633, 2005. doi: 10.1126/science.1115581. [DOI] [PubMed] [Google Scholar]
- 22.Duez H, Staels B. Rev-erb α gives a time cue to metabolism. FEBS Lett : 19–25, 2008. doi: 10.1016/j.febslet.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 23.Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, Lindgren CM, Mägi R, Morris AP, Randall J, Johnson T, Elliott P, Rybin D, Thorleifsson G, Steinthorsdottir V, Henneman P, Grallert H, Dehghan A, Hottenga JJ, Franklin CS, Navarro P, Song K, Goel A, Perry JRB, Egan JM, Lajunen T, Grarup N, Sparsø T, Doney A, Voight BF, Stringham HM, Li M, Kanoni S, Shrader P, Cavalcanti-Proença C, Kumari M, Qi L, Timpson NJ, Gieger C, Zabena C, Rocheleau G, Ingelsson E, An P, O’Connell J, Luan J, Elliott A, McCarroll SA, Payne F, Roccasecca RM, Pattou F, Sethupathy P, Ardlie K, Ariyurek Y, Balkau B, Barter P, Beilby JP, Ben-Shlomo Y, Benediktsson R, Bennett AJ, Bergmann S, Bochud M, Boerwinkle E, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Böttcher Y, Brunner E, Bumpstead SJ, Charpentier G, Chen YD, Chines P, Clarke R, Coin LJ, Cooper MN, Cornelis M, Crawford G, Crisponi L, Day IN, de Geus EJ, Delplanque J, Dina C, Erdos MR, Fedson AC, Fischer-Rosinsky A, Forouhi NG, Fox CS, Frants R, Franzosi MG, Galan P, Goodarzi MO, Graessler J, Groves CJ, Grundy S, Gwilliam R, Gyllensten U, Hadjadj S, Hallmans G, Hammond N, Han X, Hartikainen AL, Hassanali N, Hayward C, Heath SC, Hercberg S, Herder C, Hicks AA, Hillman DR, Hingorani AD, Hofman A, Hui J, Hung J, Isomaa B, Johnson PR, Jørgensen T, Jula A, Kaakinen M, Kaprio J, Kesaniemi YA, Kivimaki M, Knight B, Koskinen S, Kovacs P, Kyvik KO, Lathrop GM, Lawlor DA, Le Bacquer O, Lecoeur C, Li Y, Lyssenko V, Mahley R, Mangino M, Manning AK, Martínez-Larrad MT, McAteer JB, McCulloch LJ, McPherson R, Meisinger C, Melzer D, Meyre D, Mitchell BD, Morken MA, Mukherjee S, Naitza S, Narisu N, Neville MJ, Oostra BA, Orrù M, Pakyz R, Palmer CN, Paolisso G, Pattaro C, Pearson D, Peden JF, Pedersen NL, Perola M, Pfeiffer AF, Pichler I, Polasek O, Posthuma D, Potter SC, Pouta A, Province MA, Psaty BM, Rathmann W, Rayner NW, Rice K, Ripatti S, Rivadeneira F, Roden M, Rolandsson O, Sandbaek A, Sandhu M, Sanna S, Sayer AA, Scheet P, Scott LJ, Seedorf U, Sharp SJ, Shields B, Sigurethsson G, Sijbrands EJ, Silveira A, Simpson L, Singleton A, Smith NL, Sovio U, Swift A, Syddall H, Syvänen AC, Tanaka T, Thorand B, Tichet J, Tönjes A, Tuomi T, Uitterlinden AG, van Dijk KW, van Hoek M, Varma D, Visvikis-Siest S, Vitart V, Vogelzangs N, Waeber G, Wagner PJ, Walley A, Walters GB, Ward KL, Watkins H, Weedon MN, Wild SH, Willemsen G, Witteman JC, Yarnell JW, Zeggini E, Zelenika D, Zethelius B, Zhai G, Zhao JH, Zillikens MC, Borecki IB, Loos RJ, Meneton P, Magnusson PK, Nathan DM, Williams GH, Hattersley AT, Silander K, Salomaa V, Smith GD, Bornstein SR, Schwarz P, Spranger J, Karpe F, Shuldiner AR, Cooper C, Dedoussis GV, Serrano-Ríos M, Morris AD, Lind L, Palmer LJ, Hu FB, Franks PW, Ebrahim S, Marmot M, Kao WH, Pankow JS, Sampson MJ, Kuusisto J, Laakso M, Hansen T, Pedersen O, Pramstaller PP, Wichmann HE, Illig T, Rudan I, Wright AF, Stumvoll M, Campbell H, Wilson JF, Bergman RN, Buchanan TA, Collins FS, Mohlke KL, Tuomilehto J, Valle TT, Altshuler D, Rotter JI, Siscovick DS, Penninx BW, Boomsma DI, Deloukas P, Spector TD, Frayling TM, Ferrucci L, Kong A, Thorsteinsdottir U, Stefansson K, van Duijn CM, Aulchenko YS, Cao A, Scuteri A, Schlessinger D, Uda M, Ruokonen A, Jarvelin MR, Waterworth DM, Vollenweider P, Peltonen L, Mooser V, Abecasis GR, Wareham NJ, Sladek R, Froguel P, Watanabe RM, Meigs JB, Groop L, Boehnke M, McCarthy MI, Florez JC, Barroso I DIAGRAM ConsortiumGIANT ConsortiumGlobal BPgen ConsortiumAnders Hamsten on behalf of Procardis Consortium; MAGIC investigators . New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet : 105–116, 2010. