Abstract
Transabdominal-US is the first-line imaging modality used to assess the whole liver parenchyma and vascularization; EUS assessment of the liver is incomplete and is not sufficient to rule out the presence of focal liver lesions. On the other hand, due the high diagnostic yield in detecting very small (< 1 cm) lesions, EUS is considered complementary to radiological imaging techniques for the investigation of liver parenchyma. Scarce data are available regarding the investigation of liver parenchyma using both EUS-elastography (EUS-E) and CH-EUS. The aim of this review is to evaluate the clinical role of image enhancement techniques, namely EUS-E and contrast harmonic-EUS (CH-EUS), for the evaluation liver diseases. Despite a potential interest for the application of EUS-E in the assessment of liver diseases, available evidence relegates this technique only to research areas, such as the differential diagnosis between benign and malignant focal liver lesions and the quantification of liver fibrosis in diffuse parenchymal diseases. With the future introduction of EUS shear-wave elastography, interesting data can be obtained for the assessment of liver fibrosis during real-time EUS evaluation. The usefulness of CH-EUS for the evaluation of liver disease is limited by the intrinsic EUS ability to explore only the left lobe and a small part of the right lobe. CH-EUS could be used to increase the diagnostic ability of EUS for the detection and characterization of small lesions and for guiding tissue sampling. Targeting EUS-guided treatments with either EUS-E or CH-EUS might represent potential future applications.
Keywords: Contrast agent, elastography, EUS, liver
INTRODUCTION
EUS has been introduced in clinical practice 40 years ago in order to overcome transabdominal ultrasound (tUS) limits related to the depth of some organs and to the presence of interposed intestinal gas. The first attention for research and technique development was focused on the evaluation of pancreatic disorders.[1] In the subsequent decades, the main areas of interest were represented by pancreatic neoplastic and inflammatory diseases, mucosal and subepithelial tumors of the gastrointestinal (GI) wall, and extrahepatic biliary tree disorders (i.e., obstruction, jaundice, biliary tree stones, and tumors).[2] Since the introduction of curvilinear array echoendoscopes in 90's, EUS was not only considered a diagnostic technique for identification and staging but also for interventional approaches, such as tissue acquisition and therapeutic maneuvers.[3]
tUS is the first-line imaging modality used to assess the whole liver parenchyma and vascularization since it is noninvasive, cheap, and does not require radiological exposure. Moreover, both diffuse and focal liver biopsy can be performed under tUS guidance. Only in a small number of cases (<10%), a focal liver lesion cannot be reached by tUS and a different imaging modality (computed tomography [CT] or magnetic resonance [MR]) or different approach (endoscopic) becomes necessary.[4] The first study on EUS-guided liver tissue acquisition reported a small experience on 14 patients with neoplastic focal liver lesions.[5]
EUS assessment of the liver is incomplete as it usually allows no >50% of the parenchyma to be seen. For this reason, EUS evaluation per se is not sufficient to rule out the presence of liver metastases during the staging of GI neoplasms. On the other hand, due to the high diagnostic yield in detecting very small (<1 cm) lesions, EUS is considered complementary to radiological imaging by CT and MR.[6]
Recently, a scoring system was proposed for the differential diagnosis between benign and malignant liver lesions found on EUS; the authors proposed a diagnostic algorithm that reaches a suboptimal predictive value (up to 90%) for neoplasms.[7]
EUS image enhancement techniques have been developed to increase the diagnostic accuracy of B-mode EUS evaluation for the differential diagnosis of solid pancreatic masses, to identify neoplastic features among pancreatic cystic lesions, and to better characterize enlarged lymph nodes or subepithelial tumors. In particular, EUS elastography (EUS-E) was used to depict mechanical characteristics and to indirectly quantify the amount of fibrosis of solid tissues; while the use of ultrasound contrast agents (UCAs) enabled the visualization of microvascular architecture under Doppler contrast-enhanced-EUS (CE-EUS) or contrast harmonic-EUS (CH-EUS) mode.[8,9,10] A detailed technical description of the basic principles and technology issues of both techniques is available elsewhere.[11,12,13,14,15,16,17,18] Unlike the above-mentioned indications, scarce data are available regarding the investigation of liver parenchyma using both EUS-E and CH-EUS. The aim of this review is to evaluate the clinical role of image enhancement techniques, namely EUS-E and CH-EUS, for the evaluation of liver diseases.
