Abstract
Lung transplantation is now a treatment option for many patients with end-stage lung disease. Now 55 years since the first human lung transplant, this is a good time to reflect upon the history of lung transplantation, to recognize major milestones in the field, and to learn from others’ unsuccessful transplant experiences. James Hardy was instrumental in developing experimental thoracic transplantation, performing the first human lung transplant in 1963. George Magovern and Adolph Yates carried out the second human lung transplant a few days later. With a combined survival of only 26 days for these first 2 lung transplant recipients, the specialty of lung transplantation clearly had a long way to go. The first “successful” lung transplant, in which the recipient survived for 10.5 months, was reported by Fritz Derom in 1971. Ten years later, Bruce Reitz and colleagues performed the first successful en bloc transplantation of the heart and one lung with a single distal tracheal anastomosis. In 1988, Alexander Patterson performed the first successful double lung transplant. The modern technique of sequential double lung transplantation and anastomosis performed at the mainstem bronchus level was originally described by Henri Metras in 1950, but was not reintroduced into the field until Pasque reported it again in 1990. Since then, lung transplantation has seen landmark changes: evolving immunosuppression regimens, clarifying the definition of primary graft dysfunction (PGD), establishing the lung allocation score (LAS), introducing extracorporeal membrane oxygenation (ECMO) as a bridge to transplant, allowing donation after cardiac death, and implementing ex vivo perfusion, to name a few. This article attempts to connect the historical dots in this field of research, with the hope that our effort helps summarize what has been achieved, and identifies opportunities for future generations of transplant pulmonologists and surgeons alike.
Keywords: Extracorporeal membrane oxygenation (ECMO), history, immunosuppression, lung transplantation, primary graft dysfunction (PGD)
Introduction
Human interest in solid organ transplantation and xenotransplantation can be traced back to Hindu mythology, when Lord Ganesha received an elephant’s head after he was accidentally decapitated (Figure 1). In figurines and iconography, Lord Ganesha is depicted showing signs of side effects from steroid-like medication, including voracious appetite, truncal obesity, abdominal striae, telangiectasia, and osteoporosis.
Figure 1.

Lord Ganesha, in one of the earliest depictions of xenotransplantation.
While working at the Rockefeller Institute in 1907, Alexis Carrel (Figure 2) provided the technical basis of solid organ transplantation by being the first to describe various blood vessel anastomosis techniques (1). He transplanted a kitten’s lung into the neck of an adult cat. Unfortunately, the cat developed sepsis 2 days later. Vladimir Demikhov refined the initial attempts at lung transplantation nearly 40 years later, when he first transplanted heart and lungs in a dog (2). He followed this experiment with the first lung-alone transplantation in a dog in 1947. Later that year, he performed a lower-lobe transplantation in a dog and achieved 10-day survival. The dog died due to bronchial disruption (3). In his laboratory at the University of Mississippi Medical Center some 16 years later, James Hardy carried out experimental studies of homo- and re-transplantations of lungs in hundreds of animals, and ultimately was successful in surgically removing lungs and re-implanting them in the same animal. These lungs retained sufficient function to sustain survival even when the in situ contralateral pulmonary artery was ligated (4). These early studies in the field of transplantation set the foundation for the first attempts at lung transplantation in human patients.
Figure 2.

Alexis Carrel, a pioneer of early blood vessel anastomosis techniques and solid organ transplantation. Reprinted with permission from the Library of Congress, Prints & Photographs Division, Reproduction Number: LC-DIG-ggbain-34418.
Early human lung transplantation
On June 11, 1963, James Hardy performed the first human lung transplant (Figure 3) (5). The patient undergoing the transplant had been diagnosed with advanced lung cancer and a lung abscess, and the odds of long-term success were not in Hardy’s favor.
Figure 3.
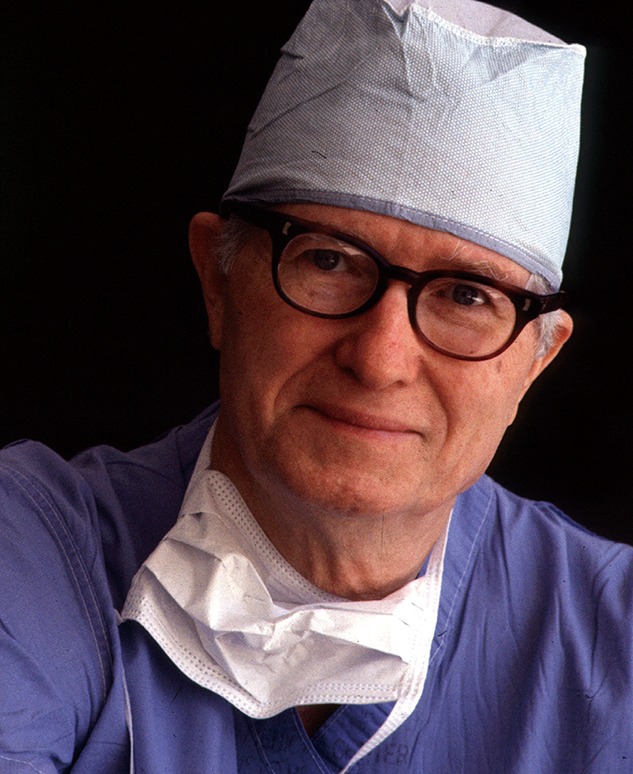
James Hardy, who performed the first human lung transplant. Reprinted with permission from University of Mississippi Medical Center.
Despite the deck being stacked against Hardy and his team, the surgery itself was successful. However, this monumental day in the history of medicine was overshadowed by the shooting of a prominent civil rights activist named Medgar Evers. Evers was shot on the driveway of his home in Jackson, Mississippi and was brought to the University of Mississippi Hospital emergency department. Hardy’s chief resident, Martin Dalton, had to excuse himself from the transplant procedure to provide emergent care to Evers, but the attempts at resuscitating Evers were sadly unsuccessful and he died. Hardy, after a successful surgery, heard a great uproar outside the operating room and later learned that it was not in reaction to his landmark achievement, but rather in pained response to Evers’ tragic death. The first-ever human lung transplantation managed to find its way to only the bottom corner of the front page of the morning newspaper. The recipient unfortunately died on day 18, but interestingly enough, the cause of death was renal failure—no graft rejection was identified at the time of the autopsy (6).
George Magovern and Adolph Yates reported the second human lung transplant just a few days later at the then-named Presbyterian-University Hospital in Pittsburgh (7). The recipient died just 1 week later. With a combined survival of only 26 days for the first 2 patients who underwent lung transplant, the specialty clearly had a long way to go. Over the next few years, 20 surgeons worldwide performed a total of 23 lung transplants, all with little or no success (8).
It was not until 1971 that the first successful lung transplantation was reported by Fritz Derom in Belgium (9). The patient, an individual with end-stage silicosis, survived 10.5 months after the procedure. The palliative benefit of the transplant was questionable, however, given that the patient spent most of his postoperative life in the hospital (10). At the time, no criteria existed that helped physicians identify which patients would obtain maximum benefit from lung transplantation, and most recipients were debilitated or had multi-organ dysfunction. By 1983, more than two decades had passed since the initial lung transplantation, and the total number of lung transplant recipients had reached 40. Derom’s patient still held the distinction of having the longest survival, but interestingly, one of the patients who declined lung transplantation during this period lived for 1.6 years (10).
Double lung transplantation
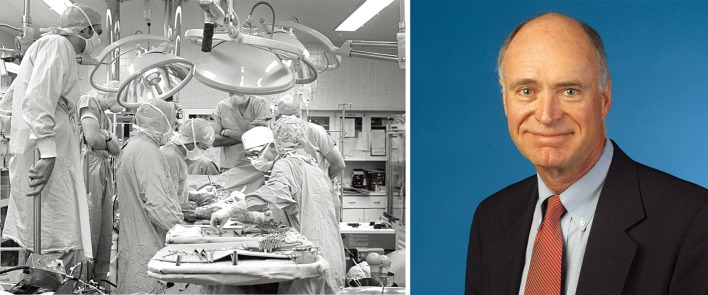
Until March 9, 1981, all human lung transplants were single-lung-only transplants. It was on this date that Bruce Reitz and colleagues performed the first successful transplantation of the heart and one lung in a 45-year-old woman diagnosed with primary pulmonary hypertension (Figure 4) (11). In this en bloc heart-lung transplant with a single distal tracheal anastomosis, the coronary-bronchial collaterals and other mediastinal tissue collaterals helped maintain the blood supply to the anastomosis (12). Five years later, Alexander Patterson (Figure 5) and Joel Cooper performed the first successful double lung transplant in a 42-year-old Toronto woman with emphysema secondary to alpha1-antitrypsin deficiency (13). The lungs were transplanted en bloc, and surgeons soon learned that this technique was likely to cause tracheal dehiscence. Thus, the modern technique of sequential double lung transplantation emerged, and anastomosis has since been performed at the mainstem bronchus level. This procedure, which is now the method of choice, was originally described by Henri Metras in 1950 (Figure 6) (14), and was reintroduced at Washington University in St. Louis by Pasque in 1990 (15).
