Abstract
Background
Non-intubated, or awake, video-assisted thoracoscopic surgery has been implemented for non-anatomical lung resection and the results obtained were encouraging to consider the approach for anatomical pulmonary resection. This study was conducted to evaluate the perioperative outcomes of the non-intubated and intubated video-assisted thoracoscopic lobectomy in lung cancer in regards to feasibility and safety.
Methods
A retrospective analysis of 62 consecutive video-assisted thoracoscopic lobectomies (31 lobectomies as non-intubated, 31 lobectomies as intubated) performed in Seoul St. Mary’s Hospital, The Catholic University of Korea between January and December 2016.
Results
Both groups share comparable clinical characteristics including the age, sex, BMI, FEV1, DLCO, smoking history, lung lobes procedure, histological type and pathological staging. There was no difference in the mean of postoperative hospitalization period (6.9 versus 7.6 days, P=0.578) and the total chest tube duration (5.6 versus 5.4 days, P=0.943) between non-intubated and intubated lobectomy respectively. Both groups had a comparable surgical outcome for the anesthesia duration, operative time, blood loss and postoperative complications. The operative time required for lobe-specific surgery was shorter in the non-intubated group except for the LLL (mean 121.7 minutes for non-intubated group versus 118.3 minutes for the intubated group). The only statistically significant surgical outcome was for the number of dissected lymph nodes between both groups (the mean number of nodes for the non-intubated group was 12.6 versus 18.0 nodes for the intubated group, P=0.003). One patient in the non-intubated group required conversion to single lung intubation and mini-thoracotomy because of bleeding with no conversion in the intubated group. No mortality encountered in either group.
Conclusions
The perioperative surgical outcomes for the non-intubated video-assisted thoracoscopic lobectomy are comparable to the intubated technique. Non-intubated video-assisted thoracoscopic lobectomy is safe and is technically feasible. However, further prospective randomized studies are needed for a better comparison between non-intubated and intubated VATS lobectomy.
Keywords: Lung cancer, video-assisted thoracic surgery (VATS), lobectomy, non-intubated surgery
Introduction
Innovation in thoracic surgical and anesthesia techniques during the last two decades has participated in having better surgical outcomes for managing non-small cell lung cancer. Video-assisted thoracoscopic surgery (VATS) approach is supported by the literature to be a safe alternative to open thoracotomy with less morbidity (1). On the other hand, double-lumen endotracheal intubation has been considered the gold standard for thoracic procedures since its introduction by Carlens in 1949 (2). However, intubated general anesthesia with one lung ventilation has recognizable complications including; a postoperative sore throat, irritating cough, nausea and vomiting, ventilator-related complications and impaired lung performance (3-5). To overcome the adverse effect of tracheal intubation and general anesthesia, the non-intubated VATS technique (also known as tubeless or awake surgery) has been implemented for non-anatomical resection surgeries for pneumothorax, peripherally located solitary pulmonary nodule and lung volume reduction surgery. The result obtained was encouraging to consider the approach for more complex surgical diseases (6-8). In 2007, Al-Abdullatief et al. reported a promising result of low morbidity, no procedure-related mortality and shorter hospital stay for a variety of major thoracic procedures performed under thoracic epidural anesthesia. Chen et al. conducted a retrospective case-control study on patients underwent a lobectomy for lung cancer and found non-intubated VATS lobectomy safe and feasible as compared to intubated VATS lobectomy (9,10).
As the evidence growing toward the safety of VATS procedure in the management of lung cancer, we performed a retrospective review on our initial experience in non-intubated VATS lobectomy for lung cancer study and compared the short-term perioperative outcomes with patients underwent intubated VATS lobectomy for lung cancer.
Methods
Sitting
Seoul St. Mary’s Hospital, the Catholic University of Korea is a tertiary academic hospital. Around 300 major lung cancer surgeries are performed per year in our department. This study cohort the surgery performed solely by a single surgeon during the study period.
Study design
Non-intubated VATS lobectomy introduced in Seoul St. Mary’s Hospital in September 2016 for the management of lung cancer patients. All non-intubated cases performed in the period between September and December 2016 were reviewed and compared to consecutive patients who had an intubated VATS lobectomy since January 2016. A total of 62 patients (31 patients in each group) were analyzed retrospectively and their clinical data pertaining the perioperative outcomes were collected. We excluded one patient from each group because of conversion to thoracotomy. All surgeries in both groups were performed by a single thoracic surgeon and thoracic anaesthesia team. Both the surgeon and the anesthetists meet the patient separately before the surgery. They explain the procedure, the risk, the conversion to general anaesthesia, and upon the acceptance, patient signed the consent.
