Abstract
Background
Complete thymectomy is recommended for thymic malignancies to reduce local recurrence and the likelihood of the long-term development of myasthenia gravis (MG). Thymus-conserving surgery (thymomectomy) seems to yield similar results, but evidence is still limited. The objective of this study was to assess if the oncological outcome, in terms of overall survival (OS) and disease-free survival (DFS), are comparable between radical thymectomy vs. conservative thymomectomy patients, and to assess if the outcome of the video-assisted thoracoscopic surgery (VATS) approach was similar to open surgery approach.
Methods
We retrospectively analyzed 157 consecutive patients with either resectable thymoma or thymic carcinoma from two Italian centers (Humanitas Research Hospital, Milan, and Humanitas Gavazzeni, Bergamo) between 1997 and 2013 who underwent thymomectomy or extended thymectomy with the VATS or open approach; the patients with Miastenia Gravis underwent radical thymectomy. The patients were followed through physical examinations and phone interviews.
Results
Thymomectomy and thymectomy were performed on 86 (54.8%) and 71 (45.2%) patients, respectively. Prognostic factors and comorbidities were comparable in the two groups. The median follow-up was 77 months. Cox proportional hazards model revealed that Masaoka advanced stage and thymic carcinoma of WHO classification were independent predictive factors for overall survival, but that the extent of surgery and the approach used (minimally invasive versus open) were not. Notably, five- and ten-year survival rates were similar in the two groups.
Conclusions
In our experience, radical thymectomy and conservative thymomectomy did not differ in terms of disease-free and overall survival rates. In nonmyasthenic patients with early-stage resectable thymic malignancy, minimally invasive thymomectomy provided equivalent results to open thymectomy. Our results should be interpreted with caution due to the retrospective nature of the study. Well-designed, adequately-powered studies should be very welcome to increase the quantity and the quality of clinical evidence before incorporating this procedure in future guidelines.
Keywords: Thymoma, surgery, radical thymectomy, thymomectomy
Introduction
Thymic tumors—typically diagnosed in middle-aged patients—are rare, comprising only 0.2–1.5% of all mediastinal neoplasms; indeed, incidence is 0.15 per 100,000 person-years in the United States (1,2). The approach to treatment is controversial, although radical thymectomy with complete tumor resection is commonly accepted as standard procedure when thymoma is associated with myasthenia gravis (MG) (3-5), an event occurring in about 45% of cases (6). Moreover, most of the investigators indicate that radical thymectomy should be standard also for non-myasthenic thymoma as well as thymic carcinoma because less-extensive surgery can be associated with increased risk of local recurrence, as thymic tissue within mediastinal fat can represent possible foci of occult cancer (3,7). However, some series indicate that conservative surgery (thymomectomy without radical thymectomy) for non-myasthenic thymoma is not associated with a worse outcome (4,8,9); indeed, in a 2016 study by Tseng et al. (4) of stage I–II nonmyasthenic thymoma patients receiving extended thymectomy via median sternotomy (n=42), thymomectomy without thymectomy via video-assisted thoracoscopic surgery (VATS) (n=22), or thoracotomy (n=31), found that recurrence rates were always low (n=1 in the thymomectomy group; n=2 in the thymectomy group) and did not differ significantly between interventional groups after a mean follow-up of 57 months (range, 6–121 months). The authors concluded that thymomectomy without thymectomy is justified for early-stage non-myasthenic thymic tumors, but acknowledged the need for a longer follow-up. To our knowledge, no prospective analyses have compared thymomectomy with radical thymectomy in non-myasthenic patients with thymic tumors.
The most appropriate surgical approach to operable thymic tumors has also become controversial. Indeed, the established trans-sternal approach (10) has been challenged by the introduction of less invasive VATS (11-13) and, more recently, robot-assisted surgery (14).
Many studies indicate reduced morbidity and mortality with similar oncological outcomes for these minimally invasive approaches compared with the trans-sternal approach for thymomectomy and for radical thymectomy (14-19). However, several authors suggest that long-term studies are required to evaluate the efficacy of these approaches in the treatment of thymic tumors (10,15,18).
In the present study, we retrospectively analyzed the outcomes of patients with thymic tumors treated over a 16-year period, with the main goal of understanding if the outcomes of radically resected versus conservatively resected groups are similar. We also assessed any difference in the outcomes of VATS and open surgery.
