Abstract
Background
Cancerous esophageal stenosis encountered during endoscopic ultrasonography before treatment was observed in about 30% of esophageal cancer patients. Since the pT stage in TNM classification measures only the depth of infiltration but not the growth to esophagus, it is interesting to know whether tumor growth into the esophagus (and eventual stenosis) is of added value in prognostic assessment. However, the impact of esophageal stenosis on survival of esophageal cancer patient remains unclear. Therefore, we conducted a meta-analysis focusing on current topic for the first time.
Methods
A systematic literature search in PubMed and EMBASE was conducted to identify relevant studies up to 14 March 2018. Data of 5-year overall survival (OS) was extracted and analysed.
Results
A total of five cohort studies consisting of 1,282 patients (278 patients with cancerous esophageal stenosis before treatment and 1,004 patients without) with esophageal cancer treated with surgery, chemoradiotherapy, or palliative therapy were included for analysis. Meta-analysis showed that patients with esophageal stenosis had significantly lower 5-year OS [22.3% and 33.0%, respectively; risk ratio (RR) =1.21; 95% CI, 1.11–1.32; P<0.001; I2=27.1%] than those without. No heterogeneity or publication bias was observed during analysis.
Conclusions
Patients with cancerous esophageal stenosis identified by endoscopy before treatment had significantly poorer survival than those without. High-quality studies with appropriate adjustments for confounding factor are needed to confirm the findings.
Keywords: Esophageal cancer, esophageal stenosis, prognosis, meta-analysis
Introduction
Esophageal cancer is the eighth most common malignant tumors and the sixth most common cause of death from cancer worldwide (1). Despite advances in treating esophageal cancer, overall survival of patients with esophageal cancer remains poor, with a 5-year survival rate of 15–34% (2). In order to plan an optimal stage-specific treatment for esophageal cancer patients, endoscopic ultrasonography (EUS) is widely applied to accurately assess the primary tumor infiltration (T stage) and lymph node status (N stage) of esophageal cancer before treatment (3,4). However, approximately 30% of esophageal cancer patients could not complete the EUS evaluation due to failure to cross esophageal tumors by EUS during examination, which is also commonly defined as cancerous esophageal stenosis (5,6). Since the pT stage in TNM classification measures only the depth of infiltration (lamina propria, submucosa, etc.), but not growth to esophagus, it is interesting to know whether tumor growth into the esophagus (and eventual stenosis) is of added value in prognostic assessment. Previously, some studies reported that esophageal stenosis was significantly correlated to poor prognosis of esophageal cancer patients (6-8) while other studies found that esophageal stenosis has no impact on the overall survival of those patients (5,9). Given the very limited sample size, it is reasonable that previous studies have drawn controversial conclusions. Therefore, in this study, we aimed to conduct a meta-analysis to figure out the role of cancerous esophageal stenosis before treatment in the survival of esophageal cancer patients by pooling all those available evidence together. To our knowledge, this is the first meta-analysis focusing on current topic.
Methods
Search strategies
Systematic computerized searches of the PubMed and EMBASE for reports dated up to March 14, 2018, were performed with the following search terms and strategies: (((((((esophageal[Title/Abstract]) OR oesophageal[Title/Abstract]) OR esophagus[Title/Abstract]) OR oesophagus[Title/Abstract])) AND (((cancer[Title/Abstract]) OR carcinoma[Title/Abstract]) OR tumor[Title/Abstract])) AND (((survival[Title/Abstract]) OR prognosis[Title/Abstract]) OR prognostic[Title/Abstract])) AND ((stricture[All Fields]) OR stenosis[All Fields]). All reference lists from the trials selected by electronic searching were scanned to further identify relevant studies.
Study selections
The following criteria were used for study inclusion: (I) either randomized controlled trials (RCTs) or observational studies evaluated the impact of cancerous esophageal stenosis before treatment on the survival of esophageal cancer patients; (II) sufficient data could be obtained for 5-year overall survival (OS); (III) the most recent one was chosen if the studies were based on overlapping patients. The exclusion criteria were as follows: (I) paper that was not published in English; (II) case reports, abstracts, conference reports, reviews and experiments; (III) paper without any relevant data that could be extracted for analysis.
