Abstract
We report the first case of epithelial-to-mesenchymal transition (EMT) as the cause of acquired resistance to the second-generation EGFR-tyrosine kinase inhibitor (TKI), afatinib in a patient with advanced non-small cell lung cancer (NSCLC) harboring a sensitizing EGFR mutation. Patients with EGFR-mutant NSCLC inevitably develop acquired resistance while on EGFR-TKI treatment. EMT which renders cancer cells more invasive and migratory is one of the mechanisms of acquired resistance to EGFR-TKIs and correlates with a poor prognosis. Possible therapeutic strategies in patients with EMT include blocking M2 muscarinic receptor signalling, targeting EMT with histone deacetylase inhibitors such as entinostat and MEK-inhibitors such as selumetinib, inhibition of microRNAs, immunotherapy and inhibiting fibroblast growth factor receptor-1.
Keywords: Epithelial-to-mesenchymal transition (EMT), acquired resistance, afatinib, EGFR mutation, lung adenocarcinoma
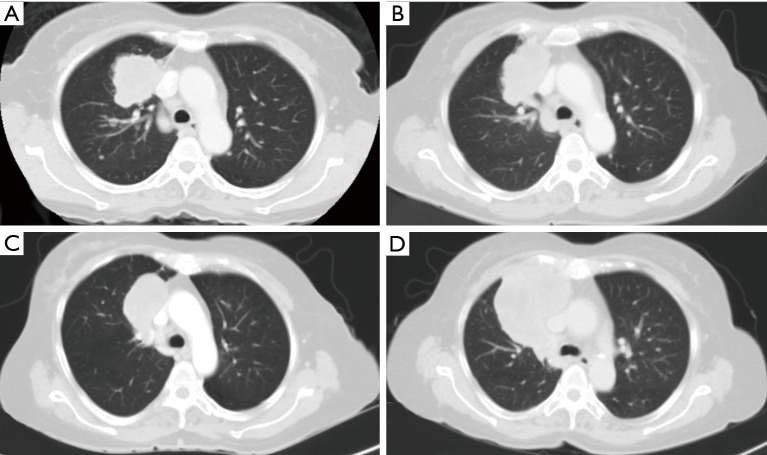
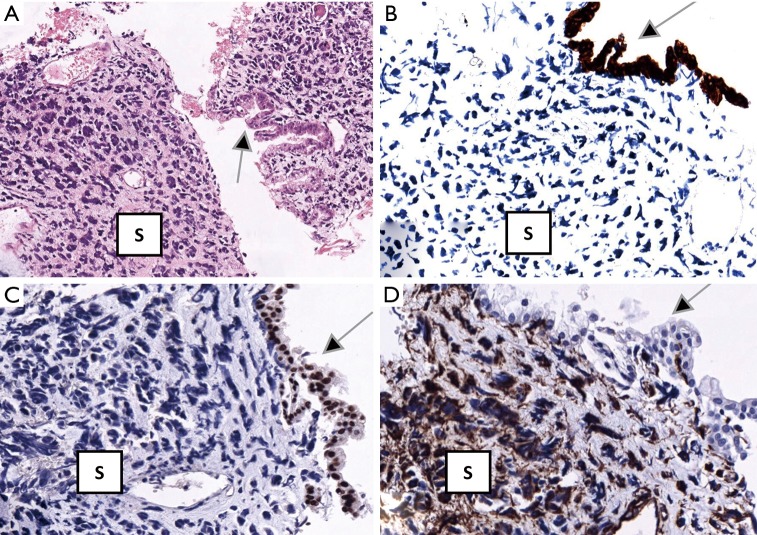
A 63-year-old woman who had never smoked, was diagnosed to have a stage IV (T2bN3M1a) right upper lobe lung adenocarcinoma with metastasis to the right hilar, subcarinal, right paratracheal and right supraclavicular lymph nodes in addition to contralateral lung nodules on computed tomography (CT) scan in July 2016 (Figure 1A). Excision biopsy of the right supraclavicular lymph node revealed metastatic adenocarcinoma with epidermal growth factor receptor (EGFR) exon 19 deletion mutation detected by Therascreen® EGFR RGQ PCR Kit (Qiagen, Manchester Ltd., UK). Despite an initial radiological response on CT scan one month after starting gefitinib 250 mg once daily, CT examination another 2 months later showed progression of disease (PD) (Figure 1B). Treatment was switched to afatinib 40 mg once daily in December 2016. After switching to afatinib, CT scan examination 1 and 3 months later, respectively showed partial response (Figure 1C). Afatinib dose was reduced to 30 mg once daily in March 2017 because of grade 2 diarrhea and drug-induced hepatitis. CT scan in June 2017 showed PD (Figure 1D). EGFR exon 19 deletion and exon 20 T790M mutations were not detected in circulating tumor DNA by droplet digital PCR (Sanomics, Hong Kong). In comparison to the metastatic adenocarcinoma found on initial excision biopsy of the right supraclavicular lymph node (Figure 2), needle biopsy of the rapidly enlarging right upper lobe tumor in early July 2017 showed a biphasic pattern of epithelial and sarcomatous differentiation (Figure 3A,B,C,D). The epithelial cells were immunoreactive to pancytokeratin and TTF-1 while the sarcomatous elements were immunoreactive to vimentin. Molecular studies on the lung biopsy specimen were negative for EGFR mutation (both exon 19 deletion and T790 mutation), anaplastic lymphoma kinase (ALK) rearrangement and HER2 mutation.