Methods
Patient recruitment and sample collection
We retrospectively evaluated all patients operated on for primary thymic epithelial tumors from 1997 to 2013 at two sister hospitals (Humanitas Research Hospital, Milan, and Humanitas Gavazzeni, Bergamo) in Italy. Cases with recurrent thymic disease or other malignancy (diagnosed previously or synchronously) were excluded. The list of the patients was obtained by the analysis of surgical registry, combined with the database of the pathology division. We extracted the following data from clinical records: age, sex, preoperative Charlson comorbidity index (20), presence of MG, thymectomy vs. thymomectomy, thoracotomy vs. VATS, extent of resection, complications [WHO-derived criteria (21)], chemotherapy, radiotherapy, Masaoka stage, histotype [WHO histological classification (22) with the highest grade defining histotype for mixed tumors (23)], recurrence, and vital status. Histology and stage were determined at the single pathology laboratory used by both hospitals.
The patients were followed with chest CT with contrast and physical examination every 6 months for about 5 years. After the first 5 years, the follow-up was made by phone call.
All patients signed an informed consent for the acquisition and the usage of clinical data for research purposes at admission. For the use of data, we followed the rules of the Helsinki declaration. The study was approved by the internal research board.
Surgical procedures
Thymomectomy was resection of the tumor with clear margins, leaving thymic tissue behind. Radical thymectomy was resection of the tumor together with the thymus and mediastinal fat above the innominate veins to the diaphragm, between the phrenic nerves.
The extended resection was used only if the thymic tumor was widely invasive and the surgeon performed an en-bloc removal of all affected structures including lung parenchyma (usually through limited resections), pericardium, great vessels, nerves and pleural implants.
Incomplete resections (R1 or R2) were defined according to the International Thymic Malignancy Interest Group guidelines (24).
Pre-treatment biopsy was not usually performed if CT and FDG-PET findings indicated stage I–II disease, or if VATS thymectomy was planned. For locally advanced disease, where more invasive surgery or induction therapy was indicated, CT-guided core biopsy was performed to confirm the diagnosis. Patients were offered primary surgery if preoperative work-up indicated immediately resectable clinical stage I–III thymoma. If complete resection was deemed not to be achievable upfront on the basis of imaging studies, as it is frequently the case in Masaoka stage IV (direct invasion of the aorta, arch vessels, the main pulmonary artery, the myocardium, the trachea, or the esophagus), induction chemotherapy, radiotherapy, or both were offered. Chemotherapy was platinum, adriamycin, and cyclophosphamide for three cycles. In the absence of response, second-line chemotherapy with platinum-etoposide (PE) for two cycles plus radiotherapy (45 Gy) was administered. The patients were then re-evaluated for surgery. Adjuvant chemotherapy was also offered to patients presenting with residual disease after surgery, unfavorable histotype, or stage III thymoma after complete resection.
For patients with non-MG thymoma the surgical approach was different in the two centers. In Milan, in most cases, the conservative thymomectomy surgery was performed via anterior muscle-sparing thoracotomy; while in Bergamo the surgeons usually performed radical thymectomy via the trans-sternal approach.
Up to 2007, all patients with MG-associated thymoma always received radical thymectomy with open approach surgery. From 2007, VATS was increasingly used to perform both for radical thymectomy and thymomectomy for early clinical stage of thymoma in both centers.
Statistical methods
Data were expressed as numbers and percentages, medians and ranges, or means and standard deviations (SD). The significance of differences in variables between groups was tested with the Wilcoxon test (continuous data) or chi-square test with Fisher correction if necessary (categorical data).
Survival time was calculated from date of surgery to death or latest contact. Deaths due to treatment complications were regarded as related to the thymic tumors. Time to recurrence or progression was calculated from date of surgery to date of first relapse for complete resections, or detection of progression for incomplete resections. Survival curves were produced by the Kaplan-Meier method.
The variables age (continuous), sex, MG (present/absent), Charlson morbidity index, thymomectomy vs. thymectomy, VATS vs. open surgery, complete resection vs. residual disease, A–B2 vs. B3 histology, carcinoma vs. thymoma, Masaoka stage I–II vs. III–IV, chemotherapy vs. none, and postoperative radiotherapy vs. none were included in univariable Cox regression models to produce hazard ratios (HR) with p values indicating associations of these variables with overall (OS) and disease-free survival (DFS). Variables with P<0.20 were included in a stepwise multivariable Cox hazards model, a procedure to select the best model, with a probability to enter of 0.05 and a probability to remove of 0.1. The analyses were performed with Stata version 13.
Results
In the studied period, a total of 222 patients were operated on for thymic epithelial tumors at the two hospitals; 39 patients were excluded because of previous surgery for recurrent tumors. Twenty-six patients were excluded from the study for synchronous or previous associated malignancy, leaving 157 cases for the present analysis. Patients’ demographic characteristics, surgical and pathological features, treatment details, and outcome are summarized in Table 1.
