Abstract
Background
Even with the advance of diagnosis and the treatment, the 5-year survival rate for esophageal cancer patients is still poor. The checkpoint protein inhibition provides another choice to improve the survival. The expression of the programmed death ligand-1 (PD-L1) was reported but the clinical relevance remained inconsistent in esophageal cancer. Besides, there were few references about the other ligand, programed death ligand-2 (PD-L2). In this study, we evaluated the expressions of PD-L1 and PD-L2 in patients with esophageal squamous cell carcinoma (ESCC) and assessed their clinical relevance.
Methods
From 1996 to 2011, 150 patients undergone complete surgical resection for ESCC were enrolled. Clinical data were recorded. Expression of PD-L1 and PD-L2 on cytoplasm in paraffin embedded tumor samples were analyzed by immunohistochemistry staining and scored with a semi-quantitative method.
Results
Of the patients, 96 (64.0%) patients had PD-L1 overexpression and 63 (42.0%) had PD-L2 overexpression. There was a correlation between the expression of PD-L1 and PD-L2 (P<0.001). Patients without overexpression of PD-L1, pathological T1–2 and N0 status, pathological stage I–II and no post-operative adjuvant treatment had a better disease free survival (DFS). In multivariate analysis, PD-L1 expression and pathological stage were the independent prognostic factors for DFS. The expression of PD-L2 did not influence the DFS. Although not statistically significant, patients without overexpression of PD-L1 and PD-L2 seem to have a better overall survival (OS).
Conclusions
The overexpression of PD-L1 on cytoplasm, not PD-L2, is an independent prognostic factor for DFS in patients with ESCC undergone esophagectomy. However, there is a trend which suggested that patients without overexpression of PD-L1 and PD-L2 had a better OS.
Keywords: Programmed death ligand-1 (PD-L1), programed death ligand-2 (PD-L2), overexpression, cytoplasm, esophageal squamous cell carcinoma (ESCC), survival
Introduction
According to the report of the World Health Organization, the incidence of esophageal cancer is increasing and it is the 6th most common cancer in males and the 13th common cancer in females (1). The main treatment methods for esophageal cancer include surgical resection, chemotherapy, and radiotherapy. However, even the treatment methods of esophageal cancer have advanced greatly in recent decades, the results are still poor, and the 5-year survival rate is less than 15% (1-4). Over the last 10 years, the development of immunotherapy provides another choice to treat the disease and improve the survival.
Programmed death-1 (PD-1) is a co-inhibitory receptor expressed on the cell surface of T cells, B cells, monocytes and natural killer cells. PD-1 is part of the CD28 signaling family with structure similar to cytotoxic T lymphocyte antigen-4 (CTLA-4), especial in extracellular domain (5,6). Programmed death ligand-1 (PD-L1, B7-H1) and programmed death-1 ligand-2 (PD-L2, B7-DC) are the ligands for PD-1; both have been identified as glycoprotein on the cell surface which belongs to the B7 receptor family (7-10). Although these two ligands have partial identity and similarity in structure, their regulation for expression is different (9,11).
Some recent studies show that the interaction between PD-1 and programmed death ligand (PD-L) inhibits T-cell receptor signaling and down-regulates T-cell response (8,10,12). Other studies further suggested an inhibitory role for the PD-1 and PD-L engagement in the responses of lymphocyte and regulation of peripheral tolerance using PD-1–deficient mice model (6,13). In addition to these basic immunologic roles, recent studies have suggested that PD-L1 in cancer cell increases apoptosis of T cells in vitro and the PD-L1 expression promotes tumor growth in vivo (14). Preclinical study suggested PD-L1 is upregulated by tumor cells and by cells in the tumor microenvironment (15). Furthermore, blockade of the PD-1 and PD-L1 interaction can restore T cell activity against tumor cells to prevent cancer metastasis and reduce tumor volume (12,13,16). These previous studies are well evaluated the function of PD-L1, but the clinical implication is still unknown (14,16). In the other part, the expression of PD-L1 is reported in various different human cancers, such as lung, liver, colon and also in melanomas, however, the association between PD-L1, patient’s clinicopathological characteristics and prognosis has not been determined, especially in esophageal cancer (14,17-20).
On the other hand for PD-L2, although PD-L2 expression has been reported in esophageal adenocarcinoma, the function in tumor cells remains unclear. There were several publications have suggested that PD-L2 expression may play a role in tumor immunity (21-23). However, the clinical relevance was still unknown.
Therefore, the aim of this study was to investigate the expression of PD-L1 and PD-L2 in patients with esophageal squamous cell carcinoma (ESCC) post resection to define the clinical significance.
