Abstract
Background
The extent of emergent surgery for acute DeBakey type I aortic dissection is discussed controversial. The frozen elephant trunk (FET) technique in addition to ascending and arch repair promotes aortic remodelling in the descending aorta and thus may provide superior long-term results in terms of less secondary re-interventions and reduced mortality linked to the downstream aorta.
Methods
Between October 2009 and December 2016, a total of 72 patients underwent emergent hybrid aortic repair using the FET for acute DeBakey type I aortic dissection at our centre. Data were analysed from our prospectively collected database and clinical and imaging mid-term follow-up was obtained.
Results
Implant success was 98.6% with an overall 30-day-mortality of 15.3%. New postoperative stroke was seen in 2.8%, new spinal cord injury in 4.2%. In follow-up (mean 37.8±21.2 months) cumulative survival was 75.0% with freedom from distal reintervention in 96.7% and aortic remodelling rate in the descending aorta in 96.5%.
Conclusions
Hybrid aortic repair using the FET in acute DeBakey type I aortic dissection does not elevate the perioperative risk of mortality and provides excellent aortic remodelling with low distal re-intervention rate in mid-term follow-up.
Keywords: Aortic dissection, total arch replacement, frozen elephant trunk technique (FET technique)
Introduction
Surgery for acute aortic dissection was focused on the proximal repair to eliminate the entry site so far. But in DeBakey type I dissection subsequent follow-up revealed a high rate of mortality linked to the distal aorta (1-4). Therefore, aortic arch inspection for re-entries and repair, if needed, is already recommended and established in international guidelines (5). Moreover, the development of the frozen elephant trunk (FET) allows for one-stage hybrid treatment of the thoracic aorta by classic Dacron prosthetic arch replacement and integrated covered stentgrafting of the proximal descending aorta. The stentgraft portion re-establishes the true and decompresses the false lumen and thus promotes aortic remodelling by inducing false lumen thrombosis (6-12). This technique may reduce the rate of reintervention of the downstream aorta. In case of secondary distal aortic dilation or re-dissection endovascular repair is facilitated as well as open surgery due to the landing zone provided by the stentgraft portion of the hybrid prosthesis. But the high complexity of the procedure requires not only expertise by the performing surgeon but also prolonged operative and circulatory arrest times. The potentially increased rates of perioperative mortality and neurologic complications raised concerns about the use of the FET in the acute dissection despite the potential long-term benefit. Aim of the study was to assess the safety and efficacy of the hybrid aortic repair using the FET in acute aortic dissection. We therefore analysed our results including mid-term follow-up.
Methods
Patients
From our institutional prospective database, counting a total of 260 patients operated on aortic dissection, 72 patients were identified who underwent emergent FET procedure for acute aortic dissection DeBakey type I between October 2009 and December 2016. Indication for FET procedure in these patients was DeBakey type I dissection, including retrograde Stanford type B dissection in two cases, especially in presence of an intimal tear reaching into the descending aorta, and a suitable anatomy in preoperative imaging. That is a minimum aortic diameter of 20 mm according to the size of hybrid prosthesis available as oversizing is not recommended, and absence of multiple re-entries or extremely kinking in the descending aorta due to increased risk of false lumen placement and stentgraft-induced aortic injury. In case of preoperative paraparesis or paraplegia individual patient centred decision was made. If symptoms were most likely due to true lumen collapse FET procedure was favoured to restore distal body perfusion. Instead, if the spinal cord was supposed to depend from false lumen perfusion conventional aortic arch procedure was considered to be superior to allow for possibly communication between true and false lumen and prevent false lumen thrombosis for spinal cord perfusion. Accordingly, exclusion criteria were DeBakey type II dissection and anatomical reasons like small aortic diameter or presence of multiple re-entries or extreme kinking in the descending aorta. Not least, as it is emergency cases, final decision was left to the discretion of the attending surgeon. All but one senior surgeons of our department are familiar with the FET procedure.
Clinical presentation of patients at admission was cardiogenic shock in 20.8%, a neurologic deficit in 26.4%, and malperfusion in 26.4%. The critically ill condition of these patients is also reflected by a mean logistic EuroSCORE of 40.0%. Haemopericardium as “impending tamponade” was even existent in roughly half of the patients (n=33, 45.8%). Of note, no patient is declined surgery as long as he reaches our centre alive. Preoperative patient characteristics are summarized in Table 1.
Table 1. Preoperative patient characteristics.
| Patient characteristics | Values |
|---|---|
| Age (years) | 59.4±12.0 |
| Male sex | 55 (76.4) |
| BMI (kg/m2) | 27.1±4.5 |
| Logistic EuroSCORE I (%) | 40.0±20.0 |
| Hypertension | 68 (94.4) |
| Diabetes | 3 (4.2) |
| COPD | 9 (12.5) |
| Chronic kidney disease | 9 (12.5) |
| Reoperation (previous sternotomy) | 4 (5.6) |
| Clinical presentation | |
| Preoperative CPR | 2 (2.8) |
| Cardiogenic shock | 15 (20.8) |
| Cardiac tamponade | 11 (15.3) |
| Haemopericardium | 33 (45.8) |
| Ventilated | 7 (9.7) |
| Neurologic deficit | 19 (26.4) |
| Malperfusion | 19 (26.4) |
Continuous variables are given as means ± standard deviation (SD), categorical variables as numbers (n) and percentages (%). BMI, body mass index; COPD, chronic obstructive pulmonary disease; CPR, cardiopulmonary resuscitation.