Table 1. Patients’ characteristics, treatments, pathology findings, and outcome according to the kind of surgery.
| Variables | All | Thymomectomy | Radical thymectomy | P |
|---|---|---|---|---|
| No. of patients | 157 (100%) | 86 (54.8%) | 71 (45.2%) | – |
| Age | 57±14 | 58±14 | 56±14 | 0.202 |
| Gender (males), n (%) | 76 (48.4) | 41 (47.7) | 35 (49.3) | 0.873 |
| MG, n (%) | 22 (14.0) | 0 | 22 (31.0) | <0.001 |
| Charlson index | 1.78±1.60 | 2.00±1.67 | 1.49±1.47 | 0.073 |
| WHO predominant histology, n (%) | 0.055 | |||
| A | 21 (13.4) | 10 (11.6) | 11 (15.5) | |
| AB | 45 (28.7) | 32 (37.2) | 13 (18.3) | |
| B1 | 27 (17.2) | 12 (14.0) | 15 (21.1) | |
| B2 | 22 (14.0) | 12 (14.0) | 10 (14.1) | |
| B3 | 27 (17.2) | 10 (11.6) | 17 (23.9) | |
| Carcinoma | 15 (9.6) | 10 (11.6) | 5 (7.0) | |
| Masaoka stage, n (%) | 0.724 | |||
| I | 41 (26.1) | 20 (23.3) | 21 (29.6) | |
| II | 74 (47.1) | 44 (51.2) | 30 (42.3) | |
| III | 29 (18.5) | 15 (17.4) | 14 (19.7) | |
| IV | 13 (8.3) | 7 (8.1) | 6 (8.5) | |
| Incomplete resection, n (%) | 16 (10.2) | 9 (10.5) | 7 (9.9) | 1.000 |
| R1 resection | 10 (6.4) | 6 (7.0) | 4 (5.6) | |
| R2 resection | 6 (3.8) | 3 (3.5) | 3 (4.2) | |
| Approach, n (%) | 0.944 | |||
| Open | 123 (78.3) | 68 (79.1) | 55 (77.5) | |
| VATS | 29 (18.5) | 15 (17.4) | 14 (19.7) | |
| Conversion | 5 (3.2) | 3 (3.5) | 2 (2.8) | |
| Extended surgery, n (%) | 55 (35.0) | 27 (31.4) | 28 (39.4) | 0.317 |
| Complications, n (%) | 33 (21.0) | 15 (17.4) | 18 (25.4) | 0.243 |
| Post-operative stay | 5 [0–30] | 5 [2–28] | 5 [0–30] | 0.227 |
| Multimodality therapy, n (%) | 50 (31.8) | |||
| Neoadjuvant chemo | 17 (10.8) | 10 (11.6) | 7 (9.9) | 0.800 |
| Neoadjuvant RT | 3 (1.9) | 2 (2.3) | 1 (1.4) | 1.000 |
| Adjuvant chemo | 11 (7.0) | 6 (7.0) | 5 (7.0) | 1.000 |
| Adjuvant RT (PORT) | 36 (22.9) | 20 (23.3) | 16 (22.5) | 1.000 |
WHO, World Health Organization; VATS, video-assisted thoracic surgery; RT, radiotherapy; PORT, post-operative radiotherapy.
All patients were staged by contrast enhanced CT-scan of the chest and, in locally advanced cases, by MRI (29 patients, 18%) as well. Preoperative FDG-PET-scan was conducted on 44 patients (27%).
No differences were observed between thymomectomy and radical thymectomy groups in terms of stage, Charlson Index, histology, and rate of extended surgery.
VATS thymectomy was attempted in 34 cases, but for 5 (14.7%) patients it was decided to switch to open thymectomy due to invasion of the great veins (n=1), lung (n=1), pericardium (n=1), or left phrenic nerve (n=2). Tumor invasion of neighboring structures (lung in n=45, pericardium in n=47, diaphragm in n=5, phrenic nerve in n=14, and great veins in n=9 cases) required extended resections in 55 (35%) patients.
Microscopically clear margins were obtained in a total of 141 cases: n=77 (89.5%) after thymomectomy and n=64 (90.1%) after radical thymectomy (P=1.000). All resections attempted by VATS achieved clear margins. The 16 patients who had an incomplete resection (R1 or R2 resection margins) received adjuvant radiotherapy and adjuvant sequential chemo-radiotherapy.
