Abstract
Background and Objectives:
Acquisition of core tissue on endoscopic ultrasound (EUS)-guided fine-needle aspiration has been regarded as important for establishing an accurate histological diagnosis. Recently, a new 20-gauge histology needle with reverse bevel (ProCore needle) and a 22-gauge needle with 3 novel symmetric heels (Acquire needle) have been developed. The aims of this animal experimental study were to assess the core tissue acquisition (TA) abilities of these new histology needles by comparing them with those of conventional 22-gauge needles and to evaluate the efficacy of suction for these needles.
Materials and Methods:
Three experienced echoendoscopists performed EUS-guided TA with and without suction using 43 types of needles. The amount of obtained tissue specimens and blood contamination was assessed using a scoring system, and the weight of the obtained tissue specimens was measured using an electronic balance.
Results:
The mean amount of core tissue score of the Acquire 22-gauge needle or ProCore 20-gauge needle was significantly higher than that of the conventional 22-gauge needles (Acquire 22-gauge needle vs. conventional 22-gauge needles: P = 0.024; ProCore 20-gauge needle vs. conventional 22-gauge needles: P = 0.001). There was no significant difference in the mean amount of core tissue score between the Acquire 22-gauge needle and the ProCore 20-gauge needle (P = 0.296). In the Acquire 22-gauge needle and ProCore 20-gauge needle, there was no significant difference between the mean amount of core tissue score with suction and that without suction (3.7 ± 0.4 vs. 3.5 ± 0.4, P = 0.734) although blood contamination increased (2.3 ± 0.7 vs. 1.6 ± 0.3, P = 0.061).
Conclusion:
The TA abilities of the ProCore 20-gauge needle and Acquire 22-gauge needle were better than those of the conventional 22-gauge needles. The efficacy of suction for the ProCore 20-gauge needle and Acquire 22-gauge needle was limited.
Keywords: Acquire needle, animal experimental study, endoscopic ultrasound-guided fine-needle aspiration, ProCore needle
INTRODUCTION
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) is a safe and accurate procedure for establishing pathological diagnosis of various intraintestinal and extraintestinal target lesions.[1,2,3,4,5] To distinguish between malignant lesions and benign lesions, cytological or histological assessment with a small fragmented tissue is sufficient in most cases. However, the accurate histological diagnosis of a particular tumor or inflammatory diseases such as malignant lymphoma or autoimmune pancreatitis requires histological assessment with a large amount of core tissue and immunohistochemical examinations.[6,7,8] Therefore, acquisition of a large amount of core tissue, so-called EUS-guided fine-needle biopsy (EUS-FNB), has been regarded as important for establishing an accurate histological diagnosis. EUS-FNA with a large-caliber 19-gauge needle is recommended for obtaining sufficient core tissue for histological analysis.[9] However, there are some technical issues regarding the use of a 19-gauge needle because its stiffness makes adequate positioning of the scope and manipulation of the needle difficult. Thus, a needle thinner than a 19-gauge needle with good maneuverability, which can obtain a large amount of core tissue with fewer needle passes, is required.
Recently, various kinds of needle for EUS-FNB such as a new 20-gauge histology needle with reverse bevel and a 22-gauge needle with novel three symmetric heels have been developed. Herein, we conducted an experiment using animal organs to assess the core tissue acquisition (TA) abilities of these new histology needles by comparing with conventional 22-gauge needles, as well as to evaluate the efficacy of additional application of negative pressure using syringe suction for these needles.
