Abstract
Background
The pathologic stages of lymph nodes usually differ from preoperatively predicted in lung cancers and it is difficult to predict the metastasis of lymph nodes for the patients diagnosed as clinical stage IA non-small cell lung cancers (NSCLC). This study aimed to investigate the patterns of lymph node metastasis and the risk factors predicting lymph node metastasis in the patients with clinical stage IA NSCLCs.
Methods
All patients diagnosed as clinical stage IA NSCLC from July 2013 to June 2017 in our center were retrospectively reviewed, and a total number of 1,543 patients who underwent anatomical lobectomy with systematic lymph node dissection were enrolled in this study. Multivariate logistic regression analysis was performed to identify the risk factors predicting lymph node metastasis, and Fisher’s exact test was used to confirm the lymph node spread mode according to the locations of primary tumors.
Results
Totally, lymph node metastases presented in 131 patients (8.5%) in this series. Sixty-three patients presented N1 diseases, 17 patients showed only skipped N2 diseases, and 51 patients had simultaneous N1 and N2 positive lymph nodes. No lymph node metastasis was found in the patients with pure ground grass opacity (GGO). When patients were arbitrarily divided into six groups by the longest tumor diameter of ≤0.5, 0.6–1, 1.1–1.5, 1.6–2.0, 2.1–2.5, 2.6–3 cm, the lymph node metastasis rates of each group were 0% (0/20), 1.5% (4/264), 4.7% (20/429), 8.6% (29/336), 13.1% (38/290), 19.6% (40/204), respectively. When the patients with pure GGO were excluded, the lymph node metastasis rates in the patients with partial or total solid tumors were 0% (0/10), 2.4% (4/164), 6.6% (20/303), 11.7% (29/249), 16.0% (38/238) and 23.1% (40/173). The cut off value showed by receiver operating characteristic (ROC) curve for tumor size was 1.95 cm, and the area under the curve (AUC) was measured as 0.681 (P<0.001, 95% CI: 0.630–0.726). Multivariate logistic regression analysis indicated that male patients [odds ratio (OR) =3.34, P=0.012], smoking history (OR =14.12, P<0.001), solid components (OR =3.34, P=0.01), large tumor size (OR =1.9, P<0.001), poor differentiation (OR =2.25, P=0.013), lymphovascular invasion (OR =58.45, P<0.001), visceral pleural invasion (OR =48.37, P<0.001) were significantly associated with lymph node metastasis in clinical stage IA NSCLC. The rate of non-lobe specific lymph node metastasis was 15.8–40.0% when any of the lobe specific lymph nodes was positive, while it was only 0–2.2% when all lobe specific lymph nodes were negative.
Conclusions
Tumor size, solid components, poor differentiation, lymphovascular invasion, visceral pleural invasion and smoking history were significant factors predicting lymph node metastasis of clinical stage IA NSCLC. Patients with negative lobe-specific lymph node have very low risk of metastasis to the non-lobe specific lymph nodes. Lobe-specific lymph node dissection may become an alternative lymph node dissection mode for clinical stage IA NSCLC, especially for tumors ≤2 cm.
Keywords: Non-small cell lung cancer (NSCLC), lymph node metastasis, lymph node dissection, lobe specific lymph node
Introduction
Lung cancer has become the most common cancer among all malignances in China and it continues to be the leading cause of cancer-related deaths worldwide (1,2). Non-small cell lung cancer (NSCLC), especially the adenocarcinoma has become the major pathological type in recent years (1,2). More and more early stage lung cancers were detected in recent years due to lung cancer screening using high-resolution computed tomography (CT) among Chinese residents.