Data extraction and quality assessment
Two authors (Han-Yu Deng & Guha Alai) independently extracted data from included studies and compared results. In order to avoid bias, discrepancies were resolved by third author (Jun Luo) adjudication. Data were carefully retrieved from full articles by using a standardized data collection form. The following data were collected from each study: first author, year of publication, number of the patients, age, study design and follow-up as well as the outcome (5-year OS). The Jadad scale (10) was used for the quality assessment of RCTs. The quality assessment and risk-of-bias analysis of observational studies was evaluated by The Newcastle-Ottawa Scale (NOS) as we previously described (11), which consists of three factors: patient selection, comparability of the study groups, and assessment of outcome. A score of 0–9 (allocated as stars) was assigned to each study, and the high-quality study was defined as a study with quality scores≥7 (Table 1). The name of the first author and the year of publication of the article were used for identification.
Table 1. Characteristics of the included studies in this meta-analysis.
| Author | Esophageal stenosis definition | Comparisons | Number | Disease stage | Treatment modalities (number) | Age (years) | Follow-up time | Study design | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|
| Morgan 2008 | Failure to cross at endoscopic ultrasonography. | Patients with esophageal stenosis | 12 | NA | Surgery: 2; dCRT: 6; palliative therapy: 4 | Median: 64 (range, 50–83) | NA | Cohort | NOS: 6 |
| Patients without | 399 | NA | Surgery: 127; dCRT: 145; palliative therapy: 127 | Median: 68 (range, 47–89) | NA | ||||
| Clavier 2014 | Non-traversable esophageal stricture by a conventional endoscope | Patients with esophageal stenosis | 40 | NA | dCRT: 40 | Median for all patients: 65 (range, 42–81) | Median follow-up for all patients: 20.8 months | Cohort | NOS: 7 |
| Patients without | 103 | NA | dCRT: 103 | ||||||
| Cho 2017 | Endoscopic ultrasonography non-traversable | Patients with esophageal stenosis | 26 | Clinical stage II: 9; clinical stage III: 17 | Multimodality therapy: 26 | Median: 61.0 | Median follow-up for all patients: 29.9 months | Cohort | NOS: 7 |
| Patients without | 63 | Clinical stage II: 39; clinical stage III: 24 | Multimodality therapy: 63 | Median: 62.0 | |||||
| Yang 2017 | Significant stenosis allowing endoscopic passage with remarkable resistance or severe stenosis preventing passage of the endoscope through the tumor site | Patients with esophageal stenosis | 139 | T1–2 disease: 16; T3–4 disease: 123 | Surgery with adjuvant therapy: 99; surgery without adjuvant therapy: 40 | Mean: 59.3 (range, 41–78) | Median follow-up for all patients: 37.5 months | Cohort | NOS: 7 |
| Patients without | 369 | T1–2 disease: 154; T3–4 disease: 215 | Surgery with adjuvant therapy: 236; surgery without adjuvant therapy: 133 | Mean: 59.8 (range, 31–80) | |||||
| Okuno 2017 | Patients who experienced dysphagia or patients whose primary lesions were too narrow for an endoscopy to be performed | Patients with esophageal stenosis | 61 | NA | CRT: 61 | NA | NA | Cohort | NOS: 6 |
| Patients without | 70 | NA | CRT: 61 | NA | NA |
NA, not available; dCRT, definitive chemoradiotherapy; CRT, chemoradiotherapy; NOS, the Newcastle-Ottawa Scale.
Statistical analysis
All analyses were performed in accordance to PRISMA guidelines (12) by using the STATA 12.0 package (StataCorp, College Station, TX, USA). Data of 5-year OS rate was extracted directly from text or Kaplan-Meier curve reported in the individual studies and for the comparison, risk ratio (RR) with 95% CI were used. For each study, the between-study heterogeneity was assessed using χ2-based Q statistics and the I2 test. Random effects models would be used if high heterogeneity of the studies (P<0.1 or I2>50%) was observed. Otherwise, fixed effects models were used. Sensitivity analysis was performed by sequential removal of each study. A funnel plot was used to estimate potential publication bias, and asymmetry of the plot was tested by Begg’s test and Egger’s test (13). Statistical significance was set at P<0.05.