Methods
From January 1996 to December 2011, patients with ESCC that underwent operation for tumor resection with reconstruction at Taipei Veterans General Hospital were included. The patients who had the history of preoperative chemoradiotherapy or death within 30 days after surgery were excluded. This study was approved by the Institutional Review Board of Taipei Veterans General Hospital. Patient informed consent was waived due to the retrospective nature of the study. Research was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments.
Clinical data collection
The medical records of patients were retrospectively reviewed. The clinical data of patients were collected from the medical records, such as age, sex, smoking status, pathological factors and the results of follow-up studies. Pathological TNM staging was determined according to the 7th ed. UICC/AJCC TNM staging system (24). Follow-up studies, including computerized tomography scans of the chest and the brain, and whole body nuclear scan studies, were performed every 3–6 months within 5 years and then annually. Disease-free survival (DFS) was calculated from the date of surgery to the time of the first relapse (recurrence or metastasis). Overall survival (OS) was defined as the period from the date of surgery to the date of death due to any cause.
Immunohistochemical staining procedures and interpretation
The paraffin blocked specimens were gained from Tissue Bank of Taipei Veterans General Hospital. Slides with 4 µm thick were used for immunohistochemical staining to examine the expression of PD-L1 and PD-L2. Briefly, tissue sections were treated initially in a decloaking chamber with a sodium citrate buffer (10 mM, pH 6.0) and then with serum blocking solution (Histostain Bulk Kit; Invitrogen, Carlsbad, CA) to remove endogenous peroxidase activity and to reduce nonspecific background staining. Mouse anti-human monoclonal antibody to PD-L1 (1:50, 329702, BioLegend) and PD-L2 (1:50, 329602, BioLegend) were then applied to the tissue sections. Slides were incubated at 4 °C overnight in a moist chamber. The tissue sections were subsequently treated with biotinylated secondary antibody for 30 min, DAB substrate solution (K4065, DakoCytomation) for 1–3 min, and then counterstained with hematoxylin for 5 min.
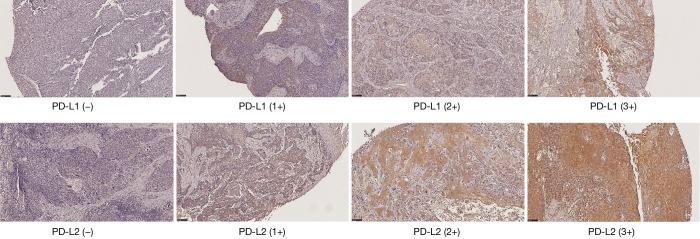
The expression of PD-L1 and PD-L2 on the cytoplasm of tumor cells was evaluated by a pathologist and semi-quantified by the predominant staining intensity of the tumor cells. The staining grading was scored a 0 (no staining), 1+, 2+, and 3+, shown in Figure 1. Comparing with 0 or 1+ staining, patients with 2+ and 3+ staining were grouped as the overexpression.
Figure 1.
Representative images of PD-L1 and PD-L2 expression in esophageal cancer tissues by immunohistochemical staining. The expression of PD-L1 (upper row) and PD-L2 (lower row) was scored from 0 to 3+ (left to right). Magnification: ×100.
Statistical analysis of immunohistochemical staining and clinicopathological data
Chi-square tests were used to compare the categorical factors. Numerical values were expressed as mean ± SD and were compared using the Student’s t-test. The survival curves were plotted by Kaplan-Meier method and compared using the log-rank test. The impacts of clinicopathological factors and expression of PD-L1/PD-L2 on survival were assessed using Cox proportioned hazards regression method. A P<0.05 is defined as statistical significance. All statistical analyses were performed using SPSS 18.0 for Windows (SPSS, Inc., Chicago, IL).
Results
A total of 160 patients who had undergone esophagectomy with reconstruction for ESCC were enrolled in this study. Six patients received pre-operative concurrent chemoradiotherapy and 4 patients died within 1 month after operation were excluded. There were 150 patients included in final analysis. The mean age of the 150 patients was 64.1±10.8 years, ranging from 36 to 88 years. Of these patients, 137 (91.3%) were male and 13 were female. Half of the patients had a history of smoking (75, 50%) with a smoking index ranging from less than 5 to 80 pack-years. Seventy (46.7%) patients had their tumor located at middle portion of their thoracic esophagus, followed by 60 patients with a lower portion tumor. A cervical or upper thoracic esophagus tumor was found in 20 patients.
All patients received an esophagectomy with reconstruction, 112 (74.7%) through open thoracotomy, 16 (10.6%) through thoracoabdominal incision, 15 with thoracoscopic assistance, which included two with robotic assistance, and 7 patients had a transhiatal approach to complete the tumor resection. Except one patient using colon for reconstruction, the other 149 patients used gastric tube for reconstruction. The route used in 105 (70.0%) patients was the retrosternal route and 45 involved the posterior mediastinal route. All patients had a cervical anastomosis except one patient had an intrathoracic anastomosis.