Up to now, surgical resection is still the major and most effective treatment for early stage NSCLC. Anatomic lobectomy combined with systematic mediastinal lymph node dissection (SMLND) has been considered to be the standard surgical treatment (3). However, the optimal lymph node resection mode for early stage NSCLC remains controversial for many years around the world. Some randomized controlled trials showed that the patients with selective lymph node dissection (SLND) based on the lobe specific lymphatic pathway presented lower perioperative morbidities and similar survival when compared with the patients who underwent SMLND, and SLND has been used as an alternative lymph node dissection procedure in patients with early stage NSCLC (<2 cm) in order to reduce perioperative complications, especially for elderly patients or the patients with impaired pulmonary functions (4-7). Therefore, the factors predicting lymph node metastasis would be valuable for making a suitable decision on lymph node resection mode for clinical stage IA NSCLC. This study aimed to investigate the predictive factors of lymph node metastasis in clinical stage IA lung cancers.
Methods
Patient population
Patients who were diagnosed as clinical stage IA NSCLCs and underwent standardized lobectomy or bilobectomy with systematic lymph node dissection at National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College from July 2014 to June 2017 were retrospectively collected from a prospectively established NSCLC database. Preoperative evaluations included: physical examination, contrast-enhanced CT of the chest and abdomen, magnetic resonance imaging (MRI) of the brain, whole-body bone scintigraphy, electrocardiogram, pulmonary function examination and blood biochemical examinations for liver and kidney as well as coagulation function. Positron-emission tomography (PET) scan was not routinely performed in early-stage lung cancer due to low cost-efficiency. When the shortest axis of the mediastinum lymph node was longer than 1 cm on CT scan, it was considered to be positive lymph node metastasis preoperatively.
The inclusion criteria of this study were: age ≤75 years; no history of malignant tumors; no preoperative treatment such as chemotherapy or radiotherapy as well as immunotherapy; no history of severe cardiovascular or pulmonary diseases. The TNM stage of the tumors was determined according to the 8th American Joint Committee on Cancer (AJCC) Edition (2015) of the TNM Classification for Lung Cancer.
Totally, 2,085 patients were preoperatively diagnosed as clinical stage IA NSCLC in the database, 61 patients who only received wedge resection and 86 patients who didn’t undergo dissection of hilar and subcarinal nodes or those with a total of harvested lymph nodes less than six were excluded from this study. Another 395 patients who underwent segmentectomy or lobectomy only with SLND were also excluded, 1,543 patients with clinical stage IA NSCLC were enrolled in the study.
This study was approved by the Ethics Committee of Cancer hospital, Chinese Academy of Medical Sciences (No. NCC2014ST-07). All informed patients’ consents were signed and collected preoperatively.
Surgical procedures
All patients received at least anatomic lobectomy combined with systematic hilar and mediastinal lymph node dissection by either thoracotomy or video-assisted thoracic surgery (VATS). Systematic lymph node dissection for right-side tumors contained 2R (the upper paratracheal), 4R (lower paratracheal), 7 (the subcarinal), 8 (para-esophageal), 9 (pulmonary ligament) and 10–14 (hilar and intrapulmonary) stations, while for left side tumors, 4 L (lower paratracheal), 5 (aorto-pulmonary window), 6 (para-aortic), 7, 8, 9 and 10–14 stations were required. Pathological evaluation of lymph node metastasis was done using hematoxylin and eosin staining. The status of lymph node involvement was defined as N0 (no lymph node metastasis), N1 (any positive metastatic lymph nodes in station 10–14) or N2 (any positive metastatic lymph nodes in station 2–9).
Statistical analysis
The Student’s t-test and χ2 test were used to test differences in clinicopathologic characteristics between two groups for statistical significance, and Fisher’s exact test was used to compare the frequencies of non-specific lymph node metastasis between positive and negative lobe specific lymph node groups. The binary logistic regression was performed to identify factors predicting lymph node metastasis in clinical stage IA NSCLC. Tumor size as a predictive factor was analyzed by the receiver operating characteristic (ROC) curve to determine the cutoff value. All statistical analysis was performed using IBM SPSS 22.0 software. A P<0.05 was considered to be statistically significant.
Results
The general characteristics of all enrolled 1,543 patients and the association between the characteristics and lymph node metastasis were showed in the Table 1.