Figure 4.
The first en bloc heart-lung transplant (left) was carried out at Stanford in 1981 by Bruce Reitz (right) et al. Reprinted with permission from the Stanford School of Medicine Office of Communication & Public Affairs.
Figure 5.

Alexander Patterson, who performed the first en bloc double lung transplant. Reprinted with permission from Washington University in St. Louis, Missouri.
Figure 6.

Henri Metras, who first described anastomosis at the mainstem bronchus level, which is still used in practice today. Reprinted with permission from Metras D. Henri Metras: a pioneer in lung transplantation. J Heart Lung Transplant, 1992;11(6):1213-5.
Bronchial anastomosis
Failure of the bronchial anastomosis was the reason for Demikhov’s unsuccessful transplant in 1947 (2), and for several decades was to blame for the failure of many other lung transplants. By the late 1970s, 16 of the 20 patients worldwide who had survived more than 1 week posttransplant had experienced some form of major bronchial anastomotic complication that either caused or contributed to the eventual failure of their transplant (16). The bronchial circulation is not re-anastomosed in this procedure, thus explaining why the anastomosis is subject to ischemic complications. One of Frank Veith’s many contributions to the field of lung transplantation was his description of the telescoping anastomosis (Figure 7). In 1979, he made the pivotal discovery that a shorter donor bronchus led to improved anastomotic healing (10). The explanation for this is that a short donor bronchus segment minimizes the portion of the airway that is supplied solely by the bronchopulmonary collaterals.
Figure 7.

Frank Veith, the pioneer of the telescoping anastomosis and the short donor bronchus. Reprinted with permission from NYU Langone Health.
Frustrated with recurrent episodes of organ rejection, many surgeons began to over-treat their patients with immunosuppressive medications. This practice not only adversely affected wound healing but also increased the likelihood of infection, and infection at the surgical site is difficult to distinguish from rejection in the first place. Joel Cooper demonstrated decreased bronchial healing in patients treated with steroids, but no such adverse effects were seen in patients treated with azathioprine and cyclosporine (17,18). In 1983, Cooper and his Toronto-based group demonstrated improved anastomotic healing and neovascularization in patients who underwent omentopexy (Figure 8) (19). Subsequently, the telescoping bronchial technique was shown to provide effective bronchial healing, which eliminated the need for omentopexy in most cases (20). In the interim, wrapping of the intercostal muscle was also attempted in an effort to reinforce the anastomosis, but this practice was later abandoned due to lack of benefit. Incidentally, today’s end-to-end anastomosis has been proven to be superior to telescoping, although it is still a useful technique when there is a large disparity in the diameter of the donor and recipient bronchus (Figure 9). Anastomotic site dehiscence, meanwhile, is now ably managed with self-expanding metallic stents by today’s interventional pulmonologists (21).
Figure 8.

Joel Cooper, who demonstrated that bronchial healing was impaired in patients treated with steroids. Reprinted with permission from Dr. Joel Cooper.
Figure 9.
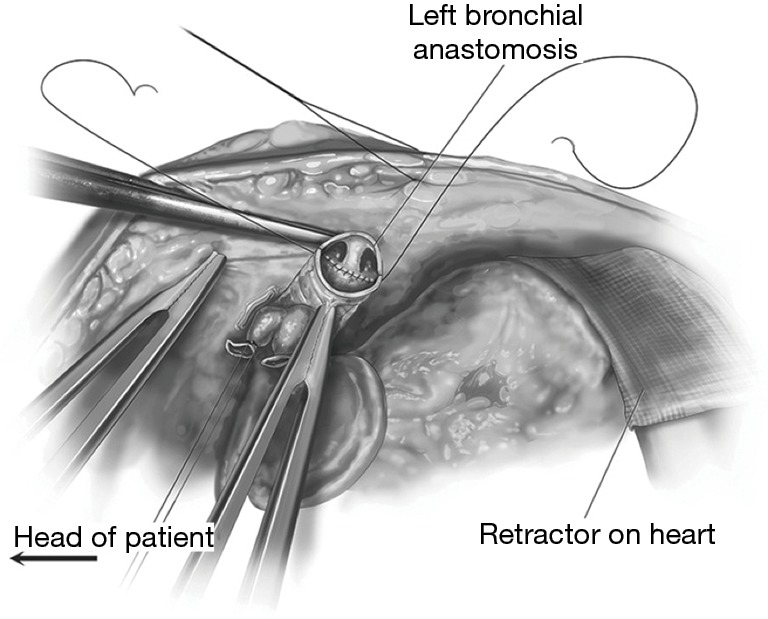
The bronchial anastomosis. Reprinted with permission from Dark JH. Median sternotomy for lung transplantation. Op Tech Thorac Cardiovasc Surg 2015;20:87-103.
Atrial cuff
In 1951, André Juvenelle published a report of a successful reimplantation of a lung autograft in a dog, which he carried out to achieve total lung denervation (22). He anastomosed the superior and inferior pulmonary veins separately and when he performed a left pneumonectomy, the re-implanted right lung could not sustain life (possibly due to pulmonary hypertension). Henri Metras in 1950 (23) described a technique (also independently described by Neptune shortly thereafter) (24) in which a cuff of the left atrium, including the pulmonary vein orifices, was transected (Figure 10). This technique helped the surgeon avoid having to anastomose veins individually, and also reduced the incidence of post-implantation pulmonary hypertension (as long as atrial cuff stenosis was avoided) (2). The physiological effect of re-implantation was found to be negligible in primates, however, and atrial cuff anastomosis was technically easier in dogs (25).
Figure 10.
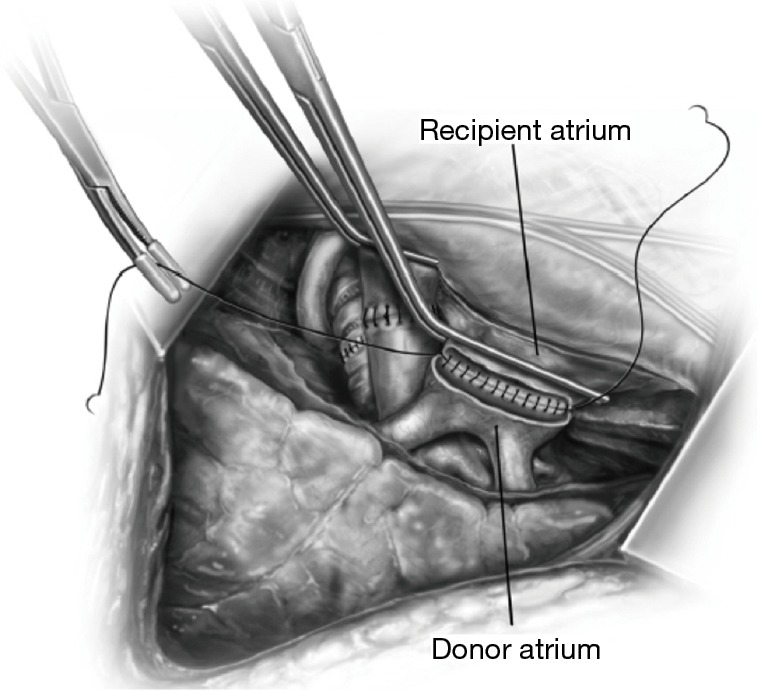
The atrial anastomosis. Reprinted with permission from Hayanga JW, D’Cunha J. The surgical technique of bilateral sequential lung transplantation. J Thoracic Dis 2014;6:1063-9.
Surgical incision
Patterson et al. pioneered the early technique of performing lung transplants through a median sternotomy with en bloc double-lung replacement (13). The limitations of this approach were soon evidenced by the substantial number of tracheal anastomotic complications, intraoperative bleeding in difficult cases, and right ventricular dysfunction in patients who required prolonged cardiopulmonary bypass (CPB) support (15).
The clamshell technique (Figure 11) was first described by Kortz in 1958 (26). A Washington University group adopted the clamshell technique in the 1980s, and Pasque et al. reported their experience with the clamshell approach (then called the “crossbow approach”) in 1990 (15). This approach involves bilateral anterior thoracotomies and transverse sternotomy. The fourth intercostal space is typically the site of entry, and the mammary arteries are ligated and divided. Compared to a median sternotomy, the clamshell thoracotomy provides better exposure of the pleural space and improves access to the posterior mediastinum. The enhanced anterolateral exposure facilitates a more successful left atrial anastomosis (27). The heart can also be elevated out of the pericardium to facilitate implantation. This incision also allows for sequential bilateral lung transplantation, which means CPB support can be avoided in many patients who would otherwise require it (15). Between October 1989 and July 1996, the Washington University group performed 193 bilateral sequential lung transplants using this approach (27).