Procedure
For FET procedure the E-vita Open Plus® (Jotec GmbH, Hechingen, Germany) hybrid prosthesis was used in all patients. It consists of a combined conventional polyester tube graft for arch replacement and an integrated covered stentgraft of self-expanding (Nitinol) zick-zack-shaped struts for deployment into the descending aorta. A sewing collar facilitates distal anastomosis to and sealing of the descending aorta. Detailed implantation technique was described earlier (13).
Standard surgical approach was median sternotomy and establishment of cardiopulmonary bypass (CPB) with preferred right axillary arterial cannulation (n=63, 87.5%). FET procedure was performed under moderate hypothermic circulatory arrest (28 °C) with bilateral selective antegrade cerebral perfusion in all patients. In the last years, additional neuromonitoring using bifrontal near infrared spectroscopy (NIRS) was established. The left subclavian artery was blocked or perfused with a separate perfusion catheter. Distal anastomosis of the FET was created distal to the left subclavian artery in Ishimaru zone 3 (14), supraaortic vessels were reimplanted as island.
Concomitant procedures were as follows: in 77.8% (n=56) the aortic root was dissected and had to be addressed by surgery; in 11.1% (n=8) replacement of the noncoronary sinus was sufficient to restore aortic root stability, but in 38.9% (n=28) aortic root repair (David procedure) and in 27.8% (n=20) prosthetic aortic root replacement was added. Reconstruction of coronary ostia was necessary in 6.9% (n=5) and coronary artery bypass grafting (CABG) in 9.7% (n=7), when coronary ostia were completely destroyed by dissection. Complete intraoperative data are depicted in Table 2.
Table 2. Intraoperative data.
| Intraoperative data | Values |
|---|---|
| Arterial cannulation for CPB, n (%) | |
| Axillary | 63 (87.5) |
| Femoral | 6 (8.3) |
| Aortic | 3 (4.2) |
| Concomitant procedures, n (%) | |
| Valve-sparing aortic root repair (David procedure) | 28 (38.9) |
| Bentall procedure | 20 (27.8) |
| CABG | 7 (9.7) |
| Intraoperative times (minutes), median [IQR] | |
| Skin-to-skin | 339 [298–392] |
| CPB | 220 [198–259] |
| Cross-clamp time | 157 [134–180] |
| Circulatory arrest | 69 [61–83] |
CPB, cardiopulmonary bypass; CABG, coronary artery bypass grafting; IQR, interquartile ranges.
Follow-up
All patients received computed tomography (CT) or magnetic resonance (MR) angiography scan of the thoracic aorta prior to discharge expect in case of non-resolved acute kidney injury. At least one further follow-up imaging scan was obtained, preferably with the same imaging method. Aortic remodelling was assumed when there was progressive false lumen thrombosis not only at the level of the stent graft but also in the distal aorta and distal aortic diameters decreased or remained stable. Aortic diameters were taken of the descending aorta at the level of pulmonary artery bifurcation (covered by stentgraft, level 1), just below the end of the stentgraft (level 2) and at the level of the coeliac trunk (level 3). In case of stable aortic remodelling and diameters or denial of angiographic imaging clinical follow-up was collected.
Statistical analysis
For this retrospective analysis statistical calculations were performed using SPSS Version 22.0 (IBM SPSS Statistics for Windows, Armonk, NY, USA: IBM Corp.). Categorical data are reported as numbers and percentages, continuous data as means ± standard deviation (SD) if normally distributed, otherwise as medians and interquartile ranges (IQR). Assumption of normal distribution was tested with the Kolmogorov-Smirnov test. Univariate models were used for risk factor analysis, i.e., Fishers exact test for categorical variables and Mann-Whitney U test for continuous variables. A multivariate model was not appropriate due to sample size and event rate. Kaplan-Meier-analysis was assessed for actuarial survival and reintervention rate.
Results
Patients
We observed an overall 30-day-mortality of 15.3% which is equivalent to in-hospital mortality. Risk factors for early mortality in univariate analysis were preoperative cardiopulmonary resuscitation (P=0.02), preoperative cardiogenic shock (P=0.008), postoperative low cardiac output syndrome (P=0.0001) and length of intensive care unit (ICU) stay (P=0.005). Interestingly, preoperative malperfusion did not have an impact on postoperative survival (P=0.46).
