Abstract
Background:
Ectopic pancreas (EP) belongs to the most frequent subepithelial lesions (SELs) of the upper gastrointestinal (GI) tract. In the majority of cases, it is detected incidentally. Differential diagnosis from mesenchymal subepithelial tumors may be difficult.
Methods:
Among 24,308 endosonographic examinations and interventions, which were prospectively enrolled in the database of the German Endoscopic Ultrasound (EUS) Registry from January 2009 to August 2013, 575 were performed for suspected SELs of the upper GI tract. Sixty three cases of EP of the upper GI tract (stomach, n = 53; duodenum, n = 10; esophagus, n = 0) were extracted and retrospectively reviewed.
Results:
In 65.1% of cases, radial echoendoscopes or radial miniprobes were used for examination. Nearly 84% of EP was found in the stomach, 16% in the duodenum, none in the esophagus. In 88.9% of cases, the EUS examination discerned the layer of origin. In 59% of cases EP was described as a heterogeneous, in 28.6% as a homogeneous-hypoechoic and in 7.9% as a homogeneous-echogenic subepithelial mass lesion. Mean diameter was 13.0 mm × 8.1 mm, the mean ratio between long and short axis diameter was 1.75. EUS-guided fine needle aspiration (EUS-FNA) was used to accomplish cytological or histological diagnosis in only 6.3% of cases.
Conclusions:
EP accounts for 11% of all EUS examinations performed for subepithelial lesions of the upper GI tract and prospectively enrolled in the German EUS registry. Rather than being an eyecatcher, EP is a chameleon with numerous differential diagnoses. In selected cases, EUS-FNA may help clarifying the diagnosis.
Keywords: Ectopic pancreas, endoscopic ultrasound, endoscopic ultrasound-guided fine-needle aspiration, pancreatic heterotopia, pancreatic rest, subepithelial tumor
INTRODUCTION
Ectopic pancreas (EP) is a developmental anomaly including normal pancreatic tissue without any vascular or anatomical relations with the anatomically normal located pancreas. In the stomach it belongs to the most common subepithelial lesions (SELs), and is also common in the duodenum, but exceedingly rare in the esophagus. Clinical implications, outcome and management of EP are different from most mesenchymal tumors, in particular from gastrointestinal stromal tumor (GIST).[1,2,3,4,5,6,7] The first case of EP, developed in an ileal diverticulum, was reported by Jean Schultz in 1727,[8] and its histological features were studied in 1859 by Klob et al.[9] The mechanism of development of ectopic pancreatic tissue is unclear. The two predominant theories involve misplacement of pancreatic tissue during development versus tissue metaplasia.[10] The misplacement theory proposes that, during rotation of the foregut, several elements of the primitive pancreas become separated and eventually form mature pancreatic tissue along the length of the gastrointestinal (GI) tract.[11] The metaplasia theory states that EP arises from areas of pancreatic metaplasia of the endoderm which migrate to the submucosa during embryogenesis.[12] The reported frequency of EP during laparoscopy is 0.5%, at panendoscopy 1%, and at autopsy 1.7%.[10] EP tissue may be functionally active, secreting pancreatic enzymes and/or insulin and glucagon.[13] However, EP is usually asymptomatic. Therefore, the majority of cases is found incidentally during routine endoscopic or radiographic studies. Symptoms are mostly related to larger EP causing GI luminal obstruction, intussusception, or mucosal ulceration and hemorrhage.[7,11,14,15,16,17,18,19] If EP is located in or near the papilla of Vater, obstructive jaundice may result.[20,21,22] Rarely, acute or chronic pancreatitis may develop in EP, and single cases with calcifications and pseudocyst formation have been observed.[23,24,25,26,27] French authors postulated cystic dystrophy of the duodenal wall to be a consequence of duodenal EP,[28,29] and relations to groove pancreatitis have been discussed.[30,31,32] Finally, case reports of nearly all distinct types of solid and cystic pancreatic neoplasms including ductal adenocarcinoma, neuroendocrine tumor, acinar cell carcinoma, serous cystadenoma, mucinous cystadenoma, and intraductal papillary mucinous neoplasia arising from EP have been published.[33,34,35,36,37,38,39,40,41]
EP may mimick other SEL, in particular GISTs, which harbor malignant potential.[42,43,44] Endoscopic ultrasound (EUS) is helpful in characterizing SEL, but cannot determine the type of lesion or whether a lesion is benign or malignant with absolute certainty.[1,2,3] EUS-guided sampling is often used for cytopathological characterization of upper GI subepithelial tumors, but diagnostic yield is limited to approximately 60%.[45] EUS provides the most useful information regarding tumor location in the gastric wall, and helps to select good candidates for endoscopic removal.[46,47,48]
The aim of this study was to evaluate the frequency of EP among SEL of the upper GI tract reported in the German EUS Registry of the German Society for Ultrasound in Medicine (DEGUM), their typical endosonographic features, and the role of EUS-guided fine needle aspiration (EUS-FNA) for diagnosis.
