Abstract
Background
Pericardiectomy is an effective treatment for constrictive pericarditis (CP). Early postoperative complications such as refractory hypotension and congestive heart failure occur in these patients and are associated with increased morbidity and mortality. We hypothesized that left ventricular (LV) myocardial strain measured by two-dimensional speckle tracking echocardiography (2DSTE) could identify early cardiac dysfunction and relate to acute postoperative adverse events in CP patients.
Methods
Forty-four CP patients with preserved left ventricular ejection fraction (LVpEF, 64%±8%) and 44 age- and sex-matched controls were enrolled. Conventional 2DSTE was performed before pericardiectomy. Global and segmental peak systolic strain values were measured. The primary endpoint was a composite of postoperative refractory hypotension, congestive heart failure and cardiogenic death. Refractory hypotension was defined as hypotension requiring prolonged usage of intravenous inotropic medication (IVIM) (≥2 days).
Results
Postoperative refractory hypotension occurred in 26 cases, and no patients experienced congestive heart failure or cardiogenic death. Compared to controls, CP patients had decreased absolute global and segmental circumferential strain (CS), radial strain (RS), and longitudinal strain (LS) except septal LS. Patients with refractory hypotension exhibited lower epicardial CS (P=0.04). Epicardial CS was an independent risk factor correlated with postoperative adverse outcome [P=0.014, OR =1.236 (1.044–1.464)] while LVEF was not. Lower absolute value of epicardial CS was related to higher (P=0.02) and longer usage of intravenous furosemide (P=0.04) to keep negative fluid balance perioperatively.
Conclusions
LV strain value is markedly reduced in patients with CP and LVpEF. Lower preoperative epicardial CS value is associated with greater risk of early refractory hypotension and more aggressive fluid management.
Keywords: Strain, constrictive pericarditis (CP), pericardiectomy, outcome
Introduction
Constrictive pericarditis (CP) is characterized by pericardia constraint due to inflammation and/or fibrosis which leads to impaired diastolic function. Pericardiectomy is the definitive treatment for chronic CP patients by relieving pericardial restraint, and is recommended once patients are diagnosed (1).
However, 1.2% to 6% patients died within 30 days after surgery, and the most common cause of perioperative death was congestive heart failure (2-5). The benefit and risk of pericardiectomy depends not only on pericardial and diastolic involvement, but also on myocardial dysfunction of the heart. Concomitant systolic myocardial dysfunction caused by pathological changes such as myocardial atrophy, fibrosis, and insufficient perfusion may impede cardiac recovery in patients with constrictive pericardium (6-9). Echocardiography is a reliable noninvasive method to assess cardiac function before surgery. A previous report suggested that reduced left ventricular ejection fraction (LVEF) was a predictor for perioperative mortality (10). However, preoperative LVEF of most CP patients is within the normal range (11,12). Compared to patients who survived during the follow-up, patients who died during follow-up did not have a lowered preoperative LVEF (2), suggesting that LVEF itself plays a limited role in identifying early cardiac dysfunction and in predicting adverse outcome in CP patients.
Two-dimensional speckle tracking echocardiography (2DSTE) is an angle-independent and accurate method to better characterize subtle changes in left ventricular (LV) performance and mechanics (13). The purpose of this study was to use 2DSTE measurements to determine whether LV strain could identify impaired cardiac mechanics and relate to early postoperative cardiac adverse outcome in CP patients with preserved LVEF (LVpEF).
Methods
Patients
Consecutive forty-five patients with CP were admitted and underwent total pericardiectomy after assessed by echocardiography via GE equipment (Vivid7, GE Healthcare, Waukesha, WI, USA) in Peking Union Medical College Hospital from 2015 to 2017. One patient was excluded due to malignancies involving the pericardium. Forty-four patients were identified. Forty-four age- and sex-matched controls were selected from our healthy volunteer database. The study was approved by the Peking Union Medical College Hospital’s Institutional Review Board (ID: S-K494).
