Abstract
EUS provides real-time images of the intramural gastrointestinal tract and adjacent structures that otherwise would not be visible. Given the anatomic proximity to the bowel, accurate identification of the major abdominal vasculature, such as the portal vein, serves as a useful landmark tool for identifying anatomy and staging of malignancies. Recently, increased reports have centered on the utility of EUS-guided vascular access of the portal vein for diagnostic and therapeutic interventions. Pilot and feasibility studies in human patients utilizing EUS-guided portal vein access for circulating tumor cell enumeration and portal pressure gradient monitoring suggest that sampling the portal vein under EUS guidance is safe and effective. This review discusses the rationale and technical aspects of EUS-guided portal vein sampling for diagnostic purposes in gastrointestinal cancer. Understanding the technical aspects of EUS-guided portal vein sampling will be critical to standardizing the procedure, developing new vascular access technologies, and increasing the safety profile.
Keywords: EUS, FNA, portal vein
INTRODUCTION
Although EUS was developed initially for diagnostic purposes in the 1980s, curvilinear array echoendoscopes containing an elevator and therapeutic channel have subsequently transformed the field. EUS now serves as a platform for minimally invasive diagnostic and therapeutic maneuvers. In particular, the advent of EUS-FNA has provided a real-time, directly visualized port of access to structures within and outside the gastrointestinal lumen and made it standard of care for diagnosing pancreatic malignancy.
Based on the safety profiles of interventional radiology and cardiology percutaneous vascular maneuvers, emerging reports have centered on the utility of EUS-guided vascular access of the portal vein for diagnostic and therapeutic interventions. We reported the first use of EUS-guided sampling of the portal vein in humans in 2015. This study demonstrated that in patients with pancreaticobiliary cancer, EUS-guided aspiration of blood from the portal vein could be done safely and yield a higher number of circulating tumor cells (CTCs) when compared to peripheral blood. In 2017, a similar safety profile and technical feasibility was achieved with EUS-guided FNA of malignant vascular thrombosis and portal pressure gradient measurement. The cumulative findings indicate that the portal vein can be safely accessed through EUS for diagnostic purposes.
EUS-guided portal vein access is an exciting new field and natural advancement for the procedure. This review will discuss the rationale and technical aspects of EUS-guided portal vein sampling for diagnostic purposes in gastrointestinal cancer.
LIQUID BIOPSIES OF THE PORTAL VEIN FOR TUMOR ANALYSIS
Distant metastases are responsible for ~90% of cancer-related mortalities.[1] Based on multiple animal models, our current understanding of metastatic colonization is that the risk of developing metastasis is proportional to the amount of injected tumor cell material[2] and that even small tumors can shed millions of cancer cells by the time a tumor is diagnosed.[3] Only subsets of these cancer cells can survive an anchorage-independent circulation, invade into foreign tissue, avoid postinvasion immune response, and subsequently proliferate.[4] From a clinical standpoint, the current standards of radiologic imaging used to determine curative surgery eligibility are limited in its ability to identify micrometastatic (<2 mm) disease.[5] Thus, there remains a clinical need not only for the early diagnosis but also for the adequate staging of cancers to determine the postoperative risk of recurrence and determine who would benefit/not benefit from aggressive neoadjuvant chemotherapy or invasive surgery.
Cancer-derived products shed into the bloodstream currently being explored as minimally invasive tools for diagnosing, staging, and assessing solid tumor characteristics and heterogeneity include circulating tumor DNA (ctDNA), CTCs, and exosomes.[6] Despite the potential of liquid biopsies, these tumor products make up a small fraction of circulating nucleic acids and cells with an estimate that a single CTC is 1 in a 100 million or 1 billion circulating cells.[7] Technologic advances in enrichment techniques and analysis tools for molecular characterization continue to increase the sensitivity of peripheral blood testing; however, the utility of these markers still remains dependent on the quantity of tumor material and depending on the malignancy, the systemic circulation may have less tumor signature material until the tumor burden is widely metastatic.