METHODS
Using the prospectively enrolled online database of the German EUS Registry, we identified 24,308 EUS examinations performed by 62 examiners from January 2009 to August 2013. The anonymized data collection of the registry included age and sex of the patient, indication for examination, type of echoendoscope used, adverse events, indication-specific quality indicators and final diagnosis. For EUS examinations of suspected SEL the following specifications were recorded: size of the lesion, echogenicity/echo-pattern (anechoic, hypoechoic, hyperechoic, mixed echogenicity), assignment of layer of origin (yes, no, infiltrating), EUS-FNA performed (yes/no). For EUS-FNA cases, information on needle size, number of needle passes, adequacy of material for cytopathological and histopathological examinations, benign/neoplastic or malignant nature of the lesion, and specific diagnosis are available. No information was collected about symptoms, specific layer, outline, or endoscopic features.
We retrospectively reviewed all cases with the final diagnosis of EP of the upper GI tract and identified localization, size, echogenicity, and results of EUS-FNA, if performed.
RESULTS
Among 575 EUS examinations performed for SEL of the upper GI tract, we identified 63 patients (33 female, 30 male) with the final diagnosis EP (15.6%; stomach, n = 53; duodenum, n = 10; esophagus, n = 0). Among defined SEL, EP was the third most frequent (11.0%), the relative frequency in the stomach being higher than in the duodenum and the esophagus [15.4% vs. 5.7% vs. 0%; Tables 1 and 2].
Table 1.
Relative frequency of different types of subepithelial lesion in the stomach and duodenum
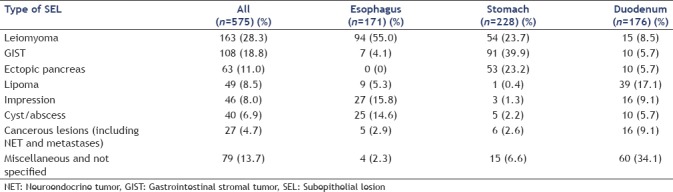
Table 2.
General characteristics of patients and subepithelial lesion with final diagnosis of ectopic pancreas of the upper gastrointestinal tract
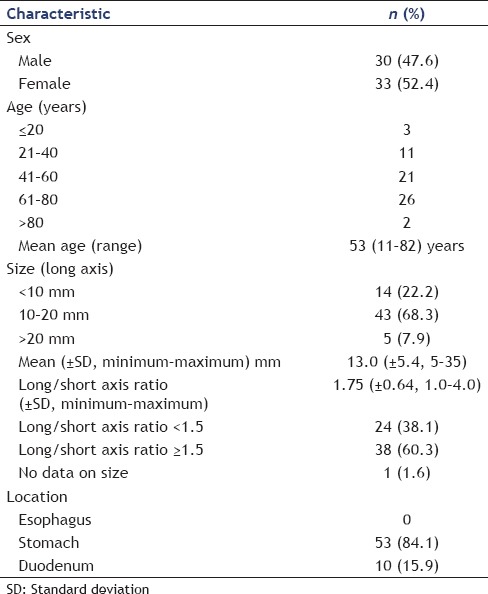
EUS was performed using curvilinear echoendoscopes (n = 20, 31.7%), radial echoendoscopes (n = 38, 60.3%), and miniprobes (n = 3, 4.8%). In two cases, no information on the type of echoendoscope was available. EUS-FNA of suspected EP was performed in three cases only (4.8%). None of the patients developed any adverse events in association with the EUS procedures.
In 58.7% of cases, EPs of the upper GI tract were described to have mixed echogenicity, in 28.6% of cases, the lesions were hypoechoic, and in 7.9% echogenic. Identification of the layer of origin was feasible in 88.9% of cases. Mean diameters were 13 ± 5.4 mm × 8.1 ± 3.8 mm (range: 5 mm × 4 mm–35 mm × 19 mm), the mean long/short axis ratio was 1.75 ± 0.64 [Table 2].
Stomach
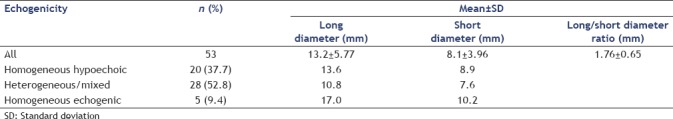
EP accounted for 23.2% of 228 gastric SEL. The 53 patients with gastric EP included 29 men and 34 women, age ranging from 11 to 82 years (mean age: 51 years). In average, the lesions measured 13.2 ± 5.8 mm (range: 5–35) × 8.1 ± 4.0 mm (range: 3–22 mm). The long/short axis ratio was 1.76 ± 0.65. Mixed echogenicity was described in 52.8% of gastric EP, homogeneous low echogenicity in 37.7%, and homogeneous high echogenicity in 9.4% of cases [Table 3].