Table 1. General characteristics and association between patients’ characteristics and lymph node metastasis.
| Variables | Total (1,543) | pN0 (n=1,412) | pN1 + pN2 (n=131) | t/χ2 | P value |
|---|---|---|---|---|---|
| Age (years) | 57.96±8.59 | 57.86±8.62 | 59.03±8.33 | −1.488 | 0.137 |
| ≤40 | 42 | 39 | 3 | 3.241 | 0.514 |
| 41–50 | 259 | 242 | 17 | ||
| 51–60 | 586 | 533 | 53 | ||
| 61–70 | 557 | 511 | 46 | ||
| ≥71 | 99 | 87 | 12 | ||
| Sex | 10.546 | 0.001 | |||
| Male | 710 | 632 | 78 | ||
| Female | 833 | 780 | 53 | ||
| Smoking history | 274.504 | <0.001 | |||
| Never | 1,374 | 1,314 | 60 | ||
| Current/former | 169 | 98 | 71 | ||
| Tumor location | 2.913 | 0.580 | |||
| RUL | 532 | 494 | 38 | ||
| RML | 100 | 93 | 7 | ||
| RLL | 311 | 283 | 28 | ||
| LUL | 367 | 330 | 37 | ||
| LLL | 233 | 212 | 21 | ||
| CT characters | 62.593 | <0.001 | |||
| GGO | 406 | 406 | 0 | ||
| P-GS | 252 | 238 | 14 | ||
| Solid | 885 | 769 | 116 | ||
| Tumor size (cm) | 67.161 | <0.001 | |||
| ≤0.5 | 20 | 20 | 0 | ||
| 0.5–1 | 264 | 260 | 4 | ||
| 1.1–1.5 | 429 | 409 | 20 | ||
| 1.6–2.0 | 336 | 307 | 29 | ||
| 2.1–2.5 | 290 | 252 | 38 | ||
| 2.6–3.0 | 204 | 164 | 40 | ||
| Histologic type | 1.322 | 0.531 | |||
| Adenocarcinoma | 1,321 | 1,213 | 108 | ||
| SCC | 189 | 170 | 19 | ||
| Others | 33 | 29 | 4 | ||
| Differentiation | 107.344 | <0.001 | |||
| Well | 477 | 471 | 6 | ||
| Moderate | 760 | 703 | 57 | ||
| Poor | 306 | 238 | 68 | ||
| VPI | 161.879 | <0.001 | |||
| Yes | 180 | 120 | 60 | ||
| No | 1,363 | 1,292 | 71 | ||
| LVI | 331.739 | <0.001 | |||
| Yes | 95 | 39 | 56 | ||
| No | 1,448 | 1,373 | 75 |
GGO, ground-glass opacity; VPI, visceral pleural invasion; LVI, lymphovascular invasion; P-GS, partially GGO-solid tumors; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; SCC, squamous cell carcinoma.
Of the 1,543 patients, 1,412 were pathologically confirmed as N0 while the other 131 as N1 and/or N2: 63 patients (4.1%) presented only N1 positive lymph nodes, 17 patients (1.1%) showed only skip N2 metastatic lymph nodes without any N1 positive nodes, and 51 patients (3.3%) had both N1 and N2 positive lymph nodes, therefore, the total lymph node metastasis rate in this series was 8.5% (131/1,543). No lymph node metastasis was found in pure ground grass opacity (GGO) tumors in this study, while the lymph node metastasis rates in partial solid-GGO tumors and solid tumors were 5.96% (15/252) and 13.1% (116/885), respectively. The rates of lymph node metastasis in each following group divided by tumor diameter of ≤0.5, 0.6–1, 1.1–1.5, 1.6–2.0, 2.1–2.5, 2.6–3 cm were 0% (0/20), 1.5% (4/264), 4.7% (20/429), 8.6% (29/336), 13.1% (38/290), 19.6% (40/204), respectively. It was 0% (0/10), 2.4% (4/164), 6.6% (20/303), 11.7% (29/249), 16.0% (38/238) and 23.1% (40/173) in the patients with partial solid or solid tumors when all pure GGO patients were excluded.