Based on the AJCC classification for esophageal cancer, 9 (6.0%) patients had pathological T1 lesions, 25 (16.7%) T2, 103 (68.7%) T3, and 13 (8.7%) T4 lesions. The average number of lymph nodes removed was 24.5 (ranging 2-87). For pathological N status, 53 (35.3%) patients had no nodal involvement, 50 (33.3%) N1, 27 (18.0%) N2, and 20 (13.3%) N3 status. For pathological stages, 14 (9.3%) patients had stage I, 44 (29.3%) stage II, 87 (58.0%) stage III, and 5 (3.3%) stage IV disease. The pathological stages I–II correlated with the pathological T1–2 status (P<0.001) and N0 status (P<0.001). In this series, the range of tumor length was 1.0–14 cm with median 4.4 cm. Tumor length was significantly shorter in the pathological early T status (P=0.001) and stage I–II (P=0.013). Due to local advance or lymph nodes involvement, 61 (40.7%) patients received adjuvant treatment postoperatively. There were 35 patients received concurrent chemoradiotherapy, 11 chemotherapy and 15 radiotherapy alone. Patients with long tumor length (P=0.001), pathological T3–4 (P=0.006), nodal involvement (P<0.001) and late stage disease (P<0.001) had a higher opportunity to receive adjuvant treatment.
According to the assessment of the immunohistochemical staining, 96 (64.0%) patients had PD-L1 overexpression and 63 (42.0%) had PD-L2 overexpression. There was a correlation between the expression of PD-L1 and PD-L2 (P<0.001). The expression of PD-L1 was also correlated with patients without post-operative adjuvant therapy (P=0.037). There were no other significant correlations between clinicopathological parameters and the expression of PD-L1 and PD-L2, as shown in Table 1.
Table 1. Characteristic correlations between PD-L1 (over vs. non-over) and PD-L2 (over vs. non-over) expression.
| Variable | PD-L1 | PD-L2 | |||||
|---|---|---|---|---|---|---|---|
| Non-over (n=54) | Over (n=96) | P | Non-over (n=87) | Over (n=63) | P | ||
| Age | 63.8 | 64.2 | 0.804 | 64.0 | 64.2 | 0.925 | |
| Gender | 0.847 | 0.365 | |||||
| Male | 49 | 88 | 81 | 56 | |||
| Female | 5 | 8 | 6 | 7 | |||
| Smoking | 0.736 | 0.870 | |||||
| No | 26 | 49 | 44 | 31 | |||
| Yes | 28 | 47 | 43 | 32 | |||
| Tumor length (cm) | 0.903 | 0.547 | |||||
| <4 | 27 | 47 | 44 | 30 | |||
| >4 | 27 | 49 | 43 | 33 | |||
| Differentiation | 0.616 | 0.083 | |||||
| Well | 5 | 9 | 10 | 4 | |||
| Moderate | 31 | 54 | 52 | 33 | |||
| Poorly | 10 | 8 | 9 | 9 | |||
| No mention | 8 | 25 | 16 | 17 | |||
| pT status | 0.759 | 0.371 | |||||
| T1–2 | 13 | 21 | 22 | 12 | |||
| T3–4 | 41 | 75 | 65 | 51 | |||
| pN status | 0.463 | 0.262 | |||||
| N0 | 17 | 36 | 34 | 19 | |||
| N1–3 | 37 | 60 | 53 | 44 | |||
| Pathological stage | 0.462 | 0.069 | |||||
| I–II | 23 | 35 | 39 | 19 | |||
| III–IV | 31 | 61 | 48 | 44 | |||
| Post-op adjuvant treatment | 0.037 | 0.588 | |||||
| No | 26 | 63 | 50 | 39 | |||
| Yes | 28 | 33 | 37 | 24 | |||
PD-L1, programmed death-1 ligand-1; PD-L2, programmed death ligand-2.