Speckle tracking echocardiographic studies
Echocardiography was performed using a standard ultrasound system (Vivid7, GE Healthcare, Waukesha, WI, USA). Two-dimensional gray-scale images were acquired in three long-axis views of the apical four-, two-, and three-chambers, and three short-axis views of the left ventricle at the levels of mitral valve, papillary muscle and apex. Global peak longitudinal strain (GLS), global circumferential strain (GCS), and global radial strain (GRS) were measured using the EchoPAC analytics software Version 113 (GE Healthcare, Waukesha, WI, USA). To assess the direct and indirect impact of the constrictive pericardium on cardiac mechanics, the left ventricle was divided into non-septal and septal sections. In particular, the system-generated region of interest in the mitral valve short-axis view was automatically divided into six segments by EchoPAC’s 2D strain analyzing system. Septal circumferential strain (CS) or radial strain (RS) was calculated by averaging segmental strain of the anterior and posterior ventricular septum. Free wall CS or RS was calculated by averaging the four non-septal segments of strain. Similarly, septal longitudinal strain (LS) was calculated by averaging six septal segments (system generated by EchoPAC) and free wall LS was determined by averaging the twelve non-septal segments in the three long-axis views (Figure 1). Epicardial CS and LS were generated from free wall CS and LS respectively. The endocardial boundary determination and strain measurement were performed by a single operator who was blinded to clinical information. To assess reliabilities of strain, 30 subjects (15 patients and 15 controls) were randomly selected and analyzed by two readers who were allowed to select the best cardiac cycle and blinded to previous measurements.
Figure 1.

Pictures of myocardia in short-axis (left) and long-axis (right) views were shown. Dark red regions indicate septal segments, and light red regions indicate epicardia of left ventricle. Septal and epicardial LS were calculated separately by averaging septal segments and epicardial segments in long-axis views (including apical two-, three-, and four-chamber views). Regional CS and RS were calculated similarly in short-axis views (including mitral valve, papillary muscle and apex levels). RV, right ventricle; MV, mitral valve; LV, left ventricle; CS, circumferential strain; RS, radial strain; LS, longitudinal strain.
Outcome
Postoperative follow-up was obtained from hospital medical records. Patients were followed up for 16.6±10.9 days’ post-pericardiectomy. The primary outcome was the composite of postoperative refractory hypotension, heart failure and cardiogenic death. Refractory hypotension was defined as hypotension (a systolic blood pressure <90 mmHg, a mean arterial blood pressure <60 mmHg, a decrease of more than 40 mmHg below the baseline, or any combination of these variables) requiring intravenous inotropic medication (IVIM) (such as dopamine, dobutamine, milrinone, or epinephrine) ≥2 days after correction of hypovolemia. Inotropic administration was initiated in the operating room if needed during pericardiectomy and weaned from their agents after recovery from anesthesia. Prolonged usage of inotropic medication due to refractory hypotension can lengthen recovery and hospitalization time. Thus we included the time on inotropic medication when evaluating the presence of refractory hypotension.
Statistical analysis
Results for continuous variables were expressed as means ± standard deviation (SD), medians (interquartile range, IQR) or frequencies as deemed appropriate. Intraclass correlation coefficient (ICC) was used to determine interobserver and intraobserver variability. The two-tailed unpaired t-test was used to evaluate differences in continuous variables between two groups, and the chi-squared test was used for categorical variables. The variables assessed as potential prognostic value of CP were evaluated with a binary logistic regression model. Multivariate analysis was performed using a stepwise forward logistic regression model to identify independent risk factors for adverse outcome. Statistical significance was set at P<0.05. Statistical analyses were performed using SPSS software (SPSS Version 23.0, Inc., Chicago, IL, USA).
Results
Patient characteristics
There were 25 male and 19 female patients included in this study (Table 1). All patients underwent total pericardiectomy via midline sternotomy. Patients, on average, had pericardial disease for 39 months (range from 1 month to 25 years). The average age was 40±17.8 years old. Sixteen patients (36%) had functional class [New York Heart Association (NYHA) III or IV]. Atrial fibrillation was present in 5 patients (11%). The most common etiology of CP was tuberculosis, in 59 percent of the cases (n=26); followed by idiopathic causes (n=16, 36%). Further etiologies included post-radiation (n=1) and bacterial infection (n=1). All patients had normal preoperative LVEF (64%±8%). Acute postoperative refractory hypotension occurred in 26 patients. No patients experienced congestive heart failure or cardiogenic death.