The association between clinical factors and lymph node metastasis was analyzed and the results showed that gender (χ2=10.55, P=0.001), smoking history (χ2=274.50, P<0.001), CT characteristics (χ2=62.59, P<0.001), tumor sizes (χ2=61.16, P<0.001), differentiation (χ2=107.34, P<0.001), visceral pleural invasion (χ2=161.88, P<0.001), and lymphovascular invasion (χ2=321.74, P<0.001) were statistically significant factors predicting lymph node metastasis, while age (t=3.241, P=0.514), tumor locations (χ2=2.91, P=0.58) and histologic type (χ2=1.32, P=0.531) were not significant factors.
When divided the 252 patients whose CT showed partial-solid tumors into GGO-predominant group (GGO component ≥50%) and solid-predominant (GGO component <50%) group, the incidence of lymph node metastasis presented 2.9% and 8.9%, respectively (χ2=4.31, P=0.037)
The results of multivariate analysis were showed in Table 2. The results indicated that male patients [odds ratio (OR) =3.34, P=0.012], smoking history (OR =14.12, P<0.001), CT characteristics (OR =3.34, P=0.01), tumor size (OR =1.9, P<0.001), visceral pleural invasion (OR =48.37, P<0.001), poor differentiation (OR =2.25, P=0.013) and lymphovascular invasion (OR =58.45, P<0.001) were statistically associated with lymph node metastasis in clinical stage IA NSCLC, their 95% CI were 1.31–8.56, 5.71–34.90, 1.59–6.99, 1.36–2.65, 15.86–147.52, 1.18–4.27, and 21.89–157.15, respectively (Table 2).
Table 2. Factors predicting lymph node metastasis in clinical stage IA NSCLC.
| Parameters | OR | P value | 95% CI |
|---|---|---|---|
| Sex | 3.34 | 0.012 | 1.31–8.56 |
| Age group | 0.84 | 0.247 | 0.72–2.08 |
| Smoking history | 14.12 | <0.001 | 5.71–34.90 |
| Tumor location | 0.88 | 0.301 | 0.68–1.13 |
| CT characteristics | 3.34 | 0.001 | 1.59–6.99 |
| Tumor size | 1.90 | <0.001 | 1.36–2.65 |
| Histologic type | 1.99 | 0.064 | 0.96–4.15 |
| Differentiation | 2.25 | 0.013 | 1.18–4.27 |
| LVI | 58.45 | <0.001 | 21.89–157.15 |
| VPI | 48.37 | <0.001 | 15.86–147.52 |
NSCLC, non-small cell lung cancers; VPI, visceral pleural invasion; LVI, lymphovascular invasion; OR, odds ratio.
Among all risk factors of lymph node metastasis, tumor size was analyzed by ROC curve. The result showed that the cut off value of 1.95 cm could be a predictive factor (P<0.001) of lymph node metastasis in clinical stage IA NSCLC. The AUC was measured as 0.681 (P<0.001, 95% CI: 0.630–0.726; Figure 1).
Figure 1.
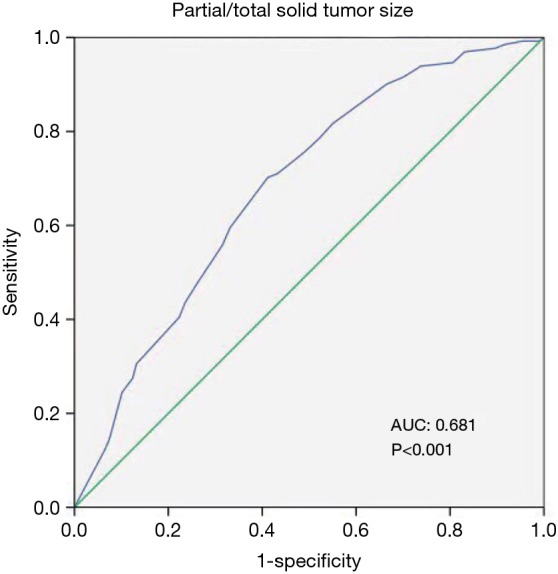
ROC curve showed the predictability of lymph lone metastasis based on tumor size. AUC, 0.681 (95% CI: 0.630–0.726), P<0.001. ROC, receiver operating characteristic; AUC, area under the curve.