Acute kidney injury requiring haemodialysis occurred in 25.0% (n=18) of patients, 5 of these were acute-on-pre-existent-chronic kidney injuries. The need for renal replacement therapy (RRT) was transient in all patients, no one needed permanent haemodialysis. Re-exploration was necessary in 25.0% (n=18), predominant indications were bleeding and tamponade. Half of the patients (50.0%) could be extubated within 24 hours, median ICU stay was 5 days (IQR 3–9 days), median hospital length of stay 19 days (IQR 12–31 days).
New postoperative stroke occurred in 2 patients (2.8%), new spinal cord injury in 3 (4.2%), one of these with delayed onset on postoperative day 2 after the patient had been extubated and mobilized without limitations on postoperative day 1. All 3 patients with paraparesis showed regressive symptoms in postoperative course and were able to walk again after accomplished rehabilitation.
Out of the 19 patients with preoperative neurologic deficit (26.4%) there were 4 in-hospital deaths, and only 7 patients with postoperative persistent neurologic deficit.
Postoperative data are summarized in Table 3.
Table 3. Postoperative data.
| Postoperative data | Values |
|---|---|
| 30-day mortality, n (%) | 11 (15.3) |
| New postoperative stroke, n (%) | 2 (2.8) |
| New postoperative spinal cord injury, n (%) | 3 (4.2) |
| Delirium, n (%) | 18 (25.0) |
| Acute kidney injury (transient RRT), n (%) | 18 (25.0) |
| Low cardiac output syndrome, n (%) | 12 (16.7) |
| Transfusions, median [IQR] | |
| Packed red blood cells | 9 [4–14] |
| Fresh frozen plasma | 12 [8–16] |
| Thrombocyte concentrate | 4 [2–5] |
| Recombinant factor VII | 32 (44.4) |
| Re-exploration, n (%) | 18 (25.0) |
| Postoperative ventilation <24 hours, n (%) | 36 (50.0) |
| ICU length of stay (days), median [IQR] | 5 [3–9] |
| Hospital length of stay (days), median [IQR] | 19 [12–31] |
RRT, renal replacement therapy; ICU, intensive care unit; IQR, interquartile ranges.
Procedure
Procedural implant success was 98.6% (71/72). In one patient with multiple re-entries in the descending aorta, retrograde guidewire placement was not possible and antegrade stenting resulted in false lumen placement, so that the FET had to be retrieved, which ended lethally. Most common FET sizes used were between 24 and 28 mm of diameter in 72.2% (n=52). Stentgraft length was 150 mm and later on during the study period 130 mm.
Follow-up
Clinical follow-up was 96.7% complete. In follow-up up to 4 years (mean 37.8±21.2 months) cumulative survival was 75.0%. Causes of late mortality were: in each two patients pneumonia and sepsis (after previous severe neurologic damage), one fatal bleeding from aorto-esophageal fistula, one cancer, one unknown. Most deaths occurred within 1 year after surgery resulting in a 1-year-survival rate of 80.6%, survival at 2 years of 76.4%, at 3 years 75%, and no late deaths beyond 3 years (Figure 1). CT-angiographic imaging showed positive aortic remodelling in the downstream aorta in terms of false lumen thrombosis at level 1 in 98.2%, at level 2 in 85.2%, and at level 3 in 18.9%, in terms of aortic diameter changes, i.e., decreased or stable diameter, at level 1 in 96.5%, at level 2 in 80.4%, and at level 3 in 82.1% (Figure 2). There was a significant decrease of median aortic diameter at level 1 (P<0.001), whereas aortic diameter kept stable at level 2 and increased significantly at level 3 (P=0.04, Figure 3). Figure 4 gives an example of the aortic remodelling process over time, Figure 5 a 3D reconstruction.
Figure 1.
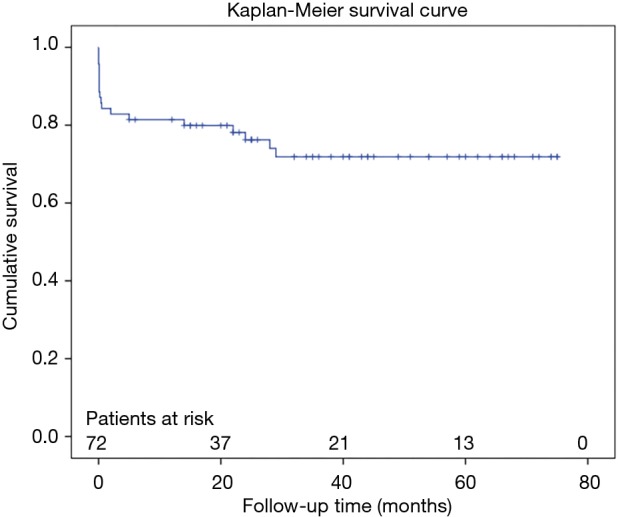
Kaplan-Meier overall survival estimate.
Figure 2.