MATERIALS AND METHODS
Animal experiment of endoscopic ultrasound-guided tissue acquisition
Three experienced echoendoscopists (T.I., A.I., and A.K.) performed EUS-guided TA on a porcine liver using a curved linear array echoendoscope (GF-UCT260; Olympus Medical Systems, Tokyo, Japan), which was connected to a processor (EU-ME1; Olympus Medical Systems). EUS-TA was performed under general anesthesia with intubation and ventilation. The following three types of needles (total 4 needles) were evaluated: two conventional 22-gauge needles (Expect SL, Boston Scientific, Natick, MA, USA; EZshot3, Olympus Medical Systems), a new 20-gauge histology needle with reverse bevel (ProCore, Wilson Cook Medical Inc., Winston-Salem, NC) [Figure 1a], and a 22-gauge histology needle with a novel Franseen tip design (Acquire, Boston Scientific) [Figure 1b]. Each echoendoscopist performed two passes of EUS-TA using each needle. One pass was performed with no suction, and the other pass was performed with 20-mL syringe suction force. A total of six tissue specimens obtained by each needle were evaluated. The number of stroke (five times) and the length of stroke in the porcine liver (20 mm) were standardized. The standard stroke method without the door knocking method and fanning technique were used. The obtained tissue specimens were immediately fixed in 10% neutral-buffered formalin solution for histological examination by releasing the syringe and reinserting the stylet. On the following puncture, the puncture point was selected, avoiding the previous puncture site.
Figure 1.
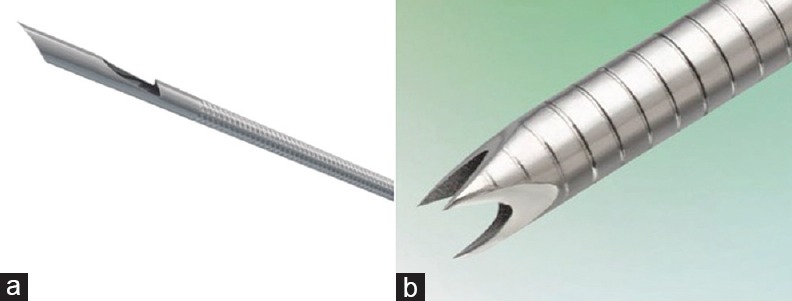
(a) The new ProCore 20-gauge histology needle with reverse bevel (Wilson Cook Medical Inc., Winston-Salem, NC). (b) The Acquire 22-gauge histology needle with three novel symmetric heels (Boston Scientific, Natick, MA, USA)
Specimen handling and histological assessment
The obtained tissue specimens fixed in formalin were brought to the Medical Research Center at Tokyo Medical University. The fixed tissue specimens were processed and embedded in paraffin. Then, the paraffin-embedded tissue specimens were cut into 3 μm slices. Only sections that contained mostly tissue specimen were processed into slides, namely one slide was made for one pass. Thereafter, the tissue specimens in the slides were stained with hematoxylin and eosin for histological examination.
In this study, only histological analyses were performed without cytological analyses by a single experienced pathologist blinded to information of used needles. The amount of obtained tissue specimens based on the amount of liver parenchyma was assessed using a scoring system classified into four phases according to the amount of obtained core tissue [Table 1 and Figure 2]. Histologic core tissue was defined as an architecturally intact piece of tissue sufficient for histologic evaluation of the targeted lesion. The core TA ability of each needle was compared in terms of the mean amount of core tissue score. The amount of blood contamination was also assessed using a scoring system classified into three phases.
Table 1.
Scoring systems for the amounts of core tissue and blood contamination
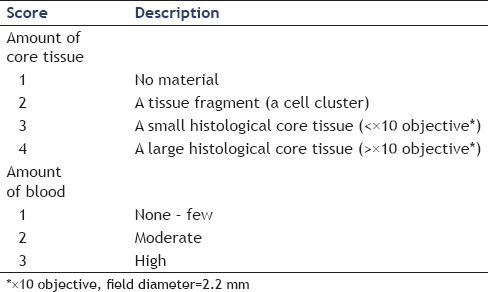
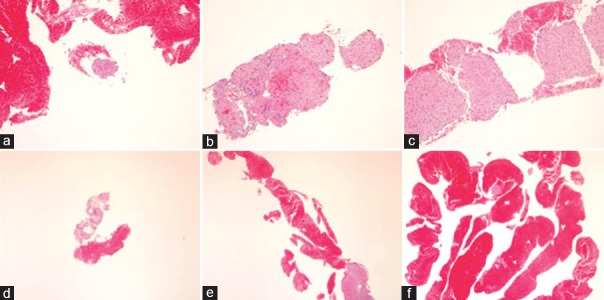
Figure 2.