The inability to routinely use peripheral blood is partially explained by the fact that intact CTCs are frequently shed into the bloodstream as 20–30 um single cells or as larger clusters/microemboli. When shedding as a single cell, the larger diameter of the CTC is nearly three times the diameter of the capillaries (~8 um), suggesting possible sequestration in first-pass organs such as the liver or lungs.[4] This theory is supported by the following: (i) animal models demonstrating that radio-labeled cancer cells injected into the portal vein and tail vein are frequently trapped in the first-pass organ and result in massive cancer cell death[8] and (ii) in the case of pancreatic and colorectal cancer, the clinical observation that the most common site of distant metastatic spread is to the liver, the first-pass organ for gastrointestinal venous blood drainage through the portal system. Thus, it is very likely that gastrointestinal ctDNA and CTCs are likely to be more abundant in the portal venous circulation before being sequestered in the liver.
In 2012, CTC enumeration was performed in the systemic circulation and mesenteric circulation of 200 and 80 patients who underwent surgical resection for colorectal cancer, respectively.[9] CTCs were found at a significantly higher rate (P = 0.01) and higher count (P = 0.006) in the mesenteric circulation. In 2015, Bissolati et al. enumerated CTCs in the systemic circulation and portal venous blood in 20 patients undergoing surgery for resectable pancreatic cancer.[10] They found CTCs in nine (45%) patients, including five patients with CTCs only in the portal vein, three patients in both systemic circulation and portal vein, and one patient in systemic circulation only. After 3 years of follow-up, portal vein CTC-positive patients presented with a higher rate of liver metastases than CTC-negative patients (53 vs. 8%, P = 0.038). Using an alternative epithelial cell adhesion molecule (EpCAM)-based enumeration method, Tien et al. evaluated paired peripheral and intraoperative portal venous blood for CTC enumeration in 60 patients with periampullary or pancreatic adenocarcinoma.[11] Similar to the colorectal cancer study, CTCs were detected at a significantly higher rate (58.3% vs. 40.0% of patients, P < 0.01) and a higher count (mean, 230.1 vs. 71.7, P < 0.001) in portal than in peripheral venous blood. Multivariate analysis confirmed a prognostic significance to portal vein CTC count with high portal CTCs predictor for liver metastases (64.7% sensitivity and 95.4% specificity) within 6 months after surgery. Out of 13, 11 patients with a high-portal CTC count developed liver metastases within 6 months after surgery, as opposed to only 6 of 47 patients with a low-portal CTC count. Interestingly, there was no statistical difference in CTC number based on the stage of cancer.
EUS-GUIDED LIQUID BIOPSY FOR PORTAL VEIN BLOOD ACQUISITION
All the aforementioned studies acquired portal venous blood in the intraoperative setting after surgical mobilization of the anatomy and accessing a directly visualized portal vein. While the prognostic significance of identifying a patient to be high risk for liver metastases may determine who should receive aggressive adjuvant chemotherapy, it would be preferable to risk stratify patients before surgery. Derived from the same concept and safety profile as islet cell transplantation by interventional radiology in which the portal vein is accessed percutaneously,[12] EUS can similarly provide minimally invasive access to the portal vein.
In 2015, our group demonstrated that in pancreatic cancer patients, blood could safely be obtained by EUS from the portal vein and that portal venous blood yields a higher number of CTCs when compared to peripheral blood.[13] Using an EpCAM-based CTC enrichment method (CellSearch System), CTCs were detected in the portal vein samples from 100% of the patients with pancreaticobiliary cancer, but < 25% of matched peripheral blood samples. In the patients with cancer, there were significantly more CTCs in the portal venous blood compared to peripheral blood (118 CTCs per 7.5 mL vs. <1 CTC per 7.5 mL). Further, in patients with nonmetastatic, borderline resectable cancer, there was a high number of portal vein CTCs (83.2 per 7.5 mL) but less CTCs than in patients with unresectable cancer (157.9 per 7.5 mL, P = 0.23). These findings are consistent with the following hypotheses: (1) tumor cells are shed into the vasculature before the clinical/radiologic detection of metastases and (2) CTCs from the primary tumor are being filtered during their transit through the hepatic portal circulation, resulting in fewer cells entering the systemic circulation. The collective data on intraoperative and EUS-guided portal vein blood CTC analysis emphasizes the importance of the blood collection site for tumor biomarker detection in gastrointestinal malignancies that drain primarily through the portal venous system.