Table 3.
Size and echogenicity of 53 cases of gastric ectopic pancreas
Duodenum
Ten patients with duodenal EP were identified among 176 examinations performed for duodenal SEL. The patients included 4 men and 6 women, age ranging from 55 to 76 years (mean age: 66 years). Mean diameters were 11.7 ± 2.4 mm (range: 7–15 mm) × 7.9 ± 2.8 mm (range: 5–13) mm (long/short axis ratio: 1.63 ± 0.63. Nine of ten duodenal EP were described as SEL of mixed echogenicity, one case as homogeneous and hypoechoic [Table 4].
Table 4.
Size and echogenicity of ten cases of duodenal ectopic pancreas

Esophagus
No EP case was identified among 171 cases of esophageal SEL.
EUS-FNA was performed in 4 cases for differential diagnosis of gastric SEL, finally diagnosed as EP. Nineteen gauge (G) aspiration needles were used in three cases, a 22 G aspiration needle in one case. Adequate material facilitating a specific diagnosis of EP was obtained in three cases of hypoechoic SEL with 2–4 needle passes using 19 G (n = 2) and 22 G (n = 1) aspiration needles. In another case of gastric EP with mixed echogenicity, EUS-FNA using a 19G aspiration needle (>4 needle passes) failed to obtain adequate material for specific diagnosis.
DISCUSSION
Heterotopic pancreatic tissue can be located anywhere along the GI tract. The most common sites are stomach, duodenum, and jejunum. EP has been also reported in the colon, spleen, liver, Meckel's diverticulum, gallbladder, bile ducts, lung, esophagus, pelvis, or fallopian tubes.[7,14,15,16,17,18,19] In one of the largest series of EP published so far (n = 184), 53% of EP were found in the stomach, 26% in the duodenum and jejunum, 13.8% in the minor and major omentum and mesenteries, 2.7% in spleen and splenic hilum, 2.2% in esophagus, and 1.5% in other rare locations.[7] In a review of 544 surgical cases from several publications, 27% of EP were localized in the gastric wall, 29% in the duodenum, 16% in the jejunum, 6% in the ileum, 6% in Meckel's diverticulum, 3% in gallbladder, and 13% at miscellaneous other sites.[15] In our series, EP was more frequently found in the stomach (23%, third most frequent defined SEL) than in the duodenum (6%, fifth most frequent SEL). No EP was found in the esophagus. The frequency distribution of upper GI SEL in the German EUS Registry database is very similar to that of other large cohorts.[5,6] As expected, the most frequent gastric SEL was GIST and the most frequent esophageal SEL was leiomyoma. Surprisingly, the most EUS examinations for duodenal SEL diagnosed lipoma. The fact, that some SEL may be diagnosed without EUS with a high degree of certainty (e.g., gastric lipoma, gastric varix), may have influenced the frequency distribution of upper GI SEL of the German EUS registry.
In the upper GI tract, EP most often presents incidentally as an SEL [Figure 1]. Rarely, specific EP-related symptoms may occur, in particular caused by luminal obstruction [Figure 2], inflammation, or, very rarely, by the occurrence of exocrine or endocrine neoplasms in the EP.[7,11,14,15,16,17,18,19,20,21,22,23,24,25,26,27,33,34,35,36,37,38,39,40,41] Endoscopically, central dimpling is a characteristic feature [Figure 1], described in 35%–90% of cases.[49,50,51,52] However, mucosal dimpling or umbilication is also observed in submucosal neuroendocrine tumors and hamartomas.[49] Occasionally, large GISTs, schwannomas and, very rarely, leiomyomas also have a similar central depression, resulting from necrosis due to insufficient blood supply.[3]
Figure 1.
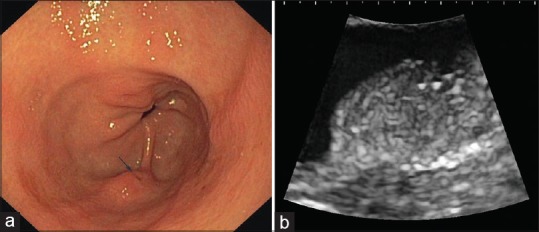
Upper gastrointestinal endoscopy revealed a flat protruding subepithelial lesion in the gastric antrum (a) with central dimpling (arrow). Endoscopic ultrasound appearance (b); curvilinear echoendoscope UG EG-3870UTK, Pentax Medical, Hamburg, Germany with HI Vision Preirus (Hitachi Medical Systems, Wiesbaden, Germany) was consistent with ectopic pancreas: a slightly heterogeneous subepithelial lesion located within the submucosal layer. Histological proof of diagnosis was obtained after endoscopic resection
Figure 2.