Figure 11.
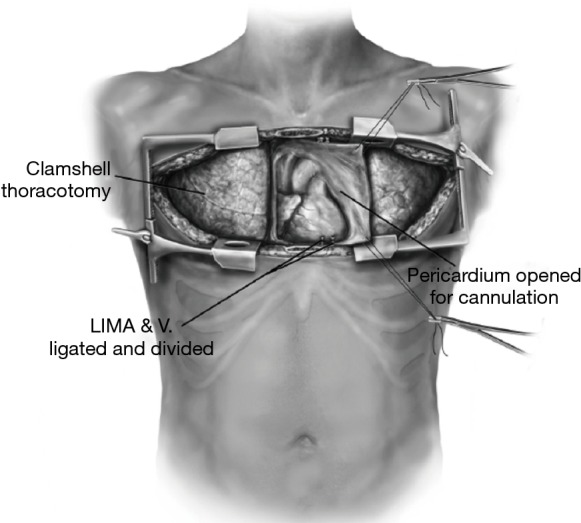
The clamshell incision, which provides improved pleural space exposure as well as sufficient posterior mediastinal access. Reprinted with permission from Hayanga JW, D’Cunha J. The surgical technique of bilateral sequential lung transplantation. J Thoracic Dis 2014;6:1063-9.
Sternal division is not without complications, however. This technique is associated with increased risk of angulation and anterior displacement of the distal sternum after closure with sutures—a problem called “sternal override.” Sternal wires may also be prominent beneath the skin of thin individuals and can even erode through the skin. Brown reported a 36% incidence of sternal disruption in lung transplant recipients who underwent bilateral thoracotomies at their institution (28). The Washington University group, meanwhile, reported deep sternal wound infections in 34% of their patients who underwent sternal division (27). Thus, starting in July 1996, the Washington University group began using bilateral anterolateral thoracotomies without sternal division. These sternal-sparing incisions are still used today and are associated with lower rates of wound infection and with better healing, but are not feasible in many cases due to limited exposure of the chest, and insertion of cannulas for bypass is more complicated (29). Although central cannulation or extracorporeal membrane oxygenation (ECMO)/CPB via the groin are possible solutions for patients with hemodynamic compromise, some surgeons today prefer a sternal-sparing approach when possible. The clamshell still has not gone out of favor (30,31).
Success & broadening the spectrum
The advent of cyclosporine in the early 1980s allowed for reduced steroid doses and for better anastomotic healing. Before the cyclosporine era, the world of lung transplantation could only claim one medium-term survivor—Fritz Derom’s patient who survived 10.5 months (12). With the use of cyclosporine, however, Joel Cooper and his group reported the first long-term survivor in 1983. A 58-year-old man with end-stage fibrosis who received a right-sided, single-lung transplant with an omental wrap (32). By 1987, Cooper had 4 long-term survivors (33). The Toronto group thus far had successfully transplanted only patients with pulmonary fibrosis. Chronic obstructive pulmonary disease (COPD), pulmonary hypertension, and cystic fibrosis were considered contraindications to lung transplantation (10).
Mal and Andreassian went on to successfully perform a single-lung transplant in a patient with COPD in 1988 (34). The most effective treatment for end-stage pulmonary hypertension was thought to be heart-lung transplantation. However, the improvement of right ventricular function with a reduction in pulmonary pressures soon led to isolated lung transplants being performed for patients with pulmonary hypertension, and even for patients with Eisenmenger’s syndrome, with the use of a corrected causative shunt (10). In 1987, at the University of Toronto, the first pediatric lung transplantation was performed in a 16-year-old boy diagnosed with pulmonary fibrosis (16).
Shinoi and his colleagues performed the world’s first lobar transplant at Tokyo Medical College in 1966 (35). The patient survived, but the transplanted lobe had to be removed on postoperative day 18. The transplant was nonetheless considered to be a success, as the transplanted lobe supplemented the recipient’s lung function for the time period in question, particularly because he was in very poor health and would not have survived without a transplant. This heralded the eventual development of lobar transplantation some 25 years later (36). Vaughn Starnes (Figure 12) carried out the first successful living related lobar transplant in 1990; the recipient was a 12-year-old girl with pulmonary fibrosis who received her 44-year-old mother’s right upper lobe. Bilateral lobar transplant from a single donor lung was now possible in small adults or in children (in cases of size discrepancy with the donor) (36,37).
Figure 12.

Vaughn Starnes, who carried out the first successful living related lobar transplant. Photograph by Richard Carrasco III, reprinted with permission from Keck School of Medicine at University of Southern California.
Lung transplantation survival was much better in the last decade that in the two preceding decades. However, the difference in survival at 5 years posttransplant has not changed much over the same period. This is likely a consequence of better surgical techniques and improved postoperative care, rather than better management of late-onset complications such as rejection or infection (38). Per the International Society for Heart and Lung Transplantation (ISHLT) registry reports, in 2017 the median posttransplant survival was 6 years; recipients of double lung transplant had better survival than recipients of single lung transplant (39).
Immunosuppression
The first lung transplant recipient in 1963 received an immunosuppressive regimen that included prednisone, azathioprine, and cytoablative irradiation focused on the thymic region. The patient died from renal insufficiency on day 18, with no evidence of rejection on the lung allograft (5,40). Between 1963 and 1980, only 2 lung transplant recipients had noteworthy posttransplant survival (6 and 10 months), and no single patient survived long enough to be discharged from the hospital (41). However, this trend was destined to change with the emergence of cyclosporine. Initially discovered in 1968 as a product of Tolypocladium inflatum, a fungus isolated from Norwegian soil (42), cyclosporine was approved by the Food and Drug Administration (FDA) in 1983 and was almost immediately put to use as an immunosuppressive agent in recipients of solid organ transplants (43). Joel Cooper reported the first long-term survivor of lung transplantation in 1983, and that patient was treated with cyclosporine (32). Tacrolimus, a macrolide antibiotic originally isolated from the actinomycete Streptomyces tsukubaensis in 1984, was granted FDA approval in 1994 (44). Whether it is more effective than cyclosporine is still not completely clear (45-49), but the weight of the evidence supports tacrolimus (50).
As experience with lung transplantation grows, early postoperative survival improves. Of course, better early survival reveals complications that arise over the long term, especially chronic rejection. The high rate of chronic rejection underscores the vital role of immunosuppression in the modern transplantation era. Unfortunately, much of our understanding of immunosuppression in lung transplantation comes from trials and practices carried out in patients who have undergone transplantation of other solid organs (43). As a consequence of the unfortunate anastomotic complications and acute rejection episodes in the early lung transplants, immunosuppressive strategies used in heart and kidney recipients were applied to lung transplant recipients (51). This meant that from the early 1980s onward, lung transplant recipients received a maintenance regimen comprising steroids, cyclosporine and azathioprine. Even today, a regimen of an antiproliferative agent, an antimetabolite, and a steroid is standard (43). Currently, tacrolimus is the most popular calcineurin inhibitor in use, while mycophenolic acid is the dominant purine synthesis inhibitor used (52).
Induction therapy in lung transplantation has been extrapolated from its observed benefits in patients who have undergone kidney transplant (53). Even though its benefits in lung transplantation are not well defined (54), most centers use induction therapy to deplete the recipient’s immune system in the immediate postoperative period. Muromonab-CD3, anti-thymocyte globulin, chimeric human-murine monoclonal antibodies against interleukin-2 receptor (e.g., daclizumab and basiliximab), and a humanized rat monoclonal directed against CD52 (i.e., alemtuzumab), have been popular induction therapy drugs over the years. In recent times, the use of anti-thymocyte globulin has declined, whereas use of interleukin-2 receptor antagonists has risen (52); although which agent best prevents rejection remains unclear (55-59).
Compared to a prior median survival of 4 years, patients who underwent lung transplantation between 1994 and 2011 had a median survival of 5.6 years (60,61). Much of the survival improvement over years has been concentrated in the first posttransplant year, but the decline in survival thereafter has shown a similar trend in successive eras, suggesting that chronic rejection remains a major limiting factor to long-term survival (52).
Defining primary graft dysfunction (PGD)
PGD is the term used to describe poor early function of the allograft, and PGD is believed to be a syndrome of acute injury to the transplanted lungs that develops within the first 72 hours of transplant (starting from the time of reperfusion following release of the second lung receipt pulmonary artery cross clamp). Its reported incidence is highly variable (generally 10% to 25%) (62,63). In an effort to streamline research on PGD, the ISHLT standardized the definition of PGD in 2005, and that definition was updated in 2016 (64,65). PGD is multifactorial, and has been associated with both cold ischemic storage and ischemia-reperfusion injury, among other possible factors (66,67). It contributes to increased morbidity and mortality (68-70), and also appears to be a risk factor for chronic rejection (71-73).