EUS-GUIDED PORTAL VEIN ACCESS FOR BLOOD ACQUISITION: METHODS
Preprocedure considerations
Given the limited data on the safety and technical methods, we adopted the following practices based on our personal experience[13] and available human investigational data.[14,15] EUS-guided portal vein sampling remains a novel and potentially risky procedure; therefore, patients should be consented under an IRB approved protocol with adequate explanation of risk and benefits. We suggest performing the procedure under monitored anesthesia care or general anesthesia, utilizing only CO2 insufflation, and only after any bleeding risks have been addressed (i.e., coagulopathy, use of anticoagulants). Some endoscopists have advocated administering a dose of intraprocedural prophylactic IV antibiotics; however, currently, there is no data to support this practice.
Confirming cancer diagnosis pre-versus post-accessing of the portal vein
One of the primary indications for EUS is to diagnose pancreaticobiliary malignancy using FNA. Patients frequently present with suggestive symptoms or cross sectional imaging suggestive of a mass, however rarely have a tissue diagnosis before their EUS. In our protocol, routine peripheral blood samples were obtained before EUS in parallel and processed identically. During the EUS, we first completed staging and/or diagnosis confirmation with onsite cytopathologic analysis of FNA samples in standard fashion. There have been concerns regarding tumor cell dissemination artificially increasing the yield of portal venous tumor material. While no published studies have directly assessed this in portal vein sampling, EUS-FNA has been demonstrated to be safe in the workup of suspicious pancreatic lesions and does not impair survival of patients with resected pancreatic cancer.[16] Over a period of 11 years, Ngamruengphong et al. examined 498 patients with pancreatic cancer who had EUS-FNA compared with 1,536 patients without FNA. The median overall survival in the EUS-FNA group was 22 months compared to 15 months in the non-EUS-FNA group (hazard ratio 0.84, 95% confidence interval 0.72–0.99). Further research is necessary to confirm whether EUS-FNA of solid malignant lesions subsequently alters the yield of tumor material from the portal vein, however, given the wide, and predominantly, lower range of CTC yield in nonmetastatic patients; in our study (median: 62.0 per 7.5 mL; range: 1–265),[13] timing of portal vein access is may not be a significant confounding variable.
EUS-FNA needle selection
The development of EUS-guided tissue acquisition techniques including FNA, and more recently, fine needle biopsy (FNB) has enabled cytologic and histologic sampling of intramural and extramural gastrointestinal lesions. The goal of EUS-FNA and EUS-FNB is to provide the largest sample size while minimizing adverse events. EUS-FNA needles are currently available in 19G, 22G, and 25G sizes with adjustable sheaths that can be advanced between 1 and 8 cm. Needle selection is often dependent on the target lesion, for example, in pancreatic cysts, a larger bore 19G needle may be required to aspirate thick, mucinous cystic fluid, while a smaller, 25G needle is frequently used to limit blood contamination when performing a cytologic examination of lymph nodes.
In EUS-guided portal vein sampling, our practice is to use a 19G EUS-FNA needle with a transgastric or transduodenal, transhepatic approach. Although there is no data on the topic, FNB needles with proprietary bevels to obtain core tissues specimens do not appear to be warranted in this situation and theoretically may be more prone to bleeding from vessel shearing. In our experience, the 19G EUS-FNA needle allows adequate blood flow to minimize time within the vessel and appears to reduce clotting compared to smaller gauge needle sizes. In 2017, Huang et al. utilized a 25G needle to access the portal vein and hepatic vein to obtain the portal pressure gradient portal vein.[14] Although successful in obtaining pressure measurements, obtaining viable blood specimens was not assessed, but would likely be challenging using the smaller gauge needle. There currently is a lack of data assessing the needle gauge size for portal venous blood acquisition; however, given the safety profile and adequate biospecimen acquisition in our feasibility trial, we encourage the use of a larger bore, 19G FNA needle.
Portal vein access point: Transhepatic versus extrahepatic portal vein
Two methods of EUS-guided accessing of the portal vein have been published. These include the most frequently utilized transgastric or transduodenal, transhepatic routes to access the intrahepatic portal vein or subsidiary branch and the transduodenal extrahepatic route for portal vein access. In humans, except for one report that performed extrahepatic EUS-guided FNA of malignant intravascular thrombi,[15] transhepatic routes have been used.