METHODS
A systematic review was conducted using PubMed library with the following search terms: Contrast (title or abstract) OR elastography (title or abstract) AND EUS (title or abstract) OR EUS (title or abstract) OR endosonography (title or abstract or MeSH terms) OR endoscopic ultrasonography (title or abstract). Original articles (randomized controlled trials and prospective or retrospective studies), meta-analyses, systematic reviews, and guidelines focused on liver diseases were included. Commentaries, non-English manuscripts, and articles in which EUS did not represent the principal matter were excluded from the study.
Role of EUS-elastography in the assessment of liver diseases
Percutaneous liver biopsy is considered the gold standard for the differential diagnosis, staging, and grading of diffuse liver diseases. Moreover, percutaneous liver biopsy is the most common technique to acquire liver tissue from focal liver lesions. In 2006, with the introduction of sonoelastography in the EUS armamentarium, Giovannini et al. hypothesized the use of EUS-E as a virtual biopsy that was able to assess tissue elasticity and to guide through the differential diagnosis.[19]
EUS-elastography for the assessment of liver fibrosis
Transient elastography was the first noninvasive transabdominal technique to be developed to quantify the “stiffness” of hepatic parenchyma and correlate with the stage of liver disease.[20] In the last decade, a significant amount of data was published to corroborate this preliminary hypothesis;[21] despite tUS-based elastography techniques have been confirmed as common modalities for the assessment of liver fibrosis, few data have been reported on the use of EUS-E in patients with diffuse liver diseases.[22,23,24]
To date, no study evaluated the usefulness of EUS-E for the assessment and quantification of liver fibrosis. In fact, only qualitative (color map) or semiquantitative (strain ratio) modalities are available in EUS. On the other hand, quantitative elastography is only available with tUS that confers the best diagnostic information compared to nonquantitative methods.[22,23,24]
We believe that more promising data will become available when quantitative shear-wave elastography is introduced into EUS. Still, it is difficult to envision that EUS-E assessment of liver fibrosis will replace tUS elastography as the first-line investigation because of the costs and albeit small risks of EUS. However, EUS-E could become an interesting tool to complete the examination in the case of patients without apparent biliary tract conditions on EUS, who underwent EUS because of altered liver function tests.[25,26]
EUS-elastography for the differential diagnosis between benign and malignant focal liver lesions
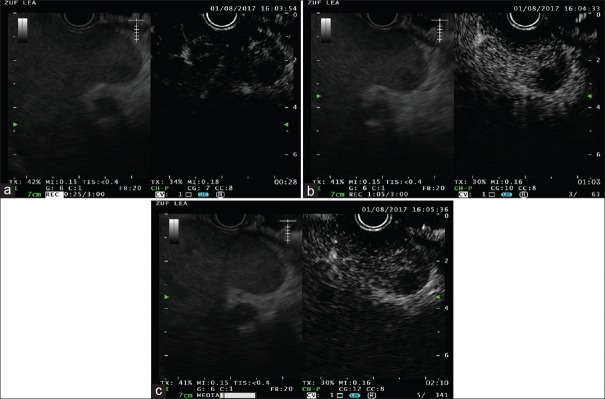
The use of EUS-E for the differential diagnosis between benign and malignant condition is based upon the observed increased hardness of malignant diseases compared to benign or normal conditions [Figure 1].[13,15] In the case of focal liver lesions, malignant neoplasms are significantly harder compared to benign ones and about 100-time stiffer than the surrounding normal tissue.[27]
Figure 1.
EUS elastography in a patient with pancreatic adenocarcinoma and multiple hypoechoic liver lesions
Saldolescu et al. retrospectively evaluated focal liver lesions by real time elastography both with tUS (27 patients) and with EUS (12 patients). The sonoelastographic videos were subsequently analyzed by a computer-based software.