Table 1. Characteristics in patients with constrictive pericarditis.
| Variables | All (n=44) | With tuberculosis (n=26) | With non-TB etiologies (n=18) |
|---|---|---|---|
| Sex | |||
| Male | 25 (57%) | 14 (54%) | 11 (61%) |
| Female | 19 (43%) | 12 (46%) | 7 (39%) |
| Age, years | 40±17.8 | 40.9±16.5 | 38.6±19.9 |
| Heart rate, bpm | 89±17 | 92±19 | 86±13 |
| Blood pressure, mmHg | |||
| Systolic | 108±17 | 108±12 | 108±23 |
| Diastolic | 72±12 | 73±9 | 73±16 |
| NYHA classification | |||
| I–II | 28 (64%) | 17 (65%) | 11 (61%) |
| III | 15 (34%) | 9 (35%) | 6 (33%) |
| IV | 1 (2%) | 0 (0%) | 1 (6%) |
| Course, month | 39.3±61.8 | 24.0±28.4 | 61.4±87.2 |
| <5 years | 35 (80%) | 23 (88%) | 12 (67%) |
| ≥5 years | 9 (20%) | 3 (12%) | 6 (33%) |
| Atrial fibrillation | 5 (11%) | 2 (8%) | 3 (17%) |
| Congenital heart disease | 5 (11%) | 3 (12%) | 2 (11%) |
| Patent foramen ovale | 2 (5%) | 0 (0%) | 2 (11%) |
| Atrial septal defect | 3 (6%) | 3 (12%) | 0 (0%) |
| Previous thoracotomy operation | 5 (11%) | 2 (8%) | 3 (17%) |
| Pericardiac calcification | 6 (13%) | 3 (12%) | 3 (17%) |
| Pericardial effusion | 23 (52%) | 13 (50%) | 10 (56%) |
| Pleural effusion | 37 (84%) | 22 (85%) | 15 (83%) |
| Etiology of tuberculosis | 26 (59%) | 26 (100%) | 0 (0%) |
| Preoperative laboratory tests | |||
| ESR, mm/h | 16.1±14.1 | 15.9±14.4 | 16.5±14.2 |
| Alb, g/L | 34.5±9 | 34.2±8.6 | 34.8±9.8 |
| PT, s | 13.8±1.2 | 13.6±0.2 | 14.2±1.5 |
| TBil, ìmol/L | 24.8±16.5 | 21.5±12.2 | 29.5±20.8 |
| Cr, ìmol/L | 76.1±40.8 | 74.6±16.7 | 78.4±61.6 |
| Pre-CVP, mmHg | 19.1±4.5 | 19.8±3.7 | 18.1±5.4 |
| ΔCVP, mmHg | 10.2±3.4 | 10.2±3.4 | 10.2±3.6 |
| Length of stay, days | 37.9±23.3 | 36.0±23.2 | 40.7±23.8 |
Each value indicates the number of patients (n) or mean ± standard deviation. TB, tuberculosis; NYHA, New York Heart Association; ESR, erythrocyte sedimentation rate; Alb, albumin; PT, prothrombin time; TBil, total bilirubin; Cr, creatinine; pre-CVP, pre-pericardiectomy central venous pressure; ÄCVP, decline central venous pressure after pericardiectomy
LV strain
Compared to healthy controls, CP patients with LVpEF had significantly decreased absolute GCS (−21.9%±5.7% vs. −26.9%±3.7%, P<0.001), GRS (11.8%±5.4% vs. 22.9%±7.1%, P<0.001), and GLS (−19.4%±4.5% vs. −21.7%±2.1%, P<0.001) (Table 2). Patients also had decreased segmental strain value in both short-axis and long-axis views. But septal LS was relatively preserved. CP patients caused by TB had significantly lower CS value (GCS, −20.4%±5.2% vs. −23.9%±5.9%; P=0.045) than patients with other etiologies, and especially deteriorated in free wall epicardial strain (−13.7%±4.1% vs. −16.4%±4.3%; P=0.036). LVEF of patients was lower than controls (64%±8% vs. 72%±5%, P<0.001) though all of patients had LVpEF. Patients with CP had significantly lower left ventricular end-diastolic volume index (LVEDVi, 42.34±14.6 vs. 58.36±10.69, P<0.001) and higher left atrial volume index (LAVi, 30.72±18.29 vs. 12.44±3.4) than controls, while left ventricular end-systolic volume index (LVESVi) was comparable to controls.
Table 2. Echo profiles in constrictive pericarditis patients and controls.