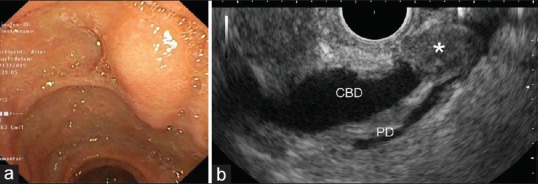
Endoscopic aspect (a) and radial endoscopic ultrasound appearance (b) of an ectopic pancreas of the papilla of Vater (*) causing obstructive jaundice (EG-3670URK and Pentax Medical, Hamburg, Germany with HI Vision Preirus, Hitachi Medical Systems, Wiesbaden, Germany). Diagnosis was confirmed histologically after surgical treatment. CBD: Common bile duct; PD: Pancreatic duct
EUS is highly accurate for clarifying the layer of origin of upper GI tract SELs.[2,3,53] EP may occur in all layers of the GI tract wall. The majority involves the submucosa (15%–70% of cases) and the muscularis propria (11%–80%), whereas mucosal or serosal localizations are rare. However, layer assignment of EP varies a lot between case series.[50,51,52,54,55,56] Mesenchymal tumors, including GISTs, leiomyomas, schwannomas are known to occur predominantly in the fourth wall layer (muscularis propria).[57] Therefore, differential diagnosis remains difficult. In a recent series of 184 EP cases from China, only 14% of EP cases were correctly diagnosed before resection or biopsy. All cases with correct diagnosis before histology were located in the upper GI tract.[7] In another study, suspected diagnosis before endoscopic resection proved to be correct in 43% of gastric EP cases. GIST and simple gastric polyp were suspected in 19% and 17% of gastric EP cases before endoscopic resection, respectively.[42]
Our data show that EUS is helpful for characterization and diagnosis of EP. In 59% of cases EP was described as heterogeneous (mixed echogenicity), in 29% as a hypoechoic, and in only 8% as echogenic mass lesion. Homogeneous low echogenicity as an important source of diagnostic uncertainty was more often observed in gastric EP (37.7%) than in duodenal EP (10%). The mean diameter was 13.0 mm × 8.1 mm with no difference between gastric and duodenal EPs. 90.5% of EPs had a long axis diameter ≤20 mm. However, variability was considerably with the smallest EP measuring 5 mm × 4 mm and the largest 35 mm × 19 mm. The shape of EP was also highly variable with a long/short axis ratio varying between 1.0 (round) and 4.0 (flat-oval) and averaging 1.75. Sixty percent of EPs had an oval shape with a long/short axis ratio ≥ 1.5. In approximately 90% of cases, the examiner was able to identify the layer of origin as an important precondition for decisions on endoscopic resection or EUS-FNA for diagnostic purposes. Several studies tried to identify typical features of EP, which may facilitate differentiation from other SELs of the upper GI tract. However, conclusive statements are limited by the small number of patients included in the published case series and by inconsistency of data [Table 5].
Table 5.
Endoscopic ultrasound features of ectopic pancreas of the upper ectopic pancreas tract in cases series
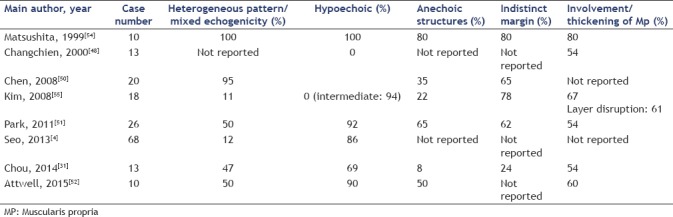
Only two studies including 15 cases evaluated endosonographic features in direct comparison with histology.[46,54] Anechoic structures were found to be dilated ductal elements, the indistinct and lobulated margins were due to the lobular structure of hypoechoic acinar tissue, which was accompanied by scattered small areas of hyperechoic fatty tissue.[54] Another study compared endosonographic features of gastric EP (n = 18) with gastric mesenchymal tumors (n = 53).[55] EP was found to occur more frequently than mesenchymal tumors in the antrum. However, the gastric body was the most common localization of both EPs (55.5%) and mesenchymal tumors (47.2%). The gastric body was the most frequent site of GISTs and schwannomas, and the cardia is known to be a common site of true leiomyomas.[4,5] Mesenchymal tumors predominantly showed an endoluminal growth pattern (43.3%) and are nearly round with a long/short axis ratio <1.5 (1.44 ± 0.36), whereas a predominantly mural growth pattern and higher longest/shortest diameter ratio were observed in EP (77.7%; 1.99 ± 0.53). Involvement of more than one layer (layer disruption) was found significantly more often in EP compared with mesenchymal tumor (61% vs. 4%).[55] A recent study compared several histological types of gastric SEL with a simple scoring system, using anatomical location, layer of origin, echogenicity, and shape as variables. EUS diagnosis of EP was possible with a sensitivity of 84.6% and a negative predictive value of 91.4%. However, specificity (73.1%) and positive predictive value (58.1%) were relatively low, reflecting significant overlap of features with hypoechoic mesenchymal subepithelial tumors.[4]