Selection criteria & data collection
Once the clinical diagnosis of lung cancer confirmed and the perioperative risk assessment obtained, the patient considered legible for surgery and informed surgical consent received. Similar inclusion and exclusion criteria applied to all patients in this study. Our inclusion criteria of VATS include NSCLC stage I & II, tumor size <6 cm, no evidence of bronchus involvement, and no regional or distant metastasis. Patients with BMI >30, bleeding diathesis, difficult airway, previous pulmonary resection or cardiac dysfunction were excluded from the non-intubated surgery. Perioperative care, protocol and postoperative orders applied in a similar way to both groups.
Data concerning the perioperative outcomes were collected from patients file that includes; patient’s demographic, pulmonary function, BMI, intraoperative complications, mortality, operative and anesthesia time, tumor location and size, histological type, pathological grade, the number of dissected lymph nodes and duration of postoperative hospital stay. We compared the clinical characteristics and surgical outcomes between both groups using SPSS for data analysis. All continuous variable such as age, BMI, postoperative hospital stays and number of dissected lymph nodes represented by mean ± standard deviations and t-test used for analysis. Categorical data such as sex, tumor location, pathological stage, histological type, and complications were represented as frequencies (%), and Chi-squared test was used for the analysis. Variables with P<0.05 were considered statistically significant. This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital.
Operative technique
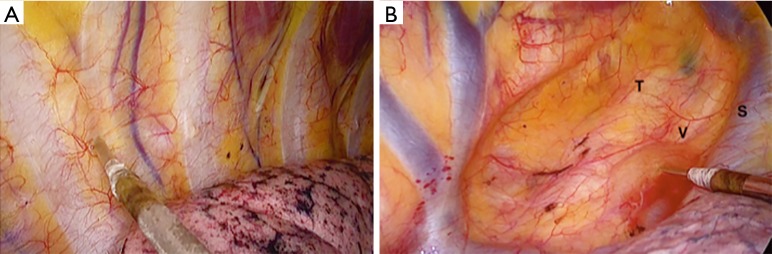
Our operative procedure for VATS lobectomy in intubated and non-intubated groups was utilizing a 4-ports approach. The patient was placed in lateral decubitus position in semi-flexion position and extended arm. Camera port set in 8th intercostal space in the mid-axillary line, utility incision at the 5th intercostal space at the anterior axillary line, 4 cm length. Two working ports inserted, first at the 7th intercostal space anteriorly and the second at the 6th intercostal space posteriorly. Moreover, in the non-intubated procedure, we perform intercostal nerve block directly under the vision of scope from the 3rd to the 8th nerves followed by intrathoracic vagal nerve block at the level of the lower paratracheal area on the right-sided operation and aortopulmonary window on the left-sided operation (Figure 1). We do not apply epidural pain management. After negative hemithorax exploration and confirmation of malignancy preoperatively or by frozen biopsy intraoperatively, complete dissection and division of the fissure, pulmonary vessels and bronchi were achieved by stapler devices. The resected lung was removed within endo-retrieval bag from the utility incision. Air-leak test performed for the bronchial stump. Dissection of multiple lymph node stations performed routinely for all patients including N1 and N2 stations. After completion of surgery and confirmation of hemostasis, a 24 or 28 French size chest tube inserted through the lowest incision. For the intubated VATS technique, there was no difference except for vagal nerve block, multi-level intercostal nerve block, and the use of intubation.
Figure 1.
Thoracoscopic intercostal nerve block and vagal block are delivered by an intra-thoracic needle using 0.5% bupivacaine. Thoracoscopic intercostal nerve block and vagal block are delivered by an intra-thoracic needle using 0.5% bupivacaine. (A) Six intercostal nerves are blocked from the third to the eighth intercostal nerves; (B) the intrathoracic vagal nerve block applied at the level of the lower trachea on the right side procedure and the aorto-pulmonary window for the left side procedure. T, trachea; V, vagus nerve; S, superior vena cava.