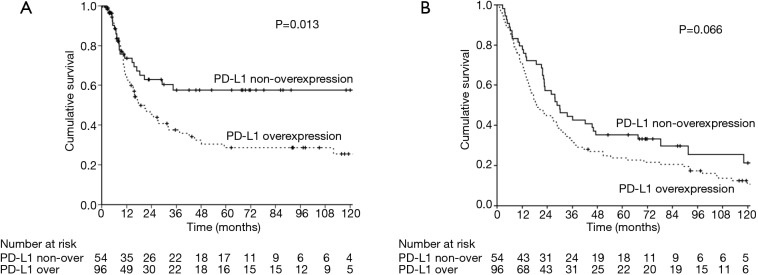
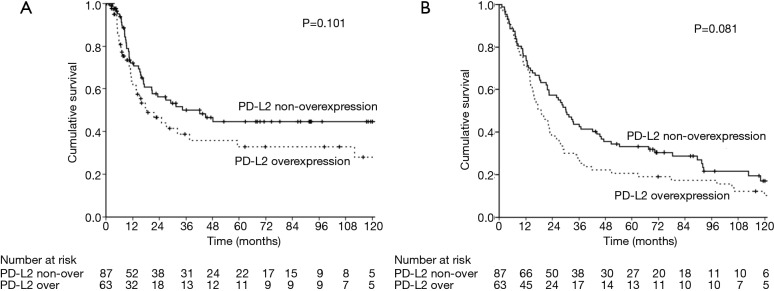
The median follow-up time was 23.3 months [interquartile range (IQR) 10.7–67.6 months]. Prior to data analysis, 92 (61.3%) patients had tumor relapse. The 3- and 5-year DFS rates were 45.4% and 39.8%, respectively. The univariate and multivariate analyses of DFS are presented in Table 2. Patients without overexpression of PD-L1 (P=0.013, Figure 2), pathological T1–2 and N0 status, pathological stage I–II and no post-operative adjuvant treatment had a better DFS. The expression of PD-L2 did not influence the DFS (P=0.101, Figure 3). In multivariate analysis, PD-L1 expression and pathological stage were the independent prognostic factors for DFS.
Table 2. Univariate and multivariate analysis of factors influencing disease free survival (DFS) after surgical resection.
| Variable | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| 5-year rate (%) | P value | HR (95% CI) | P value | ||
| PD-L1 expression | 0.013 | 0.042 | |||
| Non-over | 57.7 | 1 (referent) | |||
| Over | 28.7 | 1.713 (1.020–2.880) | |||
| PD-L2 expression | 0.101 | 0.277 | |||
| Non-over | 44.6 | 1 (referent) | |||
| Over | 32.7 | 1.319 (0.801–2.173) | |||
| Age (years) | 0.275 | ||||
| <65 | 34.1 | ||||
| >65 | 47.0 | ||||
| Gender | 0.334 | ||||
| Male | 39.3 | ||||
| Female | 48.0 | ||||
| Smoking | 0.091 | 0.097 | |||
| No | 49.4 | 1 (referent) | |||
| Yes | 30.1 | 1.485 (0.931–2.369) | |||
| Tumor length (cm) | 0.166 | ||||
| <4 | 45.0 | ||||
| >4 | 34.1 | ||||
| Differentiation | 0.147 | ||||
| Well | 41.0 | ||||
| Moderate | 40.1 | ||||
| Poorly | 23.7 | ||||
| No mention | 48.5 | ||||
| pT status | 0.045 | 0.988 | |||
| T1–2 | 55.9 | 1 (referent) | |||
| T3–4 | 34.2 | 0.994 (0.437–2.260) | |||
| pN status | 0.002 | 0.531 | |||
| N0 | 56.7 | 1 (referent) | |||
| N1–3 | 29.2 | 1.351 (0.527–3.461) | |||
| Pathological stage | <0.001 | <0.001 | |||
| I–II | 60.1 | 1 (referent) | |||
| III–IV | 22.5 | 2.507 (1.499–4.194) | |||
| Post-op adjuvant treatment | 0.039 | 0.279 | |||
| No | 46.7 | 1 (referent) | |||
| Yes | 30.5 | 1.340 (0.789–2.274) | |||
HR, hazard ratio; CI, confidence interval; PD-L1, programmed death ligand-1; PD-L2, programmed death ligand-2.
Figure 2.
The survival curves in patients with esophageal squamous cell carcinoma, solid line: PD-L1 non-overexpression; dotted line: PD-L1 overexpression. (A) Disease free survival; (B) overall survival
Figure 3.
The survival curves in patients with esophageal squamous cell carcinoma, solid line: PD-L2 non-overexpression; dotted line: PD-L2 overexpression. (A) Disease free survival; (B) overall survival.
A total of 86 (57.3%) patients those died of the esophageal cancer disease within five years. Another 38 patients died of other disease, most reasons were poor nutrition and pulmonary problems. The 3- and 5-year OS rates were 36.7% and 27.9%, respectively. The univariate and multivariate analyses of OS are presented in Table 3. Patients with a well differentiation tumor, pathological nodal negative status and pathological early stage had a better OS. Patients without overexpression of PD-L1 and PD-L2 had a better OS, but not significant (Figures 2,3). Pathological stage was the independent prognostic factor for OS in multivariate analysis.