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckel-Mahan KL, Patel VR, de Mateo S, Orozco-Solis R, Ceglia NJ, Sahar S, Dilag-Penilla SA, Dyar KA, Baldi P, Sassone-Corsi P. Reprogramming of the circadian clock by nutritional challenge. Cell : 1464–1478, 2013. doi: 10.1016/j.cell.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eckel RH, Depner CM, Perreault L, Markwald RR, Smith MR, McHill AW, Higgins J, Melanson EL, Wright KP Jr. Morning circadian misalignment during short sleep duration impacts insulin sensitivity. Curr Biol : 3004–3010, 2015. doi: 10.1016/j.cub.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Edgar RS, Green EW, Zhao Y, van Ooijen G, Olmedo M, Qin X, Xu Y, Pan M, Valekunja UK, Feeney KA, Maywood ES, Hastings MH, Baliga NS, Merrow M, Millar AJ, Johnson CH, Kyriacou CP, O’Neill JS, Reddy AB. Peroxiredoxins are conserved markers of circadian rhythms. Nature : 459–464, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng D, Liu T, Sun Z, Bugge A, Mullican SE, Alenghat T, Liu XS, Lazar MA. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science : 1315–1319, 2011. doi: 10.1126/science.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA : 18664–18669, 2010. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forni D, Pozzoli U, Cagliani R, Tresoldi C, Menozzi G, Riva S, Guerini FR, Comi GP, Bolognesi E, Bresolin N, Clerici M, Sironi M. Genetic adaptation of the human circadian clock to day-length latitudinal variations and relevance for affective disorders. Genome Biol : 499, 2014. doi: 10.1186/s13059-014-0499-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallant AR, Lundgren J, Drapeau V. The night-eating syndrome and obesity. Obes Rev : 528–536, 2012. doi: 10.1111/j.1467-789X.2011.00975.x. [DOI] [PubMed] [Google Scholar]
- 31.Garaulet M, Gómez-Abellán P. Timing of food intake and obesity: a novel association. Physiol Behav : 44–50, 2014. doi: 10.1016/j.physbeh.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee YC, Ordovás JM, Scheer FAJL. Timing of food intake predicts weight loss effectiveness. Int J Obes : 604–611, 2013. doi: 10.1038/ijo.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong C, Li C, Qi X, Song Z, Wu J, Hughes ME, Li X. The daily rhythms of mitochondrial gene expression and oxidative stress regulation are altered by aging in the mouse liver. Chronobiol Int : 1254–1263, 2015. doi: 10.3109/07420528.2015.1085388. [DOI] [PubMed] [Google Scholar]
- 34.Grosbellet E, Dumont S, Schuster-Klein C, Guardiola-Lemaitre B, Pevet P, Criscuolo F, Challet E. Circadian phenotyping of obese and diabetic db/db mice. Biochimie : 198–206, 2016. doi: 10.1016/j.biochi.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 35.Guillemot-Legris O, Mutemberezi V, Muccioli GG. Oxysterols in metabolic syndrome: from bystander molecules to bioactive lipids. Trends Mol Med : 594–614, 2016. doi: 10.1016/j.molmed.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Gunn PJ, Middleton B, Davies SK, Revell VL, Skene DJ. Sex differences in the circadian profiles of melatonin and cortisol in plasma and urine matrices under constant routine conditions. Chronobiol Int : 39–50, 2016. doi: 10.3109/07420528.2015.1112396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanlon EC, Van Cauter E. Quantification of sleep behavior and of its impact on the cross-talk between the brain and peripheral metabolism. Proc Natl Acad Sci USA , Suppl 3: 15,609–15,616, 2011. doi: 10.1073/pnas.1101338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harfmann BD, Schroder EA, Kachman MT, Hodge BA, Zhang X, Esser KA. Muscle-specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skelet Muscle : 12, 2016. doi: 10.1186/s13395-016-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab : 848–860, 2012. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He B, Nohara K, Park N, Park YS, Guillory B, Zhao Z, Garcia JM, Koike N, Lee CC, Takahashi JS, Yoo SH, Chen Z. The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metab : 610–621, 2016. doi: 10.1016/j.cmet.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho A, Chin A. Circadian feeding and drinking patterns of genetically obese mice fed solid chow diet. Physiol Behav : 651–656, 1988. doi: 10.1016/0031-9384(88)90221-1. [DOI] [PubMed] [Google Scholar]
- 42.Huang G, Zhang Y, Shan Y, Yang S, Chelliah Y, Wang H, Takahashi JS. Circadian oscillations of NADH redox state using a heterologous metabolic sensor in mammalian cells. J Biol Chem : 23,906–23,914, 2016. doi: 10.1074/jbc.M116.728774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacobi D, Liu S, Burkewitz K, Kory N, Knudsen NH, Alexander RK, Unluturk U, Li X, Kong X, Hyde AL, Gangl MR, Mair WB, Lee C-H. Hepatic Bmal1 regulates rhythmic mitochondrial dynamics and promotes metabolic fitness. Cell Metab : 709–720, 2015. doi: 10.1016/j.cmet.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnston JD, Ordovás JM, Scheer FA, Turek FW. Circadian rhythms, metabolism, and chrononutrition in rodents and humans. Adv Nutr : 399–406, 2016. doi: 10.3945/an.115.010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kettner NM, Mayo SA, Hua J, Lee C, Moore DD, Fu L. Circadian dysfunction induces leptin resistance in mice. Cell Metab : 448–459, 2015. doi: 10.1016/j.cmet.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kil IS, Ryu KW, Lee SK, Kim JY, Chu SY, Kim JH, Park S, Rhee SG. Circadian oscillation of sulfiredoxin in the mitochondria. Mol Cell : 651–663, 2015. doi: 10.1016/j.molcel.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 47.Kitayama Y, Nishiwaki T, Terauchi K, Kondo T. Dual KaiC-based oscillations constitute the circadian system of cyanobacteria. Genes Dev : 1513–1521, 2008. doi: 10.1101/gad.1661808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knutsson A. Health disorders of shift workers. Occup Med (Lond) : 103–108, 2003. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- 49.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab : 414–421, 2007. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Kornmann B, Schaad O, Bujard H, Takahashi JS, Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol : e34, 2007. doi: 10.1371/journal.pbio.0050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lamia KA, Storch K-F, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA : 15,172–15,177, 2008. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Landgraf D, Tsang AH, Leliavski A, Koch CE, Barclay JL, Drucker DJ, Oster H. Oxyntomodulin regulates resetting of the liver circadian clock by food. eLife : e06253, 2015. doi: 10.7554/eLife.06253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lennernäs M, Hambraeus L, Akerstedt T. Shift-related dietary intake in day and shift workers. Appetite : 253–266, 1995. doi: 10.1006/appe.1995.0060. [DOI] [PubMed] [Google Scholar]