Anesthesia technique of non-intubated surgery
All patients were oxygenated via facial mask with O2 5 L/min and premedicated with intravenous glycopyrrolate (0.2 mg). Electrocardiography, pulse oximetry, and arterial blood pressure were monitored. Bispectral index sensor was attached to the forehead of patients to monitor their consciousness levels. Capnometer was inserted inside one of the nostrils to detect end-tidal carbon dioxide. Anesthesia was induced and maintained by infusion of propofol (2–4 mcg/mL) and dexmedetomidine (0.6–0.8 mcg/kg/min). Incremental adjustment of anesthetics was provided to maintain a BIS value between 40 and 60.
Results
A total of 62 patients post VATS lobectomy for non-small cell lung cancer were reviewed between the period of January and December 2016. We compared the clinical characteristics and operative outcomes of 31 patients with non-intubated VATS (September to December 2106) to the first 31 consecutive patients in the control group (January to August 2016). All patients were involved in the study, excluding the converted open thoracotomy patients in each cluster for better evaluation of the safety and feasibility of the non-intubated VATS approach.
Both groups share comparable clinical characteristics including the age, sex, BMI, FEV1, DLCO, smoking history, lung lobes procedure, histological type and pathological staging without any statistical significances findings (Table 1). The majority of patients included in the study had an early stage lung cancer based on our inclusion criteria, and that might directly influence the validity of retrieved perioperative outcomes. Adenocarcinoma histology was the predominant cell type in both groups (NI-VATS 86.7%, I-VATS 83.3%). The operative and anesthesia results are summarized in Table 2. No significant statistical differences noticed during the anesthesia time, operative time and in blood loss between both groups. However, there was a trend of shorter anesthesia time (the duration from induction to self-respiration) and operation time (duration from incision to closure) in the non-intubated group. The time required for the lobectomy for each segment was shorter in non-intubated VATS except for the LLL in intubated VATS group (121.7 min for non-intubated VATS, 118.3 for intubated VATS). Intra-operative blood loss was approximate in both groups. One patient had dense adhesions and intraoperative bleeding from the right lower lobe pulmonary vein among the non-intubated group required conversion into intubation on the lateral decubitus position and thoracotomy to control the source of bleeding. Table 3 is summarizing the post-operative outcomes. Despite the postoperative hospital stay was shorter in non-intubated VATS group 6.9±3.8 days compared to the intubated VATS group 7.6±5.3 days, the chest tube duration in the non-intubated group was 5.6 days while in the intubated group was 5.4 days. The incidence of air-leak in the non-intubated group compared to intubated group were 5 patients (16.7%) and 4 patients (13.3%), respectively. Although there were differences in the findings of the patient’s outcomes, however, none of them were statistically significant. We did not encounter any mortality among the patients involved in this study. Though there were no statistical differences found in the pathological stage, 70.0% of stage IA was in the non-intubated group, while only 43.3% in the intubated group. Probably because this stems from the selection process before the surgery, patients with small nodules tend to receive the non-intubated surgery approach. We analysed the pathological stage between the groups and they were statistically comparable. The only statistical significance difference obtained was related to the number of dissected lymph nodes (P=0.003). Despite the fact that we routinely examine, at least, 3 mediastinal stations we retrieved fewer lymph nodes in non-intubated VATS group in comparison to intubated VATS group (12.6±6.0 lymph nodes and 18.0±7.4 lymph nodes, respectively).
Table 1. Patent’s baseline clinical characteristics.
| Variable | Non-intubated VATS (n=30) | Intubated VATS (n=30) | P value |
|---|---|---|---|
| Age | 64.9±10.5 | 66.1±9.5 | 0.654 |
| Sex | |||
| Male | 10 (33.3%) | 12 (40.0%) | |
| Female | 20 (66.7%) | 18 (60.0%) | 0.592 |
| BMI | 23.8±3.2 | 23.5±2.9 | 0.634 |
| Smoking history | |||
| Smoker | 7 (23.3%) | 9 (30.0%) | 0.559 |
| Non-smoker | 23 (76.7%) | 21 (70.0%) | |
| FEV1 | 94.8±9.3 | 95.1±16.8 | 0.948 |
| DLOC | 88.9±17.9 | 91.1±27.3 | 0.711 |
| Lobe % | |||
| RUL | 8 (26.7%) | 11 (36.7%) | |
| RML | 5 (16.7%) | 5 (16.7%) | |
| RLL | 6 (20.0%) | 4 (13.3%) | 0.861 |
| LUL | 8 (26.7%) | 6 (20.0%) | |
| LLL | 3 (10.0%) | 4 (13.3%) | |
| Cell type % | |||
| Adenocarcinoma | 26 (86.7%) | 25 (83.3%) | |
| Squamous cell carcinoma | 4 (13.3%) | 4 (13.3%) | 0.601 |
| Adenosquamous cell carcinoma | 0 | 1 (3.3%) |
VATS, video-assisted thoracoscopic surgery.