Postoperative complications were experienced by 28/123 (22.8%) open surgery patients, by 4/29 (13.8%) VATS patients, and by 1/5 (20%) patients converted from VATS to open (P=0.447). Two deaths occurred perioperatively after extended resections for bulky stage III and IVa thymoma (mortality rate 1.3%) and one after chemotherapy.
Complementary pre- or post-operative treatments were administered to 47 (29.9%) patients overall: 26 received chemotherapy, and 39 received radiotherapy.
Altogether, 115 patients (73.3%) had Masaoka stage I-II disease, 142 patients (90.4%) had WHO thymoma histology, and 15 had thymic carcinoma (9.6%). There was a significant association between higher Masaoka stage and higher-grade WHO histology (P<0.001).
After a median follow-up time of 77 (range, 0–212) months, 22 patients (14%) experienced a recurrence: 7 had local recurrences (involving the residual mediastinal structures or nodes), 5 had parietal pleura or chest-wall involvement alone, 4 had isolated pulmonary metastasis, and 6 had distant metastasis. Surgical treatment of the recurrence (either alone or in combination with chemo-radiotherapy) was possible in 9 cases, while 9 received radiotherapy or chemo-radiotherapy only. In 4 cases, the treatment administered is unknown.
There were a total of 27 deaths, including the 3 post-treatment deaths: 6 (3.8%) due to disease progression, 4 from other cancers, 1 from MG, 12 from non-neoplastic conditions, and 1 from an unknown cause. Five and ten-year DFS rates were 91.1% and 81.8%, respectively, while the OS rates were 87.8% and 79.8%.
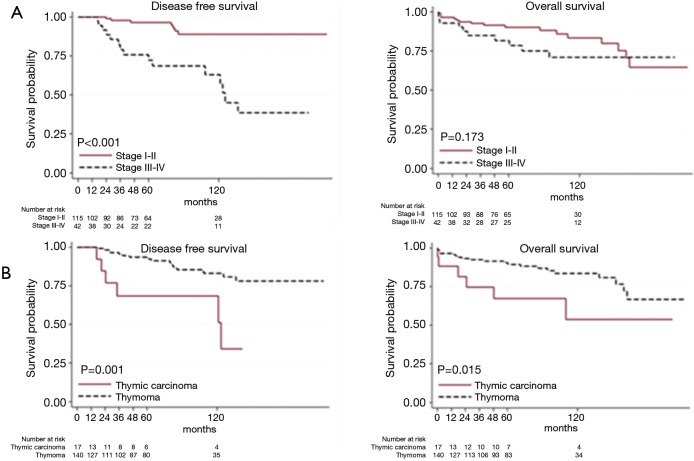
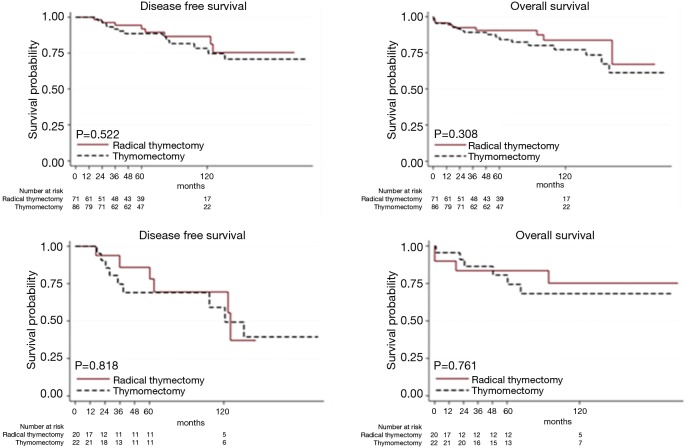
At univariable analysis, incomplete resection, stage III–IV disease, B3-carcinoma histology, and multimodality treatments were associated with lower DFS, while carcinoma WHO histology alone was negatively associated with OS (Table 2, Figure 1A,B). Multivariable analysis (Table 2) indicated advanced Masaoka III–IV stage and incomplete resection as significant predictors of recurrence. Analysis of outcome according to mode of resection showed no differences between thymomectomy and radical thymectomy groups. Five- and ten-year OS were 85.8% and 77.1% in the former, and 90.6% and 83.8% in the latter. Respectively, the DFS were 88.6% and 78.3% in the first group and 94.4% and 86.6% in the second one (Figure 2A). There were no differences in survival between the two modes of resection in the subgroup of patients with stage III and IV thymoma (DFS: HR, 1.12, 95% CI: 0.40–3.19, P=0.818; OS: HR, 1.21, 95% CI: 0.34–4.34, P=0.761) (Figure 2B).