- 54.Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, Pierre JF, Heneghan AF, Nadimpalli A, Hubert N, Zale E, Wang Y, Huang Y, Theriault B, Dinner AR, Musch MW, Kudsk KA, Prendergast BJ, Gilbert JA, Chang EB. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe : 681–689, 2015. doi: 10.1016/j.chom.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leproult R, Holmbäck U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes : 1860–1869, 2014. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang X, Bushman FD, FitzGerald GA. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc Natl Acad Sci USA : 10,479–10,484, 2015. doi: 10.1073/pnas.1501305112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu C-T, Raghavan S, Maruthur N, Kabagambe EK, Hong J, Ng MC, Hivert M-F, Lu Y, An P, Bentley AR, Drolet AM, Gaulton KJ, Guo X, Armstrong LL, Irvin MR, Li M, Lipovich L, Rybin DV, Taylor KD, Agyemang C, Palmer ND, Cade BE, Chen W-M, Dauriz M, Delaney JA, Edwards TL, Evans DS, Evans MK, Lange LA, Leong A, Liu J, Liu Y, Nayak U, Patel SR, Porneala BC, Rasmussen-Torvik LJ, Snijder MB, Stallings SC, Tanaka T, Yanek LR, Zhao W, Becker DM, Bielak LF, Biggs ML, Bottinger EP, Bowden DW, Chen G, Correa A, Couper DJ, Crawford DC, Cushman M, Eicher JD, Fornage M, Franceschini N, Fu Y-P, Goodarzi MO, Gottesman O, Hara K, Harris TB, Jensen RA, Johnson AD, Jhun MA, Karter AJ, Keller MF, Kho AN, Kizer JR, Krauss RM, Langefeld CD, Li X, Liang J, Liu S, Lowe WL Jr, Mosley TH, North KE, Pacheco JA, Peyser PA, Patrick AL, Rice KM, Selvin E, Sims M, Smith JA, Tajuddin SM, Vaidya D, Wren MP, Yao J, Zhu X, Ziegler JT, Zmuda JM, Zonderman AB, Zwinderman AH, Adeyemo A, Boerwinkle E, Ferrucci L, Hayes MG, Kardia SL, Miljkovic I, Pankow JS, Rotimi CN, Sale MM, Wagenknecht LE, Arnett DK, Chen YD, Nalls MA, Province MA, Kao WH, Siscovick DS, Psaty BM, Wilson JG, Loos RJ, Dupuis J, Rich SS, Florez JC, Rotter JI, Morris AP, Meigs JB; AAAG Consortium; CARe Consortium; COGENT-BP Consortium; eMERGE Consortium; MEDIA Consortium; MAGIC Consortium . Trans-ethnic meta-analysis and functional annotation illuminates the genetic architecture of fasting glucose and insulin. Am J Hum Genet : 56–75, 2016. doi: 10.1016/j.ajhg.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Z, Huang M, Wu X, Shi G, Xing L, Dong Z, Qu Z, Yan J, Yang L, Panda S, Xu Y. PER1 phosphorylation specifies feeding rhythm in mice. Cell Reports : 1509–1520, 2014. doi: 10.1016/j.celrep.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 59.Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spégel P, Bugliani M, Saxena R, Fex M, Pulizzi N, Isomaa B, Tuomi T, Nilsson P, Kuusisto J, Tuomilehto J, Boehnke M, Altshuler D, Sundler F, Eriksson JG, Jackson AU, Laakso M, Marchetti P, Watanabe RM, Mulder H, Groop L. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet : 82–88, 2009. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature : 627–631, 2010. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Masri S, Rigor P, Cervantes M, Ceglia N, Sebastian C, Xiao C, Roqueta-Rivera M, Deng C, Osborne TF, Mostoslavsky R, Baldi P, Sassone-Corsi P. Partitioning circadian transcription by SIRT6 leads to segregated control of cellular metabolism. Cell : 659–672, 2014. doi: 10.1016/j.cell.2014.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, Andrews JL, Antoch MP, Walker JR, Esser KA, Hogenesch JB, Takahashi JS. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci USA : 3342–3347, 2007. doi: 10.1073/pnas.0611724104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mirzaei K, Xu M, Qi Q, de Jonge L, Bray GA, Sacks F, Qi L. Variants in glucose- and circadian rhythm-related genes affect the response of energy expenditure to weight-loss diets: the POUNDS LOST Trial. Am J Clin Nutr : 392–399, 2014. doi: 10.3945/ajcn.113.072066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mistlberger RE, Lukman H, Nadeau BG. Circadian rhythms in the Zucker obese rat: assessment and intervention. Appetite : 255–267, 1998. doi: 10.1006/appe.1997.0134. [DOI] [PubMed] [Google Scholar]