Regardless of method, before advancing the EUS-FNA needle into the portal vein or subsidiary branch, color Doppler evaluation of the liver should be performed to confirm patency of the hepatic artery, portal vein, and hepatic veins. Once a baseline of the major hepatic vasculature is obtained, the care must be taken to the following: (i) not go through any visible metastatic lesions, including hepatic parenchymal lesions or lymph nodes, (ii) ensure an absence of interposed vasculature using Color Doppler, (iii) identify the left and right portal vein branches with an angle and scope position to allow maximum stability (minimizing scope torque) for blood aspiration without movement and shearing of the vessel, (iv) ensure the target vessel has flow and a venous waveform with Doppler, and (v) minimize the number of passes into the target vessel [Figure 1].
Figure 1.
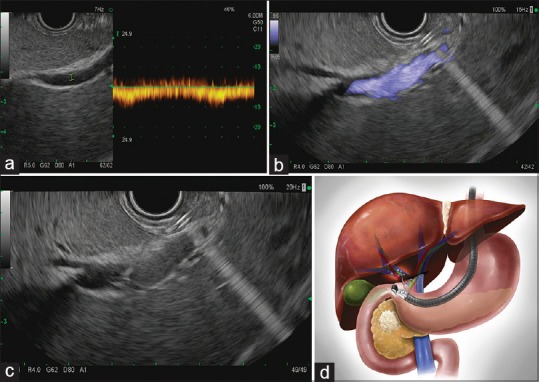
EUS-guided access of the portal vein. The portal vein is identified under EUS guidance with Doppler (a). Wave verification and (b) flow verification. (c) EUS-guided, transhepatic, FNA puncture of the portal vein with a 19G EUS-FNA needle for portal venous blood acquisition (d) diagram representing EUS-guided transgastric, transhepatic portal vein puncture
While identifying and stabilizing the echoendoscope position with the extrahepatic portal vein offers less technical difficulty, we strongly favor transhepatic routes due to the theoretical benefit of liver parenchyma tamponading the FNA needle track and the extensive safety data from percutaneous, transhepatic access by interventional radiology. In the study of 18 patients undergoing EUS-guided transgastric or transduodenal, transhepatic portal vein access in cases of suspected pancreaticobiliary malignancies, there were no episodes of immediate or delayed postprocedural bleeding or perforation.[13] In 2017, Huang et al. published the first report of measuring the portal pressure gradient in 28 patients with liver disease.[14] In this safety and feasibility trial, the intrahepatic portal vein was targeted with a 25G needle using a transgastric or transduodenal transhepatic access point. Similarly, there were no complications including no episodes of immediate or delayed postprocedural bleeding.
Contrarily, in 2004, Lai et al. published the first report of EUS-guided portal vein access in a swine model.[17] Using a 21G EUS-FNA needle, the extrahepatic portal vein was accessed through a transduodenal approach to obtain EUS-guided portal pressure measurements. At necropsy 4 days postintervention, there were small subserosal hematomas at the EUS puncture site in every pig. In one anticoagulated pig, there was a small (approximately 25 mL) collection of blood between the portal vein and duodenum. Further, in the intraoperative portal vein CTC acquisition study by Tien et al., a 21G needle (PrecisionGlide Needle 21G 1 1/2 TW; BD Becton, Dickinson) was used to directly puncture the extrahepatic portal vein.[11] The authors noted bleeding that stopped after digital compression in 65 of 66 patients; however, one patient did require placement of a 6-0 Prolene suture to stop bleeding. Thus, due to the published safety profile to date, the risk of bleeding and the lack of an endoscopic approach to arrest it, transhepatic approaches are recommended.
Negative suction
Negative suction is a controversial adjunctive method used by some interventional endoscopists to enhance FNA tissue acquisition. In aspirating blood from the portal vein, negative suction is definitively required. Once the EUS-FNA needle has accessed the portal vein or subsidiary branch, the stylet is removed and a 10 mL negative suction syringe is applied to the FNA needle. Blood is aspirated up the shaft of the EUS-FNA needle into the negative suction syringe. Immediately after aspirating 8–10 mL of blood, an assistant should: (i) apply a second preprepared negative suction syringe to aspirate a second 8–10 mL volume and (ii) place the first aspirated volume into a vacutainer tube for downstream application and repeatedly invert to mix the blood.