Results
Description of studies
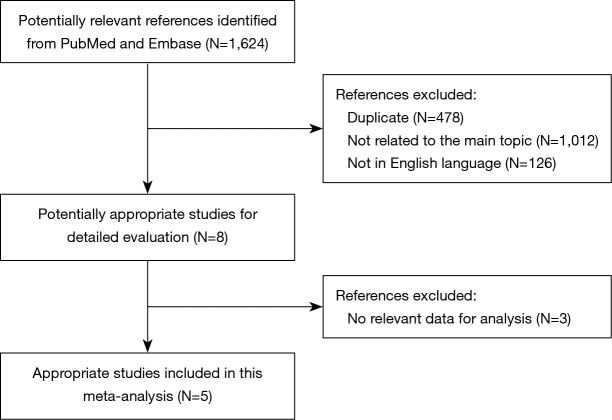
A flow chart of our analyses is shown in Figure 1. A total of 1,624 papers were found after initial search. After initial evaluation, we found 8 papers for detailed evaluation. Three studies (14-16) were excluded due to lacking of relevant data which could be obtained for analysis after detailed evaluation. Finally, no RCTs but 5 cohort studies (5-9) with a total of 1,282 patients (278 patients with cancerous esophageal stenosis before treatment and 1,004 patients without) were included for analysis. The main data extracted from these included studies are listed in Table 1. Most of those studies defined cancerous esophageal stenosis as failure to cross the tumor by EUS. Patients in those studies received either surgery, or chemoradiotherapy, or palliative therapy with a relatively short follow-up time. The mean age between patients with cancerous esophageal stenosis and those without was comparable in most of those studies. However, as reported in only two studies (6,7), more patients with cancerous esophageal stenosis were at a relatively advanced disease stage compared to those without. Data of 5-year OS for analysis could be obtained from all those studies (5-9) (Table 2).
Figure 1.
A flow chart showed the progress of studies through the review.
Table 2. Main outcomes extracted from the studies included in our meta-analysis.
| Author | Comparisons | Number | 5-year OSa |
|---|---|---|---|
| Morgan 2008 | Patients with esophageal stenosis | 12 | 3/9 |
| Patients without | 399 | 96/303 | |
| Clavier 2014 | Patients with esophageal stenosis | 40 | 7/33 |
| Patients without | 103 | 23/80 | |
| Cho 2017 | Patients with esophageal stenosis | 26 | 8/18 |
| Patients without | 63 | 31/32 | |
| Yang 2017 | Patients with esophageal stenosis | 139 | 39/100 |
| Patients without | 369 | 166/203 | |
| Okuno 2017 | Patients with esophageal stenosis | 61 | 5/56 |
| Patients without | 70 | 15/55 |
a, expressed as no. alive/no. dead. OS, overall survival.
Quality assessment and risk of bias
The quality assessment and risk-of-bias analysis of the included studies were evaluated by NOS for those cohort studies (shown in Table 1). Three studies with NOS score of 7 were ranked as studies with high quality while other two studies had a NOS score of 6, indicating potential risk of bias for comparability.