Table 2. Univariate and multivariate survival analysis.
| Variables | Disease-free | Overall | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | ||||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||||
| Age (continuous) | 0.98 (0.95–1.01) | 0.312 | 1.02 (1.00–1.06) | 0.074 | 0.857 | ||||||
| Gender (males) | 1.53 (0.66–3.59) | 0.324 | 1.50 (0.70–3.24) | 0.298 | |||||||
| MG | 1.41 (0.52–3.83) | 0.500 | 0.82 (0.28–2.37) | 0.711 | |||||||
| Charlson index | 0.85 (0.63–1.15) | 0.283 | 1.21 (0.96–1.53) | 0.100 | 1.24 (0.98–1.58) | 0.076 | |||||
| Thymomectomy | 1.33 (0.56–3.17) | 0.523 | 1.51 (0.68–3.37) | 0.312 | |||||||
| VATS resection | 0.60 (0.14–2.62) | 0.494 | 1.06 (0.36–3.17) | 0.911 | |||||||
| Incomplete resection | 3.33 (1.35–8.19) | 0.009 | 2.51 (0.99–6.33) | 0.052 | 0.57 (0.13–2.41) | 0.445 | |||||
| WHO histology | |||||||||||
| A–B2 | 1 | 1 | 1 | ||||||||
| B3 | 5.46 (2.15–13.87) | <0.001 | 0.256 | 1.90 (0.73–4.91) | 0.187 | 2.54 (0.94–6.88) | 0.067 | ||||
| Carcinoma | 5.27 (1.61–17.24) | 0.006 | 0.198 | 3.10 (1.13–8.49) | 0.028 | 3.54 (1.26–9.92) | 0.016 | ||||
| Stage III–IV | 6.78 (2.76–16.65) | <0.001 | 6.11 (2.47–15.14) | <0.001 | 1.51 (0.67–3.37) | 0.317 | |||||
HR, hazard ratio; CI, confidence interval; MG, myasthenia gravis; WHO, World Health Organization; VATS, video-assisted thoracic surgery.
Figure 1.
The Kaplan-Meier overall and disease free survival curves: (A) outcome analysis by Masaoka stage; (B) outcome analysis by WHO histology.
Figure 2.
The Kaplan-Meier overall and disease free survival curves: (A) oncological outcome by extent of resection in all stage of disease; (B) oncological outcome by extent of resection in patients with stage III–IV.
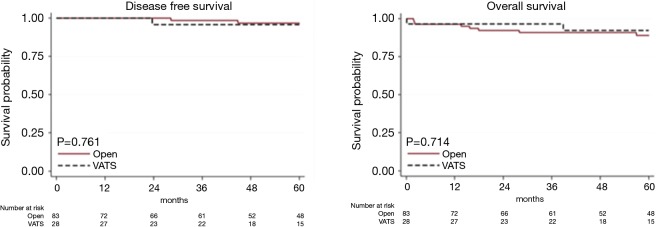
Table 3 gives patient characteristics stratified by approach (VATS vs. open surgery) and outcome, excluding 5 patients switched from VATS to open anterior thoracotomy. OS and DFS for stage I–II were similar after VATS and the open approach (OS: HR, 0.75, 95% CI: 0.16–3.53, P=0.714; DFS: HR, 1.45, 95% CI: 0.13–15.98, P=0.761). The observation is limited to 5 years because VATS was introduced only recently (Figure 3).
Table 3. Characteristics and oncological outcomes between VATS and open surgery approach.
| Variables | Total (n=152) | VATS (n=29) | Open (n=123) | P |
|---|---|---|---|---|
| Charlson index | 1.74±1.55 | 1.92±1.83 | 1.70±1.48 | 0.728 |
| Complications | 32 (21.1%) | 4 (13.8%) | 28 (22.8%) | 0.447 |
| Masaoka | ||||
| Masaoka (I–II) | 111 (73.0%) | 28 (96.6%) | 83 (67.5%) | 0.001 |
| Masaoka (III–IV) | 41 (27.0%) | 1 (3.4%) | 40 (32.5%) | 0.001 |
| WHO (thymoma) | 135 (88.8%) | 28 (96.6%) | 107 (87.0%) | 0.197 |
| CT neo | 17 (11.2%) | 0 | 17 (13.8%) | 0.044 |
| MG | 21 (13.8%) | 7 (24.1%) | 14 (11.4%) | 0.129 |
| Overall (5 years) | 87.5% | 92.5% | 86.3% | 0.959 |
| Disease-free (5 years) | 90.8% | 96.0% | 89.4% | 0.459 |
| Masaoka (stage I–II) | N=111 | N=28 | N=83 | – |
| Overall (5 years) | 89.8% | 92.2% | 89.0% | 0.406 |
| Disease-free (5 years) | 96.5% | 95.8% | 96.7% | 0.603 |
MG, myasthenia gravis; WHO, World Health Organization; VATS, video-assisted thoracic surgery.