Table 2. Operative and anesthesia results.
| Results | Non-intubated VATS (n=30) | Intubated VATS (n=30) | P value |
|---|---|---|---|
| Anesthesia time (min) | 176±32.5 | 194±41.9 | 0.071 |
| Operative time (min) | 130.9±30.1 | 146.0±47.4 | 0.144 |
| Conversion to thoracotomy | 1 | 1 | |
| Conversion to intubation | 1 | NA | |
| Blood loss (mL) | 82.3±124.4 | 78.3±48.0 | 0.870 |
VATS, video-assisted thoracoscopic surgery.
Table 3. Postoperative outcomes.
| Outcomes | Non-intubated VATS (n=30) | Intubated VATS (n=30) | P value |
|---|---|---|---|
| Chest tube duration (day) | 5.6±7.0 | 5.4±5.4 | 0.943 |
| Post-operative hospital stay (day) | 6.9±3.8 | 7.6±5.3 | 0.578 |
| Pathological stage | 0.160 | ||
| IA | 21 (70.0%) | 13 (43.3%) | |
| IB | 3 (10.0%) | 6 (20.0%) | |
| IIA | 1 (3.3%) | 5 (16.7%) | |
| IIB | 1 (3.3%) | 3 (10.0%) | |
| IIIA | 4 (13.3%) | 3 (10.0%) | |
| Number of dissected LN | 12.6±6.0 | 18.0±7.4 | 0.003 |
| Complication | 6 (20.0%) | 6 (20.0%) | 0.862 |
| Prolonged air leak | 5 | 4 | |
| Pneumonia | 0 | 1 | |
| Atrial fibrillation | 1 | 0 | |
| Hoarseness | 0 | 1 | |
| Mortality | 0 | 0 |
VATS, video-assisted thoracoscopic surgery.
Discussion
Minimally invasive surgical strategies have recently gained popularity among the thoracic surgeons. The elemental spirit of utilizing the non-intubated VATS (NIVATS) technique is to minimize the surgical stress result from thoracotomy and general anesthesia. Moreover, such strategy results in faster postoperative recovery, shortening the hospital stay and reducing the cost and with the lowest expectations, provides similar surgical outcomes to intubated VATS technique (11).
The principal advantage of non-intubation VATS is to evade the perioperative adverse effect acquired from the influence of general anaesthesia, intubation trauma, mechanical single-lung ventilation and the impact of muscle relaxants. On the other hand, performing video-assisted thoracoscopic surgery under non-intubated techniques carries its own risk related to the movements of the lung and diaphragm, coughing and the ability to perform a proper lymph node dissection for a complete oncological resection (5,12). In nonintubated surgery, the patient is doing a self-respiration, and after the insertion of the working ports, the atmospheric pressure applies to the lung. Therefore, the targeted lung is collapsed and deflated enough to perform anatomic lung resection. Also, because there is no “positive pressure ventilation,” the lung is softer than the intubated group, which makes more comfortable handling of the lungs for both operator and the assistant.
Patients' respiratory patterns during NIVATS should satisfy both oxygenation maintenance and fair view of the surgical field. In the case of light anesthesia, the patient's respiration is increased, and oxygenation is well-maintained. However, it is difficult to obtain a proper view of the surgical field due to extensive lung movement. If the depth of anesthesia is too broad, the opposite situation arises. Therefore, the surgeon and the anesthesiologist are required to cooperate with each other continuously during surgery to find an appropriate depth of anesthesia that maintains oxygenation and ensures adequate surgical field.
Knowing the minute details to perform non-intubated VATS lobectomy requires defining a safe track starting from setting the indications, contraindications, inclusion and exclusion criteria, patient’s awareness of the benefits and potential complications, knowing the most appropriate anesthetic technique and the criteria for conversion to general anesthesia. Moreover, the cornerstone of this management pathway is to have an expert surgeon who has the knowledge and extensive skills in oncological VATS lobectomies to overcome the potential complications.