(a) Amount of core tissue score 2 (a tissue fragment; a cell cluster). (b) Amount of core tissue score 3 (a small histological core tissue; <×10 objective, field diameter = 2.2 mm). (c) Amount of core tissue score 4 (a large histological core tissue; >×10 objective, filed diameter = 2.2 mm). (d) Amount of blood score 1 (none– few). (e) Amount of blood score 2 (moderate). (f) Amount of blood score 3 (high)
Laboratory test of endoscopic ultrasound-guided tissue acquisition
Three experienced echoendoscopists (T.I., A.I., and A.K.) performed EUS-TA on a chicken gizzard placed on a stainless steel tray using the four needles. One pass was performed using each needle following the same method described earlier in the animal experiment of EUS-TA section. The obtained tissue specimens were mounted entirely onto a laboratory dish by reinsertion of the stylet and then weighed using an electronic balance to assess the difference in the weight of the obtained tissue specimens for each needle.
Statistical analysis
The amounts of obtained tissue specimens and blood contamination in the animal experiments were presented as a mean ± standard deviation, and data were compared using the Student's t-test. The differences in the weights of the obtained tissue specimens for the laboratory test were presented as medians and ranges, and these data were analyzed using the Mann–Whitney U-test. Statistical analyses were performed using StatMate III (ATMS, Tokyo, Japan). A P < 0.05 was considered to indicate a statistically significant difference.
RESULTS
The results of the histological assessment for EUS-TA using each needle were as follows. The mean amount of core tissue scores was 2.2 ± 0.5 in the conventional 22-gauge needles (Expect and EZshot3), 3.3 ± 0.5 in the 22-gauge histology needle with a novel Franseen tip design (Acquire 22-gauge needle), and 3.8 ± 0.2 in the new 20-gauge histology needle with reverse bevel (ProCore 20-gauge needle) [Figure 3]. The mean amount of core tissue score of the Acquire 22-gauge needle or ProCore 20-gauge needle was significantly higher than that of the conventional 22-gauge needles (Acquire 22-gauge needle vs. conventional 22-gauge needles: P = 0.024; ProCore 20-gauge needle vs. conventional 22-gauge needles: P = 0.001). On the other hand, there was no significant difference in the mean amount of core tissue score between the Acquire 22-gauge needle and the ProCore 20-gauge needle (P = 0.296).
Figure 3.
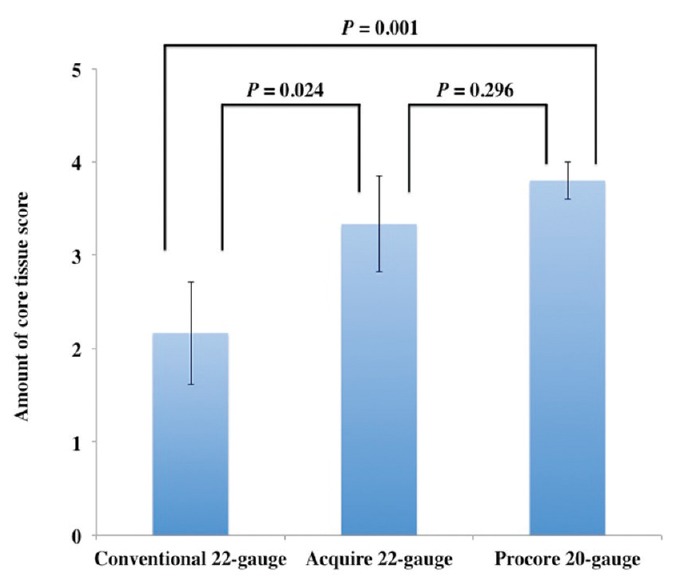
Comparison of the amount of core tissue scores and standard error among three types of needles for endoscopic ultrasound-guided tissue acquisition
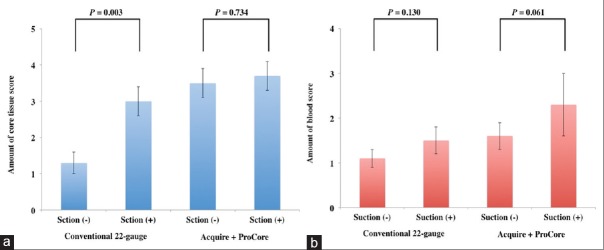
Regarding the efficacy of additional application of negative pressure using syringe suction, in the conventional 22-gauge needles, the mean amount of core tissue score of EUS-TA with suction was significantly higher than that of EUS-TA without suction (3.0 ± 0.5 vs. 1.3 ± 0.4, P = 0.03) [Figure 4a], although the mean blood score was comparable (1.5 ± 0.3 vs. 1.1 ± 0.2, P = 0.130) [Figure 4b]. On the other hand, in the Acquire 22-gauge needle and ProCore 20-gauge needle, there was no significant difference in the mean amount of core tissue score between EUS-TA with suction and EUS-TA without suction (3.7 ± 0.4 vs. 3.5 ± 0.4, P = 0.734) [Figure 4a], although the blood contamination increased (2.3 ± 0.7 vs. 1.6 ± 0.3, P = 0.061) [Figure 4b].