Figure 1.
Serial computed tomography images. (A) Right upper lobe tumor measuring 5.6 cm × 4.4 cm × 4.1 cm, enlarged right paratracheal lymph nodes and bilateral lung nodules in July 2016; (B) enlargement of primary tumor and mediastinal lymph nodes showing PD 3 months after initiation of gefitinib therapy; (C) partial response with smaller primary tumor and mediastinal lymph nodes 3 months after switching to afatinib; (D) larger primary tumor with new pericardial invasion and increasing lung nodules in June 2017. PD, progression of disease.
Figure 2.
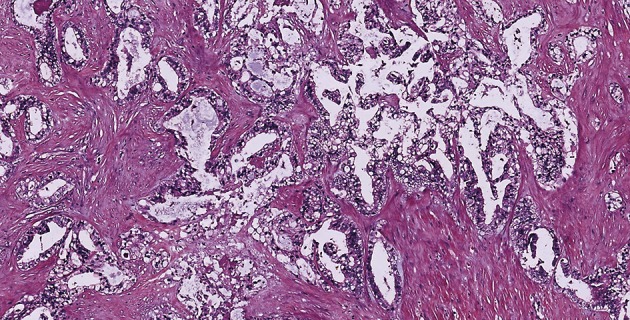
Hematoxylin and eosin staining of initial supraclavicular lymph node biopsy showing moderately differentiated adenocarcinoma with good acinar differentiation in a desmoplastic stroma (magnification: ×40).
Figure 3.
Hematoxylin and eosin staining of needle biopsy of enlarging right upper lobe tumor shows epithelial elements (arrow) juxtaposed with sarcomatous stroma [S] (A); epithelial elements (arrows) are stained positive for pancytokeratin (MNF116) (B) and TTF-1 (C) but negative for vimentin (D) (immunohistochemical staining). The sarcomatous stroma [S] is only immunoreactive for vimentin (D). Magnification: (A) ×10; (B-D) ×20.
She succumbed to rapid PD in August 2017 before the initiation of systemic chemotherapy.
Discussion
To our knowledge, this is the first reported case of EMT causing acquired resistance to the second-generation EGFR-TKI, afatinib in a patient with advanced NSCLC harboring a sensitizing EGFR mutation. EMT has by far only been reported as a likely mechanism of acquired resistance in afatinib-resistant cell lines but not in patients (1).
In EMT, epithelial cells transform to mesenchymal stem cells by losing their cell polarity and cell-to-cell adhesion, gaining invasive and migratory properties, accompanied by upregulation of anti-apoptotic signals and changes in the expression of molecular marker proteins, i.e., down-regulation of E-cadherin and up-regulation of vimentin, fibronectin, and N-cadherin (2). Expression of mesenchymal markers correlates with a poor prognosis and an inferior response to EGFR-TKIs in non-small cell lung cancer (NSCLC) (2). The sarcomatous elements in this patient were immunoreactive to vimentin, a canonical marker consistent with EMT despite N-cadherin and fibronectin not performed.
Acquired resistance due to EMT has rarely been reported in patients treated with gefitinib and erlotinib (3). In 37 EGFR-mutated patients treated with erlotinib or gefitinib, EMT was detected in only three patients on rebiopsies at disease progression (3). Interestingly, EMT has not been described as a resistance mechanism to afatinib in NSCLC patients (4). EMT which may co-exist with other resistance mechanisms causing EGFR-TKI treatment failure may be underdiagnosed due to the infrequent performance of tissue rebiopsies and overreliance on liquid biopsies.
There is a high possibility however that there was much tumor heterogeneity in the original lung tumor, as evidenced by a relatively short response period to first-generation TKI (2 months with gefitinib) and second-generation TKI (6 months with afatinib) in comparison to the reported time-to-treatment failure of 13.7 months with afatinib and 11.5 months with gefitinib (5).
In line with tumor heterogeneity, it would be almost impossible to ascertain if this case represented a case of pure acquired resistance to afatinib due to EMT as an acquired resistance mechanism, or the emergence of a distinct subclone of tumor cell population which was resistant to afatinib and exhibiting EMT after initial response to afatinib by the original subclone of tumors with EGFR exon 19 deletion. The tumor was not tested for other reported afatinib-acquired resistance mechanisms such as MET gene amplification, KRAS gene amplification, and EGFR C797S, L792F and V843I mutations.
