Abstract
Background:
Neuromyelitis optica spectrum disorder (NMOSD) is a central nervous system inflammatory disorder in which immunoglobulin G (IgG) autoantibodies possibly play a pathogenic role against the aquaporin-4 water channel protein. Vitamin D may modulate B-cell function and decrease the IgG synthesis and may play a role in NMOSD as a crucial factor. The aim of this study was to investigate the relation between Vitamin D intakes from food, Vitamin D intake from sunlight exposure, blood Vitamin D levels, and IgG-neuromyelitis optica (NMO) level in serum of patients with NMOSD and NMO.
Method:
In this cross-sectional study, food Frequency Questionnaires (FFQ) and Sun Exposure Questionnaire (SEQ) were completed to evaluate of vitamin D intakes from food and sun light exposure. Moreover, serum levels of 25(OH) vitamin D3 and IgG-NMO were assessed in patients with NMOSD and NMO.
Results:
We assessed IgG-NMO levels in 29 patients with NMOSD that nine patients (n = 31%) were positive and for the rest it was negative. Sunlight exposure scale (P = 0.01) and 25(OH) D3 (P = 0.04) in IgG-NMO-negative patients were significantly more than patients with positive IgG-NMO. Age, gender, and latitude were not confounder variables. A positive significant correlation was observed between the sun exposure scale and serum levels of 25(OH) D3 in all participants (r = 0.747, P ≤ 0.001).
Conclusions:
Physiological variation in Vitamin D may apply a significant effect on IgG-NMO synthesis in patients with NMO. Vitamin D may have significant role in pathogenesis of NMOSD and NMO.
Keywords: Immunoglobulin G, neuromyelitis optica spectrum disorder, Vitamin D
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) or Devic's syndrome is a relapse disease of the central nervous system (CNS) that basically affects the optic nerves and spinal cord.[1] Based on the reports of epidemiological and population-based studies, the prevalence of neuromyelitis optica (NMO) is between <1/100,000 and 44/100,000 in Europe and North America.[2,3,4,5,6] In more than 90% of patients, NMOSD is an unpredictable relapsing illness with the optic neuritis (ON) attacks, myelitis, or both.[7] The most abundant water channel in the CNS is aquaporin-4 (AQP4), which is limited to astrocytes and ependymal cells and located at glial–pial and glial–endothelial interfaces and surrounds Ranvier nodes and paranodes, adjacent oligodendroglia loops, and synapses.[8]
In recent years, scientists and clinical neurologists have recognized a highly specific serum immunoglobulin G (IgG)[9] autoantibody (NMO-IgG) targeting the most affluent astrocytic water channel AQP4 as a key biomarker for this disorder.[10,11,12,13] In general, NMO is recognized as an IgG-mediated autoimmune astrocytopathy. Before discovering this antibody, NMOSD was misclassified as multiple sclerosis (MS). NMO-IgG is basically involved in the pathogenesis of NMO spectrum disorders.[14]
Vitamin D3 is a prohormone made by the action of ultraviolet (UV) on 7-dehydrocholesterol in skin. It is metabolized to 25-hydroxyvitamin [25(OH)] D3 in liver and then is changed to the biologically active form 1,25-dihydroxyvitamin 1,25(OH)2] D3 in kidney. The main role of Vitamin D in human is to regulate the bone homeostasis and calcium metabolism. Furthermore, it is also vital for activating immune defense systems and preventing the pathologies of immunological disease.[15] By recognizing the Vitamin D receptor (VDR) on lymphocytes, the immunological importance of Vitamin D was detected for the first time.[16,17] Previous studies have investigated the effects of Vitamin D on human adaptive immune cells and showed an expression of the nuclear VDR as well as Vitamin D-activating enzymes in both T- and B-cells.[18] Vitamin D prevents B-cell sudden proliferation and differentiation and therefore reduces Ig secretion. Moreover, Vitamin D affects sudden increase of T-cell and maturation which results in reduction in the amounts of T-cells with the phenotypes of Th1 and Th17.[15,19] Many studies have revealed that Vitamin D levels are low in patients with autoimmune disorders, such as MS, systemic lupus erythematosus, rheumatoid arthritis, and type 1 diabetes.[20] Moreover, Vitamin D levels have been reported to be associated with illness disability or activity in these disorders.[21,22,23]
To the best of our knowledge, association between sun exposure, Vitamin D intake, serum Vitamin D level, and IgG-NMO levels in patients with NMOSD has not been examined. Therefore, the aim of this study was to examine the association between sun exposure, Vitamin D intake, and serum levels of 25(OH) D and NMO-IgG in newly diagnosed patients with Devic's syndrome.