PGD is characterized by pulmonary edema with diffuse alveolar damage, and it manifests (and is graded by) hypoxemia and infiltrates visible on radiography of the chest (74,75). A diagnosis of exclusion, practitioners must rule out common mimickers of PGD, such as infection, edema, and antibody-mediated rejection (64). Patients with PGD are managed with lung-protective ventilation and volume status optimization (76,77). Inhaled nitric oxide is often used to improve ventilation/perfusion matching, while ECMO has been successfully used in severe or refractory cases (78,79). Retransplantation in patients with PGD has been associated with poor outcomes (80). “Marginal donor” transplants do not seem to produce higher rates of PGD, and the advent of ex vivo lung perfusion (EVLP) may in fact reduce its incidence (81,82).
Lung allocation score (LAS)
Until 2005, the main variable used to prioritize lung transplant recipients was the amount of time spent on the waitlist. Before 2005, more patients died on the waitlist than received transplants, as this first-come-first-served system did not account for severity of illness at the time of listing. The LAS was implemented in 2005 to identify the best candidates for transplant. It is a complex algorithm based on both acuity of illness as well as an individual’s potential for successful long-term outcomes. Patients over the age of 12 years are given a score (i.e., their LAS) based on estimated risk of death with or without transplant. Since the introduction of LAS, IPF has replaced COPD as the most common indication for lung transplantation (83). The LAS has decreased waitlist times without having a detrimental effect on posttransplant survival (84). The number of transplants performed has increased and the number of inactive or deceased candidates on the waiting list has decreased (85), which is an indication that candidates for lung transplantation are being selected more appropriately (86).
ECMO as a bridge to transplant
Presently, the median wait time for lung transplant in the United States is 3.6 months, and the average 5-year survival is roughly 50%. However, a large number of deaths still occur in patients on the waitlist—a problem stemming mainly from inadequate donor availability (87). Based on current data, waitlist mortality is estimated at 15.4 per 100 waitlist years (85). This has raised interest in alternative “bridge therapy” strategies for patients with end-stage lung disease who are awaiting transplantation (88).
ECMO provides cardiorespiratory support for critically ill patients. Hill et al. (89) first reported using ECMO to manage a patient with cardiorespiratory failure in 1972. For decades, aside from sporadic use with mixed outcomes, studies did not demonstrate any survival benefit of the then-controversial ECMO in patients with acute respiratory failure (90). However, technological improvements and successful study data accumulated over the last decade challenged the previous concern regarding the utility of ECMO (91,92). Initially used in patients with severe PGD, ECMO is now an acceptable bridge-to-transplant (BTT) therapy (93). The shorter waitlist times after the implementation of the LAS (86) coupled with renewed interest in ECMO BTT has results in more frequent ECMO use. Reports from Hoopes, Lang, and Biscotti highlight the good outcomes that can be obtained with ECMO BTT in appropriately selected patients (94-96). When applied to patients whose pulmonary function has rapidly declined (as with IPF exacerbation) long-term outcomes and functional status after transplant have been reported to be comparable to those who did not receive ECMO BTT (97). Although no universally accepted indications for ECMO BTT have been officially established, factors such as age, functional status, disease process, infection, other organ failure, and anticipated time on the waitlist are taken into account when determining who would benefit from ECMO BTT (94,98,99). Contraindications for ECMO are also based on institutional experience, although traditional contraindications for lung transplant should likewise be considered as contraindications for ECMO (99,100).
Only 1% of patients in the United Network for Organ Sharing database between 2005 and 2011 underwent ECMO BTT (101), but there has been a 200% increase in this practice between 2009 and 2013 (88). The use of ECMO BTT should still be limited to centers with sufficient experience to optimize outcomes (99).
Despite the surge in ECMO BTT use, we must proceed with caution. Studies by Bermudez et al. and Mason et al. discuss how patients with ECMO BTT tend to be younger, suggesting a selection bias (98,102). Most patients who are on ECMO are also on mechanical ventilation, so whether ECMO is an adjunct therapy or a true alternative to mechanical ventilation remains unclear (103). Active participation in physical therapy results in faster recovery posttransplant (104). Fuehner et al. published results on 26 spontaneously breathing patients placed on “awake ECMO,” and found shorter hospital stays and better survival at 6 months than a historical control group managed with conventional mechanical ventilation (105). Awake ECMO avoids complications related to mechanical ventilation, sedation, and immobilization. In 2014, the first pediatric case of successful ambulatory ECMO BTT was reported in a 16-year-old girl (106).
The way forward: meeting the demand
Trummer’s collective review is a reminder of the countless challenges of lung transplantation that have already been solved by its pioneers, who were limited mainly by the lack of effective, safe immunosuppression (107). Progress in lung transplantation has lagged behind that of other solid organ transplantations. Explanations for this may be the extensive surface of interaction of the lung with the environment, heavy reliance on the creation and healing of a bronchial anastomosis, and the lung’s uniqueness as a transplanted organ in which the systemic arterial supply is unattached at the time of implantation (16).
One of the limitations to the success of lung transplantation is the low number of available braindead donors and the low percentage of lung procurement in such donors (15–20%) (108,109). Brain death and other complications associated with patients in an intensive care unit lead to lungs being declined at the time of allocation (110). This continued donor shortage has made it necessary to expand the donor pool, with many centers using “marginal donors” who meet extended criteria for age, infiltrates, and smoking history, among other things. The most important step in expanding donor eligibility has been optimizing the donor management. This refers to a global care strategy that preserves or improves lung quality, recommendations for which were published by Munshi et al. in 2013 (111). Expansion of the donor pool is today best exemplified by donation after cardiac death (DCD) and the implementation of EVLP.
Donation after cardiac or brain death
Donation after brain death (DBD) and DCD are the two main types of cadaveric organ donations (110). The gas-exchange systems of the lungs are thought to tolerate approximately one hour of warm ischemia without significant loss in function after cardiac arrest. The previous debate and low clinical use of DCD donors were driven by various injuries to which the lungs are prone in DCD (e.g., hypoxia, hypotension, aspiration), which are lesser threats in organs procured from DBD donors.
Tom Egan and his group first revisited the preclinical foundations for DCD in 1991 (Figure 13). They demonstrated at least 8 hours of life sustenance after transplanting a non-ventilated canine lung, which was procured 1 hour after cardiac arrest, into a recipient with a ligated contralateral pulmonary artery (112). In 1995, D’Alessandro et al. reported the first successful case of lung transplantation using a controlled DCD donor (113). More recently, several series have described the growing experience with DCD (102,114). Today, controlled DCD is used in most countries. In this type of organ procurement, life-sustaining therapies are withdrawn, triggering cardiac arrest. At this time, the recovery team procures and cold-preserves the organs in a usual fashion. Although the number of DCD donors in the United States has risen from 0 in 2000 to 32 in 2012, DCD accounts for just 1.9% of all deceased donor lung transplants (115). Patients who receive organs from DCD and DBD have similar outcomes, as demonstrated by the results of the first meta-analysis (116) and an ISHLT DCD registry review comparing these groups (117).
Figure 13.

Tom Egan, who refined the preclinical foundations of donation after cardiac death. Reprinted with permission from Tom Egan.
Uncontrolled DCD is reported mainly by centers located in Spain (118,119). Uncontrolled DCD involves continuation of cardiopulmonary resuscitation maneuvers during transport. Once death is certified and after an intravenous heparin bolus, the lungs are cooled in situ using two chest tubes, after which resuscitation and mechanical ventilation are stopped. While mid- and long-term survival and chronic rejection rates seem to be acceptable, uncontrolled DCD transplants seem to have higher initial mortality and PGD rates. With EVLP promising to be more commonplace, uncontrolled DCD activities and studies are expected to increase (110).
EVLP
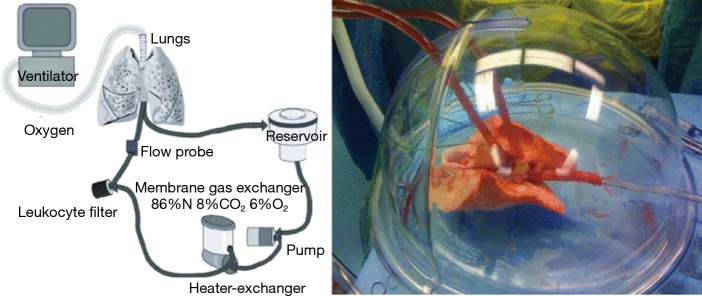
In the modern era of lung transplantation, perhaps no innovation has garnered as much excitement as EVLP. Alexis Carrel and Charles Lindbergh first envisioned whole-organ perfusion in the 1930s, and performed several experiments with different organs (120). Up to the 1990s, lung perfusion experiments were simply a useful means to study pulmonary physiology (121). Stig Steen, at the University Hospital of Lund, first described the clinical application of lung perfusion in 2001, using it to assess the short-term function of the DCD lung that he eventually successfully transplanted (Figures 14,15) (122). Thus, EVLP allowed for assessment of pre-implant identification of poorly preserved lungs, but normothermic EVLP also allows preservation of lung homeostasis and metabolic function. Its use could be extended to ex vivo preservation or even treatment of poorly functioning lungs, as first presented by the Toronto group in 2007 (123). They reported its use on initially rejected lungs, and 4 of their 6 recipients did well (124). They updated their experience in 2012, reporting on 50 transplants from 58 EVLPs, obtaining comparable outcomes to those of lungs that had been conventionally preserved (125). At the 2013 ISHLT meeting, groups from Toronto, Vienna, and Paris presented their combined experience, demonstrating similar results (126).