| Variables | Patients | Controls | ||
|---|---|---|---|---|
| All | TB | Non-TB | ||
| Count (male/female) | 44 (25/19) | 26 (14/12) | 18 (11/7) | 44 (20/24) |
| Age, years | 40±17.8 | 40.9±16.5 | 38.6±19.9 | 41±8.9 |
| CS global (%) | −21.9±5.7 | −20.4±5.2# | −23.9±5.9 | −26.9±3.7* |
| Septal | −25.9±6.6 | −24.3±6.1 | −28.1±6.8 | −30.6±4.6* |
| Epicardial | −14.8±4.4 | −13.7±4.1# | −16.4±4.3 | −18.7±3.7* |
| RS global (%) | 11.8±5.4 | 11.6±5.7 | 12±5.1 | 22.9±7.1* |
| Septal | 12.6±6.2 | 12±6.5 | 13.4±5.8 | 23.3±7* |
| Free wall | 11.3±5.8 | 11.4±6 | 11.3±5.6 | 22.8±7.6* |
| LS global (%) | −19.4±4.5 | −18.8±4.5 | −20.4±4.4 | −21.7±2.1* |
| Septal | −21.5±4.7 | −20.7±4.8 | −22.5±4.5 | −21.3±2.3 |
| Epicardial | −15.9±4.4 | −15.3±4.3 | −16.6±4.5 | −19.5±2* |
| LVEF (%) | 64±8 | 63±9 | 66±8 | 72±5* |
| LVESV index | 14.97±7.65 | 16.52±8.75 | 12.73±5.12 | 16.22±3.97 |
| LVEDV index | 42.34±14.6 | 44.47±16.55 | 39.27±10.92 | 58.36±10.69* |
| LAV index | 30.72±18.29 | 28.44±13.19 | 34.02±23.89 | 12.44±3.4* |
The unit for strain is percentage (%). Asterisk (*) indicates significant difference between all patients and controls (P<0.05); pound sign (#) indicates significant difference (P<0.05) between patients with TB and the other etiologies (non-TB). TB, tuberculosis; CS, circumferential strain; RS, radial strain; LS, longitudinal strain; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; LVEDV, left ventricular end-diastolic volume; LAV, left atrial volume.
Interobserver and intraobserver variability
ICC of interobserver variability for GCS was 0.91, and they were 0.83 for GRS and 0.92 for GLS. ICC of intraobserver variability for GCS was 0.89, and they were 0.91 for GRS and 0.89 for GLS.
Pericardiectomy outcome
Compared to CP patients without postoperative refractory hypotension, patients who suffered from acute refractory hypotension had lower absolute value of GCS (−20.1%±5.0% vs. −24.4%±5.9%, P=0.014) and segmental CS. Specifically, patients with worse outcome had significantly lower septal CS value (−23.9%±6.2% vs. −28.6%±6.1%, P=0.018) and epicardial CS value (−13.4%±3.6% vs. −16.9%±4.6%, P=0.007) (Figure 2).
Figure 2.
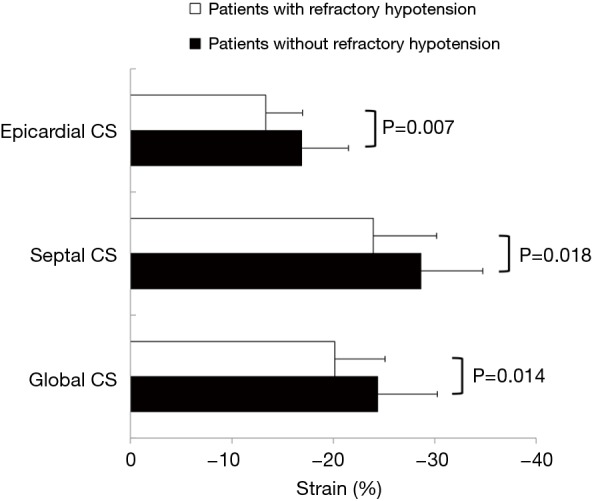
Comparison of baseline circumferential strain between patients with or without refractory hypotension. Patients with constrictive pericarditis who suffered from postoperative refractory hypotension had lower absolute value of global and epicardial circumferential strain than who didn’t. CS, circumferential strain.
Univariate analysis identified GCS, septal CS, free wall midcardial and epicardial CS as significant risk factors for postoperative refractory hypotension, while clinical parameters (showed in Table 1) and conventional echo measurements evaluating CP patients’ myocardial function such as LVEF (showed in Table 2), didn’t have significant association. Multivariate logistic regression analysis revealed that free wall epicardial CS was independent risk factor correlated with postoperative adverse cardiac outcome of CP [free wall epicardial CS, P=0.014, OR =1.236 (1.044–1.464)] (Table 3). We used receiver operating characteristic (ROC) curve analysis to estimate value of CS to distinguish CP patients with postoperative outcome from those without. The results showed the area under the curve (AUC) of free wall epicardial CS was 0.70 (P=0.02). Additionally in tuberculous patients, the AUC of epicardial CS was 0.80 (P=0.01), and GCS was 0.77 (P=0.03) (Figure 3).