Methods
Study design and patients
The present research is a cross-sectional study carried out in Isfahan, Iran, in June 2015 that was approved by the Ethics Committee of Isfahan University of Medical Sciences (Project Code: 393886). Sixty-three patients with newly diagnosed NMOSD and NMO with magnetic resonance imaging assessment of spinal cord were recruited from MS clinic in Ayatollah Kashani Hospital of Isfahan University of Medical Sciences, Isfahan, Iran. Inclusion criteria included having NMOSD, lack of medical comorbidities, or any other basic chronic illnesses. Exclusion criteria were using cholecalciferol, calcium, multivitamin, mineral supplementation, or Vitamin D-fortified foods during the past 3 months and lived in other city with different latitude. Thirty-four patients excluded from the study because they consumed cholecalciferol and were living in other city with different latitude. A 49-item semi-quantitative food frequency questionnaire was used for the assessment of dietary intake, particularly calcium and Vitamin D intake. Food intake frequency over the past 3 months was reported in day, week, or month. Calcium content of foods was calculated from Nutritionist 4 software published by N-squared computing (FIRM) modified for Iranian foods, and reliability and relative validity were surveyed in the previous study.[24] Vitamin D was calculated based on the “provisional table on the Vitamin D foods’ content” released by the United States Department of Agriculture.[25]
Sunshine exposure assessment
Average sunshine exposure was measured using a questionnaire to quantify the amount of time patients spent in the sun and other sun-related habits during the past 3 months of the patients’ recruitment. The questions were about the amount of time that patients spent outdoors, time spending outdoors, wearing sunscreen, sun protection factor, type of clothing, and the skin pigment. It was a modified version of the sun exposure questionnaire made by Glanz et al.,[26] and it was used in previous study in Iran.[9]
The score of sun index was determined as hours that were spent under sun exposure during the week, sun-protective behaviors (i.e., clothing and sunscreen use), and patients’ skin color. Possible scores ranged from 4 to 14. Higher scores showed higher sunlight exposure. The sun scores were examined regarding their relationship to serum Vitamin D levels.
Clinical characteristics
All 29 participants were Iranian and had been living in Isfahan city since their birth (latitude: 26°42’N). We collected demographic and clinical characteristics of participants such as sex and age, body mass index (BMI), illness duration, the first clinical sign, and history of other autoimmune disease. Patients were stable in remission and experienced no attack and did not take any pulse therapy during the past 3 months. All of the patients have received a low dose of oral prednisolone and/or oral azathioprine as maintenance therapy.
Laboratory procedures
25(OH) D was assessed with LIAISON® (LIAISON® 25 OH Vitamin D TOTAL Specimen Diluent Set, REF 310602) method at the Milad Laboratory in Isfahan. This method is a direct competitive chemiluminescence immunoassay for quantitative determination of total 25(OH) D in serum. During the first incubation, 25(OH) D is separated from its binding protein and binds to the specific antibody in the solid phase. The tracer is added after 10 min (Vitamin D linked to an isoluminol derivative). After 10 min of incubation, the unbound material is removed with a wash cycle. Subsequently, the beginner reagents are added to initiate a flash chemiluminescent reaction. The light signal is measured by a photomultiplier as relative light units and is inversely proportional to the 25(OH) D concentration present in calibrators, controls, or samples.