Figure 4.
(a) Comparison of the amount of core tissue scores and standard error between endoscopic ultrasound-guided tissue acquisition with suction and without suction. (b) Comparison of the amount of blood scores and standard error between endoscopic ultrasound-guided tissue acquisition with suction and without suction
Regarding the laboratory test of EUS-TA, the average median weights of the obtained tissue were 2.3 mg (range: 0.7–8.5 mg) in the conventional 22-gauge needles, 4.2 mg (range: 4.0–20.9 mg) in the Acquire 22-gauge needle, and 2.8 mg (range: 2.4–6.8 mg) in the ProCore 20-gauge needle [Figure 5]. There was no significant difference between the weights of tissue obtained using each needle, although those obtained using the Acquire 22-gauge needle and ProCore 20-gauge needle tended to be higher than those obtained using the conventional 22-gauge needles.
Figure 5.
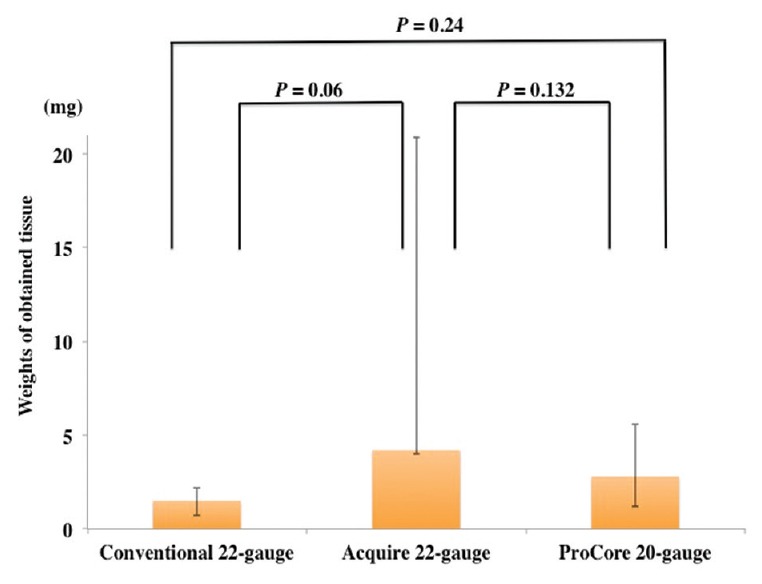
Comparison of the weights of the obtained tissue and range among three types of needles for endoscopic ultrasound-guided tissue acquisition
DISCUSSION
EUS-FNA with cytology has been used as a safe and accurate procedure for establishing a pathological diagnosis of intraluminal or extraluminal lesions since the first report by Vilmann et al. in 1992.[10] Although the high accuracy of EUS-FNA with cytology has been reported, it remains imperfect because of the limited pathological evaluation. Recently, the acquisition of a large amount of core tissue and its histological assessment has become increasingly important to further improve the diagnostic yield of EUS-FNA and to establish an accurate diagnosis with fewer FNA passes.