Table 3. Relation between strain and LVEF to postoperative refractory hypotension.
| Variables | Outcome | ||||
|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | ||||
| P value | OR (95% CI) | P value | OR (95% CI) | ||
| CS global | 0.022 | 1.16 (1.022–1.316) | |||
| Septal | 0.025 | 1.134 (1.016–1.264) | |||
| Epicardial | 0.014 | 1.236 (1.044–1.464) | 0.014 | 1.236 (1.044–1.464) | |
| RS global | 0.751 | 0.982 (0.877–1.099) | |||
| Septal | 0.717 | 0.982 (0.89–1.084) | |||
| Free wall | 0.802 | 0.987 (0.888–1.096) | |||
| LS global | 0.450 | 1.055 (0.918–1.213) | |||
| Septal | 0.332 | 1.069 (0.934–1.222) | |||
| Epicardial | 0.493 | 1.050 (0.913–1.209) | |||
| LVEF | 0.969 | 1.159 (0.001–1,823.122) | |||
CS, circumferential strain; RS, radial strain; LS, longitudinal strain; LVEF, left ventricular ejection fraction.
Figure 3.
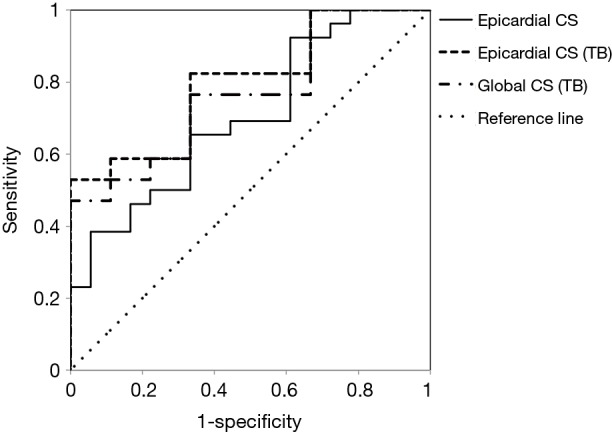
Receiver operating characteristic curves of circumferential strain (CS) to distinguish constrictive pericarditis patients with acute postoperative refractory hypotension from those without. The results showed the area under the curve (AUC) of free wall epicardial CS was 0.70 (P=0.02). Additionally in tuberculous patients, the AUC of epicardial CS was 0.80 (P=0.01), and GCS was 0.77 (P=0.03). CS, circumferential strain; TB, tuberculosis.
Furthermore, we investigated preliminarily correlation between preoperative epicardial CS and clinical treatment after surgery (n=22). Patients with relatively lower preoperative absolute epicardial CS (cut-off point was −14.8%, the mean value of CS) need more time to keep negative fluid balance during 7 days after surgery (69%±5% vs. 51%±7%, P=0.04). Higher postoperative intravenous furosemide usage (≥300 vs. <300 mg) was related to lower absolute value of baseline epicardial CS (−13.6%±0.9% vs. −18.0%±1.5%, P=0.02).
Discussion
In this study, we evaluated relationship between preoperative strain and early prognosis of CP patients following pericardiectomy. We found that CS, RS and LS of CP patients with LVpEF were markedly reduced than healthy controls. Compared to patients with the other etiologies, patients due to TB had lower CS value. We identified strain as an important parameter correlating with postoperative refractory hypotension, specifically lower epicardial CS was an independent risk factor for early postoperative complication.
It was reported that 30-day perioperative mortality was 1.2–6% among CP patients who underwent pericardiectomy (2,3), and it cannot be ignored considering the great beneficial role of surgery that 5-year postoperative survival among those patients was 78–95% (2,3,14). Low cardiac output syndrome was the most common complication during the early postoperative phase, and heart failure accounts for nearly half of all deaths (4,5). In addition to incomplete epicardial resection, pathological changes to the myocardium can lead to post-pericardiectomy complications (15). Pathological changes to the pericardium and myocardium help to elucidate CP disease progression. Gradual pericardial restriction leads to disuse atrophy (6,7) while direct penetration of pathogens or concomitant pathologic processes to both the myocardium and pericardium can cause myocardial fibrosis (8,9). Therefore, the extent of preoperative myocardial involvement may affect the effectiveness of pericardiectomy and contribute to severe postoperative complications and cardiac dysfunction even death. Busch and colleagues (10) found reduced LVEF (between 35% and 55%) was an independent predictor for perioperative mortality, and preserved LVEF (>56%) was protective in CP patients. However, according to our study, early postoperative refractory hypotension occurred in a high proportion of CP patients with LVpEF (59%), which may due to long disease course and TB as the primary etiology (59%) of the patients, because tuberculosis related CP was associated with high mortality and the incidence of severe complications (16). In our study, we found TB patients had lower CS absolute value compared to non-TB patients, indicating more severe myocardial involvement. However, occurrence of refractory hypotension in tuberculous CP patients was not significantly higher than non-TB patients in our study (TB vs. non-TB, 65.4% vs. 50%, P=0.361), which may result from the small sample size.