Interestingly, upon PD to afatinib, both circulating tumour DNA (ctDNA) and lung biopsy were negative for the original EGFR mutation. Concordance rates between ctDNA and tumor tissues for common EGFR mutations in the literature have a wide variation ranging from 27.5% to 100% (6) with pooled sensitivities from two meta-analyses reported to be 67.4% and 62%, and specificities of 93.5% and 95.9%, respectively (7,8). Despite epithelial cells being present in the lung specimen, these cells do not exhibit the original EGFR mutation, thus suggesting that these cells have developed acquired resistance, possibly through the loss of the mutant EGFR gene allele which has been reported in cell lines resistant to first-generation TKIs (9).
Therapeutic strategies to reverse EMT in patients with acquired resistance to EGFR-TKI include blocking M2 muscarinic receptor signalling (10), targeting EMT with histone deacetylase inhibitors such as entinostat (11) and MEK-inhibitors such as selumetinib (2), inhibition of microRNAs (12), immunotherapy (13); and inhibiting fibroblast growth factor receptor-1 (14).
Conclusions
This is the first report of EMT causing acquired resistance to afatinib treatment in a patient with a sensitizing EGFR-mutant NSCLC.
Acknowledgements
None.
Informed Consent: The patient’s son has given informed consent for the case report to be written and published without any identifying information.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Hashida S, Yamamoto H, Shien K, et al. Acquisition of cancer stem cell-like properties in non-small cell lung cancer with acquired resistance to afatinib. Cancer Sci 2015;106:1377-84. 10.1111/cas.12749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jakobsen KR, Demuth C, Sorensen BS, et al. The role of epithelial to mesenchymal transition in resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Transl Lung Cancer Res 2016;5:172-82. 10.21037/tlcr.2016.04.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. 10.1126/scitranslmed.3002003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Wekken AJ, Saber A, Hiltermann TJ, et al. Resistance mechanisms after tyrosine kinase inhibitors afatinib and crizotinib in non-small cell lung cancer, a review of the literature. Crit Rev Oncol Hematol 2016;100:107-16. 10.1016/j.critrevonc.2016.01.024 [DOI] [PubMed] [Google Scholar]
- 5.Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577-89. 10.1016/S1470-2045(16)30033-X [DOI] [PubMed] [Google Scholar]
- 6.Sun W, Yuan X, Tian Y, et al. Non-invasive approaches to monitor EGFR-TKI treatment in non-small-cell lung cancer. J Hematol Oncol 2015;8:95. 10.1186/s13045-015-0193-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu M, Wang J, Xu Y, et al. Circulating tumor DNA is effective for the detection of EGFR mutation in non-small cell lung cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2015;24:206-12. 10.1158/1055-9965.EPI-14-0895 [DOI] [PubMed] [Google Scholar]
- 8.Luo J, Shen L, Zheng D. Diagnostic value of circulating free DNA for the detection of EGFR mutation status in NSCLC: a systematic review and meta-analysis. Sci Rep 2014;4:6269. 10.1038/srep06269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabara K, Kanda R, Sonoda K, et al. Loss of activating EGFR mutant gene contributes to acquired resistance to EGFR tyrosine kinase inhibitors in lung cancer cells. PLoS One 2012;7:e41017. 10.1371/journal.pone.0041017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Q, Gu X, Zhang C, et al. Blocking M2 muscarinic receptor signaling inhibits tumor growth and reverses epithelial-mesenchymal transition (EMT) in non-small cell lung cancer (NSCLC). Cancer Biol Ther 2015;16:634-43. 10.1080/15384047.2015.1029835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witta SE, Jotte RM, Konduri K, et al. Randomized phase II trial of erlotinib with and without entinostat in patients with advanced non-small-cell lung cancer who progressed on prior chemotherapy. J Clin Oncol 2012;30:2248-55. 10.1200/JCO.2011.38.9411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Legras A, Pécuchet N, Imbeaud S, et al. Epithelial-to-Mesenchymal Transition and MicroRNAs in Lung Cancer. Cancers (Basel) 2017;9. doi: . 10.3390/cancers9080101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lou Y, Diao L, Cuentas ER, et al. Epithelial-Mesenchymal Transition Is Associated with a Distinct Tumor Microenvironment Including Elevation of Inflammatory Signals and Multiple Immune Checkpoints in Lung Adenocarcinoma. Clin Cancer Res 2016;22:3630-42. 10.1158/1078-0432.CCR-15-1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jakobsen KR, Demuth C, Madsen AT, et al. .MET amplification and epithelial-to-mesenchymal transition exist as parallel resistance mechanisms in erlotinib-resistant, EGFR-mutated, NSCLC HCC827 cells. Oncogenesis 2017;6:e307. 10.1038/oncsis.2017.17 [DOI] [PMC free article] [PubMed] [Google Scholar]