- 65.Mukherji A, Kobiita A, Damara M, Misra N, Meziane H, Champy M-F, Chambon P. Shifting eating to the circadian rest phase misaligns the peripheral clocks with the master SCN clock and leads to a metabolic syndrome. Proc Natl Acad Sci USA : E6691–E6698, 2015. doi: 10.1073/pnas.1519807112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell : 812–827, 2013. doi: 10.1016/j.cell.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 67.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell : 329–340, 2008. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science : 654–657, 2009. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakajima M, Imai K, Ito H, Nishiwaki T, Murayama Y, Iwasaki H, Oyama T, Kondo T. Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science : 414–415, 2005. doi: 10.1126/science.1108451. [DOI] [PubMed] [Google Scholar]
- 70.Neufeld-Cohen A, Robles MS, Aviram R, Manella G, Adamovich Y, Ladeuix B, Nir D, Rousso-Noori L, Kuperman Y, Golik M, Mann M, Asher G. Circadian control of oscillations in mitochondrial rate-limiting enzymes and nutrient utilization by PERIOD proteins. Proc Natl Acad Sci USA : E1673–E1682, 2016. doi: 10.1073/pnas.1519650113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O’Neill JS, Reddy AB. Circadian clocks in human red blood cells. Nature : 498–503, 2011. doi: 10.1038/nature09702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Neill JS, van Ooijen G, Dixon LE, Troein C, Corellou F, Bouget FY, Reddy AB, Millar AJ. Circadian rhythms persist without transcription in a eukaryote. Nature : 554–558, 2011. doi: 10.1038/nature09654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Orozco-Solis R, Aguilar-Arnal L, Murakami M, Peruquetti R, Ramadori G, Coppari R, Sassone-Corsi P. The circadian clock in the ventromedial hypothalamus controls cyclic energy expenditure. Cell Metab : 467–478, 2016. doi: 10.1016/j.cmet.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ouyang Y, Andersson CR, Kondo T, Golden SS, Johnson CH. Resonating circadian clocks enhance fitness in cyanobacteria. Proc Natl Acad Sci USA : 8660–8664, 1998. doi: 10.1073/pnas.95.15.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Panda S. Circadian physiology of metabolism. Science : 1008–1015, 2016. doi: 10.1126/science.aah4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell : 307–320, 2002. doi: 10.1016/S0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 77.Paschos GK, Ibrahim S, Song W-L, Kunieda T, Grant G, Reyes TM, Bradfield CA, Vaughan CH, Eiden M, Masoodi M, Griffin JL, Wang F, Lawson JA, Fitzgerald GA. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat Med : 1768–1777, 2012. doi: 10.1038/nm.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) : 643–653, 2008. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, Levine DC, Bacsik DJ, Gius D, Newgard CB, Goetzman E, Chandel NS, Denu JM, Mrksich M, Bass J. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science : 1243417, 2013. doi: 10.1126/science.1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perelis M, Marcheva B, Moynihan Ramsey KM, Schipma MJ, Hutchison AL, Taguchi A, Peek CB, Hong H, Huang W, Omura C, Allred AL, Bradfield CA, Dinner AR, Barish GD, Bass J. Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science : aac4250, 2015. doi: 10.1126/science.aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pezuk P, Mohawk JA, Yoshikawa T, Sellix MT, Menaker M. Circadian organization is governed by extra-SCN pacemakers. J Biol Rhythms : 432–441, 2010. doi: 10.1177/0748730410385204. [DOI] [PubMed] [Google Scholar]