Parameters are needed to early detect and evaluate myocardial dysfunction of CP patients in clinical decision-making, despite some clinical and laboratory indicators currently being used as predictors of poor overall survival (1). Ha et al. used catheterization to identify hemodynamic parameters to assess the risk of cardiac dysfunction and mortality in CP patients (17). They found more frequent administration of postoperative inotropic support and higher postoperative mortality in patients with both abnormal contractility and abnormal relaxation. But cardiac catheterization was not often used due to its invasiveness and associated costs. Echocardiography is widely applied as a promising technique to provide potential valuable parameters to predict adverse outcome. Kang and colleagues (2) found that small LV end-systolic dimension index was a significant predictor of mortality after pericardiectomy. Additionally, LVEF was reported to be a risk factor of early (30 days) mortality (10). However, our study did not show correlations between conventional echo methods and postoperative refractory hypotension. This suggested variables based on geometric assumptions may not be always applicable to a distorted heart from CP because of the anomalous tethering by inflamed pericardium.
There are multiple reports that 2DSTE imaging is a reliable method to determine ventricular myocardial function (18,19). Decreased global CS and RS in CP patients compared with healthy controls was reported (20-22), while LS value reduced less (21). Shell-like, fibrotic and even calcified pericardium tethering in CP patients may predominantly affect subepicardial function thus impact LV circumferential mechanics rather than radial mechanics (23). Impaired radial and LS is associated with transmural myocardial injury (23). In our study, CP patients had lower levels of CS, RS and LS compared with healthy controls, indicating our patients were relatively severe. Additionally, patients with tuberculous had worse CS in epicardia reflecting outside-in involvement and possible concomitant myocarditis. Considering those patients with LVpEF, our results supported the consensus that 2DSTE imaging could provide more comprehensive information about heart mechanics so that it may be able to detect subclinical myocardial dysfunction at an earlier stage as compared with conventional imaging modalities (24-26).
An improvement in LV CS and LS after surgery suggested the cardiac mechanics (27,28). The relation of LV mechanics using 2DSTE imaging to postoperative clinical status was reported by Li et al. (29), showing that an increase of global CS between post- and pre-pericardiectomy was associated with improved heart function. Under the circumstances that pericardiectomy is the only curative treatment for patients with CP, it highlights the potential value of strain- a parameter to recognize possible reversible deformation of the myocardium and a predictive parameter for early myocardial dysfunction in CP patients. In our study, we found that CP patients with LVpEF had reduced strain value, which was associated with acute postoperative refractory hypotension.
Our results showed that compared to septal strain, epicardial strain declined more. Regional peri-myocardial adhesions and tethering may account for the difference between septal and free wall strain. Kusunose and associates (27) found similar results that strain was depressed in the LV anterolateral wall but preserved in the LV septal wall. Our study reported for the first time that regional strain-lower level of epicardial CS was a risk factor for detecting poor postoperative condition of patients with CP. For the tuberculous patients, epicardial CS could also distinguish patients who suffered acute refractory hypotension from those who didn’t. The relationship between epicardial CS and postoperative diuretic treatment suggested that patients with reduced baseline epicardial CS might benefit from more aggressive fluid management.
According to our study, reduced strain could help us evaluate ventricular myocardial contraction and was associated with acute postoperative refractory hypotension in patients with LVpEF, which may give us a new perspective on early identification of high-risk CP patients. A prospective study with a longer term of follow-up is needed in the future.
Limitation
Our study had a few known limitations. First, the sample size was small due to the selection criteria. We included not only patients with CP who underwent solitary pericardiectomy but also patients requiring concomitant cardiac procedures (n=5, 2 atrioseptopexy, 1 bipolar radiofrequency ablation, 1 mitral valvuloplasty and 1 aortic valve replacement), which may add extra risk to patients who had isolated CP. However we did not find the differences in postoperative condition between patients undergoing concomitant procedures and solitary pericardiectomy. Also other vendors and software were not included for speckle tracking analysis, which may impact the generalizability. Second, we considered acute postoperative refractory hypotension as outcome in this study. This limits the ability to understand long-term cardiac adverse outcome of these patients. In further studies, a longer term of follow-up and analysis of strain will be required to verify the prognostic value of 2DSTE for CP patients.