The authors observed that benign liver lesions (hemangiomas) presented hue histograms with significantly lower stiffness values than neoplastic lesions (hepatocellular carcinoma, cholangiocarcinoma, and metastases). They identified a hue histogram cutoff value of 170, which was able to discriminate between benign and malignant tumors with 92.5% sensitivity, 88.8% specificity, 88.6% accuracy, 86.7% positive predictive value, and 92.3% negative predictive value.[28]
Characterization of inflammatory and fibrotic biliary lesions
Rustemovic et al. compared EUS linear array findings and EUS-E in 21 patients with primary sclerosing cholangitis (PSC) and 20 healthy controls who underwent EUS because of suspected common bile duct (CBD) stones. The authors observed that PSC patients presented with increased CBD wall thickness (0.89 mm vs. 0.39 mm) despite similar diameter. Hard or mixed EUS-E patterns were found in 16/21 PSC patients and in 4/20 healthy control. The authors demonstrated that EUS-E had a sensitivity of 80%, specificity of 81%, and accuracy of 81% for the detection of PSC and suggested that EUS-E could be useful for the noninvasive identification of PSC.[29]
Role of contrast harmonic-EUS in the assessment of liver diseases
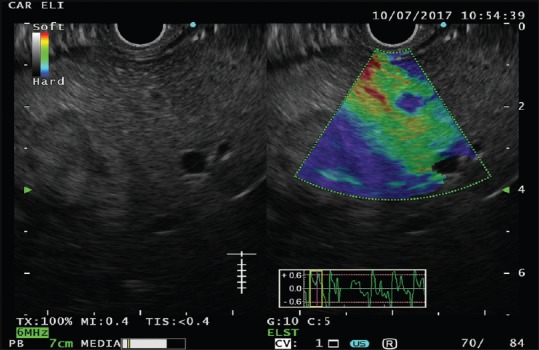
The use of UCAs allows an enhanced depiction of liver vascular architecture, leading to better identification and characterization of focal lesions [Figures 2 and 3].[4,30]
Figure 2.
Contrast harmonic-EUS in a patient with pancreatic adenocarcinoma; (a) arterial phase (0–30 s), (b) portal phase (30–120 s), (c) late venous phase (>120 s)
Figure 3.
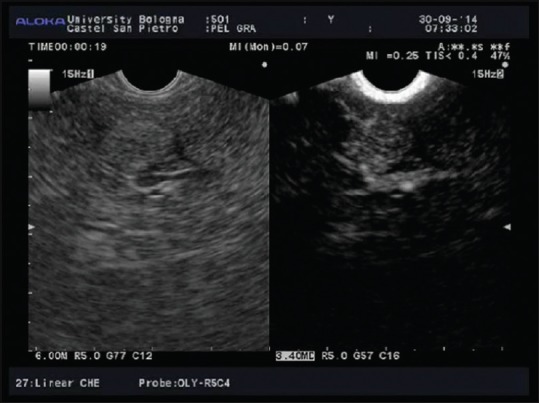
Contrast harmonic-EUS arterial phase of hepatocellular carcinoma in a patient with hepatitis C virus-related cirrhosis
Contrast-enhanced tUS and CH-EUS imaging reflect the dual hepatic blood systems, supplied by the hepatic artery and the portal vein. The CH-EUS study of the liver could be divided in three different phases: the arterial phase, starting few seconds after the injection and lasting for 30 s; the portal phase (from 30 to 120 s); and the late, venous phase (after 120 s from injection).[4,30,31]
Contrast harmonic-EUS for the identification of liver metastases
A recent preliminary report by Minaga et al.[32] reported the results of CH-EUS for the identification of liver metastases in patients who underwent EUS because of pancreatic cancer. The authors hypothesized that the evaluation of liver parenchyma 15 min after UCAs injection (sonazoid) could be enhanced by the phagocytosis of the contrast agent by liver Kupffer cells (also called “Kupffer phase”). The authors observed that CH-EUS showed higher accuracy for the detection of left-lobe liver metastases, compared to B-mode EUS and multidetector CT scan (accuracy of diagnosis 98.5% for CH-EUS, 91.1% for B-mode EUS, and 90.5% for CT scan). Moreover, CH-EUS-guided tissue acquisition led to excellent diagnostic accuracy, even in the case of small (<10 mm) lesions.[32]
Other indications of CH-EUS in different fields such as differential diagnosis of the gallbladder and biliary conditions were recently reported.[33,34]
Contrast-enhanced color Doppler EUS for the assessment of portal hypertension and esophageal varices
Three studies[35,36,37] evaluated the ability of color Doppler EUS, after infusion of a galactose-based UCAs (Levovist) for the assessment of esophageal varices hemodynamic characteristics.