The incidence of non-lobe specific lymph node metastasis between lobe-specific lymph nodes positive and negative groups were analyzed by Fisher’s exact test and showed in Table 3. When any of the lobe-specific lymph nodes had metastasis, 15.8–40.0% patients presented non-lobe specific lymph nodes metastasis, but only 0–2.2% patients had non-lobe specific lymph node metastasis when all lobe-specific lymph nodes were negative.
Table 3. Comparison of the rates of non-lobe specific lymph node metastasis between the lobe specific lymph node positive and negative groups according to the locations of primary tumors.
| Tumor locations | LSLNS (+) N-LSLNS (+) | LSLNS (−) N-LSLNS (+) | P value |
|---|---|---|---|
| RUL | 15.79% (6/38) | 0 | <0.001 |
| RML | 40% (2/5) | 2.15% (2/93) | 0.012 |
| RLL | 21.42% (6/28) | 0 | <0.001 |
| LUL | 22.86% (8/35) | 0.61% (2/330) | <0.001 |
| LLL | 22.22% (4/18) | 1.42% (3/212) | 0.001 |
LSLNS, lobe specific lymph node stations; N-LSLNS, non-lobe specific lymph node stations; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe.
Discussion
More and more early stage lung cancers without any symptoms have been discovered and diagnosed in recent years due to cancer screening programs for high risk people and annual health check-up using high-resolution CT in China (8). Surgical treatment is still the most effective way to cure the diseases. Up to now, the anatomical lobectomy with systematic lymph nodes dissection either by VATS or thoracotomy is still the most frequently used procedures for early stage lung cancer. Therefore, accurate preoperative assessment of lymph node status is important in order to design the most appropriate treatment regimen for the early lung cancer patients. Unfortunately, it is really difficult to predict the precise lymph node status preoperatively and the postoperative pathologic status of lymph nodes may be different from preoperative prediction (9). For the advanced stage NSCLCs, lobectomy with systematic mediastinal lymph node dissection has been considered to be the standard treatment, however, for the early stage NSCLCs, the optimal lymph node dissection mode has remained to be controversial in clinical practice for many years.
This study focused on the association of primary tumor characteristics on the CT scan and other clinicopathological factors with lymph node metastasis in clinical stage IA NSCLC. The overall lymph node metastasis rate in this series was 8.5%, which was similar to 7.5% in 894 patients with clinical stage IA disease reported by Koike and colleagues (10). No lymph node metastasis was found in the patients with pure GGO lesions in this series. Haruki reported that the incidence of lymph node metastasis was 9.1% and no lymph node metastasis occurred in GGO patients, which was also similar to other reports (11,12). The lymph node metastasis rate was 5.96% in partial solid-GGO tumors and 13.1% in the solid tumors in this study. In the patients with partial GGO lesion, the frequency of lymph node metastasis increased significantly (P=0.037) as the solid components increased in this series and this was consistent with the results reported by other authors (13,14).
It was reported that the incidence of lymph node metastasis in lung cancer increased as the tumor size increased (15-17). Seok (18) reported that when the patients were divided into six groups based on the tumor diameter: ≤0.5, 0.6–1, 1.1–1.5, 16–2.0, 2.1–2.5, 2.6–3 cm, the rates of lymph node metastasis were 0%, 0%, 7%, 14%, 27% and 31%, respectively. Our study also presented a similar result that the frequency of lymph node metastasis increased sequentially as the tumor size increased. Multivariate analysis also confirmed that tumor size and CT characteristics were significantly associated with lymph node metastasis in clinical stage IA NSCLC.