The anti-AQP4 test was conducted at the Milad Laboratory in Isfahan. Anti-AQP4 antibodies (AQP4) were determined in the patients’ serum by indirect immunofluorescence (EUROIMMUN IIFT, Germany) on a cell line which had been molecular biologically modified (AQP4 transfected cells) to produce large quantities of AQP4. Biochip slides containing AQP4-transfected cells and nontransfected cells (EU-90) were incubated with diluted patient's samples. In the positive reactions case, specific antibodies of the classes IgA, IgG, and IgM would bind to the antigens. In the second step, the attached antibodies are stained with fluorescein-labeled anti-human antibodies and became visible with the fluorescence microscope.
Ethics statement
Written informed consent was obtained from all the participants. This study was approved by School of Nutrition and Food Sciences, Isfahan University of Medical Sciences (Ethic Code: 393886).
Statistical analysis
Normal distribution of variables among IgG-NMO positive and negative participants was determined by Shapiro–Wilk test. All variables including sun exposure scale, Vitamin D intake, and 25(OH) D3 levels were normally distributed. Independent sample t-test was applied to compare the means of variables between IgG-NMO positive and negative groups. Pearson's correlation test was used to assess the association between sun exposure scale, Vitamin D intake, and 25(OH) D3 levels among all the participants and in subgroups of IgG-NMO positive and negative patients. All values were presented as mean (standard deviation [SD]). Statistical analyses were performed using SPSS version 20 published by IBM Crop, Armonk, New York. P < 0.05 was considered statistically significant.
Results
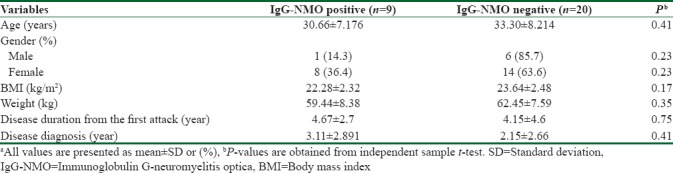
General characteristics of participants are demonstrated in Table 1. According to the presented data, age, sex, BMI, weight, disease duration, and disease diagnosis had no significant differences between NMO-IgG positive and negative group.
Table 1.
General characteristics of the study populationa
Mean ± SD of sun exposure scale, Vitamin D intake, and serum levels of 25(OH) D3 is shown in Table 2. NMO-IgG-negative participants had significantly higher scores of sun exposure and 25(OH) D3 levels in comparison with NMO-IgG-positive participants. Furthermore, the participants with negative NMO-IgG consumed marginally higher amounts of Vitamin D compared to NMO-IgG-positive participants.
Table 2.
Mean±standard deviation values of variables between neuromyelitis optica-immunoglobulin G positive and negative patients
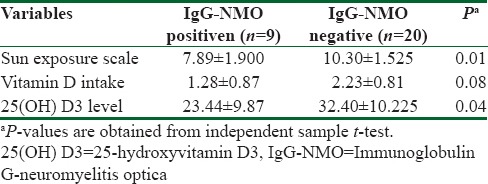
Table 3 shows the results of Pearson's correlation test between studied variables and serum levels of 25(OH) D3.
Table 3.
Correlation between serum levels of 25-hydroxyvitamin D3 level and studied variables among all participants and neuromyelitis optica-immunoglobulin G positive and negative persons

Discussion
This study suggests that negative patients had higher scales of sun exposure, dietary Vitamin D, and serum levels of 25(OH) D3 in comparison with NMO-IgG-positive participants. In addition, there was a significant direct association between scales of sun exposure and serum concentrations of 25(OH) D3 among all the participants (n = 29), which was the first report of the association between the NMO-IgG levels and sun exposure scale, dietary Vitamin D, and serum levels of 25(OH) D3, determined among patients with NMOSD and NMO.