Postprocedure monitoring
As the EUS-FNA needle is being withdrawn into the sheath, the intrahepatic needle track should be observed with color Doppler to assess for persistent flow. The puncture site is monitored under direct EUS color Doppler visualization for complications for a minimum of 5 min in the endoscopy suite and for 45 min after the procedure in the recovery area. We routinely made telephone calls 24 h and 7 days after the procedure to further assess recovery. Similarly in the pilot study of portal pressure gradient measurement, Huang et al. completed postprocedural interview of all patients in person in recovery and by telephone within the subsequent 48 h.[14] In both cases, there were no cases of immediate or delayed complications from EUS-guided portal vein sampling, including hematoma formation or gastrointestinal bleeding.
EUS-GUIDED PORTAL VEIN ACCESS FOR BLOOD ACQUISITION: TROUBLESHOOTING
Bleeding
A significant concern with EUS-guided portal vein access is the risk of immediate or delayed gastrointestinal hemorrhage. Prior to any EUS-guided portal venous access procedure, patients should be optimized by maintaining an international normalized ratio <1.5, platelet count >50 × 10^9/L, and discontinuation of anticoagulation medications. Furthermore, selecting patients without evidence posthepatic/sinusoidal portal hypertension, may reduce the risk of needle track bleeding.[14]
To reduce the likelihood of bleeding events in interventional radiology guided portal vein islet cell transplantation, metal coils or gelfoam plugs are used to embolize the track at the point of portal vein entry.[12] While this has not been proven to be necessary in EUS-guided portal vein sampling to date, the slow withdrawal of the EUS-FNA needle into the sheath under direct EUS color Doppler visualization may identify high risk tracks for bleeding. Prior to completely removing the needle from the liver capsule, no flow should be identified in the track. However, if flow is persistent, as has been previously demonstrated for variceal bleeding, sclerosants, cyanoacrylate, thrombin, and coils could theoretically be applied to the site of portal entry under EUS guidance.[18]
Blood sample clotting
EUS is frequently used for fluid aspiration including pancreatic cystic lesions and ascites, however when EUS-FNA is used for blood aspiration, yield may be lower due to clotting of the blood sample. After access to the portal vein is obtained and negative suction is applied, blood has to travel the length of the echoendoscope before reaching a FNA needle not designed to prevent thrombosis. Further, vascular access devices can also exert shear forces during aspiration, creating a predilection to cell lysis.[19]
To help lower the risk of developing blood clots in the aspirated blood sample, it should be immediately transferred from the negative suction syringe into vacutainer tube containing cell preservatives for downstream application. Additional methods that may help reduce clotting include priming the negative suction syringe or EUS-FNA needle by flushing a small amount of (1 mL) of anticoagulant solution (e.g., EDTA or citrate). However, the choice of anticoagulation solution must be carefully considered and ensured to be safe and compatible for downstream applications.[20]
CONCLUSIONS
EUS has become standard of care for preoperative tissue acquisition particularly in pancreaticobiliary malignancies and offers a high-resolution, minimally invasive imaging method to evaluate major abdominal vessels. The entire portal venous system, including its main tributaries, can easily be identified offering a minimally invasive window for diagnostic and therapeutic applications. Pilot and feasibility studies in human patients utilizing EUS-guided portal vein access for CTC enumeration and more recently, portal pressure gradient monitoring, suggest that EUS-guided access of the portal vein procedure is safe and effective. Understanding the technical aspects of EUS-guided portal vein sampling will be critical to standardizing the procedure, developing new vascular access technologies, and increasing the safety profile. These current small trials were completed in small cohorts in a nonrandomized or controlled fashion; however, in the future, with continued research and advancement in EUS-specific vascular access technologies, the diagnostic and therapeutic opportunities with EUS-guided portal vein access will continue to expand and likely become an addition to the diagnostic standard.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Esmaeilsabzali H, Beischlag TV, Cox ME, et al. Detection and isolation of circulating tumor cells: Principles and methods. Biotechnol Adv. 2013;31:1063–84. doi: 10.1016/j.biotechadv.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Mizuno N, Kato Y, Izumi Y, et al. Importance of hepatic first-pass removal in metastasis of colon carcinoma cells. J Hepatol. 1998;28:865–77. doi: 10.1016/s0168-8278(98)80238-9. [DOI] [PubMed] [Google Scholar]