Several studies reported that visceral pleural invasion, poor differentiation and lymphovascular invasion also associated with lymph node metastasis in NSCLC (19,20). These factors were also proved in this study by univariate and multivariate analysis as significant risk factors affecting lymph node metastasis.
Smoking history was found to be one of the risk factors affecting lymph node metastasis in this study. However, other factors such as age, tumor location and histological type were not associated with lymph node metastasis significantly. Gender was found to be a risk factor by univariate and multivariate analysis in this study, which showed that male (10.99%, 78/710) patients were more likely to suffer lymph node involvement compared with female patients (6.36%, 53/833). This might attribute to more smokers in male patients in this group,(18.17% of male patients in this series were smokers while only 4.80% of female patients had smoking history)and more female patients presented pure GGO tumors.
Some studies reported that systematic lymph node dissection in NSCLC could achieve accurate pathological staging and better overall survival (OS) (21,22). It was also reported that the lymph node stations were divided into lobe-specific and non-lobe specific lymph node stations, and the location of primary tumors significantly influenced the pathway of lymph node spread (23,24). The lobe specific lymph lode stations for right upper lobe tumors are 2R, 4R, and station 10–12, for right middle lobe tumors are station 7 and 10–12, for right lower lobe tumors are station 7, 8, 9 and station 10–12. Station 2L, 4L, 5, 6 and 10–12 are deemed to be the lobe specific lymph node stations for left upper lobe tumors, while station 7, 8, 9 and station 10–12 are considered to be the lobe specific lymph node stations for left lower lobe tumors (23,24). It was reported that the rate of non-lobe specific lymph node metastasis was less than 3% when all lobe-specific lymph nodes were negative (25,26). Therefore, some surgeons try to perform lobe-specific lymph node dissection in early stage NSCLC in recent years based on the above patterns of lobe-specific lymph node metastases (27,28). The rate of non-lobe specific lymph node metastasis was 15.8–40.0% when any of the lobe-specific lymph nodes had metastasis, while it was only 0–2.2% when all the lobe-specific lymph node were negative in our study. It showed a significant difference in non-lobe specific lymph nodes status between lobe-specific node positive and negative groups regardless of the primary tumor locations. Therefore, based on the results of our study and other reports (4,7,29,30), it may be practicable to perform a SLND procedure (lobe-specific lymph node dissection) for clinical stage IA NSCLC if intraoperative frozen sections show that all lobe-specific lymph nodes are negative microscopically, especially for those patients with a tumor <2 cm.
However, there were some limitations in this study. Firstly, this was a single-center, retrospective study. Secondly, only patients with age of ≤75 years were enrolled into this study. Therefore, patient-selection bias may be existed. Prospective multi-institutional studies may be required to validate the risk factors of lymph node metastasis and the practical usage of lobe specific lymph node dissection in clinical stage IA NSCLC in the future.
Conclusions
Patients with pure GGO nodules usually has no lymph node metastasis, and the rate of lymph nodes metastasis increases as the tumor size increases in partial-solid or solid tumors. Tumor size, solid component, poor differentiation, lymphovascular invasion, visceral pleural invasion and smoking history are independent risk factors of lymph node metastasis in clinical stage IA NSCLC, while the influence of gender on lymph node metastasis may need further evaluation. Patients with negative lobe-specific lymph nodes rarely have lymph node metastasis in non-specific lymph node stations. Therefore, lobe-specific lymph node dissection may be performed safely for patients with ≤2 cm stage IA NSCLC when all the lobe-specific lymph nodes are confirmed negative by intraoperative frozen section examination.
Acknowledgements
Funding: This work was supported by Beijing Municipal Science & Technology Commission (Research on the application of clinical characteristics in the capital, grant number: Z141107002514047).