NMOSD and NMO are demyelinating diseases of the CNS with inflammatory lesions that have been seen chiefly in spinal cord and optic nerves.[27] Particularly, NMO patients often suffer from recurrent ON and longitudinally wide transverse myelitis (LETMs) that sometimes results in blindness and paraplegia. The discovery of AQP4 antibody (NMO-IgG), the most important laboratory feature, has made it easy to diagnose of NMO from MS. While NMO-IgG was considered only as a biomarker of NMO at first, common fulminant attacks caused investigators suggest NMO-IgG that may be a marker of destructive demyelination rather than an illness-specific biomarker.[28]
25(OH) D is the main circulating metabolite of Vitamin D.[29] Although the biologically active form of Vitamin D is 1,25(OH) vitamin D3 that is synthesized in the kidney. It is generally believed that measuring 25(OH) D provides better information regarding the patients’ Vitamin D status and therefore it is a useful marker for the recognition of hypovitaminosis.[29,30,31] In general, the presence of VDR is an indicator for cells response to Vitamin D. In addition to osteoblasts, enterocytes, and distal renal tubular cells, it is shown that VDR is found in many other cell types, such as parathyroid gland cells, keratinocytes of skin, colon cells, pituitary gland cells, and ovarian cells. The VDR is also widely found in several cell types of the immune system, that is, T-cells, B-cells, monocytes, macrophages, dendritic cells, and natural killing cells.[16,32,33,34] Experimental, epidemiological, and clinical studies have revealed contrary associations between low Vitamin D status and longevity as well as impeding immunosenescence.[35]
It seems that Vitamin D may be associated with the human immune dysfunction often originated from geographic variation observations in illness occurrence. In many parts of the world, Vitamin D is principally synthesized in the skin following sun exposure (specifically UV B [UVB] irradiation). Levels of UVB radiation fluctuate strongly according to the distance from the equator (latitude) and time of year. Higher levels of Vitamin D are observed in the following circumstances: a higher radiation intensity (the sun is directly overhead or closer to the equator), in summer, and during the middle of the day. Thus, higher latitude is often considered as the main cause of both lower levels of UV radiation and lower Vitamin D status. Latitudinal gradients, an expression which describes the incidence or prevalence of an illness increases simultaneously with increasing distance from the equator (lower UVB radiation), are applied for MS, type 1 diabetes, autoimmune vasculitis, inflammatory bowel illnesses, and asthma.[36,37,38,39,40]
Research on Vitamin D and NMO is infancy. Some studies have shown reduced levels of Vitamin D in patients with NMO or NMOSD.[41,42] In another study, the authors have seen an inverse association between levels of Vitamin D and disability in these patients.[43]
Although studies about Vitamin D and NMO are low, a large body of studies has been carried out on Vitamin D and MS or other autoimmune illnesses.
For the first time in 1922, geographical variations were introduced as a potential reason for the association between Vitamin D and human immune dysfunctions as well as MS.[41] Regarding the influence of Vitamin D on illness risk, we should differentiate between Vitamin D dependent (Vitamin D supplementation) and independent pathways (Vitamin D produced from sunlight). Limited success in Vitamin D supplementation trials suggests that independent pathway may associate more strongly with illness risk. The incidence of autoimmune encephalomyelitis (EAE) in experimental models (a mouse model for MS) was significantly delayed following chronic irradiation with suberythemal doses of UVB radiation.[42] 25(OH) D serum levels were only slightly elevated and remission of illness was not occurred following 25(OH) D oral administration.[42] However, the development of EAE was suppressed through hypercalcemia by dietary 1,25(OH)2 D administration. It was assumed that it was lonely important to illness suppression. TReg-inducing tolerogenic dendritic cells that motivated by UVB irradiation in the skin were required for the amelioration of EAE. Serum 25(OH) D levels and sun exposure have been shown to be independently associated with the CNS demyelination onset in humans as well as brain and spinal cord lesions in people with MS.[43,44]
Some involved factors in Vitamin D deficiency are living in north or south latitudes and sun exposure avoidance. Although the prevalence of MS is high in north and south latitudes, the prevalence of MS declines in northern latitudes probably due to adaptation to high Vitamin D-including diets (for instance: oily fish products).[45,46] A recent study has revealed that low current Vitamin D status and low past or current sun exposure are independent risk factors for the development of MS and CNS demyelination.[43]
A number of biological mechanisms may explain the association between Vitamin D status and MS. VDRs and a VDR-dependent enzyme that is responsible to form the active form of 1,25(OH) D are widely expressed in human brains and are responsible for the formation of the highly active Vitamin D metabolite. Several studies have investigated the effects of Vitamin D on VDR gene transcription in neural cells and have demonstrated that VDRs and Vitamin D are key molecules in developing brain, preventing anxiety, inducing glial-derived neurotropic factor, and induction of nerve growth factor synthesis.[47,48]
Recently, a study has demonstrated that Vitamin D could also have myelin-protective effects independent of T-lymphocyte activation and infiltration. The high amount of dietary Vitamin D was significantly associated with weaker white matter microglia activation/macrophage infiltration during oligodendrocyte death and demyelination. Moreover, it is revealed that the active metabolic of Vitamin D has immune modulatory affected through inhibiting the differentiation of dendritic cells and desensitization of them to maturing stimuli.[49]
Conclusions
The present study is the first report of association between Vitamin D and IgG-NMO in NMOSD and NMO patients, which shows that Vitamin D may have a major impact on IgG-NMO synthesis in these patients and play an important role in the pathogenesis of NMOSD and NMO. Further studies with larger sample sizes are needed to prove this result.