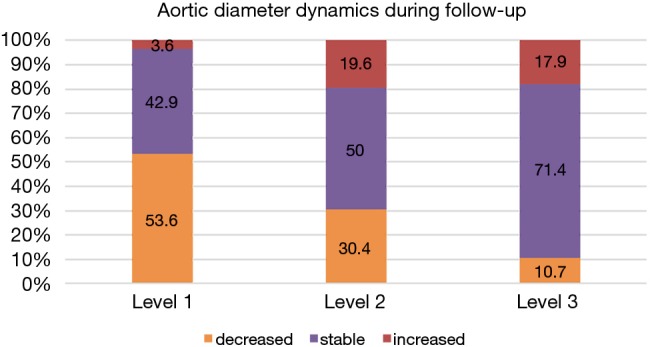
Aortic diameter dynamics in follow-up. Level 1: descending aorta at the level of pulmonary bifurcation (level of stentgraft); level 2: descending aorta distal of stentgraft; level 3: abdominal aorta at the level of celiac trunk.
Figure 3.
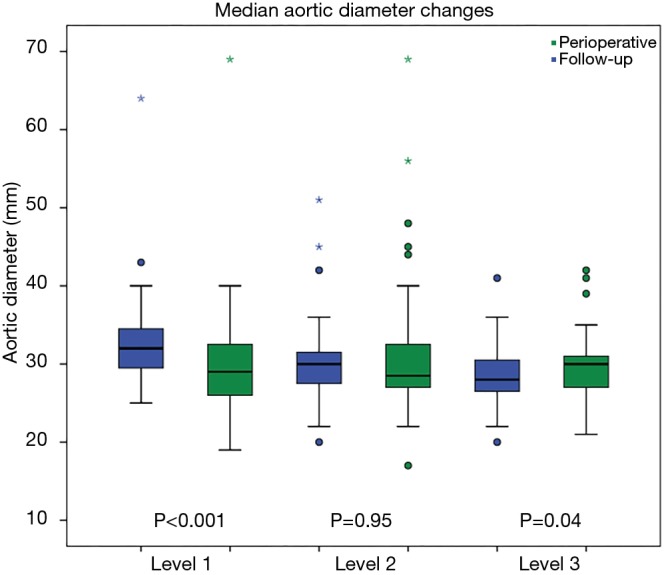
Median aortic diameter changes. Level 1: descending aorta at the level of pulmonary bifurcation (level of stentgraft); level 2: descending aorta distal of stentgraft; level 3: abdominal aorta at the level of celiac trunk. Asterixes and dots: outliers.
Figure 4.
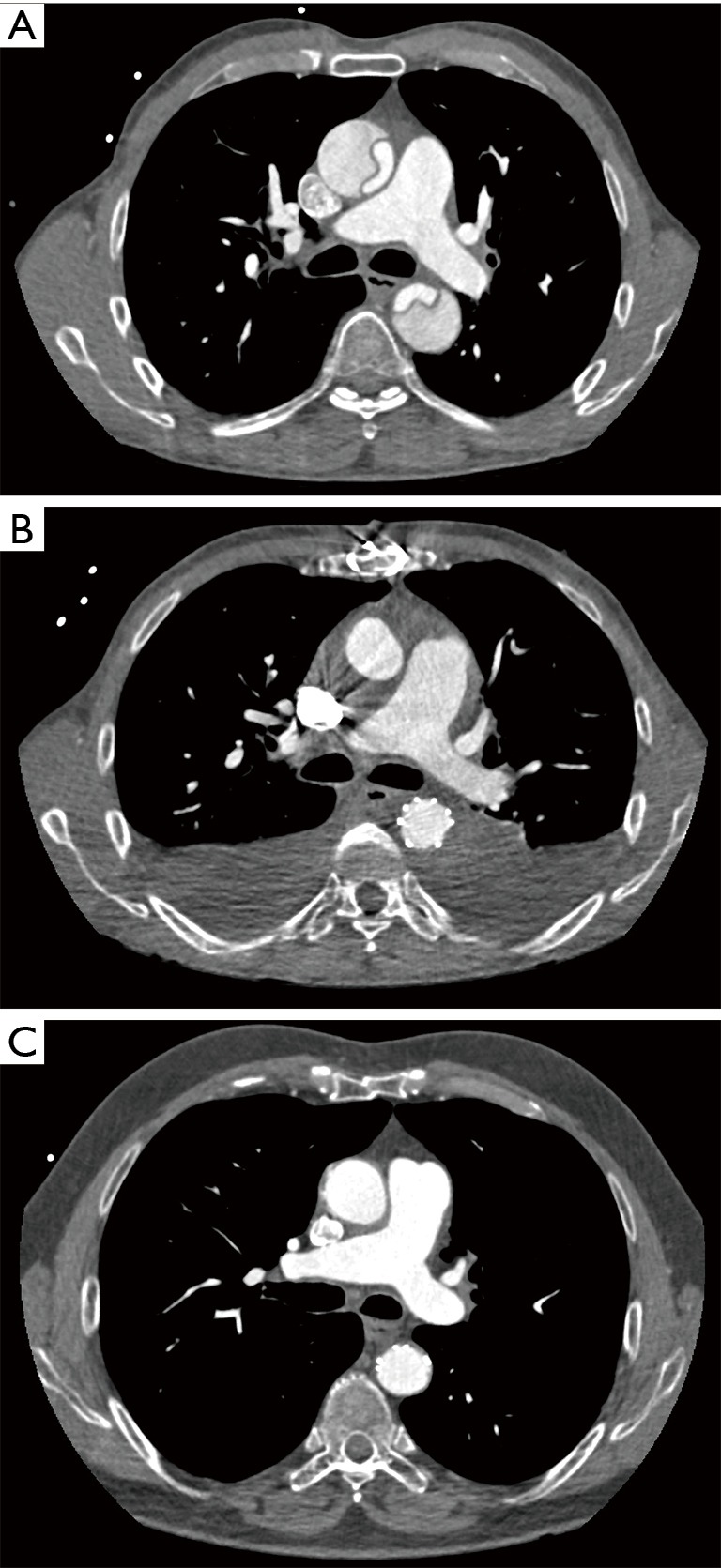
CT documentation of aortic remodelling over time in the same patient. (A) Preoperative CT scan; (B) postoperative CT scan one week after surgery; (C) follow-up CT scan after 3 years. CT, computed tomography.