Figure 14.

Stig Steen, a pioneer in ex vivo lung perfusion. Reprinted with permission from Igelösa Life Science.
Figure 15.
The ex vivo lung perfusion circuit. Reprinted with permission from Cypel M, Rubacha M, Yeung J, et al. Normothermic ex vivo perfusion prevents lung injury compared to extended cold preservation for transplantation. Am J Transplant 2009;9:2262-9.
There has always been intense interest on the role of EVLP in reconditioning lungs previously rejected for transplant. The HELP Trial reported the successful transplantation of 20 high-risk donor lungs that were physiologically stabilized using EVLP; the results of those transplants were comparable to the results of transplants carried out with conventionally selected lungs (108). The 2013 FDA-mandated NOVEL trial (127) and its 2014 update (128) confirmed the satisfactory outcomes of lungs transplanted from marginal donors, comparing them to contemporary controls.
Today, EVLP is a safe technique that has increased the number of DCD lung transplantations, but further studies are needed to better define its role in this context (129). The future of EVLP lies not only in the ability to rescue, revive and rejuvenate lungs (130), but also has the potential to deliver specific therapies to injured lungs (110).
The future of lung transplantation
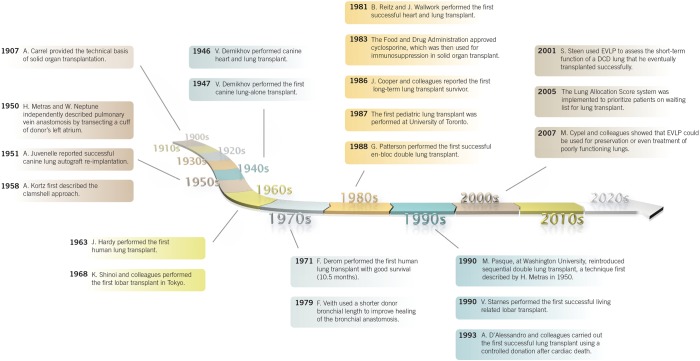
Lung transplantation has come a long way from its beginnings (Figure 16) and will remain a viable modality for treatment of end-stage lung disease, and indications for it will continue to broaden. Anticipated advancements in the pre-transplant realm include a broader donor pool and greater candidate eligibility through better and innovative medical care, as well as the use of alternative lung sources (131). Future efforts in lung transplantation should concentrate on improving posttransplant survival, possibly by finding ways to prevent or manage chronic rejection. Interestingly, newer therapies for select conditions (e.g., pulmonary arterial hypertension, IPF, and even COPD) may eliminate the need for lung transplantation. Continued increases in the costs of medications and medical care certainly pose a challenge to the future of lung transplantation.
Figure 16.
Major milestones in the field of lung transplantation. Reprinted with permission from Norton Thoracic Institute, Phoenix, Arizona.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Carrel A. The surgery of blood vessels, etc. Johns Hopkins Hospital Bulletin 1907;18:18-28. [Google Scholar]
- 2.Veith FJ, Blumenstock DA. Current research review. Lung transplantation. J Surg Res 1971;11:33-55. 10.1016/0022-4804(71)90047-3 [DOI] [PubMed] [Google Scholar]
- 3.Demikhov VP. Experimental transplantation of vital organs. New York: Consultants Bureau, 1962. [Google Scholar]
- 4.Hardy JD, Eraslan S, Dalton ML, Jr, et al. Re-implantation and homotransplantation of the lung: laboratory studies and clinical potential. Ann Surg 1963;157:707-18. 10.1097/00000658-196305000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy JD, Webb WR, Dalton ML, Jr, et al. Lung Homotransplantation in Man. JAMA 1963;186:1065-74. 10.1001/jama.1963.63710120001010 [DOI] [PubMed] [Google Scholar]
- 6.Aru GM, Call KD, Creswell LL, et al. James D. Hardy: a pioneer in surgery (1918 to 2003). J Heart Lung Transplant 2004;23:1307-10. 10.1016/j.healun.2004.05.031 [DOI] [PubMed] [Google Scholar]
- 7.Magovern GJ, Yates AJ. Human Homotransplantation of Left Lung: Report of a Case. Ann N Y Acad Sci 1964;120:710-28. 10.1111/j.1749-6632.1965.tb30696.x [DOI] [PubMed] [Google Scholar]
- 8.Wildevuur CR, Benfield JR. A review of 23 human lung transplantations by 20 surgeons. Ann Thorac Surg 1970;9:489-515. 10.1016/S0003-4975(10)65544-0 [DOI] [PubMed] [Google Scholar]
- 9.Derom F, Barbier F, Ringoir S, et al. Ten-month survival after lung homotransplantation in man. J Thorac Cardiovasc Surg 1971;61:835-46. [PubMed] [Google Scholar]
- 10.Grover FL, Fullerton DA, Zamora MR, et al. The past, present, and future of lung transplantation. Am J Surg 1997;173:523-33. 10.1016/S0002-9610(97)00004-4 [DOI] [PubMed] [Google Scholar]
- 11.Reitz BA, Wallwork JL, Hunt SA, et al. Heart-lung transplantation: successful therapy for patients with pulmonary vascular disease. N Engl J Med 1982;306:557-64. 10.1056/NEJM198203113061001 [DOI] [PubMed] [Google Scholar]
- 12.Shumway NE. Thoracic transplantation. World J Surg 2000;24:811-4. 10.1007/s002680010129 [DOI] [PubMed] [Google Scholar]
- 13.Patterson GA, Cooper JD, Goldman B, et al. Technique of successful clinical double-lung transplantation. Ann Thorac Surg 1988;45:626-33. 10.1016/S0003-4975(10)64763-7 [DOI] [PubMed] [Google Scholar]
- 14.Metras D. Henri Metras: a pioneer in lung transplantation. J Heart Lung Transplant 1992;11:1213-5; discussion 1215-6. [PubMed] [Google Scholar]
- 15.Pasque MK, Cooper JD, Kaiser LR, et al. Improved technique for bilateral lung transplantation: rationale and initial clinical experience. Ann Thorac Surg 1990;49:785-91. 10.1016/0003-4975(90)90023-Y [DOI] [PubMed] [Google Scholar]
- 16.Mendeloff EN. The history of pediatric heart and lung transplantation. Pediatr Transplant 2002;6:270-9. 10.1034/j.1399-3046.2002.00217.x [DOI] [PubMed] [Google Scholar]
- 17.Goldberg M, Lima O, Morgan E, et al. A comparison between cyclosporin A and methylprednisolone plus azathioprine on bronchial healing following canine lung autotransplantation. J Thorac Cardiovasc Surg 1983;85:821-6. [PubMed] [Google Scholar]
- 18.Lima O, Cooper JD, Peters WJ, et al. Effects of methylprednisolone and azathioprine on bronchial healing following lung autotransplantation. J Thorac Cardiovasc Surg 1981;82:211-5. [PubMed] [Google Scholar]
- 19.Morgan E, Lima O, Goldberg M, et al. Improved bronchial healing in canine left lung reimplantation using omental pedicle wrap. J Thorac Cardiovasc Surg 1983;85:134-9. [PubMed] [Google Scholar]
- 20.Miller JD, DeHoyos A. An evaluation of the role of omentopexy and of early perioperative corticosteroid administration in clinical lung transplantation. The University of Toronto and Washington University Lung Transplant Programs. J Thorac Cardiovasc Surg 1993;105:247-52. [PubMed] [Google Scholar]
- 21.Mughal MM, Gildea TR, Murthy S, et al. Short-term deployment of self-expanding metallic stents facilitates healing of bronchial dehiscence. Am J Respir Crit Care Med 2005;172:768-71. 10.1164/rccm.200410-1388OC [DOI] [PubMed] [Google Scholar]
- 22.Juvenelle AA, Citret C, Wiles CE, Jr, et al. Pneumonectomy with replantation of the lung in the dog for physiologic study. J Thorac Surg 1951;21:111-5. [PubMed] [Google Scholar]
- 23.Metras H. Preliminary report of lung grafts in the dog. C R Acad Sci (Paris) 1950;231:1176. [Google Scholar]