- 3.Massagué J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. 2016;529:298–306. doi: 10.1038/nature17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–64. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Z, Qutaish M, Han Z, et al. MRI detection of breast cancer micrometastases with a fibronectin-targeting contrast agent. Nat Commun. 2015;6:7984. doi: 10.1038/ncomms8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Speicher MR, Pantel K. Tumor signatures in the blood. Nat Biotechnol. 2014;32:441–3. doi: 10.1038/nbt.2897. [DOI] [PubMed] [Google Scholar]
- 7.Yu M, Stott S, Toner M, et al. Circulating tumor cells: Approaches to isolation and characterization. J Cell Biol. 2011;192:373–82. doi: 10.1083/jcb.201010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss L, Ward PM, Holmes JC. Liver-to-lung traffic of cancer cells. Int J Cancer. 1983;32:79–83. doi: 10.1002/ijc.2910320113. [DOI] [PubMed] [Google Scholar]
- 9.Rahbari NN, Bork U, Kircher A, et al. Compartmental differences of circulating tumor cells in colorectal cancer. Ann Surg Oncol. 2012;19:2195–202. doi: 10.1245/s10434-011-2178-1. [DOI] [PubMed] [Google Scholar]
- 10.Bissolati M, Sandri MT, Burtulo G, et al. Portal vein-circulating tumor cells predict liver metastases in patients with resectable pancreatic cancer. Tumour Biol. 2015;36:991–6. doi: 10.1007/s13277-014-2716-0. [DOI] [PubMed] [Google Scholar]
- 11.Tien YW, Kuo HC, Ho BI, et al. A high circulating tumor cell count in portal vein predicts liver metastasis from periampullary or pancreatic cancer: A high portal venous CTC count predicts liver metastases. Medicine (Baltimore) 2016;95:e3407. doi: 10.1097/MD.0000000000003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funaki B. Islet cell transplantation. Semin Intervent Radiol. 2006;23:295–7. doi: 10.1055/s-2006-948762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catenacci DV, Chapman CG, Xu P, et al. Acquisition of portal venous circulating tumor cells from patients with pancreaticobiliary cancers by endoscopic ultrasound. Gastroenterology. 2015;149:1794–803.e4. doi: 10.1053/j.gastro.2015.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang JY, Samarasena JB, Tsujino T, et al. EUS-guided portal pressure gradient measurement with a simple novel device: A human pilot study. Gastrointest Endosc. 2017;85:996–1001. doi: 10.1016/j.gie.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rustagi T, Gleeson FC, Chari ST, et al. Remote malignant intravascular thrombi: EUS-guided FNA diagnosis and impact on cancer staging. Gastrointest Endosc. 2017;86:150–5. doi: 10.1016/j.gie.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Ngamruengphong S, Swanson KM, Shah ND, et al. Preoperative endoscopic ultrasound-guided fine needle aspiration does not impair survival of patients with resected pancreatic cancer. Gut. 2015;64:1105–10. doi: 10.1136/gutjnl-2014-307475. [DOI] [PubMed] [Google Scholar]
- 17.Lai L, Poneros J, Santilli J, et al. EUS-guided portal vein catheterization and pressure measurement in an animal model: A pilot study of feasibility. Gastrointest Endosc. 2004;59:280–3. doi: 10.1016/s0016-5107(03)02544-6. [DOI] [PubMed] [Google Scholar]
- 18.Binmoeller KF, Sendino O, Kane SD. Endoscopic ultrasound-guided intravascular therapy. J Hepatobiliary Pancreat Sci. 2015;22:44–50. doi: 10.1002/jhbp.183. [DOI] [PubMed] [Google Scholar]
- 19.Bowen RA, Remaley AT. Interferences from blood collection tube components on clinical chemistry assays. Biochem Med (Zagreb) 2014;24:31–44. doi: 10.11613/BM.2014.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warton K, Yuwono NL, Cowley MJ, et al. Evaluation of streck BCT and PAXgene stabilised blood collection tubes for cell-free circulating DNA studies in plasma. Mol Diagn Ther. 2017;21:563–70. doi: 10.1007/s40291-017-0284-x. [DOI] [PubMed] [Google Scholar]