Figure 5.
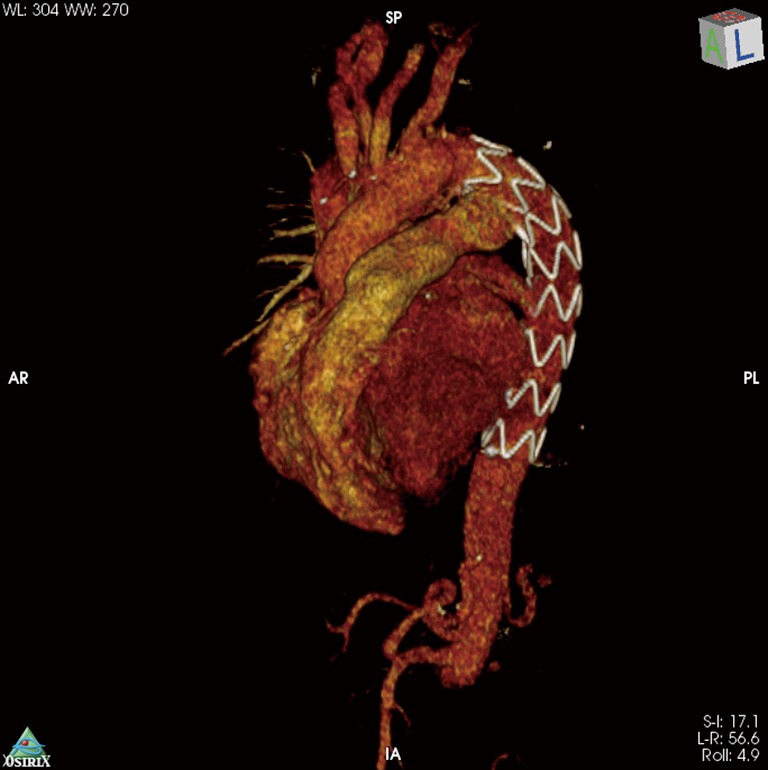
3D-CT reconstruction after FET implantation in acute aortic dissection. CT, computed tomography; FET, frozen elephant trunk.
In survivors freedom from distal reintervention was 96.7% (Figure 6). Two patients required reintervention within 2 and 3 years after initial surgery, respectively. Both received open thoraco-abdominal surgery due to progressive aneurysm growth distal of stentgraft. One of these was turned out to be a Marfan.
Figure 6.
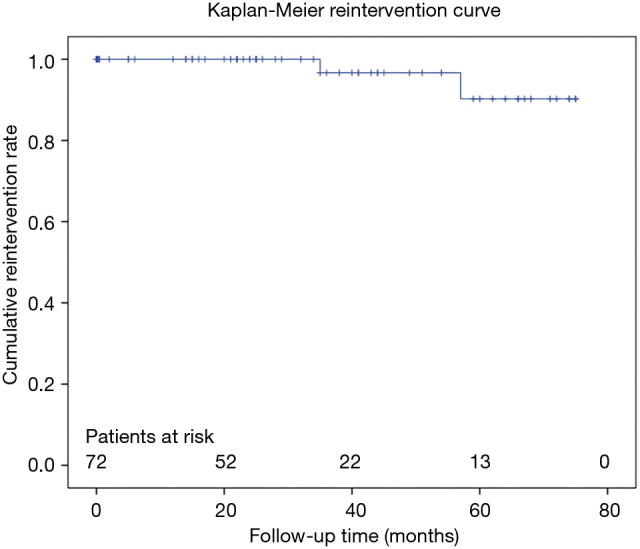
Kaplan-Meier freedom from distal reintervention estimate.