- 24.Neptune WB, Weller R, Bailey CP. Experimental lung transplantation. J Thorac Surg 1953;26:275-89. [PubMed] [Google Scholar]
- 25.Nigro SL, Evans RH, Benfield JR, et al. Physiologic Alterations of Cardiopulmonary Function in Dogs Living One and One-Half Years on Only a Reimplanted Right Lung. J Thorac Cardiovasc Surg 1963;46:598-605. [PubMed] [Google Scholar]
- 26.Kortz AB. Experimental bilateral transsternal thoracotomy: factors improving survival. J Thorac Surg 1958;35:305-8. [PubMed] [Google Scholar]
- 27.Meyers BF, Sundaresan RS, Guthrie T, et al. Bilateral sequential lung transplantation without sternal division eliminates posttransplantation sternal complications. J Thorac Cardiovasc Surg 1999;117:358-64. 10.1016/S0022-5223(99)70434-4 [DOI] [PubMed] [Google Scholar]
- 28.Brown RP, Esmore DS, Lawson C. Improved sternal fixation in the transsternal bilateral thoracotomy incision. J Thorac Cardiovasc Surg 1996;112:137-41. 10.1016/S0022-5223(96)70188-5 [DOI] [PubMed] [Google Scholar]
- 29.Arndt G, Granger E, Glanville A, et al. Clamshell Incision vs. Sternal-Sparing Incision in Lung Transplantation. J Heart Lung Transplant 2013;32:S265 10.1016/j.healun.2013.01.691 [DOI] [Google Scholar]
- 30.Boasquevisque CH, Yildirim E, Waddel TK, et al. Surgical techniques: lung transplant and lung volume reduction. Proc Am Thorac Soc 2009;6:66-78. 10.1513/pats.200808-083GO [DOI] [PubMed] [Google Scholar]
- 31.Hayanga JW, D'Cunha J. The surgical technique of bilateral sequential lung transplantation. J Thorac Dis 2014;6:1063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toronto Lung Transplant Group Unilateral lung transplantation for pulmonary fibrosis. N Engl J Med 1986;314:1140-5. 10.1056/NEJM198605013141802 [DOI] [PubMed] [Google Scholar]
- 33.Cooper JD, Pearson FG, Patterson GA, et al. Technique of successful lung transplantation in humans. J Thorac Cardiovasc Surg 1987;93:173-81. [PubMed] [Google Scholar]
- 34.Mal H, Andreassian B, Pamela F, et al. Unilateral lung transplantation in end-stage pulmonary emphysema. Am Rev Respir Dis 1989;140:797-802. 10.1164/ajrccm/140.3.797 [DOI] [PubMed] [Google Scholar]
- 35.Shinoi K, Hayata Y, Aoki H, et al. Pulmonary lobe homotransplantation in human subjects. Am J Surg 1966;111:617-28. 10.1016/0002-9610(66)90028-6 [DOI] [PubMed] [Google Scholar]
- 36.Starnes VA, Lewiston NJ, Luikart H, et al. Current trends in lung transplantation. Lobar transplantation and expanded use of single lungs. J Thorac Cardiovasc Surg 1992;104:1060-5; discussion 1065-6. [PubMed] [Google Scholar]
- 37.Couetil JP, Tolan MJ, Loulmet DF, et al. Pulmonary bipartitioning and lobar transplantation: a new approach to donor organ shortage. J Thorac Cardiovasc Surg 1997;113:529-37. 10.1016/S0022-5223(97)70366-0 [DOI] [PubMed] [Google Scholar]
- 38.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Lung and Heart-Lung Transplantation Report--2015; Focus Theme: Early Graft Failure. J Heart Lung Transplant 2015;34:1264-77. 10.1016/j.healun.2015.08.014 [DOI] [PubMed] [Google Scholar]
- 39.Chambers DC, Yusen RD, Cherikh WS, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Lung And Heart-Lung Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant 2017;36:1047-59. 10.1016/j.healun.2017.07.016 [DOI] [PubMed] [Google Scholar]
- 40.Blumenstock DA, Lewis C. The first transplantation of the lung in a human revisited. Ann Thorac Surg 1993;56:1423-4; discussion 1424-5. 10.1016/0003-4975(93)90706-N [DOI] [PubMed] [Google Scholar]
- 41.Veith FJ, Kamholz SL, Mollenkopf FP, et al. Lung transplantation 1983. Transplantation 1983;35:271-8. 10.1097/00007890-198304000-00001 [DOI] [PubMed] [Google Scholar]
- 42.Kostakis A. Early experience with cyclosporine: a historic perspective. Transplant Proc 2004;36:22S-4S. 10.1016/j.transproceed.2004.01.066 [DOI] [PubMed] [Google Scholar]
- 43.Bhorade SM, Stern E. Immunosuppression for lung transplantation. Proc Am Thorac Soc 2009;6:47-53. 10.1513/pats.200808-096GO [DOI] [PubMed] [Google Scholar]
- 44.Hausen B, Morris RE. Review of immunosuppression for lung transplantation. Novel drugs, new uses for conventional immunosuppressants, and alternative strategies. Clin Chest Med 1997;18:353-66. 10.1016/S0272-5231(05)70384-1 [DOI] [PubMed] [Google Scholar]
- 45.Hachem RR, Yusen RD, Chakinala MM, et al. A randomized controlled trial of tacrolimus versus cyclosporine after lung transplantation. J Heart Lung Transplant 2007;26:1012-8. 10.1016/j.healun.2007.07.027 [DOI] [PubMed] [Google Scholar]
- 46.Keenan RJ, Konishi H, Kawai A, et al. Clinical trial of tacrolimus versus cyclosporine in lung transplantation. Ann Thorac Surg 1995;60:580-4; discussion 584-5. 10.1016/0003-4975(95)00407-C [DOI] [PubMed] [Google Scholar]
- 47.Penninga L, Penninga EI, Moller CH, et al. Tacrolimus versus cyclosporin as primary immunosuppression for lung transplant recipients. Cochrane Database Syst Rev 2013;(5):CD008817. [DOI] [PubMed] [Google Scholar]
- 48.Treede H, Glanville AR, Klepetko W, et al. Tacrolimus and cyclosporine have differential effects on the risk of development of bronchiolitis obliterans syndrome: results of a prospective, randomized international trial in lung transplantation. J Heart Lung Transplant 2012;31:797-804. 10.1016/j.healun.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 49.Zuckermann A, Reichenspurner H, Birsan T, et al. Cyclosporine A versus tacrolimus in combination with mycophenolate mofetil and steroids as primary immunosuppression after lung transplantation: one-year results of a 2-center prospective randomized trial. J Thorac Cardiovasc Surg 2003;125:891-900. 10.1067/mtc.2003.71 [DOI] [PubMed] [Google Scholar]
- 50.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant 2012;31:1073-86. 10.1016/j.healun.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 51.Knoop C, Haverich A, Fischer S. Immunosuppressive therapy after human lung transplantation. Eur Respir J 2004;23:159-71. 10.1183/09031936.03.00039203 [DOI] [PubMed] [Google Scholar]
- 52.Trulock EP, Christie JD, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult lung and heart-lung transplantation report-2007. J Heart Lung Transplant 2007;26:782-95. 10.1016/j.healun.2007.06.003 [DOI] [PubMed] [Google Scholar]
- 53.Shield CF, Edwards EB, Davies DB, et al. Antilymphocyte induction therapy in cadaver renal transplantation: a retrospective, multicenter United Network for Organ Sharing Study. Transplantation 1997;63:1257-63. 10.1097/00007890-199705150-00011 [DOI] [PubMed] [Google Scholar]
- 54.Penninga L, Moller CH, Penninga EI, et al. Antibody induction therapy for lung transplant recipients. Cochrane Database Syst Rev 2013;(11):CD008927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ailawadi G, Smith PW, Oka T, et al. Effects of induction immunosuppression regimen on acute rejection, bronchiolitis obliterans, and survival after lung transplantation. J Thorac Cardiovasc Surg 2008;135:594-602. 10.1016/j.jtcvs.2007.10.044 [DOI] [PubMed] [Google Scholar]