Ethical Statement: This study was approved by the Ethics Committee of Cancer hospital, Chinese Academy of Medical Sciences (No. NCC2014ST-07). All informed patients’ consents were signed and collected preoperatively.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Zuo T, et al. National cancer incidence and mortality in China, 2012. Chin J Cancer Res 2016;28:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Izbicki JR, Passlick B, Pantel K, et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non-small cell lung cancer: results of a prospective randomized trial. Ann Surg 1998;227:138-44. 10.1097/00000658-199801000-00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okada M, Sakamoto T, Yuki T, et al. Selective mediastinal lymphadenectomy for clinico-surgical stage I non-small cell lung cancer. Ann Thorac Surg 2006;81:1028-32. 10.1016/j.athoracsur.2005.09.078 [DOI] [PubMed] [Google Scholar]
- 5.Kates M, Perez X, Gribetz J, et al. Validation of a model to predict perioperative mortality from lung cancer resection in the elderly. Am J Respir Crit Care Med 2009;179:390-5. 10.1164/rccm.200808-1342OC [DOI] [PubMed] [Google Scholar]
- 6.Sugi K, Nawata K, Fujita N, et al. Systematic lymph node dissection for clinically diagnosed peripheral non-small-cell lung cancer less than 2 cm in diameter. World J Surg 1998;22:290-4; discussion 294-5. 10.1007/s002689900384 [DOI] [PubMed] [Google Scholar]
- 7.Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. 10.1016/j.jtcvs.2010.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerfolio RJ, Bryant AS, Ojha B, et al. Improving the inaccuracies of clinical staging of patients with NSCLC: a prospective trial. Ann Thorac Surg 2005;80:1207-13; discussion 1213-4. 10.1016/j.athoracsur.2005.04.019 [DOI] [PubMed] [Google Scholar]
- 10.Koike T, Koike T, Yamato Y, et al. Predictive risk factors for mediastinal lymph node metastasis in clinical stage IA non-small-cell lung cancer patients. J Thorac Oncol 2012;7:1246-51. 10.1097/JTO.0b013e31825871de [DOI] [PubMed] [Google Scholar]
- 11.Cho S, Song IH, Yang HC, et al. Predictive factors for node metastasis in patients with clinical stage I non-small cell lung cancer. Ann Thorac Surg 2013;96:239-45. 10.1016/j.athoracsur.2013.03.050 [DOI] [PubMed] [Google Scholar]
- 12.Haruki T, Aokage K, Miyoshi T, et al. Mediastinal nodal involvement in patients with clinical stage I non-small-cell lung cancer: possibility of rational lymph node dissection. J Thorac Oncol 2015;10:930-6. 10.1097/JTO.0000000000000546 [DOI] [PubMed] [Google Scholar]
- 13.Tsutani Y, Miyata Y, Nakayama H, et al. Prognostic significance of using solid versus whole tumor size on high-resolution computed tomography for predicting pathologic malignant grade of tumors in clinical stage IA lung adenocarcinoma: a multicenter study. J Thorac Cardiovasc Surg 2012;143:607-12. 10.1016/j.jtcvs.2011.10.037 [DOI] [PubMed] [Google Scholar]
- 14.Murakawa T, Konoeda C, Ito T, et al. The ground glass opacity component can be eliminated from the T-factor assessment of lung adenocarcinoma. Eur J Cardiothorac Surg 2013;43:925-32. 10.1093/ejcts/ezs467 [DOI] [PubMed] [Google Scholar]
- 15.Li GL, Zhu Y, Zheng W, et al. Analysis of factors influencing skip lymphatic metastasis in pN(2) non-small cell lung cancer. Chin J Cancer Res 2012;24:340-5. 10.1007/s11670-012-0273-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oda M, Watanabe Y, Shimizu J, et al. Extent of mediastinal node metastasis in clinical stage I non-small-cell lung cancer: the role of systematic nodal dissection. Lung Cancer 1998;22:23-30. 10.1016/S0169-5002(98)00070-1 [DOI] [PubMed] [Google Scholar]