Financial support and sponsorship
This study was supported by School of Nutrition and Food Sciences, Isfahan University of Medical Sciences, Isfahan, Iran.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This study was extracted from M. Sc. dissertation which was approved by School of Nutrition and Food Sciences, Isfahan University of Medical Sciences (Code 393886).
References
- 1.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–15. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- 2.Jacob A, Panicker J, Lythgoe D, Elsone L, Mutch K, Wilson M, et al. The epidemiology of neuromyelitis optica amongst adults in the Merseyside county of United Kingdom. J Neurol. 2013;260:2134–7. doi: 10.1007/s00415-013-6926-y. [DOI] [PubMed] [Google Scholar]
- 3.Bizzoco E, Lolli F, Repice AM, Hakiki B, Falcini M, Barilaro A, et al. Prevalence of neuromyelitis optica spectrum disorder and phenotype distribution. J Neurol. 2009;256:1891–8. doi: 10.1007/s00415-009-5171-x. [DOI] [PubMed] [Google Scholar]
- 4.Ketelslegers IA, Catsman-Berrevoets CE, Neuteboom RF, Boon M, van Dijk KG, Eikelenboom MJ, et al. Incidence of acquired demyelinating syndromes of the CNS in Dutch children: A nationwide study. J Neurol. 2012;259:1929–35. doi: 10.1007/s00415-012-6441-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cossburn M, Tackley G, Baker K, Ingram G, Burtonwood M, Malik G, et al. The prevalence of neuromyelitis optica in South East Wales. Eur J Neurol. 2012;19:655–9. doi: 10.1111/j.1468-1331.2011.03529.x. [DOI] [PubMed] [Google Scholar]
- 6.Mealy MA, Wingerchuk DM, Greenberg BM, Levy M. Epidemiology of neuromyelitis optica in the United States: A multicenter analysis. Arch Neurol. 2012;69:1176–80. doi: 10.1001/archneurol.2012.314. [DOI] [PubMed] [Google Scholar]
- 7.Jarius S, Ruprecht K, Wildemann B, Kuempfel T, Ringelstein M, Geis C, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: A multicentre study of 175 patients. J Neuroinflammation. 2012;9:14. doi: 10.1186/1742-2094-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: High-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–80. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ataie-Jafari A, Hossein-Nezhad A, Maghbooli Z, Karimi F, Rahmani M, Shahbazi S, et al. The influence of sunlight exposure on serum Vitamin D concentration and bone turnover; a controlled clinical trial. Iran J Public Health. 2008;37:41–8. [Google Scholar]
- 10.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–7. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: Distinction from multiple sclerosis. Lancet. 2004;364:2106–12. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 12.Jarius S, Franciotta D, Bergamaschi R, Wright H, Littleton E, Palace J, et al. NMO-IgG in the diagnosis of neuromyelitis optica. Neurology. 2007;68:1076–7. doi: 10.1212/01.wnl.0000256822.01222.bd. [DOI] [PubMed] [Google Scholar]
- 13.Paul F, Jarius S, Aktas O, Bluthner M, Bauer O, Appelhans H, et al. Antibody to aquaporin 4 in the diagnosis of neuromyelitis optica. PLoS Med. 2007;4:e133. doi: 10.1371/journal.pmed.0040133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinson SR, Pittock SJ, Lucchinetti CF, Roemer SF, Fryer JP, Kryzer TJ, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69:2221–31. doi: 10.1212/01.WNL.0000289761.64862.ce. [DOI] [PubMed] [Google Scholar]