Meta-analysis of the effects of esophageal stenosis before treatment on the survival of esophageal cancer patients
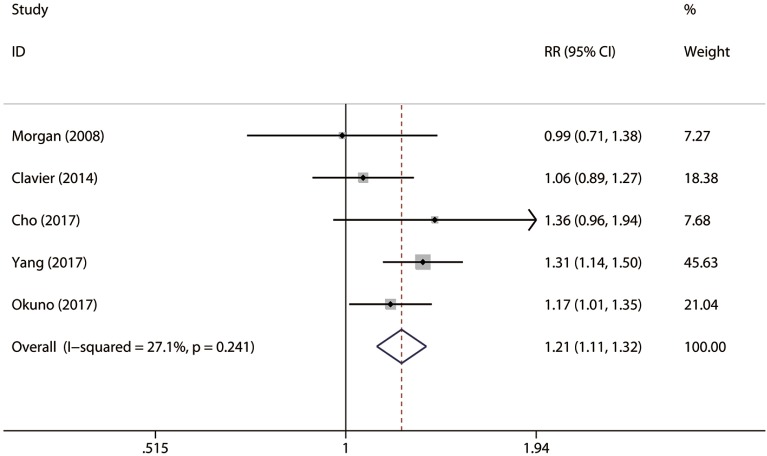
A total of five studies consisting of 1,282 patients reported 5-year OS rate (shown in Table 2). There were 278 patients with cancerous esophageal stenosis before treatment while there were 1,004 patients without. Patients with cancerous esophageal stenosis yielded a 5-year OS rate of 22.3% while patients without had a 5-year OS rate of 33.0%. Patients with esophageal stenosis had significantly lower 5-year OS than those without (fixed effects: RR =1.21; 95% CI, 1.11–1.32; P<0.001; I2=27.1%) (Figure 2). No obvious heterogeneities were observed during the analysis.
Figure 2.
Forest plot of 5-year overall survival rate of patients with esophageal stenosis compared with those without.
Sensitivity analysis and publication bias
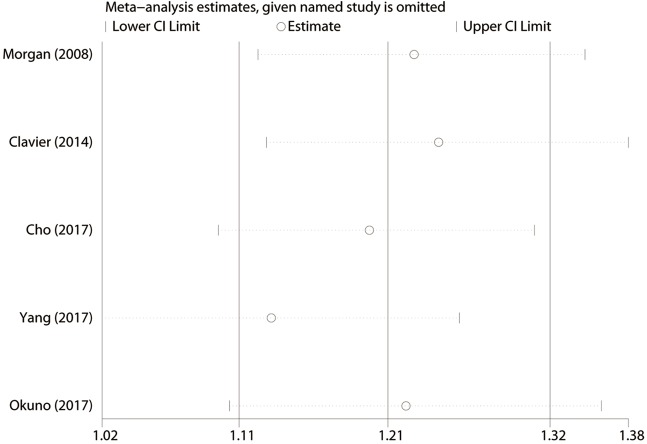
We conducted the sensitivity analysis by sequential removal of each study to evaluate the stability of our result, and it showed that sequential removal of each study did not change the survival outcome of primary analysis (Figure 3). Moreover, after removal of the two studies with a relatively low quality, the survival outcome of the primary analysis did not change either. Publication bias was tested and the funnel plot for the analysis of 5-year OS had a symmetrical appearance (Begg’s test: P=0.624; Egger’s test: P=0.682) (Figure 4), which indicated no publication bias.
Figure 3.
Sensitivity analysis of the results of overall analysis conducted by omitting each study sequentially for 5-year survival rate.
Figure 4.
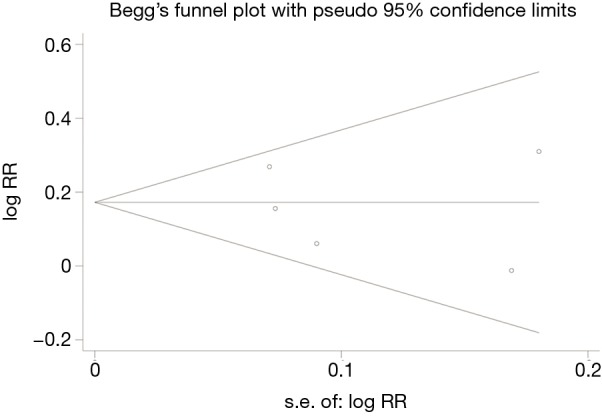
Funnel plot of the included studies for the analysis of 5-year overall survival rate. Begg’s test: P=0.624; Egger’s test: P=0.682.