The authors observed that contrast-enhanced color Doppler EUS improved the quality of color Doppler images in 60 out of 62 cases. In particular, three patterns of perforating veins were described: type 1 showed inflow from the paraesophageal veins, type 2 showed outflow to paraesophageal veins, and type 3 presented mixed inflow and outflow.[35] However, it was not clear whether the different patterns of venous flow correlated with the risk of bleeding in patients with/without medical and/or endoscopic therapy of esophageal varices.
Nevertheless, in subsequent studies, the authors observed that contrast-enhanced color Doppler EUS demonstrated the presence of perforating veins in 22 out of 29 (75.9%) patients with recurrent esophageal varices[36] and arterial flow in patients with high-risk esophageal varices.[37]
CONCLUSIONS
In many centers worldwide, the use of EUS-E and CH-EUS is considered as an integral part of the entire EUS procedure. On this basis, when available and depending on personal expertise, these techniques should be integrated into the whole diagnostic and therapeutic EUS process.
EUS imaging in hepatology can be considered a new diagnostic challenge, including EUS-E and CH-EUS. Despite a potential interest for the application of EUS-E in the assessment of liver diseases, available evidence relegates this technique only to research areas. With the future introduction of EUS shear-wave elastography, interesting data can be obtained for the assessment of liver fibrosis during real-time EUS evaluation.
The usefulness of CH-EUS for the evaluation of liver disease is limited by the intrinsic EUS ability to explore only the left lobe and a small part of the right lobe. CH-EUS could be used to increase the diagnostic ability of EUS for the detection and characterization of small lesions and for guiding tissue sampling. Targeting EUS-guided treatments with either EUS-E or CH-EUS might represent potential future applications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Fontana G, Bolondi L, Conti M, et al. An evaluation of echography in the diagnosis of pancreatic disease. Gut. 1976;17:228–34. doi: 10.1136/gut.17.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fusaroli P, Kypreos D, Alma Petrini CA, et al. Scientific publications in endoscopic ultrasonography: Changing trends in the third millennium. J Clin Gastroenterol. 2011;45:400–4. doi: 10.1097/MCG.0b013e3181fbde42. [DOI] [PubMed] [Google Scholar]
- 3.Srinivasan I, Tang SJ, Vilmann AS, et al. Hepatic applications of endoscopic ultrasound: Current status and future directions. World J Gastroenterol. 2015;21:12544–57. doi: 10.3748/wjg.v21.i44.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sidhu PS, Brabrand K, Cantisani V, et al. EFSUMB guidelines on interventional ultrasound (INVUS), part II. Diagnostic ultrasound-guided interventional procedures (Long version) Ultraschall Med. 2015;36:E15–35. doi: 10.1055/s-0035-1554036. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen P, Feng JC, Chang KJ. Endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration (FNA) of liver lesions. Gastrointest Endosc. 1999;50:357–61. doi: 10.1053/ge.1999.v50.97208. [DOI] [PubMed] [Google Scholar]
- 6.Dumonceau JM, Deprez PH, Jenssen C, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline – Updated January 2017. Endoscopy. 2017;49:695–714. doi: 10.1055/s-0043-109021. [DOI] [PubMed] [Google Scholar]