- 82.Pittendrigh CS, Minis DH. Circadian systems: longevity as a function of circadian resonance in Drosophila melanogaster. Proc Natl Acad Sci USA : 1537–1539, 1972. doi: 10.1073/pnas.69.6.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, Loos RJ, Manning AK, Jackson AU, Aulchenko Y, Potter SC, Erdos MR, Sanna S, Hottenga JJ, Wheeler E, Kaakinen M, Lyssenko V, Chen WM, Ahmadi K, Beckmann JS, Bergman RN, Bochud M, Bonnycastle LL, Buchanan TA, Cao A, Cervino A, Coin L, Collins FS, Crisponi L, de Geus EJ, Dehghan A, Deloukas P, Doney AS, Elliott P, Freimer N, Gateva V, Herder C, Hofman A, Hughes TE, Hunt S, Illig T, Inouye M, Isomaa B, Johnson T, Kong A, Krestyaninova M, Kuusisto J, Laakso M, Lim N, Lindblad U, Lindgren CM, McCann OT, Mohlke KL, Morris AD, Naitza S, Orrù M, Palmer CN, Pouta A, Randall J, Rathmann W, Saramies J, Scheet P, Scott LJ, Scuteri A, Sharp S, Sijbrands E, Smit JH, Song K, Steinthorsdottir V, Stringham HM, Tuomi T, Tuomilehto J, Uitterlinden AG, Voight BF, Waterworth D, Wichmann HE, Willemsen G, Witteman JC, Yuan X, Zhao JH, Zeggini E, Schlessinger D, Sandhu M, Boomsma DI, Uda M, Spector TD, Penninx BW, Altshuler D, Vollenweider P, Jarvelin MR, Lakatta E, Waeber G, Fox CS, Peltonen L, Groop LC, Mooser V, Cupples LA, Thorsteinsdottir U, Boehnke M, Barroso I, Van Duijn C, Dupuis J, Watanabe RM, Stefansson K, McCarthy MI, Wareham NJ, Meigs JB, Abecasis GR. Variants in MTNR1B influence fasting glucose levels. Nat Genet : 77–81, 2009. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, Burris LL, Khorasanizadeh S, Burris TP, Rastinejad F. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nat Struct Mol Biol : 1207–1213, 2007. doi: 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong H-K, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai S, Bass J. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science : 651–654, 2009. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reutrakul S, Hood MM, Crowley SJ, Morgan MK, Teodori M, Knutson KL, Van Cauter E. Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care : 2523–2529, 2013. doi: 10.2337/dc12-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rey G, Valekunja UK, Feeney KA, Wulund L, Milev NB, Stangherlin A, Ansel-Bollepalli L, Velagapudi V, O’Neill JS, Reddy AB. The pentose phosphate pathway regulates the circadian clock. Cell Metab : 462–473, 2016. doi: 10.1016/j.cmet.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science : 1241214, 2013. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol : 939–943, 2012. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 90.Roenneberg T, Kantermann T, Juda M, Vetter C, Allebrandt KV. Light and the human circadian clock. Handb Exp Pharmacol : 311–331, 2013. doi: 10.1007/978-3-642-25950-0_13. [DOI] [PubMed] [Google Scholar]
- 91.Rudic RD, McNamara P, Curtis A-M, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol : e377, 2004. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rutter J, Reick M, Wu LC, McKnight SL. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science : 510–514, 2001. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- 93.Salgado-Delgado R, Angeles-Castellanos M, Saderi N, Buijs RM, Escobar C. Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology : 1019–1029, 2010. doi: 10.1210/en.2009-0864. [DOI] [PubMed] [Google Scholar]