Table 3. Univariate and multivariate analysis of factors influencing overall survival (OS) after surgical resection.
| Variable | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| 5-year rate (%) | P value | HR (95% CI) | P value | ||
| PD-L1 expression | 0.066 | 0.166 | |||
| Non-over | 35.2 | 1 (referent) | |||
| Over | 23.8 | 1.316 (0.892–1.941) | |||
| PD-L2 expression | 0.081 | 0.536 | |||
| Non-over | 33.1 | 1 (referent) | |||
| Over | 20.6 | 1.129 (0.770–1.655) | |||
| Age (years) | 0.374 | ||||
| <65 | 30.3 | ||||
| >65 | 25.6 | ||||
| Gender | 0.644 | ||||
| Male | 26.9 | ||||
| Female | 38.5 | ||||
| Smoking | 0.090 | 0.063 | |||
| No | 33.3 | 1 (referent) | |||
| Yes | 22.3 | 1.425 (0.981–2.070) | |||
| Tumor length (cm) | 0.310 | ||||
| <4 | 32.2 | ||||
| >4 | 23.7 | ||||
| Differentiation | 0.042 | 0.084 | |||
| Well | 42.9 | 1 (referent) | |||
| Moderate | 31.6 | 1.233 (0.655–2.320) | 0.516 | ||
| Poorly | 16.7 | 1.634 (0.763–3.500) | 0.206 | ||
| No mention | 18.2 | 2.010 (1.012–3.994) | 0.046 | ||
| pT status | 0.090 | 0.574 | |||
| T1–2 | 43.7 | 1 (referent) | |||
| T3–4 | 23.2 | 0.853 (0.490–1.485) | |||
| pN status | 0.001 | 0.839 | |||
| N0 | 47.0 | 1 (referent) | |||
| N1–3 | 17.5 | 0.922 (0.421–2.018) | |||
| Pathological stage | <0.001 | <0.001 | |||
| I–II | 49.8 | 1 (referent) | |||
| III–IV | 14.1 | 2.173 (1.476–3.197) | |||
| Post-op adjuvant treatment | 0.381 | ||||
| No | 31.3 | ||||
| Yes | 23.0 | ||||
HR, hazard ratio; CI, confidence interval; PD-L1, programmed death ligand-1; PD-L2, programmed death ligand-2.
Discussion
Immunotherapy has been heralded as a major breakthrough in cancer treatment in recent years. As the basic principle of modern immunotherapy, immune check-point blockage using PD-1 and PD-Ls antibodies appears to be one of the most popular immunotherapy approaches (25).
PD-L1 and PD-L2 are the two known ligands for PD-1. PD-L1 expression has been reported in many different human neoplasms, such as lung, breast, gastric, pancreatic, kidney, bladder, ovarian cancers, and melanoma (26). PD-L1 expression has been correlated with rapid cancer progression, higher recurrence rates and poorer survival rates. Recently, there are several publications that have shown the relationship between PD-L1 expression and esophageal cancer, including both ESCC and esophageal adenocarcinoma, but the results were inconsistent (27). Investigations focused on PD-L2 in malignant tumors are still rare. Thus, most of the studies have focused on the expression of PD-L1 with inconsistent results and the influence of the expression of PD-L2 in tumor tissues remains unknown.
In this study, 64.0% of patients had PD-L1 overexpression and 42.0% had PD-L2 overexpression. There was a significant correlation between the expression of PD-L1 and PD-L2 (P<0.001), but there were complete no correlations between the clinicopathological parameters and the expression of PD-L1 and PD-L2. Only postoperative adjuvant therapy status was negatively correlated with PD-L1 expression. These findings are similar to some previous studies, shown in Table 4. However, it has been reported in different studies that overexpression of PD-L1 is correlated with age, tumor site, tumor differentiation, tumor length, tumor depth, pathological T, N, stage status, and history of pre-operative treatment or response, but without full consistence (28-43).
Table 4. Comparisons of clinical implications and PD-L1 expression in patients with esophageal squamous cell carcinoma.