Discussion
EUS has been widely applied in daily practice for evaluating the extent of local tumor infiltration and local lymph node status of esophageal cancer with excellent accuracy before treatment (17). However, it is reported that due to esophageal stenosis, EUS could not be performed successfully in about 30% of esophageal cancer patients before treatment (5,6). So far, only a small number of studies have been carried out evaluating the impact of endoscopic finding of cancerous esophageal stenosis before treatment on the survival of esophageal cancer patients but drawn controversial conclusions because of the limited sample size (5-9). Therefore, in this study, we conducted a meta-analysis to generate a relatively valid conclusion of the impact of cancerous esophageal stenosis before treatment on survival of esophageal cancer patients by pooling all those evidence available. To our knowledge, this is the first meta-analysis focusing on current topic.
In our meta-analysis, we included five cohort studies with a total of 1,282 patients. Patients were divided into two groups by the status of cancerous esophageal stenosis before treatment. We found that the 5-year OS rate of esophageal cancer patients with stenosis was significantly lower than that of patients without stenosis (22.3% vs. 33.0%, respectively; P<0.001), suggesting that cancerous esophageal stenosis before treatment has a significantly unfavorable impact on the survival of esophageal cancer patients.
In 1997, Hiele et al. (14) explored the relation between EUS findings and outcomes of patients with tumors of the esophagus or esophagogastric junction for the first time. They found that patients with esophageal stenosis which could not be passed by EUS yielded significantly shorter median survival time than those whose stenosis could be passed (10 and 20 months, respectively; P=0.02), indicating that esophageal stenosis had a negative impact on the prognosis of esophageal cancer patients. Later, Mariette et al. (15) also found that the median survival time of patients with esophageal stenosis was significantly shorter than that of patients without stenosis (23 and 54 months, respectively; P=0.004). Thereafter, several similar studies (5-9) have been carried out but have drawn controversial conclusions. Therefore we conducted the first meta-analysis by pooling the evidence from those studies (5-9). Our meta-analysis added to the evidence that patients with esophageal stenosis before treatment had significantly poor OS than those without. Moreover, du Rieu et al. (16) found that patients with esophageal stenosis had significantly lower 5-year recurrence free survival than patients without (31.8% and 77.9%, respectively; P<0.001). Clavier et al. (9) even found that esophageal stenosis was significantly correlated to poor recurrence free survival of esophageal cancer patients (HR =1.35; 95% CI, 1.05–1.75; P=0.021). Therefore, taken together, we believe that endoscopic finding of cancerous esophageal stenosis before treatment was significantly correlated to poor prognosis of esophageal cancer patients. The reasons why cancerous esophageal stenosis had unfavorable impact on prognosis of esophageal cancer patients may be explained by the facts that esophageal stenosis was associated with higher tumor stage, larger tumor burden and poorer tumor differentiation as well as poor nutrition status (6,7,16). Therefore, for patients with cancerous esophageal stenosis, neoadjuvant therapy may be more emphasized before surgical treatment.
Our study has several limitations. First, lacking of well-conducted RCTs, retrospective cohort studies with a relatively short follow-up time might reduce the statistical power. Second, even though this is the first meta-analysis, our study suffered from limited sample size. Third, due to limited number of study, we could only focus on 5-year OS, but data of short-term outcomes and other long-term outcomes such as recurrence free survival could not be obtained from those original studies and thus were omitted for analysis. Fourth, the baseline characteristics (for example, disease stage) between patients with cancerous esophageal stenosis and those without were not well balanced in some studies, which could influence the validity of our results. Moreover, the definition of stenosis by Okuno et al. (8) included both dysphagia and failure to cross the tumor by EUS, which is slightly different from other studies. Finally, even though no obvious heterogeneity was observed in our study, patients included in our meta-analysis received various treatments including surgery, chemoradiotherapy, and palliative therapy, which could cause heterogeneity among studies. Therefore, more well conducted studies are needed to confirm and update our conclusions.