- 94.Santhi N, Lazar AS, McCabe PJ, Lo JC, Groeger JA, Dijk D-J. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc Natl Acad Sci USA : E2730–E2739, 2016. doi: 10.1073/pnas.1521637113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sato M, Murakami M, Node K, Matsumura R, Akashi M. The role of the endocrine system in feeding-induced tissue-specific circadian entrainment. Cell Reports : 393–401, 2014. doi: 10.1016/j.celrep.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 96.Scheer FAJL, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA : 4453–4458, 2009. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sengupta S, Yang G, O’Donnell JC, Hinson MD, McCormack SE, Falk MJ, La P, Robinson MB, Williams ML, Yohannes MT, Polyak E, Nakamaru-Ogiso E, Dennery PA. The circadian gene Rev-erbα improves cellular bioenergetics and provides preconditioning for protection against oxidative stress. Free Radic Biol Med : 177–189, 2016. doi: 10.1016/j.freeradbiomed.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z, Froy O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J : 3493–3502, 2012. doi: 10.1096/fj.12-208868. [DOI] [PubMed] [Google Scholar]
- 99.Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature : 56–64, 2016. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet : 1435–1439, 1999. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 101.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med : 846–850, 2004. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 102.Spoelstra K, Wikelski M, Daan S, Loudon AS, Hau M. Natural selection against a circadian clock gene mutation in mice. Proc Natl Acad Sci USA : 686–691, 2016. doi: 10.1073/pnas.1516442113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science : 490–493, 2001. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 104.Storch K-F, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature : 78–83, 2002. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 105.Stringari C, Wang H, Geyfman M, Crosignani V, Kumar V, Takahashi JS, Andersen B, Gratton E. In vivo single-cell detection of metabolic oscillations in stem cells. Cell Reports : 1–7, 2015. doi: 10.1016/j.celrep.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stunkard AJ, Grace WJ, Wolff HG. The night-eating syndrome: a pattern of food intake among certain obese patients. Am J Med : 78–86, 1955. doi: 10.1016/0002-9343(55)90276-X. [DOI] [PubMed] [Google Scholar]
- 107.Thaiss CA, Levy M, Korem T, Dohnalová L, Shapiro H, Jaitin DA, David E, Winter DR, Gury-BenAri M, Tatirovsky E, Tuganbaev T, Federici S, Zmora N, Zeevi D, Dori-Bachash M, Pevsner-Fischer M, Kartvelishvily E, Brandis A, Harmelin A, Shibolet O, Halpern Z, Honda K, Amit I, Segal E, Elinav E. Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell : 1495–1510, 2016. doi: 10.1016/j.cell.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 108.Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, Abramson L, Katz MN, Korem T, Zmora N, Kuperman Y, Biton I, Gilad S, Harmelin A, Shapiro H, Halpern Z, Segal E, Elinav E. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell : 514–529, 2014. doi: 10.1016/j.cell.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 109.Tsai L-L, Tsai Y-C, Hwang K, Huang Y-W, Tzeng J-E. Repeated light-dark shifts speed up body weight gain in male F344 rats. Am J Physiol Endocrinol Metab : E212–E217, 2005. doi: 10.1152/ajpendo.00603.2004. [DOI] [PubMed] [Google Scholar]
- 110.Tsai TH, Scheving LE, Scheving LA, Pauly JE. Sex differences in circadian rhythms of several variables in lymphoreticular organs, liver, kidney, and corneal epithelium in adult CD2F1 mice. Anat Rec : 263–270, 1985. doi: 10.1002/ar.1092110306. [DOI] [PubMed] [Google Scholar]