| First author, year | Case number | Pre-operative treatment | Staining location | PD-L1 overexpression (%) | Correlated factor | DFS impact of PD-L1 overexpression | OS impact of PD-L1 overexpression |
|---|---|---|---|---|---|---|---|
| This study | 150 | No | Cytoplasm | 64.0 | No factor | Worseb | ND |
| Ohigashi, 2005 (28) | 31 | Unknown | Membrane/cytoplasm | 41.9 | N.A. | NA | Worsea |
| Chen, 2014 (29) | 99 | No | Membrane/cytoplasm | 82.8 | N.A. | NA | Worsea |
| Lim, 2016 (30) | 73 | Yes | Membrane/cytoplasm | 56.2 | No factor | ND | Worseb |
| Chen, 2016 (31) | 536 | No | Membrane/cytoplasm | 41.4 | Tumor site, differentiation, N status, stage | Improveda | ND |
| Ito, 2016 (32) | 90 | No | Membrane/cytoplasm | 18.9 | Tumor site, tumor depth, N status | ND | Worsea |
| Hatogai, 2016 (33) | 196 | No | Membrane | 18.4 | Age | NA | Improvedb |
| Tanaka, 2016 (34) | 180 | Some | Cytoplasm | 29.4 | N status, pre-operative treatment | NA | Worseb |
| Chen, 2016 (35) | 162 | Yes | Membrane/cytoplasm | 45.7 | Tumor depth, nodal status, pre-op treatment response | Worsea | Worseb |
| Leng, 2016 (36) | 106 | No | Membrane/cytoplasm | 46.2 | No factor | NA | Worsea |
| Kim, 2016 (37) | 200 | Some | Membrane/cytoplasm | 33.5 | No factor | Worsea | ND |
| Zhu, 2017 (38) | 133 | No | Membrane/cytoplasm | 51.1* | Age, tumor length, differentiation, | Worseb | Worseb |
| Zhang, 2017 (39) | 344 | No | No mention | 14.5 | T status, stage | ND | ND |
| Jiang, 2017 (40) | 428 | No | Membrane | 79.7 | N status | Worseb | Worseb |
| Momose, 2017 (41) | 251 | Some | Membrane | 15.5 | No factor | NA | Worsea |
| Tsutsumi, 2017 (42) | 90 | No | Membrane/cytoplasm | 63.3 | T status | Worsea | Worsea |
| Jesinghaus, 2017 (43) | 125 | No | Membrane | 30.4 | No factor | Improvedb | Improvedb |
a, by univariate analysis; b, by multivariate analysis; *, including tumor cells and tumor infiltrating lymphocyte. PD-L1, programmed death ligand-1; PD-L2, programmed death ligand-2; DFS, disease free survival; OS, overall survival; ND, no difference; NA, not applicable.
The overexpression rates of PD-L1 in various studies are different, between 14.5% in the study of Zhang and his colleagues in Tianjin, China and 82.8% in the study of Chen et al. in Jiangsu, China (29,39). The overexpression rate of PD-L1 in our study was 64.0%, just between their reports. There were five studies that included patients with pre-operative chemo/radiotherapy, while our study had only chemo/radiotherapy-naive patients. Interestingly, PD-L1 expression negatively influenced the survival in these five studies, including OS in 4 studies and distal failure rate only in the other study (30,34,35,37,41).
The overexpression rate of PD-L2 (42.0%) in our study is little lower than other three studies (42.5% to 48.4%), but not much different. There was no survival impact of PD-L2 expression on both disease free survival (DFS) and OS. The result was the same as those of Leng and his coworkers in OS (36). In contrast, two studies from Ohigashi et al. and Tanaka et al. showed that expression of PD-L2 negatively influenced the OS in univariate analysis (28,34).
In the analysis of DFS, overexpression of PD-L1 is an independent prognostic factor for worse survival using multivariate analysis in this study. There were ten studies previously that investigated the influence of PD-L1 in DFS in patients with ESCC. Five studies had the same results as ours, even though only univariate analysis was used in two studies (35,37,38,40,42). However, two studies had the opposite result; in these studies the patients with positive expression of PD-L1 had a better DFS rate (31,43). The other studies showed the expression of PD-L1 had no influence on DFS.
In the OS analysis, there was not significantly different between patients with and without overexpression of PD-L1 using the Kaplan-Meier method in this study (P=0.066). In the past, there were three of 16 studies had the same results as our study (31,37,39). Most studies showed that expression of PD-L1 was negatively influencing the OS with four studies using multivariate analysis. Only two studies in Japan and Germany disclosed that expression of PD-L1 was a better independent predictor for prognosis (33,43), shown in Table 4.
In general, the heterogeneity in patient populations in the different studies, such as age, gender, pathological factors and the percentage of chemo/radiotherapy-naive patients may influence the results of PD-Ls, such as positive rate and the impaction of survival. But, the difference in the criteria for “Positive” staining in tissue samples across studies is the major reason with the inconsistent results. This has been pointed out by Dhupar and his Pittsburgh group (27). Several different antibodies that recognize the different part of PD-Ls would influence the reading of staining. A significant variation of cutoffs for positive staining, including intensity and tumor percentage, would also make the results confuse and would make the comparisons between studies quite difficult. There is a need for a standard scoring system to consider and compare the clinical outcomes in the future.
In addition, the staining in different cells of tissue samples, the tumor cells vs. the tumor infiltrating immune cells, or in different locations of tumor cells (cytoplasm and membrane) may represent different effects that may have the different meaning. Although PD-L1 is a membranous protein, the production of PD-L1 comes from the cytoplasm of cells. The membranous and/or cytoplasmic staining of PD-L1 was common presented in most of the previous studies (28-32,35-38,42). There were also focused on membranous staining only in several studies (33,40,41,43). In this study, we only focused on the expression of PD-L1 on the cytoplasm of tumor cells, not the membranous or cytoplasmic/membranous staining, that was the same as the study of Tanaka et al. (34), but differed with other studies.