The inclusion criteria for non-intubated VATS lobectomy included all patients fit for intubated VATS lobectomy and those whom the conventional thoracotomy or general anesthesia will add to their morbidity. Our exclusion criteria involved patients with expected challenging airway management, BMI >30, coagulopathy (INR >1.5), patients with a persistent cough or unusual airway secretion, hypoxemia (PaO2 <60%) or hypercarbia (PCO2 >50) and extensive pleural adhesion. We considered patients with previous pulmonary resection as a relative contraindication for the non-intubated approach.
To optimize patient condition intraoperatively, a clear preoperative protocol for conversion to general anesthesia should be apparent to surgical and anesthesia team during an elective or emergency situations to minimize the risk on the patient side. Without a doubt, effective communication between both teams is a fundamental part (13).
Conversion to intubation while a patient on lateral decubitus position is technically demanding and challenging. The rate of conversion reported between 2.3% and 10% depending on the type of procedure and team experience. The anesthesiologist must be skilled to place double-lumen tube and to perform fibreoptic intubation in a timely fashion to secure the patient airway. We recommend conversion to general anesthesia once the patient had one of the situations listed in Table 4 (12).
Table 4. Intraoperative causes for conversion to general anesthesia.
| Surgery-related causes | Safety-related causes | Anesthesia-related causes |
|---|---|---|
| Major intraoperative bleeding | Persistent cough | Severe hypoxemia (PaO2 <60%) |
| Extensive pleural adhesions | Excessive diaphragm or mediastinum movements | Hypercapnia (PaCO2 >80) |
| Large tumors size | Inadequate lung collapse | Acidosis (pH <7.1) |
| Lack of progress during surgery | Hemodynamic instability | |
| Insufficient vagus nerve block |
Conversion to intubated general anesthesia is a significant difficulty during the procedure, and we had to convert to GA and thoracotomy in one patient (3%) secondary to dense adhesions and bleeding from the pulmonary vessels of the right lower lobe. Concerning this case, the surgeon was able to control the bleeding by direct pressure, and immediately the anesthesiologist informed about the bleeding event. Anesthesiologist succeeded to intubate the patient with the double lumen while the patient in lateral position. He managed to maintain the stability of patient’s hemodynamic status. The surgeon managed the bleeding quite safely after converting to thoracotomy, and there was no major problem for the patient at the end. With the accumulation of the experience, the surgeon and anesthesiologist overcome some of the difficulties during the operation and operate with more confidence to reduce the need for conversion.
The operative time, intraoperative blood loss, length of hospital stay, duration of a chest tube, postoperative complications and mortality were equally in both groups. These results support the feasibility and safety of non-intubated VATS. We noticed that the operative time was shorter in the non-intubated group for each lobe, however for the left lower lobe operation time were the same. We relate this finding to the effect of mediastinal and diaphragmatic movement.
Some concerns may arise with regards to the quality of lymph node dissection. In our study, we found the total number of lymph nodes obtained was less in the non-intubated group (12.6±6.0) compared to intubated group (18.0±7.4) with statistical significance (P=0.003). We attribute this finding to the early experience and technical difficulties in non-intubated approach. Moreover, the proportion of patient with early lung cancer with ground glass opacity (GGO) in the non-intubated group was more in comparison to the intubated group. We found 14 patients (45%) presented with GGO and in NIVATS while only 7 patients (22%) presented with GGO in intubated VATS group. That explains the preoperative surgeon intention to perform lymph node sampling rather than complete lymph node dissection. However, all patients had sampling or dissection of the required lymph node stations and accordingly, the quantity of dissected lymph node was affected yet the quality of oncological resection maintained. Other studies investigated the completeness of VATS lymph node dissection (number of nodes and stations) under spontaneous breathing for non-small lung cancer, and there were no significant differences when compared to intubated anesthesia (10,14-17).
Our study is limited by being a retrospective study with a small volume of patient and single center experience. We showed the result of the short and immediate outcomes. Long-term effects are needed to have a solid conclusion about the safety and efficacy of non-intubated VATS.