The acquisition of a large amount of histological core tissue has some advantages. First, it enables macroscopic on-site evaluation (MOSE). Although many reports have suggested that rapid on-site evaluation (ROSE) improved the diagnostic accuracy and limited the number of FNA passes required to establish a diagnosis,[11,12,13] the presence of a cytopathologist is not routinely guaranteed because of labor shortages in many centers and even at high-volume centers. Iwashita et al. reported the efficacy of MOSE as an alternative to ROSE.[14] In MOSE, the number of FNA passes is decided on the basis of the macroscopically visible core, which is defined as whitish or yellow pieces of obtained tissue with an apparent bulk. MOSE is useful for estimating specimen adequacy and for reducing the number of FNA passes without the need for ROSE if a visible amount of histological core tissue is obtained.
Second, regarding EUS-FNA for solid pancreatic masses, the cytological or histological assessment of a small tissue specimen is generally adequate for the diagnosis of benign or malignant tumors. However, it is occasionally difficult to distinguish between malignancy and chronic pancreatitis or autoimmune pancreatitis, including atypical cells with stromal fibrosis, by cytology or histology of a cell cluster, especially for pathologists who do not specialize in biliopancreatic diseases. A large amount of core tissue including an increase in invasive atypical cells with desmoplastic fibrosis readily enables a pathologist to make a pathological diagnosis of neoplasm.
Third, to make an accurate pathological diagnosis of nonepithelial tumors or inflammatory benign diseases such as gastrointestinal stromal tumor, malignant lymphoma, or autoimmune pancreatitis, tissue architecture evaluation and immunohistochemical staining assessment are required.[15,16] A large amount of core tissue enables not only hematoxylin and eosin staining but also additional immunohistochemical examinations or flow cytometric and cytogenetic assessments.
Fourth, current advances in basic medical science have enabled the development and clinical application of molecular targeting and the assessment of molecular markers or DNA profiling, indicating resistance and the effects of anticancer chemotherapy or patient prognosis.[17] A small amount of obtained tissue is not suitable for the assessment of molecular expression by immunohistochemical staining or DNA sequencing. If personalized medical treatment according to individualized molecular profiling will be developed in the future, the importance of accurately obtaining a sufficiently large amount of core tissue by EUS-FNA in 1 session would increase.
Recent developments of biopsy needles or the FNA technique have been initiated to obtain a large amount of core tissue.[18,19] EUS-FNA with a 19-gauge needle has been reported to obtain a histological core tissue and yield a significantly higher diagnostic accuracy. However, a technical limitation of a 19-gauge needle is that it is stiffer than a 22-gauge needle or a 25-gauge needle which can restrict the positioning and angulation of the scope and elevator function.[20] To adequately obtain a core tissue, the ProCore needle has been developed. This is a uniquely designed needle with reverse bevel that hooks and cuts the tissue and traps it in the needle during the FNA motion. However, in their systematic review and meta-analysis comparing the performance of the ProCore needle with that of conventional FNA needles, Bang et al. found no difference in sample adequacy, diagnostic accuracy, or core TA ability.[21] Thus, a new 20-gauge ProCore needle has been designed, which may be a good compromise between the ease of use of a 22-gauge needle and the high TA ability of a 19-gauge needle.
In the present animal experiments, the amount of core tissue obtained by the ProCore 20-guage needle was larger than that by the other 22-gauge needles. However, the flexibility of the ProCore 20-guage needle was not evaluated because only transgastric puncture was performed. In our subsequent clinical trial, the technical acceptability of transduodenal puncture would be evaluated.
The recently developed Acquire 22-gauge needle, which is called a Franseen needle, has three symmetric heels designed to maximize tissue capture and minimize fragmentation. This needle was developed to adequately obtain a core tissue and improve the diagnostic yield. Although many different types of needles are currently available, the most widely used needle type is the end-cut type needle with beveled tips. To appropriately acquire core tissue, it is important to not only cut the tissue but also collect the tissue in the needle tract. Therefore, in core TA using an end-cut type needle, the tissue should be cut with not only the beveled side but also the buffed heel. As it may be more difficult to cut the tissue with the buffed heel, higher puncture speed and axial force are required.[18] In this respect, the three symmetric heels of the Acquire 22-gauge needle are very well designed enabling easy tissue collection in the needle tract even if only the needle penetrates the target lesion. In the present animal experiments, the mean amount of core tissue score of the Acquire 22-gauge needle was comparable to that of the ProCore 20-gauge needle and significantly higher than that of the conventional 22-gauge needles. Although an increase in adverse events such as bleeding may be a concern, the Acquire 22-gauge needle is promising as a first choice needle for EUS-FNA.