Conclusions
This study found LV strain was markedly reduced in CP patients with LVpEF, and showed the value of evaluating myocardial function for disease prognosis. A lower preoperative CS values were associated with higher risk of postoperative refractory hypotension. Our results indicated high-risk of patients with LVpEF could be identified by 2DSTE imaging, and more aggressive fluid management may be needed in these patients.
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation of China (grant number 81470426); and the Peking Union Medical College Hospital Young and Middle-aged Investigation Fund (grant number PUMCH-2016-1.12).
Ethical Statement: The study was approved by the Peking Union Medical College Hospital’s Institutional Review Boar (No. S-K49).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Adler Y, Charron P, Imazio M, et al. 2015 ESC Guidelines for the Diagnosis and Management of Pericardial Diseases. Rev Esp Cardiol (Engl Ed) 2015;68:1126. 10.1016/j.rec.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 2.Kang SH, Song JM, Kim M, et al. Prognostic predictors in pericardiectomy for chronic constrictive pericarditis. J Thorac Cardiovasc Surg 2014;147:598-605. 10.1016/j.jtcvs.2013.01.022 [DOI] [PubMed] [Google Scholar]
- 3.Ling LH, Oh JK, Schaff HV, et al. Constrictive Pericarditis in the Modern Era. Circulation 1999;100:1380. 10.1161/01.CIR.100.13.1380 [DOI] [PubMed] [Google Scholar]
- 4.Ghavidel AA, Gholampour M, Kyavar M, et al. Constrictive pericarditis treated by surgery. Tex Heart Inst J 2012;39:199-205. [PMC free article] [PubMed] [Google Scholar]
- 5.Vistarini N, Chen C, Mazine A, et al. Pericardiectomy for Constrictive Pericarditis: 20 Years of Experience at the Montreal Heart Institute. Ann Thorac Surg 2015;100:107-13. 10.1016/j.athoracsur.2015.02.054 [DOI] [PubMed] [Google Scholar]
- 6.Sawyer CG, Burwell CS, Dexter L, et al. Chronic constrictive pericarditis: further consideration of the pathologic physiology of the disease. Am Heart J 1952;44:207-30. 10.1016/0002-8703(52)90146-4 [DOI] [PubMed] [Google Scholar]
- 7.Dines DE, Edwards JE, Burchell HB. Myocardial atrophy in constrictive pericarditis. Proc Staff Meet Mayo Clin 1958;33:93-9. [PubMed] [Google Scholar]
- 8.Somerville W. Constrictive pericarditis. With special reference to the change in natural history brought about by surgical intervention. Circulation 1968;38:102-10. 10.1161/01.CIR.38.1S5.V-102 [DOI] [PubMed] [Google Scholar]
- 9.Levine HD. Myocardial Fibrosis in Constrictive Pericarditis. Circulation 1973;48:1268. 10.1161/01.CIR.48.6.1268 [DOI] [PubMed] [Google Scholar]
- 10.Busch C, Penov K, Amorim PA, et al. Risk factors for mortality after pericardiectomy for chronic constrictive pericarditis in a large single-centre cohort. Eur J Cardiothorac Surg 2015;48:e110-6. [DOI] [PubMed] [Google Scholar]
- 11.Tokuda Y, Miyata H, Motomura N, et al. Outcome of Pericardiectomy for Constrictive Pericarditis in Japan: A Nationwide Outcome Study. Ann Thorac Surg 2013;96:571-6. 10.1016/j.athoracsur.2013.04.054 [DOI] [PubMed] [Google Scholar]
- 12.Lin Y, Zhou M, Xiao J, et al. Treating Constrictive Pericarditis in a Chinese Single-Center Study: A Five-Year Experience. Ann Thorac Surg 2012;94:1235-40. 10.1016/j.athoracsur.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 13.Madeira M, Teixeira R, Costa M, et al. Two-dimensional speckle tracking cardiac mechanics and constrictive pericarditis: systematic review. Echocardiography 2016. 10.1111/echo.13293 [DOI] [PubMed] [Google Scholar]
- 14.Avgerinos D, Rabitnokov Y, Worku B, et al. Fifteen-year experience and outcomes of pericardiectomy for constrictive pericarditis. J Card Surg 2014;29:434-8. 10.1111/jocs.12344 [DOI] [PubMed] [Google Scholar]