There are several potential reasons for the high variability of endosonographic features of EP in different case series. First, echogenicity and homogeneous/heterogeneous pattern are relative terms requiring comparison to reference structures with well-defined echogenicity and echo-pattern (e.g., hypoechoic muscularis propria and hyperechoic submucosa).[58] Moreover, in contrast to SEL with very clearly defined endosonographic phenotypes (submucosal cysts, lipoma, leiomyoma), the interobserver agreement for other submucosal solid lesions in one study was poor (κ =0.34)[59] Finally, the variability of endosonographic descriptions of EP between published case series may reflect different frequencies of the pathological types of EP, which have been described by Heinrich as early as 1909.[60] and modified by Gaspar Fuentes[61] and Jochimsen.[62] All pathological classifications reflect the different contents of normal components of pancreatic tissue in EPs [Tables 6 and 7]. At pathology, the gross appearance of EP includes the presence of a characteristic central ductal orifice. Histological structure is very similar to normal pancreatic tissue without any anatomical relation or direct connection by blood vessel with the pancreas. Exocrine acinar and ductal structures are nearly always present, and islet of Langerhans are found in the majority of cases.[17,18] When smooth muscle and duct-like structures are the only components, the term adenomyoma has been used.[63] In the study of Chou et al. 38.4% of cases were Heinrich Type I and 61.5% Heinrich Type II,[56] in the study of Matsushita 42% were Type I and II each, and 16% Type III.[54] Moreover, the layer assignment was shown in one study to influence endosonographic pattern: EP in the muscularis propria were more common to exhibit both hypoechoic and homogeneous feature than those not in the fourth layer (85.7% vs. 16.6%).[31] Differentiation from leiomyoma or GIST in these cases may be impractical without cytology or histology.
Table 6.
Classification of ectopic pancreas (Heinrich, 1909)

Table 7.
Modified classification of ectopic pancreas (Gaspar Fuentes, 1973)

An endosonographic classification of EP was proposed by Hase et al.[46] and modified by other groups.[51,54] The M-type (D-type or fusion type) includes cases with involvement of the thickened proper muscle layer, whereas in the S-type (superficial or separate type) the ectopic pancreatic tissue is located with the submucosal and mucosal layer separate from the deep muscle layer.[46,54] This classification may be used to guide decisions on endoscopic resection.[46,51,54] In cases with uncertain diagnosis endoscopic cap or band ligation assisted resection or endoscopic submucosal dissection are appropriate and safe treatment procedures, in particular for lesions not involving the muscularis propria.[6,42,64,65,66]
The role of EUS-FNA for diagnosis of EP is limited. Successful EUS-FNA diagnosis of gastroduodenal EP was reported only in single cases and one small case series.[52,67,68,69,70,71,72] In our series, EUS-FNA was performed in only 4 of 63 cases (6.3%) and facilitated diagnosis of EP in 3 cases (4.8%). Interestingly, these 3 cases were described to be hypoechoic and therefore difficult to distinguish from mesenchymal subepithelial tumors. This is consistent with data from the literature. In a series of 10 EP cases, in which EUS-FNA was attempted, 9 cases were hypoechoic.[52] However, two cases of heterotopic pancreatitis as a consequence of EUS-FNA of EP have been reported.[52,71] In the 4 cases included in our study, no adverse events have been reported. In conclusion, EUS-FNA should not be performed in typical cases of EP (origin in the submucosa, mixed echogenicity, indistinct margin, long/short diameter ratio >1.5, central dimpling) and in atypical cases of the S-type, in which endoscopic resection can be performed safely to afford tissue diagnosis. EUS-FNA may be considered in rare cases of homogeneous-hypoechoic tumors involving the deep muscle layer in order to enforce differentiation of M-type EP from other hypoechoic subepithelial tumors like leiomyoma, GIST, inflammatory fibroid polyp, or schwannoma.
Mostly due to its retrospective nature, our study has several limitations. Most importantly, data on important features of SEL as the specific layer of origin, borders of the lesion, and the occurrence of tubular or cystic anechoic structures, are lacking. Acquisition of these data was not requested in the German EUS Registry. A further weakness is lacking information on histological confirmation. The decision to perform EUS-FNA, other techniques of tissue acquisition, or resection of the lesion was at the discretion of the examiners. Moreover, examinations have been performed by several examiners in different hospitals.
CONCLUSIONS
EP accounts for 11% of all EUS examinations performed for upper GI SEL and prospectively enrolled in the German EUS registry. Nearly 84% were found in the stomach. In most cases, EP is an incidental finding, and specifically related symptoms or complications are rare. Using EUS, layer assignment is possible in more than 90% of cases. The majority of EP cases exhibit a heterogeneous echotexture, reflecting its histological structure, have a long axis diameter ≤20 mm and an oval shape. According to data from the literature, indistinct borders, localization in the gastric antrum, central dimpling, a relatively flat (mucosal) growth pattern, ductal or cystic anechoic structures, origin in the submucosal layer, and involvement of more than one layer are further endosonographic and endoscopic features substantiating the suspicion of EP. Therefore, careful assessment of the EUS findings is a useful aid in the differentiation of EP in particular from mesenchymal tumors in the stomach and duodenum.