What remains controversial is the efficacy of negative pressure by suction during EUS-FNA. It has been reported that negative pressure by suction can increase the amount of obtained specimen.[22] However, it has potential to increase blood contamination, which may hinder pathological interpretation.[23] In a previous bench-top experiment of suction forces generated through FNA needles, Katanuma et al. demonstrated that suction force was applied to a needle according to the needle diameter or syringe size.[24] The clinical efficacy of suction might differ depending on the needle type or the target lesions. In the present animal experiments, the mean amount of core tissue score of the conventional 22-gauge needles with suction was significantly higher than that of the conventional 22-gauge needles without suction. This indicates that in the conventional 22-gauge needles, suction is useful for increasing the amount of tissue obtained from the target lesion (i.e., porcine liver), although blood contamination may increase to some extent. On the other hand, the mean amount of core tissue score of the ProCore 20-gauge needle or Acquire 22-gauge needle was the same between EUS-FNA with suction and EUS-FNA without suction. From these results, it is considered that the efficacy of suction may be limited to needles with a high TA ability. Suction may also have the potential to produce an opposite effect because of an increase in blood contamination.
CONCLUSION
The present animal experiments showed that the TA abilities of the ProCore 20-gauge needle and Acquire 22-gauge needle were better than those of the conventional 22-gauge needles. However, the efficacy of additional application of negative pressure using syringe suction of the ProCore 20-gauge needle and Acquire 22-gauge needle was limited. As a limitation, this study involved animal experiments in which the conditions might differ from those in clinical practice. Subsequent studies should investigate the efficacy and safety of the ProCore 20-gauge needle and Acquire 22-gauge needle in the clinical setting.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thank Dr. Edward Barroga, Associate Professor and Senior Medical Editor from the Department of International Medical Communications of Tokyo Medical University for editing the manuscript.
REFERENCES
- 1.Chang KJ, Katz KD, Durbin TE, et al. Endoscopic ultrasound-guided fine-needle aspiration. Gastrointest Endosc. 1994;40:694–9. [PubMed] [Google Scholar]
- 2.Yamao K, Ohashi K, Mizutani S, et al. Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) for the diagnosis of digestive diseases. Endoscopy. 1998;30(Suppl 1):A176–8. doi: 10.1055/s-2007-1001513. [DOI] [PubMed] [Google Scholar]
- 3.Binmoeller KF, Thul R, Rathod V, et al. Endoscopic ultrasound-guided, 18-gauge, fine needle aspiration biopsy of the pancreas using a 2.8 mm channel convex array echoendoscope. Gastrointest Endosc. 1998;47:121–7. doi: 10.1016/s0016-5107(98)70343-8. [DOI] [PubMed] [Google Scholar]
- 4.Itoi T, Tsuchiya T, Itokawa F, et al. Histological diagnosis by EUS-guided fine-needle aspiration biopsy in pancreatic solid masses without onsite cytopathologist: A single-center experience. Dig Endosc. 2011;23(Suppl 1):34–8. doi: 10.1111/j.1443-1661.2011.01142.x. [DOI] [PubMed] [Google Scholar]
- 5.Dumonceau JM, Polkowski M, Larghi A, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2011;43:897–912. doi: 10.1055/s-0030-1256754. [DOI] [PubMed] [Google Scholar]
- 6.Levy MJ, Wiersema MJ. EUS-guided trucut biopsy. Gastrointest Endosc. 2005;62:417–26. doi: 10.1016/j.gie.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 7.Varadarajulu S, Fraig M, Schmulewitz N, et al. Comparison of EUS-guided 19-gauge trucut needle biopsy with EUS-guided fine-needle aspiration. Endoscopy. 2004;36:397–401. doi: 10.1055/s-2004-814316. [DOI] [PubMed] [Google Scholar]
- 8.Wittmann J, Kocjan G, Sgouros SN, et al. Endoscopic ultrasound-guided tissue sampling by combined fine needle aspiration and trucut needle biopsy: A prospective study. Cytopathology. 2006;17:27–33. doi: 10.1111/j.1365-2303.2006.00313.x. [DOI] [PubMed] [Google Scholar]