In conclusion, the expression of PD-L1 on cytoplasm is an independent prognostic factor for DFS in patients undergoing esophagectomy for ESCC, but PD-L2 expression is not. There is a trend which suggested that patients without overexpression of PD-L1 and PD-L2 had a better OS.
Acknowledgements
Funding: CC Hsieh was supported by the Veterans General Hospitals and the University System of the Taiwan Joint Research Program (VGHUST 107-G7-2-2).
Ethical Statement: This study was approved by the Institutional Review Board of Taipei Veterans General Hospital. Patient informed consent was waived due to the retrospective nature of the study. Research was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.WHO website. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- 2.Ries LAG, Eisner MP, Kosary C, et al, editors. SEER cancer statistics review, 1973–1999. Bethesda, MD.: National Cancer Institute, 2002. [Google Scholar]
- 3.Lightdale CJ. Esophageal cancer. Am J Gastroenterol 1999;94:20-9. 10.1111/j.1572-0241.1999.00767.x [DOI] [PubMed] [Google Scholar]
- 4.Faivre J, Forman D, Esteve J. Gatta G and the EUROCARE working group. Survival of patients with oesophageal and gastric cancers in Europe. Eur J Cancer 1998;34:2167-75. 10.1016/S0959-8049(98)00329-3 [DOI] [PubMed] [Google Scholar]
- 5.Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992;11:3887-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishimura H, Honjo T. PD-1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol 2001;22:265-8. 10.1016/S1471-4906(01)01888-9 [DOI] [PubMed] [Google Scholar]
- 7.Dong H, Zhu G, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999;5:1365-9. 10.1038/70932 [DOI] [PubMed] [Google Scholar]
- 8.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027-34. 10.1084/jem.192.7.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tseng SY, Otsuji M, Gorski K, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med 2001;193:839-46. 10.1084/jem.193.7.839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1and inhibits T cell activation. Nat Immunol 2001;2:261-8. 10.1038/85330 [DOI] [PubMed] [Google Scholar]
- 11.Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci USA 2003;100:5336-41. 10.1073/pnas.0931259100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDermott DF, Atkins MB. PD-1 as a potential target in cancer therapy. Cancer Med 2013;2:662-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishimura H, Nose M, Hiai H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif carrying immunoreceptor. Immunity 1999;11:141-51. 10.1016/S1074-7613(00)80089-8 [DOI] [PubMed] [Google Scholar]
- 14.Dong H, Strome SE, Salomao DR, et al. Tumor associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793-800. 10.1038/nm730 [DOI] [PubMed] [Google Scholar]
- 15.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167-75. 10.1200/JCO.2009.26.7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1blockade. Proc Natl Acad Sci U S A 2002;99:12293-7. 10.1073/pnas.192461099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang CY, Lin MW, Chang YL, et al. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer 2014;50:1361-9. 10.1016/j.ejca.2014.01.018 [DOI] [PubMed] [Google Scholar]
- 18.Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009;15:971-9. 10.1158/1078-0432.CCR-08-1608 [DOI] [PubMed] [Google Scholar]
- 19.Shi SJ, Wang LJ, Wang GD, et al. B7-H1 expression is associated with poor prognosis in colorectal carcinoma and regulates the proliferation and invasion if HCT116 colorectal cancer cells. PLoS One 2013;8:e76012. 10.1371/journal.pone.0076012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hino R, Kabashima K, Kato Y, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 2010;116:1757-66. 10.1002/cncr.24899 [DOI] [PubMed] [Google Scholar]
- 21.Derks S, Nason KS, Liao X, et al. Epithelial PD-L2 expression marks Barrett’s esophagus and esophageal adenocarcinoma. Cancer Immunol Res 2015;3:1123-9. 10.1158/2326-6066.CIR-15-0046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Gao JX, Wen J, et al. B7DC/PDL2 promotes tumor immunity by a PD-1-independent mechanism. J Exp Med 2003;197:1721-30. 10.1084/jem.20022089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radhakrishnan S, Nguyen LT, Ciric B, et al. Immunotherapeutic potential of B7-DC (PD-L2) cross-linking antibody in conferring antitumor immunity. Cancer Res 2004;64:4965-72. 10.1158/0008-5472.CAN-03-3025 [DOI] [PubMed] [Google Scholar]
- 24.Rice TW, Blackstone EH, Rusch VW. 7th Edition of the AJCC Cancer Staging Manual: Esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721-4. [DOI] [PubMed] [Google Scholar]