- 7.Fujii-Lau LL, Abu Dayyeh BK, Bruno MJ, et al. EUS-derived criteria for distinguishing benign from malignant metastatic solid hepatic masses. Gastrointest Endosc. 2015;81:1188–960. doi: 10.1016/j.gie.2014.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ignee A, Atkinson NS, Schuessler G, et al. Ultrasound contrast agents. Endosc Ultrasound. 2016;5:355–62. doi: 10.4103/2303-9027.193594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitano M, Kamata K. Contrast-enhanced harmonic endoscopic ultrasound: Future perspectives. Endosc Ultrasound. 2016;5:351–4. doi: 10.4103/2303-9027.195852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietrich CF, Dong Y, Froehlich E, et al. Dynamic contrast-enhanced endoscopic ultrasound: A quantification method. Endosc Ultrasound. 2017;6:12–20. doi: 10.4103/2303-9027.193595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitano M, Sakamoto H, Matsui U, et al. A novel perfusion imaging technique of the pancreas: Contrast-enhanced harmonic EUS (with video) Gastrointest Endosc. 2008;67:141–50. doi: 10.1016/j.gie.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 12.Iglesias-Garcia J, Lariño-Noia J, Domínguez-Muñoz JE. Contrast harmonic endoscopic ultrasound: Instrumentation, echoprocessors, and echoendoscopes. Endosc Ultrasound. 2017;6:37–42. doi: 10.4103/2303-9027.200214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seicean A, Mosteanu O, Seicean R. Maximizing the endosonography: The role of contrast harmonics, elastography and confocal endomicroscopy. World J Gastroenterol. 2017;23:25–41. doi: 10.3748/wjg.v23.i1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fusaroli P, Saftoiu A, Dietrich CF. Contrast-enhanced endoscopic ultrasound: Why do we need it?. A foreword. Endosc Ultrasound. 2016;5:349–50. doi: 10.4103/2303-9027.193596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietrich CF, Săftoiu A, Jenssen C. Real time elastography endoscopic ultrasound (RTE-EUS), a comprehensive review. Eur J Radiol. 2014;83:405–14. doi: 10.1016/j.ejrad.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 16.Seicean A, Jinga M. Harmonic contrast-enhanced endoscopic ultrasound fine-needle aspiration: Fact or fiction? Endosc Ultrasound. 2017;6:31–6. doi: 10.4103/2303-9027.196917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fusaroli P, Saftoiu A, Mancino MG, et al. Techniques of image enhancement in EUS (with videos) Gastrointest Endosc. 2011;74:645–55. doi: 10.1016/j.gie.2011.03.1246. [DOI] [PubMed] [Google Scholar]
- 18.Fusaroli P, Kypraios D, Mancino MG, et al. Interobserver agreement in contrast harmonic endoscopic ultrasound. J Gastroenterol Hepatol. 2012;27:1063–9. doi: 10.1111/j.1440-1746.2012.07115.x. [DOI] [PubMed] [Google Scholar]
- 19.Giovannini M, Hookey LC, Bories E, et al. Endoscopic ultrasound elastography: The first step towards virtual biopsy?. Preliminary results in 49 patients. Endoscopy. 2006;38:344–8. doi: 10.1055/s-2006-925158. [DOI] [PubMed] [Google Scholar]
- 20.Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–13. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Friedrich-Rust M, Poynard T, Castera L. Critical comparison of elastography methods to assess chronic liver disease. Nat Rev Gastroenterol Hepatol. 2016;13:402–11. doi: 10.1038/nrgastro.2016.86. [DOI] [PubMed] [Google Scholar]
- 22.Saftoiu A, Vilman P. Endoscopic ultrasound elastography– A new imaging technique for the visualization of tissue elasticity distribution. J Gastrointestin Liver Dis. 2006;15:161–5. [PubMed] [Google Scholar]
- 23.Iglesias García J, Lariño Noia J, Souto R, et al. Endoscopic ultrasound (EUS) elastography of the liver. Rev Esp Enferm Dig. 2009;101:717–9. doi: 10.4321/s1130-01082009001000007. [DOI] [PubMed] [Google Scholar]