- 56.Brock MV, Borja MC, Ferber L, et al. Induction therapy in lung transplantation: a prospective, controlled clinical trial comparing OKT3, anti-thymocyte globulin, and daclizumab. J Heart Lung Transplant 2001;20:1282-90. 10.1016/S1053-2498(01)00356-4 [DOI] [PubMed] [Google Scholar]
- 57.Burton CM, Andersen CB, Jensen AS, et al. The incidence of acute cellular rejection after lung transplantation: a comparative study of anti-thymocyte globulin and daclizumab. J Heart Lung Transplant 2006;25:638-47. 10.1016/j.healun.2006.01.011 [DOI] [PubMed] [Google Scholar]
- 58.Hachem RR, Chakinala MM, Yusen RD, et al. A comparison of basiliximab and anti-thymocyte globulin as induction agents after lung transplantation. J Heart Lung Transplant 2005;24:1320-6. 10.1016/j.healun.2004.09.002 [DOI] [PubMed] [Google Scholar]
- 59.Mullen JC, Oreopoulos A, Lien DC, et al. A randomized, controlled trial of daclizumab vs anti-thymocyte globulin induction for lung transplantation. J Heart Lung Transplant 2007;26:504-10. 10.1016/j.healun.2007.01.032 [DOI] [PubMed] [Google Scholar]
- 60.Hertz MI, Taylor DO, Trulock EP, et al. The registry of the international society for heart and lung transplantation: nineteenth official report-2002. J Heart Lung Transplant 2002;21:950-70. 10.1016/S1053-2498(02)00498-9 [DOI] [PubMed] [Google Scholar]
- 61.Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report--2013; focus theme: age. J Heart Lung Transplant 2013;32:965-78. 10.1016/j.healun.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 62.Diamond JM, Lee JC, Kawut SM, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 2013;187:527-34. 10.1164/rccm.201210-1865OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prekker ME, Nath DS, Walker AR, et al. Validation of the proposed International Society for Heart and Lung Transplantation grading system for primary graft dysfunction after lung transplantation. J Heart Lung Transplant 2006;25:371-8. 10.1016/j.healun.2005.11.436 [DOI] [PubMed] [Google Scholar]
- 64.Christie JD, Carby M, Bag R, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2005;24:1454-9. 10.1016/j.healun.2004.11.049 [DOI] [PubMed] [Google Scholar]
- 65.Snell GI, Yusen RD, Weill D, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2017;36:1097-103. 10.1016/j.healun.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 66.Fiser SM, Kron IL, Long SM, et al. Influence of graft ischemic time on outcomes following lung transplantation. J Heart Lung Transplant 2001;20:1291-6. 10.1016/S1053-2498(01)00355-2 [DOI] [PubMed] [Google Scholar]
- 67.Thabut G, Mal H, Cerrina J, et al. Graft ischemic time and outcome of lung transplantation: a multicenter analysis. Am J Respir Crit Care Med 2005;171:786-91. 10.1164/rccm.200409-1248OC [DOI] [PubMed] [Google Scholar]
- 68.Arcasoy SM, Fisher A, Hachem RR, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part V: predictors and outcomes. J Heart Lung Transplant 2005;24:1483-8. 10.1016/j.healun.2004.11.314 [DOI] [PubMed] [Google Scholar]
- 69.Christie JD, Sager JS, Kimmel SE, et al. Impact of primary graft failure on outcomes following lung transplantation. Chest 2005;127:161-5. 10.1378/chest.127.1.161 [DOI] [PubMed] [Google Scholar]
- 70.King RC, Binns OA, Rodriguez F, et al. Reperfusion injury significantly impacts clinical outcome after pulmonary transplantation. Ann Thorac Surg 2000;69:1681-5. 10.1016/S0003-4975(00)01425-9 [DOI] [PubMed] [Google Scholar]
- 71.Daud SA, Yusen RD, Meyers BF, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 2007;175:507-13. 10.1164/rccm.200608-1079OC [DOI] [PubMed] [Google Scholar]
- 72.Fiser SM, Tribble CG, Long SM, et al. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg 2002;73:1041-7; discussion 1047-8. 10.1016/S0003-4975(01)03606-2 [DOI] [PubMed] [Google Scholar]
- 73.Whitson BA, Prekker ME, Herrington CS, et al. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant 2007;26:1004-11. 10.1016/j.healun.2007.07.018 [DOI] [PubMed] [Google Scholar]
- 74.Christie JD, Kotloff RM, Pochettino A, et al. Clinical risk factors for primary graft failure following lung transplantation. Chest 2003;124:1232-41. 10.1378/chest.124.4.1232 [DOI] [PubMed] [Google Scholar]
- 75.Suzuki Y, Cantu E, Christie JD. Primary graft dysfunction. Semin Respir Crit Care Med 2013;34:305-19. 10.1055/s-0033-1348474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Date H, Triantafillou AN, Trulock EP, et al. Inhaled nitric oxide reduces human lung allograft dysfunction. J Thorac Cardiovasc Surg 1996;111:913-9. 10.1016/S0022-5223(96)70364-1 [DOI] [PubMed] [Google Scholar]
- 77.Shargall Y, Guenther G, Ahya VN, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part VI: treatment. J Heart Lung Transplant 2005;24:1489-500. 10.1016/j.healun.2005.03.011 [DOI] [PubMed] [Google Scholar]
- 78.Bermudez CA, Adusumilli PS, McCurry KR, et al. Extracorporeal membrane oxygenation for primary graft dysfunction after lung transplantation: long-term survival. Ann Thorac Surg 2009;87:854-60. 10.1016/j.athoracsur.2008.11.036 [DOI] [PubMed] [Google Scholar]
- 79.Fischer S, Bohn D, Rycus P, et al. Extracorporeal membrane oxygenation for primary graft dysfunction after lung transplantation: analysis of the Extracorporeal Life Support Organization (ELSO) registry. J Heart Lung Transplant 2007;26:472-7. 10.1016/j.healun.2007.01.031 [DOI] [PubMed] [Google Scholar]
- 80.Aigner C, Jaksch P, Taghavi S, et al. Pulmonary retransplantation: is it worth the effort? A long-term analysis of 46 cases. J Heart Lung Transplant 2008;27:60-5. 10.1016/j.healun.2007.09.023 [DOI] [PubMed] [Google Scholar]
- 81.Schiavon M, Falcoz PE, Santelmo N, et al. Does the use of extended criteria donors influence early and long-term results of lung transplantation? Interact Cardiovasc Thorac Surg 2012;14:183-7. 10.1093/icvts/ivr079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zych B, Garcia Saez D, Sabashnikov A, et al. Lung transplantation from donors outside standard acceptability criteria--are they really marginal? Transpl Int 2014;27:1183-91. 10.1111/tri.12410 [DOI] [PubMed] [Google Scholar]
- 83.George TJ, Arnaoutakis GJ, Shah AS. Lung transplant in idiopathic pulmonary fibrosis. Arch Surg 2011;146:1204-9. 10.1001/archsurg.2011.239 [DOI] [PubMed] [Google Scholar]
- 84.Egan TM, Edwards LB. Effect of the lung allocation score on lung transplantation in the United States. J Heart Lung Transplant 2016;35:433-9. 10.1016/j.healun.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 85.U.S. Department of Health and Human Services HRaSA. Organ Procurement and Transplantation Network & Scientific Registry of Transplant Recipient Annual Data Report 2012. 2014.