Figure 3.
Oncological outcome at 5 years by approach in stage I–II.
Discussion
Surgery is the cornerstone of thymic tumor management at early and advanced stages (6,7,10). The National Comprehensive Cancer Network guidelines clearly state that surgical resection, which consists of radical thymectomy, is recommended for resectable thymic tumors according to the technique and results described by Jaretzky (25,26). Conversely, statements by the National Cancer Institute and ITMIG consider recommending complete surgical resection only for patients affected by either stage I or stage II thymoma (27,28), but do not refer to the optimal mode of resection.
The main result of our analysis is that the oncologic outcomes of thymomectomy and radical thymectomy are superimposable. This is in line with the findings of Tseng (4) and other authors (3,5), suggesting that partial thymomectomy is as effective as radical thymectomy for stage I–II thymoma.
The second result of our study is that oncological outcome is similar in the two modes of resection, not only in early stages, but also in advanced stages (III and IV). This is the first time that a long-term comparison has shown equivalent outcomes of radical thymectomy versus thymomectomy for advanced-stage thymomas and carcinoma; indeed, previous reports have focused only on stage I and II disease (3-5).
Arguments in favor of routine maximal thymectomy via a median sternotomy, even for early-stage thymoma, are based on the necessity of: obtaining wide resection margins, removing as much ectopic thymus tissue as possible—dispersed in the anterior mediastinal fat—even in non-MG patients, avoiding unexpected myasthenic crisis, and on the hypothetical risk that a second primary thymoma may develop in the residual thymus gland (10,28,29). Conversely, in our experience, local recurrences in the two groups were similar, and the only fatal myasthenic crisis occurred in a patient with MG subjected to radical thymectomy.
Likewise, similar oncologic results have been obtained irrespective of whether the procedure was carried out via open surgery (sternotomy or anterior thoracotomy) or VATS. This observation is in agreement with several other recent reports (15-19) and suggests that adequate margins can reliably be obtained, even though a less invasive approach, and that the traditional Jaretzky approach—with its accompanying burden of potential complications and unsightly cosmetic results—may not be routinely indicated, at least in early-stage disease. In particular, as stage I–II thymoma may be identified with reasonable accuracy based on contrast-enhanced CT scan results (30), VATS thymectomy may be planned with reasonable chances of success in these patients, with conversion to open surgery decided in difficult cases after the initial VATS exploration, as occurred in 5 (14%) patients in our series. Today, with the introduction of robotic approaches, resection of neighboring structures (i.e., lung and pericardium) can be easier.
Besides the main results, multivariate analysis of our series confirmed that WHO histology, Masaoka stage, and complete resection are independent prognostic factors of outcome for thymic tumors, in accordance with other similarly unselected surgical series (8,9,31-33), including the recent review of the European Society of Thoracic Surgery, in which a large multicenter series of patients were analyzed (34,35).
The main limitation of this study is its retrospective design, as selection bias can play a role that cannot be reliably controlled. This limitation is common to all other pooled analyses available in the literature and single center studies (3-5,12,13,15-19). In addition, due to the long natural history of thymoma, despite a median follow-up of 77 months, the number of events observed was limited: 22 recurrences (14.0%), with 9 patients (5.7%) dying of thymoma altogether, including 3 treatment-related deaths. For these reasons, the reliability of our Cox model in identifying prognostic factors related with DFS is low, and with OS even lower.
Another limitation may be due to the dual origin of patients from two units with differing surgical approaches. In Bergamo, a traditional open approach was preferred, whereas in Milan minimally invasive surgery has been pursued in recent years, even for MG.
Nonetheless, this is a relatively large study with comorbidity assessed with a validated index (20,21), and that excluded patients with a history of previous or synchronous malignancy, avoiding the confounding effects of associated cancers on survival. Moreover, pathology was uniformly assessed by a single laboratory, and the variety of approaches offers some insight into the different aspects of surgical management of thymic tumors.
In conclusion, radical thymectomy and conservative thymomectomy do not differ in terms of DFS and OS. In non-MG patients with resectable thymic tumors, minimally invasive thymomectomy provided equivalent results to open thymectomy. The results of this study should be interpreted with caution due to the retrospective nature of the study. Well-designed, adequately-powered studies should be very welcome to increase the quantity and the quality of clinical evidence before incorporating this procedure in future guidelines.
Acknowledgements
None.