Discussion
Acute aortic dissection Stanford type A is still affected with a considerable high-risk of pre- and perioperative mortality. Thus, surgical efforts are primarily aimed to save life and to avert damage. Several developments over the past years have helped to improve short-term outcomes like perfusion, hypothermia or surgical strategies. Contemporary series report short-term mortality and neurologic deficit rates of 2.8–25% and 3.4–11%, respectively (15-22). In general, these do not differentiate between dissections limited to the ascending aorta and those reaching beyond. But in the long-term this distinction is of paramount importance. In DeBakey type II dissection usually all diseased tissue can be resected and replaced. In contrast, in DeBakey type I dissection the residual downstream dissection after proximal repair carries the risk of dilation, re-dissection and rupture, with remarkable impact on long-term survival (3). It has been shown that a patent false lumen in the descending aorta is a predictor for both mortality and the need for reintervention due to aortic diameter growth (1,2,4). The FET implantation in the setting of acute aortic dissection effectively promotes aortic remodelling with thrombosis of the false lumen in over 90% of cases which could be confirmed by our results (7-9). Furthermore, it stabilizes the true lumen and decompresses the false lumen in the descending aorta. Not least, it gives a substrate or landing zone in case subsequent downstream reoperation or stentgrafting is necessary. The FET can effectively prevent the descending aorta from further growth and therefore reduce the risk of descending rupture.
But potential long-term benefits have to be weighed against periprocedural risks. Mortality rates are accused to be higher than in conventional, more limited surgery. Our data show that this is not the case. In our series we report a 30-day mortality rate of 15.3%, which is well comparable to contemporary results. The latest reports of the GERAADA and IRAD registries showed an overall 30-day mortality of 16.9% and 18% in all, mostly conventional operated, dissection patients (15,16). Specialized aortic centers do even report superior results with mortality rates between 8% and 12% with the use of the FET (6,23). And risk factor analysis reveals predominantly the status of preoperative presentation accounting for perioperative survival. Of note, we don’t have any pre-selection other than death before hospital arrival due to our all-comers treatment policy, which is also reflected by the high percentage of patients with unfavourable clinical presentation. In addition, this rate is equivalent to hospital mortality without “hidden” deaths transferred to external clinics. As we have observed a dismal outcome in our patients with preoperative CPR we changed our surgical strategy to a more limited approach in this condition.
Stroke rates in acute type A aortic dissection repair range from 3.4% to 15% and a more aggressive approach regarding replacement of the aortic arch and supraaortic vessels might be even protective (6,19-22). The rate of new-onset postoperative stroke in our series is as low as 2.8%. We think that it is not only dependent on the extent of arch surgery but also on perfusion and hypothermia management. Our strategy is the consequent use of selective antegrade cerebral perfusion monitored by near-infrared spectroscopy (NIRS) of cerebral oxygenation. With the right axillary arterial cannulation, we ensure a continuous cerebral perfusion throughout CPB and circulatory arrest times. Additional perfusion of the left common carotid and subclavian artery plus the lower body might further improve organ protection of spinal cord and viscera (24-27). Selective cerebral perfusion ensures safe organ protection of the brain and allows for moderate systemic hypothermia (28 °C) avoiding the negative side effects of deep hypothermia.
One major drawback of the FET technique is the elevated risk of spinal cord injury which has been reported to be from 2% to as high as 24% (24). In our series there were 3 patients (4.2%) with postoperative paraparesis, all showing regressive symptoms in follow-up. We also had six patients with a preoperative neurologic deficit who went out without persistent neurologic damage. And also, in limited proximal aortic repair for type I dissection spinal cord injury can be observed, but is, in our opinion, underreported. The genesis of spinal cord injury is multifactorial and false lumen thrombosis can also be seen after conventional aortic repair. We are moving somewhat between Scylla and Charybdis: on the one hand we want the false lumen in the descending aorta to thrombose for prevention of subsequent dilation and rupture. On the other hand, we want to have the spinal cord perfused, even if it is perfused from the false lumen. So, if we put the FET to facilitate false lumen thrombosis we have to face the risk of spinal cord injury when collaterals are not sufficient. One strategy to reduce the risk of spinal cord injury has been to shorten the stentgraft portion of the FET as a stentgraft below Th 7 has been identified as a risk factor for spinal cord injury. We have therefore switched to the shorter 13 mm stentgraft prosthesis. Other centers changed to a more proximal implantation, i.e., in Ishimaru zone 2. Other hypothesized spinal cord saving measures are lower body perfusion, either by a prosthetic sidegraft or by a Foley catheter placed in the stentgraft portion after completion of the distal anastomosis, cerebrospinal fluid drainage, which in our opinion is not an option in acute dissection due to coagulation disorder caused elevated risk of bleeding, and a permissive elevated postoperative blood pressure (24-27). The controversy remains to put or not a FET in a patient already presenting with paraparesis.