However, owing to the variability of acinar, ductal and endocrine structural elements and possible subclinical inflammatory changes, endosonographic appearance is far from being uniform. Rather than being an eyecatcher, EP is a chameleon with numerous differential diagnoses. In particular, hypoechoic EP involving the muscularis propria may be the source of diagnostic uncertainty. In selected cases, EUS-FNA or endoscopic resection may be helpful techniques to clarify the diagnosis.
Financial support and sponsorship
The German EUS Registry was supported by a financial grant of the German Society for Ultrasound in Medicine (DEGUM).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Polkowski M, Butruk E. Submucosal lesions. Gastrointest Endosc Clin N Am. 2005;15:33–54. doi: 10.1016/j.giec.2004.07.005. viii. [DOI] [PubMed] [Google Scholar]
- 2.Jenssen C, Dietrich CF. Endoscopic ultrasound of gastrointestinal subepithelial lesions. Ultraschall Med. 2008;29:236–56. doi: 10.1055/s-2008-1027388. [DOI] [PubMed] [Google Scholar]
- 3.Eckardt AJ, Jenssen C. Current endoscopic ultrasound-guided approach to incidental subepithelial lesions: Optimal or optional? Ann Gastroenterol. 2015;28:160–72. [PMC free article] [PubMed] [Google Scholar]
- 4.Seo SW, Hong SJ, Han JP, et al. Accuracy of a scoring system for the differential diagnosis of common gastric subepithelial tumors based on endoscopic ultrasonography. J Dig Dis. 2013;14:647–53. doi: 10.1111/1751-2980.12099. [DOI] [PubMed] [Google Scholar]
- 5.Min YW, Park HN, Min BH, et al. Preoperative predictive factors for gastrointestinal stromal tumors: Analysis of 375 surgically resected gastric subepithelial tumors. J Gastrointest Surg. 2015;19:631–8. doi: 10.1007/s11605-014-2708-9. [DOI] [PubMed] [Google Scholar]
- 6.He G, Wang J, Chen B, et al. Feasibility of endoscopic submucosal dissection for upper gastrointestinal submucosal tumors treatment and value of endoscopic ultrasonography in pre-operation assess and post-operation follow-up: A prospective study of 224 cases in a single medical center. Surg Endosc. 2016;30:4206–13. doi: 10.1007/s00464-015-4729-1. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Sun X, Gold JS, et al. Heterotopic pancreas: A clinicopathological study of 184 cases from a single high-volume medical center in China. Hum Pathol. 2016;55:135–42. doi: 10.1016/j.humpath.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Hsia CY, Wu CW, Lui WY. Heterotopic pancreas: A difficult diagnosis. J Clin Gastroenterol. 1999;28:144–7. doi: 10.1097/00004836-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Klob J. Pancreas accessorium. Zeitschrift der Kaiserl. Königl. Gesellschaft der Aerzte zu Wien. 1859;15:732. [Google Scholar]
- 10.Gupta MK, Karlitz JJ, Raines DL, et al. Clinical case of the month. Heterotopic pancreas. J La State Med Soc. 2010;162:310–3. [PubMed] [Google Scholar]
- 11.Armstrong CP, King PM, Dixon JM, et al. The clinical significance of heterotopic pancreas in the gastrointestinal tract. Br J Surg. 1981;68:384–7. doi: 10.1002/bjs.1800680606. [DOI] [PubMed] [Google Scholar]
- 12.Chandan VS, Wang W. Pancreatic heterotopia in the gastric antrum. Arch Pathol Lab Med. 2004;128:111–2. doi: 10.5858/2004-128-111-PHITGA. [DOI] [PubMed] [Google Scholar]
- 13.Hammock L, Jorda M. Gastric endocrine pancreatic heterotopia. Arch Pathol Lab Med. 2002;126:464–7. doi: 10.5858/2002-126-0464-GEPH. [DOI] [PubMed] [Google Scholar]
- 14.De Castro Barbosa JJ, Dockerty MB, Waugh JM. Pancreatic heterotopia; review of the literature and report of 41 authenticated surgical cases, of which 25 were clinically significant. Surg Gynecol Obstet. 1946;82:527–42. [PubMed] [Google Scholar]
- 15.Jacobs LG, Richmond TE, Tucker JM. Aberrant pancreatic tissue in the gastric wall. Calif Med. 1951;75:221–3. [PMC free article] [PubMed] [Google Scholar]
- 16.Dolan RV, ReMine WH, Dockerty MB. The fate of heterotopic pancreatic tissue. A study of 212 cases. Arch Surg. 1974;109:762–5. doi: 10.1001/archsurg.1974.01360060032010. [DOI] [PubMed] [Google Scholar]