- 17.Ohta Y, Oda M, Wu J, et al. Can tumor size be a guide for limited surgical intervention in patients with peripheral non-small cell lung cancer? Assessment from the point of view of nodal micrometastasis. J Thorac Cardiovasc Surg 2001;122:900-6. 10.1067/mtc.2001.117626 [DOI] [PubMed] [Google Scholar]
- 18.Seok Y, Yang HC, Kim TJ, et al. Frequency of lymph node metastasis according to the size of tumors in resected pulmonary adenocarcinoma with a size of 30 mm or smaller. J Thorac Oncol 2014;9:818-24. 10.1097/JTO.0000000000000169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukui T, Katayama T, Ito S, et al. Clinicopathological features of small-sized non-small cell lung cancer with mediastinal lymph node metastasis. Lung Cancer 2009;66:309-13. 10.1016/j.lungcan.2009.03.004 [DOI] [PubMed] [Google Scholar]
- 20.Kudo Y, Saji H, Shimada Y, et al. Impact of visceral pleural invasion on the survival of patients with non-small cell lung cancer. Lung Cancer 2012;78:153-60. 10.1016/j.lungcan.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 21.Wu Y, Huang ZF, Wang SY, et al. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer 2002;36:1-6. 10.1016/S0169-5002(01)00445-7 [DOI] [PubMed] [Google Scholar]
- 22.Massard G, Ducrocq X, Kochetkova EA, et al. Sampling or node dissection for intraoperative staging of lung cancer: a multicentric cross-sectional study. Eur J Cardiothorac Surg 2006;30:164-7. 10.1016/j.ejcts.2006.04.010 [DOI] [PubMed] [Google Scholar]
- 23.Okada M, Tsubota N, Yoshimura M, et al. Proposal for reasonable mediastinal lymphadenectomy in bronchogenic carcinomas: role of subcarinal nodes in selective dissection. J Thorac Cardiovasc Surg 1998;116:949-53. 10.1016/S0022-5223(98)70045-5 [DOI] [PubMed] [Google Scholar]
- 24.Asamura H, Nakayama H, Kondo H, et al. Lobe-specific extent of systematic lymph node dissection for non-small cell lung carcinomas according to a retrospective study of metastasis and prognosis. J Thorac Cardiovasc Surg 1999;117:1102-11. 10.1016/S0022-5223(99)70246-1 [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Mao Y, He J, et al. Exploration of lymph node metastasis and appropriate lymph node dissection modes in patients with clinical stage I non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi 2014;36:536-40. [PubMed] [Google Scholar]
- 26.Aokage K, Yoshida J, Ishii G, et al. Subcarinal lymph node in upper lobe non-small cell lung cancer patients: is selective lymph node dissection valid? Lung Cancer 2010;70:163-7. 10.1016/j.lungcan.2010.02.009 [DOI] [PubMed] [Google Scholar]
- 27.Watanabe S, Asamura H. Lymph node dissection for lung cancer: significance, strategy, and technique. J Thorac Oncol 2009;4:652-7. 10.1097/JTO.0b013e31819cce50 [DOI] [PubMed] [Google Scholar]
- 28.Watanabe S, Asamura H, Suzuki K, et al. The new strategy of selective nodal dissection for lung cancer based on segment-specific patterns of nodal spread. Interact Cardiovasc Thorac Surg 2005;4:106-9. 10.1510/icvts.2004.098814 [DOI] [PubMed] [Google Scholar]
- 29.Ishiguro F, Matsuo K, Fukui T, et al. Effect of selective lymph node dissection based on patterns of lobe-specific lymph node metastases on patient outcome in patients with resectable non-small cell lung cancer: a large-scale retrospective cohort study applying a propensity score. J Thorac Cardiovasc Surg 2010;139:1001-6. 10.1016/j.jtcvs.2009.07.024 [DOI] [PubMed] [Google Scholar]
- 30.Yang XN, Zhao ZR, Zhong WZ, et al. A lobe-specific lymphadenectomy protocol for solitary pulmonary nodules in non-small cell lung cancer. Chin J Cancer Res 2015;27:538-44. [DOI] [PMC free article] [PubMed] [Google Scholar]