- 15.Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–47. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 16.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–3. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 17.Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: Presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57:1308–10. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 18.Lemire JM, Adams JS, Sakai R, Jordan SC. 1 alpha, 25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J Clin Invest. 1984;74:657–61. doi: 10.1172/JCI111465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O’Garra A. 1alpha, 25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–80. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 20.Agmon-Levin N, Theodor E, Segal RM, Shoenfeld Y. Vitamin D in systemic and organ-specific autoimmune diseases. Clin Rev Allergy Immunol. 2013;45:256–66. doi: 10.1007/s12016-012-8342-y. [DOI] [PubMed] [Google Scholar]
- 21.Smolders J, Menheere P, Kessels A, Damoiseaux J, Hupperts R. Association of Vitamin D metabolite levels with relapse rate and disability in multiple sclerosis. Mult Scler. 2008;14:1220–4. doi: 10.1177/1352458508094399. [DOI] [PubMed] [Google Scholar]
- 22.Mok CC, Birmingham DJ, Ho LY, Hebert LA, Song H, Rovin BH. Vitamin D deficiency as marker for disease activity and damage in systemic lupus erythematosus: A comparison with anti-dsDNA and anti-C1q. Lupus. 2012;21:36–42. doi: 10.1177/0961203311422094. [DOI] [PubMed] [Google Scholar]
- 23.Rossini M, Maddali Bongi S, La Montagna G, Minisola G, Malavolta N, Bernini L, et al. Vitamin D deficiency in rheumatoid arthritis: Prevalence, determinants and associations with disease activity and disability. Arthritis Res Ther. 2010;12:R216. doi: 10.1186/ar3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 2010;13:654–62. doi: 10.1017/S1368980009991698. [DOI] [PubMed] [Google Scholar]
- 25.Mahan LK, Escott-Stump S, Raymond JL. Missuri: Elsevier Health Sciences/Saunders; 2012. Krause's Food and the Nutrition Care Process. [Google Scholar]
- 26.Glanz K, Yaroch AL, Dancel M, Saraiya M, Crane LA, Buller DB, et al. Measures of sun exposure and sun protection practices for behavioral and epidemiologic research. Arch Dermatol. 2008;144:217–22. doi: 10.1001/archdermatol.2007.46. [DOI] [PubMed] [Google Scholar]
- 27.Magaña SM, Pittock SJ, Lennon VA, Keegan BM, Weinshenker BG, Lucchinetti CF. Neuromyelitis optica IgG serostatus in fulminant central nervous system inflammatory demyelinating disease. Arch Neurol. 2009;66:964–6. doi: 10.1001/archneurol.2009.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papadopoulos MC, Verkman AS. Aquaporin 4 and neuromyelitis optica. Lancet Neurol. 2012;11:535–44. doi: 10.1016/S1474-4422(12)70133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nasri H, Baradaran A. The association of 25-hydroxyvitamin D levels with secondary hyperparathyroidism in end-stage renal failure patients undergoing regular hemodialysis. Arch Med Sci. 2005;4:236–4. [Google Scholar]
- 30.Hollis BW. Assessment of Vitamin D nutritional and hormonal status: What to measure and how to do it. Calcif Tissue Int. 1996;58:4–5. doi: 10.1007/BF02509538. [DOI] [PubMed] [Google Scholar]
- 31.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–83. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 32.Brennan A, Katz DR, Nunn JD, Barker S, Hewison M, Fraher LJ, et al. Dendritic cells from human tissues express receptors for the immunoregulatory Vitamin D3 metabolite, dihydroxycholecalciferol. Immunology. 1987;61:457–61. [PMC free article] [PubMed] [Google Scholar]