Conclusions
This first meta-analysis on current topic suggests that patients with esophageal stenosis due to esophageal cancer might have poorer prognosis compared to those without stenosis. High-quality studies with appropriate adjustments for confounding are needed to confirm the findings.
Acknowledgements
Funding: This work was supported by National Natural Science Foundation of China [No. 81672291; No. 31071210] (to YD Lin).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400-12. 10.1016/S0140-6736(12)60643-6 [DOI] [PubMed] [Google Scholar]
- 2.Deng HY, Wang WP, Wang YC, et al. Neoadjuvant chemoradiotherapy or chemotherapy? A comprehensive systematic review and meta-analysis of the options for neoadjuvant therapy for treating oesophageal cancer. Eur J Cardiothorac Surg 2017;51:421-31. [DOI] [PubMed] [Google Scholar]
- 3.Lightdale CJ, Kulkarni KG. Role of endoscopic ultrasonography in the staging and follow-up of esophageal cancer. J Clin Oncol 2005;23:4483-9. 10.1200/JCO.2005.20.644 [DOI] [PubMed] [Google Scholar]
- 4.Smith BR, Chang KJ, Lee JG, et al. Staging accuracy of endoscopic ultrasound based on pathologic analysis after minimally invasive esophagectomy. Am Surg 2010;76:1228-31. [PubMed] [Google Scholar]
- 5.Morgan MA, Twine CP, Lewis WG, et al. Prognostic significance of failure to cross esophageal tumors by endoluminal ultrasound. Dis Esophagus 2008;21:508-13. 10.1111/j.1442-2050.2008.00809.x [DOI] [PubMed] [Google Scholar]
- 6.Cho CJ, Song HJ, Lee GH, et al. Clinical implications of endoscopic ultrasonography non-traversability in patients with locoregional esophageal cancer receiving multimodality therapy. Korean J Intern Med 2017;32:443-51. 10.3904/kjim.2015.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang YS, Hu WP, Ni PZ, et al. Esophageal luminal stenosis is an independent prognostic factor in esophageal squamous cell carcinoma. Oncotarget 2017;8:43397-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okuno T, Wakabayashi M, Kato K, et al. Esophageal stenosis and the Glasgow Prognostic Score as independent factors of poor prognosis for patients with locally advanced unresectable esophageal cancer treated with chemoradiotherapy (exploratory analysis of JCOG0303). Int J Clin Oncol 2017;22:1042-9. 10.1007/s10147-017-1154-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clavier JB, Antoni D, Atlani D, et al. Baseline nutritional status is prognostic factor after definitive radiochemotherapy for esophageal cancer. Dis Esophagus 2014;27:560-7. 10.1111/j.1442-2050.2012.01441.x [DOI] [PubMed] [Google Scholar]
- 10.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. 10.1016/0197-2456(95)00134-4 [DOI] [PubMed] [Google Scholar]
- 11.Deng HY, Wang YC, Ni PZ, et al. Radiotherapy, lobectomy or sublobar resection? A meta-analysis of the choices for treating stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2017;51:203-10. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Bmj 1997;315:629-34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiele M, De Leyn P, Schurmans P, et al. Relation between endoscopic ultrasound findings and outcome of patients with tumors of the esophagus or esophagogastric junction. Gastrointest Endosc 1997;45:381-6. 10.1016/S0016-5107(97)70148-2 [DOI] [PubMed] [Google Scholar]
- 15.Mariette C, Balon JM, Maunoury V, et al. Value of endoscopic ultrasonography as a predictor of long-term survival in oesophageal carcinoma. Br J Surg 2003;90:1367-72. 10.1002/bjs.4307 [DOI] [PubMed] [Google Scholar]
- 16.du Rieu MC, Filleron T, Beluchon B, et al. Recurrence risk after Ivor Lewis oesophagectomy for cancer. J Cardiothorac Surg 2013;8:215. 10.1186/1749-8090-8-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puli SR, Reddy JB, Bechtold ML, et al. Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol 2008;14:1479-90. 10.3748/wjg.14.1479 [DOI] [PMC free article] [PubMed] [Google Scholar]