- 15.Bozbuga N, Erentug V, Eren E, et al. Pericardiectomy for chronic constrictive tuberculous pericarditis: risks and predictors of survival. Tex Heart Inst J 2003;30:180-5. [PMC free article] [PubMed] [Google Scholar]
- 16.Yangni-Angate KH, Tanauh Y, Meneas C, et al. Surgical experience on chronic constrictive pericarditis in African setting: review of 35 years' experience in Cote d'Ivoire. Cardiovasc Diagn Ther 2016;6:S13-9. 10.21037/cdt.2016.09.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ha JW, Oh JK, Schaff HV, et al. Impact of left ventricular function on immediate and long-term outcomes after pericardiectomy in constrictive pericarditis. J Thorac Cardiovasc Surg 2008;136:1136-41. 10.1016/j.jtcvs.2008.03.065 [DOI] [PubMed] [Google Scholar]
- 18.Amundsen BH, Helle-Valle T, Edvardsen T, et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol 2006;47:789-93. 10.1016/j.jacc.2005.10.040 [DOI] [PubMed] [Google Scholar]
- 19.Korinek J, Wang J, Sengupta PP, et al. Two-dimensional strain--a Doppler-independent ultrasound method for quantitation of regional deformation: validation in vitro and in vivo. J Am Soc Echocardiogr 2005;18:1247-53. 10.1016/j.echo.2005.03.024 [DOI] [PubMed] [Google Scholar]
- 20.Dal-Bianco JP, Sengupta PP, Mookadam F, et al. Role of echocardiography in the diagnosis of constrictive pericarditis. J Am Soc Echocardiogr 2009;22:24-33; quiz 103-4. 10.1016/j.echo.2008.11.004 [DOI] [PubMed] [Google Scholar]
- 21.Amaki M, Savino J, Ain DL, et al. Diagnostic concordance of echocardiography and cardiac magnetic resonance-based tissue tracking for differentiating constrictive pericarditis from restrictive cardiomyopathy. Circ Cardiovasc Imaging 2014;7:819-27. 10.1161/CIRCIMAGING.114.002103 [DOI] [PubMed] [Google Scholar]
- 22.Sengupta PP, Krishnamoorthy VK, Abhayaratna WP, et al. Disparate patterns of left ventricular mechanics differentiate constrictive pericarditis from restrictive cardiomyopathy. JACC Cardiovasc Imaging 2008;1:29-38. 10.1016/j.jcmg.2007.10.006 [DOI] [PubMed] [Google Scholar]
- 23.Geyer H, Caracciolo G, Abe H, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr 2010;23:351-69; quiz 453-5. 10.1016/j.echo.2010.02.015 [DOI] [PubMed] [Google Scholar]
- 24.Aurigemma GP, Silver KH, Priest MA, et al. Geometric changes allow normal ejection fraction despite depressed myocardial shortening in hypertensive left ventricular hypertrophy. J Am Coll Cardiol 1995;26:195-202. 10.1016/0735-1097(95)00153-Q [DOI] [PubMed] [Google Scholar]
- 25.Vinereanu D, Ionescu AA, Fraser AG. Assessment of left ventricular long axis contraction can detect early myocardial dysfunction in asymptomatic patients with severe aortic regurgitation. Heart 2001;85:30-6. 10.1136/heart.85.1.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Menyar AA, Galzerano D, Asaad N, et al. Detection of myocardial dysfunction in the presence of normal ejection fraction. J Cardiovasc Med (Hagerstown) 2007;8:923-33. 10.2459/JCM.0b013e328014daf2 [DOI] [PubMed] [Google Scholar]
- 27.Kusunose K, Dahiya A, Popovic ZB, et al. Biventricular mechanics in constrictive pericarditis comparison with restrictive cardiomyopathy and impact of pericardiectomy. Circ Cardiovasc Imaging 2013;6:399-406. 10.1161/CIRCIMAGING.112.000078 [DOI] [PubMed] [Google Scholar]
- 28.Negishi K, Popovic ZB, Negishi T, et al. Pericardiectomy is Associated with Improvement in Longitudinal Displacement of Left Ventricular Free Wall Due to Increased Counterclockwise Septal-to-Lateral Rotational Displacement. J Am Soc Echocardiogr 2015;28:1204-13, e2. [DOI] [PubMed]
- 29.Li L, Deng YB, Liu K, et al. Long-Term Effects of Pericardiectomy on Left Ventricular Mechanics Evaluated by Using Speckle Tracking Echocardiography in Patients with Constrictive Pericarditis. Ultrasound Med Biol 2016;42:421-9. 10.1016/j.ultrasmedbio.2015.10.025 [DOI] [PubMed] [Google Scholar]