- 25.Lote H, Cafferkay C, Chau I. PD-1 and PD-L1 blockade in gastrointestinal malignancies. Cancer Treat Rev 2015;41:893-903. 10.1016/j.ctrv.2015.09.004 [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Teng F, Kong L, et al. PD-L1 expression in human cancers and its association with clinical outcomes. OncoTargets Therapy 2016;9:5023-39. 10.2147/OTT.S105862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhupar R, Van Der Kraak L, Pennathur A, et al. Targeting immune checkpoints in esophageal cancer: A high mutational load tumor. Ann Thorac Surg 2017;103:1340-9. 10.1016/j.athoracsur.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 28.Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 2005;11:2947-53. 10.1158/1078-0432.CCR-04-1469 [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Deng H, Lu M, et al. B7-H1 expression associates with tumor invasion and predicts patient's survival in human esophageal cancer. Int J Clin Exp Pathol 2014;7:6015-23. [PMC free article] [PubMed] [Google Scholar]
- 30.Lim SH, Hong M, Ahn S, et al. Changes in tumour expression of programmed death-ligand 1 after neoadjuvant concurrent chemoradiotherapy in patients with squamous oesophageal cancer. Eur J Cancer 2016;52:1-9. 10.1016/j.ejca.2015.09.019 [DOI] [PubMed] [Google Scholar]
- 31.Chen K, Cheng G, Zhang F, et al. Prognostic significance of programmed death-1 and programmed death-ligand 1 expression in patients with esophageal squamous cell carcinoma. Oncotarget 2016;7:30772-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ito S, Okano S, Morita M, et al. Expression of PD-L1 and HLA Class I in Esophageal Squamous Cell Carcinoma: Prognostic Factors for Patient Outcome. Ann Surg Oncol 2016;23:508-15. 10.1245/s10434-016-5376-z [DOI] [PubMed] [Google Scholar]
- 33.Hatogai K, Kitano S, Fujii S, et al. Comprehensive immunohistochemical analysis of tumor microenvironment immune status in esophageal squamous cell carcinoma. Oncotarget 2016;7:47252-64. 10.18632/oncotarget.10055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka K, Miyata H, Sugimura K, et al. Negative influence of programmed death-1-ligands on the survival of esophageal cancer patients treated with chemotherapy. Cancer Sci 2016;107:726-33. 10.1111/cas.12938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen MF, Chen PT, Chen WC, et al. The role of PD-L1 in the radiation response and prognosis for esophageal squamous cell carcinoma related to IL-6 and T-cell immunosuppression. Oncotarget 2016;7:7913-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leng C, Li Y, Qin J, et al. , Relationship between expression of PD-L1 and PD-L2 on esophageal squamous cell carcinoma and the antitumor effects of CD8+ T cells.+ Oncol Rep 2016;35:699-708. 10.3892/or.2015.4435 [DOI] [PubMed] [Google Scholar]
- 37.Kim R, Keam B, Kwon D, et al. Programmed death ligand-1 expression and its prognostic role in esophageal squamous cell carcinoma. World J Gastroenterol 2016;22:8389-97. 10.3748/wjg.v22.i37.8389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Y, Zang Y, Zhao F, et al. Inhibition of HIF-1α by PX-478 suppresses tumor growth of esophageal squamous cell cancer in vitro and in vivo. Am J Cancer Res 2017;7:1198-212. [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang W, Pang Q, Zhang X, et al. Programmed death-ligand 1 is prognostic factor in esophageal squamous cell carcinoma and is associated with epidermal growth factor receptor. Cancer Sci 2017;108:590-7. 10.1111/cas.13197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang Y, Lo AW, Wong A, et al. Prognostic significance of tumor-infiltrating immune cells and PD-L1 expression in esophageal squamous cell carcinoma. Oncotarget 2017;8:30175-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Momose K, Yamasaki M, Tanaka K, et al. MLH1 expression predicts the response to preoperative therapy and is associated with PD-L1 expression in esophageal cancer. Oncol Lett 2017;14:958-64. 10.3892/ol.2017.6215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsutsumi S, Saeki H, Nakashima Y, et al. Programmed death-ligand 1 expression at tumor invasive front is associated with epithelial-mesenchymal transition and poor prognosis in esophageal squamous cell carcinoma. Cancer Sci 2017;108:1119-27. 10.1111/cas.13237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jesinghaus M, Steiger K, Slotta-Huspenina J, et al. Increased intraepithelial CD3+ T-lymphocytes and high PD-L1 expression on tumor cells are associated with a favorable prognosis in esophageal squamous cell carcinoma and allow prognostic immunogenic subgrouping. Oncotarget 2017;8:46756-68. 10.18632/oncotarget.18606 [DOI] [PMC free article] [PubMed] [Google Scholar]