- 17.Pang LC. Pancreatic heterotopia: A reappraisal and clinicopathologic analysis of 32 cases. South Med J. 1988;81:1264–75. [PubMed] [Google Scholar]
- 18.Ormarsson OT, Gudmundsdottir I, Mårvik R. Diagnosis and treatment of gastric heterotopic pancreas. World J Surg. 2006;30:1682–9. doi: 10.1007/s00268-005-0669-6. [DOI] [PubMed] [Google Scholar]
- 19.Chen HL, Chang WH, Shih SC, et al. Changing pattern of ectopic pancreas: 22 years of experience in a medical center. J Formos Med Assoc. 2008;107:932–6. doi: 10.1016/S0929-6646(09)60016-4. [DOI] [PubMed] [Google Scholar]
- 20.Biswas A, Husain EA, Feakins RM, et al. Heterotopic pancreas mimicking cholangiocarcinoma. Case report and literature review. JOP. 2007;8:28–34. [PubMed] [Google Scholar]
- 21.Hsu SD, Chan DC, Hsieh HF, et al. Ectopic pancreas presenting as ampulla of Vater tumor. Am J Surg. 2008;195:498–500. doi: 10.1016/j.amjsurg.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 22.Rao RN, Kamlesh Y, Pallav G, et al. Ectopic pancreas presenting as periampullary tumor with obstructive jaundice and pruritus is a rare diagnostic and therapeutic dilemma. A case report. JOP. 2011;12:607–9. [PubMed] [Google Scholar]
- 23.Chung JP, Lee SI, Kim KW, et al. Duodenal ectopic pancreas complicated by chronic pancreatitis and pseudocyst formation – A case report. J Korean Med Sci. 1994;9:351–6. doi: 10.3346/jkms.1994.9.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oka R, Okai T, Kitakata H, et al. Heterotopic pancreas with calcification: A lesion mimicking leiomyosarcoma of the stomach. Gastrointest Endosc. 2002;56:939–42. doi: 10.1067/mge.2002.129587. [DOI] [PubMed] [Google Scholar]
- 25.Mulholland KC, Wallace WD, Epanomeritakis E, et al. Pseudocyst formation in gastric ectopic pancreas. JOP. 2004;5:498–501. [PubMed] [Google Scholar]
- 26.Sharma DK, Agarwal S, Saran RK, et al. Pseudocyst of ectopic pancreas of the duodenal wall masquerading as malignant cystic tumor of pancreas. Saudi J Gastroenterol. 2009;15:271–3. doi: 10.4103/1319-3767.56101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe K, Irisawa A, Hikichi T, et al. Acute inflammation occurring in gastric aberrant pancreas followed up by endoscopic ultrasonography. World J Gastrointest Endosc. 2012;4:331–4. doi: 10.4253/wjge.v4.i7.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potet F, Duclert N. Cystic dystrophy on aberrant pancreas of the duodenal wall. Arch Fr Mal App Dig. 1970;59:223–38. [PubMed] [Google Scholar]
- 29.Fléjou JF, Potet F, Molas G, et al. Cystic dystrophy of the gastric and duodenal wall developing in heterotopic pancreas: An unrecognised entity. Gut. 1993;34:343–7. doi: 10.1136/gut.34.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adsay NV, Zamboni G. Paraduodenal pancreatitis: A clinico-pathologically distinct entity unifying “cystic dystrophy of heterotopic pancreas”, “para-duodenal wall cyst”, and “groove pancreatitis”. Semin Diagn Pathol. 2004;21:247–54. doi: 10.1053/j.semdp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Rebours V, Lévy P, Vullierme MP, et al. Clinical and morphological features of duodenal cystic dystrophy in heterotopic pancreas. Am J Gastroenterol. 2007;102:871–9. doi: 10.1111/j.1572-0241.2007.01091.x. [DOI] [PubMed] [Google Scholar]
- 32.Wagner M, Vullierme MP, Rebours V, et al. Cystic form of paraduodenal pancreatitis (cystic dystrophy in heterotopic pancreas (CDHP)): A potential link with minor papilla abnormalities?. A study in a large series. Eur Radiol. 2016;26:199–205. doi: 10.1007/s00330-015-3799-8. [DOI] [PubMed] [Google Scholar]
- 33.Satake K, Uchima K, Yamashita K, et al. Pancreatic cystadenoma of the spleen. Am J Surg. 1979;137:670–2. doi: 10.1016/0002-9610(79)90045-x. [DOI] [PubMed] [Google Scholar]
- 34.Jeong HY, Yang HW, Seo SW, et al. Adenocarcinoma arising from an ectopic pancreas in the stomach. Endoscopy. 2002;34:1014–7. doi: 10.1055/s-2002-35836. [DOI] [PubMed] [Google Scholar]