- 24.Rimbaş M, Gheonea DI, Săndulescu L, et al. EUS elastography in evaluating chronic liver disease. Why not from inside? Curr Health Sci J. 2009;35:225–7. [PMC free article] [PubMed] [Google Scholar]
- 25.Nieto J, Khaleel H, Challita Y, et al. EUS-guided fine-needle core liver biopsy sampling using a novel 19-gauge needle with modified 1-pass, 1 actuation wet suction technique. Gastrointest Endosc. 2018;87:469–75. doi: 10.1016/j.gie.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Shahshahan M, Gertz H, Fakhreddine AY, et al. Mo1285 endoscopic ultrasound-guided liver biopsy versus percutaneous and trans-jugular liver biopsy for evaluation of liver parenchyma. Gastrointest Endosc. 2017;85:AB490. [Google Scholar]
- 27.Rustemovic N, Hrstic I, Opacic M, et al. EUS elastography in the diagnosis of focal liver lesions. Gastrointest Endosc. 2007;66:823–4. doi: 10.1016/j.gie.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 28.Sandulescu L, Padureanu V, Dumitrescu C, et al. A pilot study of real time elastography in the differentiation of focal liver lesions. Curr Health Sci J. 2012;38:32–5. [PMC free article] [PubMed] [Google Scholar]
- 29.Rustemovic N, Cukovic-Cavka S, Opacic M, et al. Endoscopic ultrasound elastography as a method for screening the patients with suspected primary sclerosing cholangitis. Eur J Gastroenterol Hepatol. 2010;22:748–53. doi: 10.1097/MEG.0b013e32832d489f. [DOI] [PubMed] [Google Scholar]
- 30.Fusaroli P, Napoleon B, Gincul R, et al. The clinical impact of ultrasound contrast agents in EUS: A systematic review according to the levels of evidence. Gastrointest Endosc. 2016;84:587–96. doi: 10.1016/j.gie.2016.06.006. e10. [DOI] [PubMed] [Google Scholar]
- 31.Nakaji S, Hirata N. Evaluation of the viability of hepatocellular carcinoma in the caudate lobe using contrast-enhanced endoscopic ultrasonography after transarterial chemoembolization. Endosc Ultrasound. 2016;5:390–92. doi: 10.4103/2303-9027.190924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minaga K, Takenaka M, Kitano M, et al. 115 improved diagnosis of liver metastases using Kupffer-phase image of contrast-enhanced harmonic endoscopic ultrasonography in patients with pancreatic cancer. Gastrointest Endosc. 2017;85(Suppl 5):AB53. doi: 10.1016/j.gie.2020.06.051. [DOI] [PubMed] [Google Scholar]
- 33.Choi JH, Seo DW. Applications of contrast-enhanced harmonic endoscopic ultrasound on biliary, focal liver lesions and vascular diseases. Endosc Ultrasound. 2017;6:21–4. doi: 10.4103/2303-9027.200211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamata K, Takenaka M, Kitano M, et al. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of localized gallbladder lesions. Dig Endosc. 2018;30:98–106. doi: 10.1111/den.12900. [DOI] [PubMed] [Google Scholar]
- 35.Sato T, Yamazaki K, Toyota J, et al. Evaluation of hemodynamics in esophageal varices. Value of endoscopic color Doppler ultrasonography with a galactose-based contrast agent. Hepatol Res. 2003;25:55–61. doi: 10.1016/s1386-6346(02)00168-7. [DOI] [PubMed] [Google Scholar]
- 36.Sato T, Yamazaki K, Toyota J, et al. Perforating veins in recurrent esophageal varices evaluated by endoscopic color Doppler ultrasonography with a galactose-based contrast agent. J Gastroenterol. 2004;39:422–8. doi: 10.1007/s00535-003-1314-5. [DOI] [PubMed] [Google Scholar]
- 37.Sato T, Yamazaki K, Toyota J, et al. Evaluation of arterial blood flow in esophageal varices via endoscopic color Doppler ultrasonography with a galactose-based contrast agent. J Gastroenterol. 2005;40:64–9. doi: 10.1007/s00535-004-1496-5. [DOI] [PubMed] [Google Scholar]