Ethical Statement: The study was approved by the internal research board. All patients signed an informed consent for the acquisition and the usage of clinical data for research purposes at admission.
Disclaimer: This study was presented at the 53rd Annual Meeting of The Society of Thoracic Surgeons, Houston, Texas, January 21–25, 2017.
Footnotes
Conflicts of Interest: G Veronesi had financial relationship with Medtronic, Abmedica and Versurgical for consultation and proctoring. The other authors have no conflicts of interest to declare.
References
- 1.Engels EA, Pfeiffer RM. Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer 2003;105:546-51. 10.1002/ijc.11099 [DOI] [PubMed] [Google Scholar]
- 2.Strollo DC, Rosado de Christenson ML, Jett JR. Primary mediastinal tumors. Part 1: tumors of the anterior mediastinum. Chest 1997;112:511-22. 10.1378/chest.112.2.511 [DOI] [PubMed] [Google Scholar]
- 3.Nakagawa K, Asamura H, Sakurai H, et al. Does the mode of surgical resection affect the prognosis/recurrence in patients with thymoma? J Surg Oncol 2014;109:179-83. 10.1002/jso.23499 [DOI] [PubMed] [Google Scholar]
- 4.Tseng YC, Hsieh CC, Huang HY, et al. Is thymectomy necessary in nonmyasthenic patients with early thymoma? J Thorac Oncol 2013;8:952-8. 10.1097/JTO.0b013e31828cb3c2 [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa K, Yokoi K, Nakajima J, et al. Is Thymomectomy Alone Appropriate for Stage I (T1N0M0) Thymoma? Results of a Propensity-Score Analysis. Ann Thorac Surg 2016;101:520-6. 10.1016/j.athoracsur.2015.07.084 [DOI] [PubMed] [Google Scholar]
- 6.Venuta F, Rendina EA, Anile M, et al. Thymoma and thymic carcinoma. Gen Thorac Cardiovasc Surg 2012;60:1-12. 10.1007/s11748-011-0814-0 [DOI] [PubMed] [Google Scholar]
- 7.Davenport E, Malthaner RA. The role of surgery in the management of thymoma: a systematic review. Ann Thorac Surg 2008;86:673-84. 10.1016/j.athoracsur.2008.03.055 [DOI] [PubMed] [Google Scholar]
- 8.Maggi G, Casadio C, Cavallo A, et al. Thymoma: results of 241 operated cases. Ann Thorac Surg 1991;51:152-6. 10.1016/0003-4975(91)90478-9 [DOI] [PubMed] [Google Scholar]
- 9.Margaritora S, Cesario A, Cusumano G, et al. Thirty-five-year follow-up analysis of clinical and pathologic outcomes of thymoma surgery. Ann Thorac Surg 2010;89:245-52; discussion 252. 10.1016/j.athoracsur.2009.08.074 [DOI] [PubMed] [Google Scholar]
- 10.Detterbeck FC, Parsons AM. Management of stage I and II thymoma. Thorac Surg Clin 2011;21:59-67, vi-vii. 10.1016/j.thorsurg.2010.08.001 [DOI] [PubMed] [Google Scholar]
- 11.Pennathur A, Qureshi I, Schuchert MJ, et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg 2011;141:694-701. 10.1016/j.jtcvs.2010.09.003 [DOI] [PubMed] [Google Scholar]
- 12.Lo CM, Lu HI, Hsieh MJ, et al. Thymectomy for myasthenia gravis: video-assisted versus transsternal. J Formos Med Assoc 2014;113:722-6. 10.1016/j.jfma.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 13.Nakagiri T, Inoue M, Shintani Y, et al. Improved procedures and comparative results for video-assisted thoracoscopic extended thymectomy for myasthenia gravis. Surg Endosc 2015;29:2859-65. 10.1007/s00464-014-3964-1 [DOI] [PubMed] [Google Scholar]
- 14.Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. 10.1016/j.jtcvs.2010.11.042 [DOI] [PubMed] [Google Scholar]
- 15.Ye B, Tantai JC, Ge XX, et al. Surgical techniques for early-stage thymoma: video-assisted thoracoscopic thymectomy versus transsternal thymectomy. J Thorac Cardiovasc Surg 2014;147:1599-603. 10.1016/j.jtcvs.2013.10.053 [DOI] [PubMed] [Google Scholar]
- 16.Sakamaki Y, Oda T, Kanazawa G, et al. Intermediate-term oncologic outcomes after video-assisted thoracoscopic thymectomy for early-stage thymoma. J Thorac Cardiovasc Surg 2014;148:1230-7.e1. 10.1016/j.jtcvs.2014.01.029 [DOI] [PubMed] [Google Scholar]