- 9.Larghi A, Verna EC, Ricci R, et al. EUS-guided fine-needle tissue acquisition by using a 19-gauge needle in a selected patient population: A prospective study. Gastrointest Endosc. 2011;74:504–10. doi: 10.1016/j.gie.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Vilmann P, Jacobsen GK, Henriksen FW, et al. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172–3. doi: 10.1016/s0016-5107(92)70385-x. [DOI] [PubMed] [Google Scholar]
- 11.Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705–10. doi: 10.1038/ajg.2011.119. [DOI] [PubMed] [Google Scholar]
- 12.Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest Endosc. 2012;75:319–31. doi: 10.1016/j.gie.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 13.Hébert-Magee S, Bae S, Varadarajulu S, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: A meta-analysis. Cytopathology. 2013;24:159–71. doi: 10.1111/cyt.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwashita T, Yasuda I, Mukai T, et al. Macroscopic on-site quality evaluation of biopsy specimens to improve the diagnostic accuracy during EUS-guided FNA using a 19-gauge needle for solid lesions: A single-center prospective pilot study (MOSE study) Gastrointest Endosc. 2015;81:177–85. doi: 10.1016/j.gie.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 15.Iwashita T, Yasuda I, Doi S, et al. Use of samples from endoscopic ultrasound-guided 19-gauge fine-needle aspiration in diagnosis of autoimmune pancreatitis. Clin Gastroenterol Hepatol. 2012;10:316–22. doi: 10.1016/j.cgh.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 16.Yasuda I, Goto N, Tsurumi H, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy for diagnosis of lymphoproliferative disorders: Feasibility of immunohistological, flow cytometric, and cytogenetic assessments. Am J Gastroenterol. 2012;107:397–404. doi: 10.1038/ajg.2011.350. [DOI] [PubMed] [Google Scholar]
- 17.Chantrill LA, Nagrial AM, Watson C, et al. Precision medicine for advanced pancreas cancer: The individualized molecular pancreatic cancer therapy (IMPaCT) trial. Clin Cancer Res. 2015;21:2029–37. doi: 10.1158/1078-0432.CCR-15-0426. [DOI] [PubMed] [Google Scholar]
- 18.Mukai S, Itoi T, Ashida R, et al. Multicenter, prospective, crossover trial comparing the door-knocking method with the conventional method for EUS-FNA of solid pancreatic masses (with videos) Gastrointest Endosc. 2016;83:1210–7. doi: 10.1016/j.gie.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 19.Kandel P, Tranesh G, Nassar A, et al. EUS-guided fine needle biopsy sampling using a novel fork-tip needle: A case-control study. Gastrointest Endosc. 2016;84:1034–9. doi: 10.1016/j.gie.2016.03.1405. [DOI] [PubMed] [Google Scholar]
- 20.Itoi T, Itokawa F, Sofuni A, et al. Puncture of solid pancreatic tumors guided by endoscopic ultrasonography: A pilot study series comparing trucut and 19-gauge and 22-gauge aspiration needles. Endoscopy. 2005;37:362–6. doi: 10.1055/s-2004-826156. [DOI] [PubMed] [Google Scholar]
- 21.Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339–49. doi: 10.1055/s-0034-1393354. [DOI] [PubMed] [Google Scholar]
- 22.Kudo T, Kawakami H, Hayashi T, et al. High and low negative pressure suction techniques in EUS-guided fine-needle tissue acquisition by using 25-gauge needles: A multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2014;80:1030–7.e1. doi: 10.1016/j.gie.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Nakai Y, Isayama H, Chang KJ, et al. Slow pull versus suction in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid masses. Dig Dis Sci. 2014;59:1578–85. doi: 10.1007/s10620-013-3019-9. [DOI] [PubMed] [Google Scholar]
- 24.Katanuma A, Itoi T, Baron TH, et al. Bench-top testing of suction forces generated through endoscopic ultrasound-guided aspiration needles. J Hepatobiliary Pancreat Sci. 2015;22:379–85. doi: 10.1002/jhbp.201. [DOI] [PubMed] [Google Scholar]