- 33.Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys. 2000;374:334–8. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- 34.Casteels K, Bouillon R, Waer M, Mathieu C. Immunomodulatory effects of 1,25-dihydroxyvitamin D3. Curr Opin Nephrol Hypertens. 1995;4:313–8. doi: 10.1097/00041552-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685–98. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simpson S, Jr, Blizzard L, Otahal P, Van der Mei I, Taylor B. Latitude is significantly associated with the prevalence of multiple sclerosis: A meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82:1132–41. doi: 10.1136/jnnp.2011.240432. [DOI] [PubMed] [Google Scholar]
- 37.Ball SJ, Haynes A, Jacoby P, Pereira G, Miller LJ, Bower C, et al. Spatial and temporal variation in type 1 diabetes incidence in Western Australia from 1991 to 2010: Increased risk at higher latitudes and over time. Health Place. 2014;28:194–204. doi: 10.1016/j.healthplace.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Gatenby PA, Lucas RM, Engelsen O, Ponsonby AL, Clements M. Antineutrophil cytoplasmic antibody-associated vasculitides: Could geographic patterns be explained by ambient ultraviolet radiation? Arthritis Rheum. 2009;61:1417–24. doi: 10.1002/art.24790. [DOI] [PubMed] [Google Scholar]
- 39.Khalili H, Huang ES, Ananthakrishnan AN, Higuchi L, Richter JM, Fuchs CS, et al. Geographical variation and incidence of inflammatory bowel disease among US women. Gut. 2012;61:1686–92. doi: 10.1136/gutjnl-2011-301574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krstic G. Asthma prevalence associated with geographical latitude and regional insolation in the United States of America and Australia. PLoS One. 2011;6:e18492. doi: 10.1371/journal.pone.0018492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davenport CB. Multiple sclerosis: From the standpcint of geographic distribution and race. Arch Neurol Psychiatry. 1922;8:51. [Google Scholar]
- 42.Becklund BR, Severson KS, Vang SV, DeLuca HF. UV radiation suppresses experimental autoimmune encephalomyelitis independent of Vitamin D production. Proc Natl Acad Sci U S A. 2010;107:6418–23. doi: 10.1073/pnas.1001119107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lucas RM, Ponsonby AL, Dear K, Valery PC, Pender MP, Taylor BV, et al. Sun exposure and Vitamin D are independent risk factors for CNS demyelination. Neurology. 2011;76:540–8. doi: 10.1212/WNL.0b013e31820af93d. [DOI] [PubMed] [Google Scholar]
- 44.Zivadinov R, Treu CN, Weinstock-Guttman B, Turner C, Bergsland N, O’Connor K, et al. Interdependence and contributions of sun exposure and Vitamin D to MRI measures in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2013;84:1075–81. doi: 10.1136/jnnp-2012-304661. [DOI] [PubMed] [Google Scholar]
- 45.Kampman MT, Brustad M. Vitamin D: A candidate for the environmental effect in multiple sclerosis – Observations from Norway. Neuroepidemiology. 2008;30:140–6. doi: 10.1159/000122330. [DOI] [PubMed] [Google Scholar]
- 46.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–8. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 47.Simon KC, Munger KL, Xing Yang, Ascherio A. Polymorphisms in Vitamin D metabolism related genes and risk of multiple sclerosis. Mult Scler. 2010;16:133–8. doi: 10.1177/1352458509355069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simpson S, Jr, Taylor B, Blizzard L, Ponsonby AL, Pittas F, Tremlett H. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol. 2010;68:193–203. doi: 10.1002/ana.22043. [DOI] [PubMed] [Google Scholar]
- 49.Griffin MD, Lutz WH, Phan VA, Bachman LA, McKean DJ, Kumar R. Potent inhibition of dendritic cell differentiation and maturation by Vitamin D analogs. Biochem Biophys Res Commun. 2000;270:701–8. doi: 10.1006/bbrc.2000.2490. [DOI] [PubMed] [Google Scholar]