In conclusion, our perioperative and short-term outcomes showed that non-intubated VATS lobectomy for lung cancer is comparable to intubated VATS lobectomy. For that, we believe that this approach is technically feasible and safe when performed by an expert surgeon and anesthesiologist. However, further prospective randomized studies are needed for a better comparison between non-intubated and intubated VATS lobectomy for oncological outcomes.
Acknowledgements
None.
Ethical Statement: The study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital (No. KC18RESI0173).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Nwogu CE, D'Cunha J, Pang H, et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance). Ann Thorac Surg 2015;99:399-405. 10.1016/j.athoracsur.2014.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlens E. A new flexible double-lumen catheter for bronchospirometry. J Thorac Surg 1949;18:742-6. [PubMed] [Google Scholar]
- 3.Guo Z, Shao W, Yin W, et al. Analysis of feasibility and safety of complete video-assisted thoracoscopic resection of anatomic pulmonary segments under non-intubated anesthesia. J Thorac Dis 2014;6:37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gothard J. Lung injury after thoracic surgery and one-lung ventilation. Curr Opin Anaesthesiol 2006;19:5-10. 10.1097/01.aco.0000192783.40021.c1 [DOI] [PubMed] [Google Scholar]
- 5.Fitzmaurice BG, Brodsky JB. Airway rupture from double-lumen tubes. J Cardiothorac Vasc Anesth 1999;13:322-9. 10.1016/S1053-0770(99)90273-2 [DOI] [PubMed] [Google Scholar]
- 6.Pompeo E, Tacconi F, Mineo D, et al. The role of awake video-assisted thoracoscopic surgery in spontaneous pneumothorax. J Thorac Cardiovasc Surg 2007;133:786-90. 10.1016/j.jtcvs.2006.11.001 [DOI] [PubMed] [Google Scholar]
- 7.Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg 2004;78:1761-8. 10.1016/j.athoracsur.2004.05.083 [DOI] [PubMed] [Google Scholar]
- 8.Pompeo E, Rogliani P, Tacconi F, et al. Randomized comparison of awake nonresectional versus nonawake resectional lung volume reduction surgery. J Thorac Cardiovasc Surg 2012;143:47-54, 54.e1. [DOI] [PubMed]
- 9.Al-Abdullatief M, Wahood A, Al-Shirawi N, et al. Awake anaesthesia for major thoracic surgical procedures: an observational study. Eur J Cardiothorac Surg 2007;32:346-50. 10.1016/j.ejcts.2007.04.029 [DOI] [PubMed] [Google Scholar]
- 10.Chen JS, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg 2011;254:1038-43. 10.1097/SLA.0b013e31822ed19b [DOI] [PubMed] [Google Scholar]
- 11.Zhao Z-R, Lau RWH, Ng CSH. Non-intubated video-assisted thoracic surgery: the final frontier? Eur J Cardiothorac Surg 2016;50:925-6. 10.1093/ejcts/ezw183 [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Rivas D, Bonome C, Fieira E, et al. Non-intubated video-assisted thoracoscopic lung resections: the future of thoracic surgery? Eur J Cardiothorac Surg 2016;49:721-31. 10.1093/ejcts/ezv136 [DOI] [PubMed] [Google Scholar]
- 13.Kiss G, Castillo M. Nonintubated anesthesia in thoracic surgery: general issues. Ann Transl Med 2015;3:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Cui F, Pompeo E, et al. The impact of non-intubated versus intubated anaesthesia on early outcomes of video-assisted thoracoscopic anatomical resection in non-small-cell lung cancer: a propensity score matching analysis. Eur J Cardiothorac Surg 2016;50:920-5. 10.1093/ejcts/ezw160 [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Cui F, He J. Non-intubated video-assisted thoracoscopic surgery anatomical resections: a new perspective for treatment of lung cancer. Ann Transl Med 2015;3:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu CY, Chen JS, Lin YS, et al. Feasibility and safety of nonintubated thoracoscopic lobectomy for geriatric lung cancer patients. Ann Thorac Surg 2013;95:405-11. 10.1016/j.athoracsur.2012.10.082 [DOI] [PubMed] [Google Scholar]
- 17.Hung MH, Chan KC, Liu YJ, et al. Nonintubated thoracoscopic lobectomy for lung cancer using epidural anesthesia and intercostal blockade: a retrospective cohort study of 238 cases. Medicine (Baltimore) 2015;94:e727. 10.1097/MD.0000000000000727 [DOI] [PMC free article] [PubMed] [Google Scholar]