Bleeding is a relevant complication in acute aortic dissection surgery as displayed by our substantial transfusion and rethoracotomy (25%) rates, lying in the upper range compared to the literature (24,26). In most cases a severe coagulation disorder can be seen. From the surgical point of view, the FET itself is protective for bleeding from the distal anastomosis as the stentgraft portion depressurizes the false lumen and therefore reduces the risk of back-bleeding from the false lumen. In order to reduce our rethoracotomy and transfusion rates we have now implemented a systematic and early point-of-care diagnostic tool (ROTEM® thromboelastometry) and specific administration of clotting factors (28,29).
Although the rate of acute kidney injury requiring RRT is quite high with 25% in our series it is worth noting that all of these patients came out with restored renal function without the need for permanent haemodialysis (26).
During mid-term follow-up we observed a cumulative survival of 75% up to 4 years which is in line with contemporary data (6-8,10). The FET promotes excellent aortic remodelling of the descending thoracic aorta with a rate of 96.5% which is, in our opinion, the base for the high rate of freedom from distal reintervention (96.7%). Furthermore, one of our two patients requiring distal reintervention was diagnosed a Marfan after initial surgery. Nevertheless, further follow-up is needed to confirm the decreased need for distal reintervention in the long-term (3,26).
Conclusions
In conclusion, our data support the use of the FET in acute DeBakey type I aortic dissection as it provides excellent aortic remodelling with low distal re-intervention rate in mid-term follow-up. Long-term data are still needed to confirm these encouraging results.
Acknowledgements
None.
Ethical Statement: In line with our local ethics committee an ethics approval was not required as this was a retrospective analysis of already existing data after pseudonymization.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Fattouch K, Sampognaro R, Navarra E. Long-term results after repair of type A acute aortic dissection according to false lumen patency. Ann Thorac Surg 2009;88:1244-50. 10.1016/j.athoracsur.2009.06.055 [DOI] [PubMed] [Google Scholar]
- 2.Halstead JC, Meier M, Etz C, et al. The fate of the distal aorta after repair of acute type A aortic dissection. J Thorac Cardiovasc Surg 2007;133:127-35. 10.1016/j.jtcvs.2006.07.043 [DOI] [PubMed] [Google Scholar]
- 3.Roselli EE, Loor G, He J, et al. Distal aortic interventions after repair of ascending dissection: the argument for a more aggressive approach. J Thorac Cardiovasc Surg 2015;149:S117-24.e3. 10.1016/j.jtcvs.2014.11.029 [DOI] [PubMed] [Google Scholar]
- 4.Evangelista A, Salas A, Ribera A, et al. Long-term outcome of aortic dissection with patent false lumen: predictive role of entry tear size and location. Circulation 2012;125:3133-41. 10.1161/CIRCULATIONAHA.111.090266 [DOI] [PubMed] [Google Scholar]
- 5.Erbel R, Aboyans V, Boileau C, et al. ESC Committee for Practice Guidelines. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. 10.1093/eurheartj/ehu281 [DOI] [PubMed] [Google Scholar]
- 6.Shrestha M, Haverich A, Martens A. Total aortic arch replacement with the frozen elephant trunk procedure in acute DeBakey type I aortic dissections. Eur J Cardiothorac Surg 2017;51:i29-34. 10.1093/ejcts/ezw341 [DOI] [PubMed] [Google Scholar]
- 7.Dohle DS, Tsagakis K, Janosi RA, et al. Aortic remodelling in aortic dissection after frozen elephant trunk. Eur J Cardiothorac Surg 2016;49:111-7. 10.1093/ejcts/ezv045 [DOI] [PubMed] [Google Scholar]
- 8.Iafrancesco M, Goebel N, Mascaro J, et al. International E-vita Open Registry Group. Aortic diameter remodelling after the frozen elephant trunk technique in aortic dissection: results from an international multicentre registry. Eur J Cardiothorac Surg 2017;52:310-8. 10.1093/ejcts/ezx131 [DOI] [PubMed] [Google Scholar]
- 9.Jakob H, Dohle D, Benedik J, et al. Long-term experience with the E-vita Open hybrid graft in complex thoracic aortic disease. Eur J Cardiothorac Surg 2017;51:329-38. [DOI] [PubMed] [Google Scholar]
- 10.Vallabhajosyula P, Gottret JP, Robb JD, et al. Hemiarch replacement with concomitant antegrade stent grafting of the descending thoracic aorta versus total arch replacement for treatment of acute DeBakey I aortic dissection with arch tear. Eur J Cardiothorac Surg 2016;49:1256-61. 10.1093/ejcts/ezv374 [DOI] [PubMed] [Google Scholar]