- 17.Liu TJ, Lin MW, Hsieh MS, et al. Video-assisted thoracoscopic surgical thymectomy to treat early thymoma: a comparison with the conventional transsternal approach. Ann Surg Oncol 2014;21:322-8. 10.1245/s10434-013-3228-7 [DOI] [PubMed] [Google Scholar]
- 18.Manoly I, Whistance RN, Sreekumar R, et al. Early and mid-term outcomes of trans-sternal and video-assisted thoracoscopic surgery for thymoma. Eur J Cardiothorac Surg 2014;45:e187-93. 10.1093/ejcts/ezu077 [DOI] [PubMed] [Google Scholar]
- 19.Jurado J, Javidfar J, Newmark A, et al. Minimally invasive thymectomy and open thymectomy: outcome analysis of 263 patients. Ann Thorac Surg 2012;94:974-81; discussion 981-2. 10.1016/j.athoracsur.2012.04.097 [DOI] [PubMed] [Google Scholar]
- 20.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245-51. 10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- 21.Seely AJ, Ivanovic J, Threader J, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg 2010;90:936-42; discussion 942. 10.1016/j.athoracsur.2010.05.014 [DOI] [PubMed] [Google Scholar]
- 22.Marx A, Ströbel P, Badve SS, et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Oncol 2014;9:596-611. 10.1097/JTO.0000000000000154 [DOI] [PubMed] [Google Scholar]
- 23.Zucali PA, Di Tommaso L, Petrini I, et al. Reproducibility of the WHO classification of thymomas: practical implications. Lung Cancer 2013;79:236-41. 10.1016/j.lungcan.2012.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Detterbeck FC, Moran C, Huang J, et al. Which way is up? Policies and procedures for surgeons and pathologists regarding resection specimens of thymic malignancy. J Thorac Oncol 2011;6:S1730-8. 10.1097/JTO.0b013e31821ea567 [DOI] [PubMed] [Google Scholar]
- 25.NCCN.org. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. Alberta Health Service. 2017. [Google Scholar]
- 26.Jaretzki A, 3rd, Wolff M. “Maximal” thymectomy for myasthenia gravis. Surgical anatomy and operative technique. J Thorac Cardiovasc Surg 1988;96:711-6. [PubMed] [Google Scholar]
- 27.NIH. Thymoma and Thymic Carcinoma Treatment (PDQ®) General Information About Thymoma and Thymic Carcinoma Treatment. 2015;1-26.
- 28.Group ITMI. Standard treatment options. About Thymoma. Options, International Thymyc Malignancies Interest Group. Standard treatment. 2015. Available online: https://www.itmig.org/node/11
- 29.Bae MK, Lee SK, Kim HY, et al. Recurrence after thymoma resection according to the extent of the resection. J Cardiothorac Surg 2014;9:51. 10.1186/1749-8090-9-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayes SA, Huang J, Plodkowski AJ, et al. Preoperative computed tomography findings predict surgical resectability of thymoma. J Thorac Oncol 2014;9:1023-30. 10.1097/JTO.0000000000000204 [DOI] [PubMed] [Google Scholar]
- 31.Rea F, Marulli G, Girardi R, et al. Long-term survival and prognostic factors in thymic epithelial tumours. Eur J Cardiothorac Surg 2004;26:412-8. 10.1016/j.ejcts.2004.04.041 [DOI] [PubMed] [Google Scholar]
- 32.Lucchi M, Ricciardi R, Melfi F, et al. Association of thymoma and myasthenia gravis: oncological and neurological results of the surgical treatment. Eur J Cardiothorac Surg 2009;35:812-6; discussion 816. 10.1016/j.ejcts.2009.01.014 [DOI] [PubMed] [Google Scholar]
- 33.Kondo K, Monden Y. Thymoma and myasthenia gravis: a clinical study of 1,089 patients from Japan. Ann Thorac Surg 2005;79:219-24. 10.1016/j.athoracsur.2004.06.090 [DOI] [PubMed] [Google Scholar]
- 34.Ruffini E, Detterbeck F, Van Raemdonck D, et al. Tumours of the thymus: a cohort study of prognostic factors from the European Society of Thoracic Surgeons database. Eur J Cardiothorac Surg 2014;46:361-8. 10.1093/ejcts/ezt649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Detterbeck F, Youssef S, Ruffini E, et al. A review of prognostic factors in thymic malignancies. J Thorac Oncol 2011;6:S1698-704. 10.1097/JTO.0b013e31821e7b12 [DOI] [PubMed] [Google Scholar]