- 86.Takahashi SM, Garrity ER. The impact of the lung allocation score. Semin Respir Crit Care Med 2010;31:108-14. 10.1055/s-0030-1249107 [DOI] [PubMed] [Google Scholar]
- 87.Punch JD, Hayes DH, LaPorte FB, et al. Organ donation and utilization in the United States, 1996-2005. Am J Transplant 2007;7:1327-38. 10.1111/j.1600-6143.2007.01779.x [DOI] [PubMed] [Google Scholar]
- 88.Gulack BC, Hirji SA, Hartwig MG. Bridge to lung transplantation and rescue post-transplant: the expanding role of extracorporeal membrane oxygenation. J Thorac Dis 2014;6:1070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hill JD, O'Brien TG, Murray JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med 1972;286:629-34. 10.1056/NEJM197203232861204 [DOI] [PubMed] [Google Scholar]
- 90.Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979;242:2193-6. 10.1001/jama.1979.03300200023016 [DOI] [PubMed] [Google Scholar]
- 91.Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011;306:1659-68. 10.1001/jama.2011.1471 [DOI] [PubMed] [Google Scholar]
- 92.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. 10.1016/S0140-6736(09)61069-2 [DOI] [PubMed] [Google Scholar]
- 93.Hartwig MG, Walczak R, Lin SS, et al. Improved survival but marginal allograft function in patients treated with extracorporeal membrane oxygenation after lung transplantation. Ann Thorac Surg 2012;93:366-71. 10.1016/j.athoracsur.2011.05.017 [DOI] [PubMed] [Google Scholar]
- 94.Biscotti M, Sonett J, Bacchetta M. ECMO as bridge to lung transplant. Thorac Surg Clin 2015;25:17-25. 10.1016/j.thorsurg.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 95.Hoopes CW, Kukreja J, Golden J, et al. Extracorporeal membrane oxygenation as a bridge to pulmonary transplantation. J Thorac Cardiovasc Surg 2013;145:862-7; discussion 867-8. 10.1016/j.jtcvs.2012.12.022 [DOI] [PubMed] [Google Scholar]
- 96.Lang G, Taghavi S, Aigner C, et al. Primary lung transplantation after bridge with extracorporeal membrane oxygenation: a plea for a shift in our paradigms for indications. Transplantation 2012;93:729-36. 10.1097/TP.0b013e318246f8e1 [DOI] [PubMed] [Google Scholar]
- 97.Todd EM, Biswas Roy S, Hashimi AS, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation: A single-center experience in the present era. J Thorac Cardiovasc Surg 2017;154:1798-809. 10.1016/j.jtcvs.2017.06.063 [DOI] [PubMed] [Google Scholar]
- 98.Bermudez CA, Rocha RV, Zaldonis D, et al. Extracorporeal membrane oxygenation as a bridge to lung transplant: midterm outcomes. Ann Thorac Surg 2011;92:1226-31; discussion 1231-2. 10.1016/j.athoracsur.2011.04.122 [DOI] [PubMed] [Google Scholar]
- 99.Toyoda Y, Bhama JK, Shigemura N, et al. Efficacy of extracorporeal membrane oxygenation as a bridge to lung transplantation. J Thorac Cardiovasc Surg 2013;145:1065-71. 10.1016/j.jtcvs.2012.12.067 [DOI] [PubMed] [Google Scholar]
- 100.Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006;25:745-55. 10.1016/j.healun.2006.03.011 [DOI] [PubMed] [Google Scholar]
- 101.George TJ, Beaty CA, Kilic A, et al. Outcomes and temporal trends among high-risk patients after lung transplantation in the United States. J Heart Lung Transplant 2012;31:1182-91. 10.1016/j.healun.2012.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mason DP, Thuita L, Nowicki ER, et al. Should lung transplantation be performed for patients on mechanical respiratory support? The US experience. J Thorac Cardiovasc Surg 2010;139:765-73.e1. 10.1016/j.jtcvs.2009.09.031 [DOI] [PubMed] [Google Scholar]
- 103.Chiumello D, Coppola S, Froio S, et al. Extracorporeal life support as bridge to lung transplantation: a systematic review. Crit Care 2015;19:19. 10.1186/s13054-014-0686-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rehder KJ, Turner DA, Hartwig MG, et al. Active rehabilitation during extracorporeal membrane oxygenation as a bridge to lung transplantation. Respir Care 2013;58:1291-8. 10.4187/respcare.02155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fuehner T, Kuehn C, Hadem J, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med 2012;185:763-8. 10.1164/rccm.201109-1599OC [DOI] [PubMed] [Google Scholar]
- 106.Turner DA, Rehder KJ, Bonadonna D, et al. Ambulatory ECMO as a bridge to lung transplant in a previously well pediatric patient with ARDS. Pediatrics 2014;134:e583-5. 10.1542/peds.2013-3435 [DOI] [PubMed] [Google Scholar]
- 107.Dark JH. 50th Anniversary Perspective on Volume 1: Trummer MJ. Experimental Transplantation of the Lung. Ann Thorac Surg 1965;1:203-19. Ann Thorac Surg 2015;100:773-4. 10.1016/j.athoracsur.2015.07.061 [DOI] [PubMed] [Google Scholar]
- 108.Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011;364:1431-40. 10.1056/NEJMoa1014597 [DOI] [PubMed] [Google Scholar]
- 109.Popov AF, Sabashnikov A, Patil NP, et al. Ex vivo lung perfusion - state of the art in lung donor pool expansion. Med Sci Monit Basic Res 2015;21:9-14. 10.12659/MSMBR.893674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reeb J, Keshavjee S, Cypel M. Expanding the lung donor pool: advancements and emerging pathways. Curr Opin Organ Transplant 2015;20:498-505. 10.1097/MOT.0000000000000233 [DOI] [PubMed] [Google Scholar]
- 111.Munshi L, Keshavjee S, Cypel M. Donor management and lung preservation for lung transplantation. Lancet Respir Med 2013;1:318-28. 10.1016/S2213-2600(12)70064-4 [DOI] [PubMed] [Google Scholar]
- 112.Egan TM, Lambert CJ, Jr, Reddick R, et al. A strategy to increase the donor pool: use of cadaver lungs for transplantation. Ann Thorac Surg 1991;52:1113-20; discussion 1120-1. 10.1016/0003-4975(91)91290-C [DOI] [PubMed] [Google Scholar]
- 113.D'Alessandro AM, Hoffmann RM, Knechtle SJ, et al. Controlled non-heart-beating donors: a potential source of extrarenal organs. Transplant Proc 1995;27:707-9. [PubMed] [Google Scholar]
- 114.Levvey BJ, Harkess M, Hopkins P, et al. Excellent clinical outcomes from a national donation-after-determination-of-cardiac-death lung transplant collaborative. Am J Transplant 2012;12:2406-13. 10.1111/j.1600-6143.2012.04193.x [DOI] [PubMed] [Google Scholar]
- 115.Israni AK, Zaun D, Rosendale JD, et al. OPTN/SRTR 2012 Annual Data Report: deceased organ donation. Am J Transplant 2014;14 Suppl 1:167-83. 10.1111/ajt.12585 [DOI] [PubMed] [Google Scholar]
- 116.Krutsinger D, Reed RM, Blevins A, et al. Lung transplantation from donation after cardiocirculatory death: a systematic review and meta-analysis. J Heart Lung Transplant 2015;34:675-84. 10.1016/j.healun.2014.11.009 [DOI] [PubMed] [Google Scholar]
- 117.Wigfield C. Donation after cardiac death for lung transplantation: a review of current clinical practice. Curr Opin Organ Transplant 2014;19:455-9. [DOI] [PubMed] [Google Scholar]
- 118.Gomez-de-Antonio D, Campo-Canaveral JL, Crowley S, et al. Clinical lung transplantation from uncontrolled non-heart-beating donors revisited. J Heart Lung Transplant 2012;31:349-53. 10.1016/j.healun.2011.12.007 [DOI] [PubMed] [Google Scholar]
- 119.Meneses JC, Gamez P, Mariscal A, et al. Development of a non-heart-beating donor program and results after the first year. Transplant Proc 2012;44:2047-9. 10.1016/j.transproceed.2012.07.092 [DOI] [PubMed] [Google Scholar]
- 120.Carrel A, Lindbergh CA. The Culture of Whole Organs. Science 1935;81:621-3. 10.1126/science.81.2112.621 [DOI] [PubMed] [Google Scholar]
- 121.Machuca TN, Cypel M. Ex vivo lung perfusion. J Thorac Dis 2014;6:1054-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Steen S, Sjoberg T, Pierre L, et al. Transplantation of lungs from a non-heart-beating donor. Lancet 2001;357:825-9. 10.1016/S0140-6736(00)04195-7 [DOI] [PubMed] [Google Scholar]
- 123.Cypel M, Rubacha M, Sato M, et al. 423: Adenoviral mediated interleukin 10(AdhIL-10) gene therapy in normothermic ex-vivo lung perfusion. J Heart Lung Transplant 2007;26:S212-3. 10.1016/j.healun.2006.11.445 [DOI] [Google Scholar]
- 124.Ingemansson R, Eyjolfsson A, Mared L, et al. Clinical transplantation of initially rejected donor lungs after reconditioning ex vivo. Ann Thorac Surg 2009;87:255-60. 10.1016/j.athoracsur.2008.09.049 [DOI] [PubMed] [Google Scholar]
- 125.Cypel M, Yeung JC, Machuca T, et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg 2012;144:1200-6. 10.1016/j.jtcvs.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 126.Cypel M, Aigner C, Sage E, et al. Three Center Experience with Clinical Normothermic Ex Vivo Lung Perfusion. J Heart Lung Transplant 2013;32:S16 10.1016/j.healun.2013.01.021 [DOI] [Google Scholar]
- 127.Sanchez PG, Davis RD, D’ovidio F, et al. Normothermic Ex Vivo Lung Perfusion as an Assessment of Marginal Donor Lungs – The NOVEL Lung Trial. J Heart Lung Transplant 2013;32:S16-7. 10.1016/j.healun.2013.01.022 [DOI] [Google Scholar]
- 128.Sanchez PG, Davis RD, D’Ovidio F, et al. The NOVEL Lung Trial One-Year Outcomes. J Heart Lung Transplant 2014;33:S71-2. 10.1016/j.healun.2014.01.226 [DOI] [Google Scholar]
- 129.Machuca TN, Mercier O, Collaud S, et al. Lung transplantation with donation after circulatory determination of death donors and the impact of ex vivo lung perfusion. Am J Transplant 2015;15:993-1002. 10.1111/ajt.13124 [DOI] [PubMed] [Google Scholar]
- 130.Bremner RM. Rescue, revive, rejuvenate: The new science of ex vivo lung perfusion. J Thorac Cardiovasc Surg 2017;153:205. 10.1016/j.jtcvs.2016.10.016 [DOI] [PubMed] [Google Scholar]
- 131.Nathan SD. The future of lung transplantation. Chest 2015;147:309-16. 10.1378/chest.14-1748 [DOI] [PubMed] [Google Scholar]