- 111.Turek FW. Circadian clocks: Not your grandfather’s clock. Science : 992–993, 2016. doi: 10.1126/science.aal2613. [DOI] [PubMed] [Google Scholar]
- 112.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science : 1043–1045, 2005. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature : 1027–1031, 2006. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 114.Van Cauter E, Knutson KL. Sleep and the epidemic of obesity in children and adults. Eur J Endocrinol , Suppl 1: S59–S66, 2008. doi: 10.1530/EJE-08-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Voigt RM, Forsyth CB, Green SJ, Mutlu E, Engen P, Vitaterna MH, Turek FW, Keshavarzian A. Circadian disorganization alters intestinal microbiota. PLoS One : e97500, 2014. doi: 10.1371/journal.pone.0097500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Voigt RM, Summa KC, Forsyth CB, Green SJ, Engen P, Naqib A, Vitaterna MH, Turek FW, Keshavarzian A. The circadian clock mutation promotes intestinal dysbiosis. Alcohol Clin Exp Res : 335–347, 2016. doi: 10.1111/acer.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci USA : 21453–21458, 2009. doi: 10.1073/pnas.0909591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Westerterp-Plantenga MS, IJedema MJW, Wijckmans-Duijsens NEG. The role of macronutrient selection in determining patterns of food intake in obese and non-obese women. Eur J Clin Nutr : 580–591, 1996. [PubMed] [Google Scholar]
- 119.Woldt E, Sebti Y, Solt LA, Duhem C, Lancel S, Eeckhoute J, Hesselink MKC, Paquet C, Delhaye S, Shin Y, Kamenecka TM, Schaart G, Lefebvre P, Nevière R, Burris TP, Schrauwen P, Staels B, Duez H. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat Med : 1039–1046, 2013. doi: 10.1038/nm.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wood TL, Bridwell-Rabb J, Kim Y-I, Gao T, Chang Y-G, LiWang A, Barondeau DP, Golden SS. The KaiA protein of the cyanobacterial circadian oscillator is modulated by a redox-active cofactor. Proc Natl Acad Sci USA : 5804–5809, 2010. doi: 10.1073/pnas.0910141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu K, DiAngelo JR, Hughes ME, Hogenesch JB, Sehgal A. The circadian clock interacts with metabolic physiology to influence reproductive fitness. Cell Metab : 639–654, 2011. doi: 10.1016/j.cmet.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, Lazar MA. Rev-erbα, a heme sensor that coordinates metabolic and circadian pathways. Science : 1786–1789, 2007. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
- 123.Yoshizaki T, Tada Y, Hida A, Sunami A, Yokoyama Y, Yasuda J, Nakai A, Togo F, Kawano Y. Effects of feeding schedule changes on the circadian phase of the cardiac autonomic nervous system and serum lipid levels. Eur J Appl Physiol : 2603–2611, 2013. doi: 10.1007/s00421-013-2702-z. [DOI] [PubMed] [Google Scholar]
- 124.Zarrinpar A, Chaix A, Yooseph S, Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab : 1006–1017, 2014. doi: 10.1016/j.cmet.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc Natl Acad Sci USA; :16,219–16,224, 2014. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang Y, Fang B, Emmett MJ, Damle M, Sun Z, Feng D, Armour SM, Remsberg JR, Jager J, Soccio RE, Steger DJ, Lazar MA. Discrete functions of nuclear receptor Rev-erbα couple metabolism to the clock. Science : 1488–1492, 2015. doi: 10.1126/science.aab3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou L, Summa KC, Olker C, Vitaterna MH, Turek FW. Altered body weight regulation in CK1ε null and tau mutant mice on regular chow and high-fat diets. Genet Res Int : 4973242, 2016. doi: 10.1155/2016/4973242. [DOI] [PMC free article] [PubMed] [Google Scholar]