- 11.Katayama A, Uchida N, Katayama K, et al. The frozen elephant trunk technique for acute type A aortic dissection: results from 15 years of experience. Eur J Cardiothorac Surg 2015;47:355-60. 10.1093/ejcts/ezu173 [DOI] [PubMed] [Google Scholar]
- 12.Di Eusanio M, Castrovinci S, Tian DH, et al. Antegrade stenting of the descending thoracic aorta during DeBakey type 1 acute aortic dissection repair. Eur J Cardiothorac Surg 2014;45:967-75. 10.1093/ejcts/ezt493 [DOI] [PubMed] [Google Scholar]
- 13.Di Bartolomeo R, Pantaleo A, Berretta P, et al. Frozen elephant trunk surgery in acute aortic dissection. J Thorac Cardiovasc Surg 2015;149:S105-9. 10.1016/j.jtcvs.2014.07.098 [DOI] [PubMed] [Google Scholar]
- 14.Ishimaru S. Endografting of the aortic arch. J Endovasc Ther 2004;11 Suppl 2:II62-71. 10.1177/15266028040110S609 [DOI] [PubMed] [Google Scholar]
- 15.Berretta P, Patel HJ, Gleason TG, et al. IRAD experience on surgical type A acute dissection patients: results and predictors of mortality. Ann Cardiothorac Surg 2016;5:346-51. 10.21037/acs.2016.05.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conzelmann LO, Weigang E, Mehlhorn U, et al. GERAADA Investigators Mortality in patients with acute aortic dissection type A: analysis of pre- and intraoperative risk factors from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothorac Surg 2016;49:e44-52. 10.1093/ejcts/ezv356 [DOI] [PubMed] [Google Scholar]
- 17.Knipp BS, Deeb GM, Prager RL, et al. A contemporary analysis of outcomes for operative repair of type A aortic dissection in the United States. Surgery 2007;142:524-8. 10.1016/j.surg.2007.07.012 [DOI] [PubMed] [Google Scholar]
- 18.Pagni S, Ganzel BL, Trivedi JR, et al. Early and midterm outcomes following surgery for acute type A aortic dissection. J Card Surg 2013;28:543-9. 10.1111/jocs.12170 [DOI] [PubMed] [Google Scholar]
- 19.Tokuda Y, Miyata H, Motomura N, et al. Japan Adult Cardiovascular Database Organization. Brain protection during ascending aortic repair for Stanford type A acute aortic dissection surgery. Nationwide analysis in Japan. Circ J 2014;78:2431-8. 10.1253/circj.CJ-14-0565 [DOI] [PubMed] [Google Scholar]
- 20.Coselli JS, Green SY. Aortic arch repair today: open repair is best for most arch lesions. J Cardiovasc Surg (Torino) 2015;56:531-46. [PubMed] [Google Scholar]
- 21.Andersen ND, Ganapathi AM, Hanna JM, et al. Outcomes of acute type a dissection repair before and after implementation of a multidisciplinary thoracic aortic surgery program. J Am Coll Cardiol 2014;63:1796-803. 10.1016/j.jacc.2013.10.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trivedi D, Navid F, Balzer JR, et al. Aggressive Aortic Arch and Carotid Replacement Strategy for Type A Aortic Dissection Improves Neurologic Outcomes. Ann Thorac Surg 2016;101:896-903. 10.1016/j.athoracsur.2015.08.073 [DOI] [PubMed] [Google Scholar]
- 23.Lin HH, Liao SF, Wu CF, et al. Outcome of frozen elephant trunk technique for acute type A aortic dissection: as systematic review and meta-analysis. Medicine (Baltimore) 2015;94:e694. 10.1097/MD.0000000000000694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian DH, Wan B, Di Eusanio M, et al. A systematic review and meta-analysis on the safety and efficacy of the frozen elephant trunk technique in aortic arch surgery. Ann Cardiothorac Surg 2013;2:581-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katayama K, Uchida N, Katayama A, et al. Multiple factors predict the risk of spinal cord injury after the frozen elephant trunk technique for extended thoracic aortic disease. Eur J Cardiothorac Surg 2015;47:616-20. 10.1093/ejcts/ezu243 [DOI] [PubMed] [Google Scholar]
- 26.Shrestha M, Bachet J, Bavaria J, et al. Current status and recommendations for use of the frozen elephant trunk technique: a position paper by the Vascular Domain of EACTS. Eur J Cardiothorac Surg 2015;47:759-69. 10.1093/ejcts/ezv085 [DOI] [PubMed] [Google Scholar]
- 27.Leontyev S, Tsagakis K, Pacini D, et al. Impact of clinical factors and surgical techniques on early outcome of patients treated with frozen elephant trunk technique by using EVITA open stent-graft: results of a multicentre study. Eur J Cardiothorac Surg 2016;49:660-6. 10.1093/ejcts/ezv150 [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Han L, Li J, et al. Consumption coagulopathy in acute aortic dissection: principles of management. J Cardiothorac Surg 2017;12:50. 10.1186/s13019-017-0613-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deppe AC, Weber C, Zimmermann J, et al. Point-of-care thromboelastography/thromboelastometry-based coagulation management in cardiac surgery: a meta-analysis of 8332 patients. J Surg Res 2016;203:424-33. 10.1016/j.jss.2016.03.008 [DOI] [PubMed] [Google Scholar]