- 35.Mourra N, Balladur P, Parc R, et al. Intrasplenic mucinous cystadenoma with mesenchymal stroma arising in pancreatic heterotopia. Histopathology. 2003;42:616–8. doi: 10.1046/j.1365-2559.2003.01608.x. [DOI] [PubMed] [Google Scholar]
- 36.Phillips J, Katz A, Zopolsky P. Intraductal papillary mucinous neoplasm in an ectopic pancreas located in the gastric wall. Gastrointest Endosc. 2006;64:814–5. doi: 10.1016/j.gie.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 37.Mizuno Y, Sumi Y, Nachi S, et al. Acinar cell carcinoma arising from an ectopic pancreas. Surg Today. 2007;37:704–7. doi: 10.1007/s00595-006-3384-5. [DOI] [PubMed] [Google Scholar]
- 38.Inoue Y, Hayashi M, Arisaka Y, et al. Adenocarcinoma arising in a heterotopic pancreas (Heinrich type III): A case report. J Med Case Rep. 2010;4:39. doi: 10.1186/1752-1947-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okasha HH, Al-Bassiouni F, El-Ela MA, et al. A retroperitoneal neuroendocrine tumor in ectopic pancreatic tissue. Endosc Ultrasound. 2013;2:168–70. doi: 10.7178/eus.06.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okamoto H, Fujishima F, Ishida K, et al. Intraductal papillary mucinous neoplasm originating from a jejunal heterotopic pancreas: Report of a case. Surg Today. 2014;44:349–53. doi: 10.1007/s00595-012-0486-0. [DOI] [PubMed] [Google Scholar]
- 41.Mohamed H, Dorra B, Hela B, et al. Pancreatic serous cystadenoma associated with pancreatic heterotopia. Pan Afr Med J. 2016;23:94. doi: 10.11604/pamj.2016.23.94.8533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Huang Q, Zhu LH, et al. Endoscopic excavation for gastric heterotopic pancreas: An analysis of 42 cases from a tertiary center. Wien Klin Wochenschr. 2014;126:509–14. doi: 10.1007/s00508-014-0563-z. [DOI] [PubMed] [Google Scholar]
- 43.Zinczuk J, Bandurski R, Pryczynicz A, et al. Ectopic pancreas imitating gastrointestinal stromal tumor (GIST) in the stomach. Pol Przegl Chir. 2015;87:268–71. doi: 10.1515/pjs-2015-0052. [DOI] [PubMed] [Google Scholar]
- 44.Subasinghe D, Sivaganesh S, Perera N, et al. Gastric fundal heterotopic pancreas mimicking a gastrointestinal stromal tumour (GIST): A case report and a brief review. BMC Res Notes. 2016;9:185. doi: 10.1186/s13104-016-1995-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang XC, Li QL, Yu YF, et al. Diagnostic efficacy of endoscopic ultrasound-guided needle sampling for upper gastrointestinal subepithelial lesions: A meta-analysis. Surg Endosc. 2016;30:2431–41. doi: 10.1007/s00464-015-4494-1. [DOI] [PubMed] [Google Scholar]
- 46.Hase S, Nakazawa S, Yoshino J, et al. A study on gastric and small intestinal aberrant pancreas by endoscopic ultrasonography – With special reference to comparison with histological appearance. Nihon Shokakibyo Gakkai Zasshi. 1989;86:1684–91. [PubMed] [Google Scholar]
- 47.Matsushita M, Hajiro K, Okazaki K, et al. Preoperative histological diagnosis of heterotopic pancreas. Dig Dis Sci. 1999;44:552. doi: 10.1023/a:1026657407644. [DOI] [PubMed] [Google Scholar]
- 48.Changchien CS, Hsiaw CM, Hu TH. Endoscopic ultrasonographic classification of gastric aberrant pancreas. Chang Gung Med J. 2000;23:600–7. [PubMed] [Google Scholar]
- 49.Gottschalk U, Casper B, Boden G. Ectopic pancreas presenting as a large gastric antral papilla. Endoscopy. 2003;35:547. doi: 10.1055/s-2003-39657. [DOI] [PubMed] [Google Scholar]
- 50.Chen SH, Huang WH, Feng CL, et al. Clinical analysis of ectopic pancreas with endoscopic ultrasonography: An experience in a medical center. J Gastrointest Surg. 2008;12:877–81. doi: 10.1007/s11605-008-0476-0. [DOI] [PubMed] [Google Scholar]
- 51.Park SH, Kim GH, Park do Y, et al. Endosonographic findings of gastric ectopic pancreas: A single center experience. J Gastroenterol Hepatol. 2011;26:1441–6. doi: 10.1111/j.1440-1746.2011.06764.x. [DOI] [PubMed] [Google Scholar]
- 52.Attwell A, Sams S, Fukami N. Diagnosis of ectopic pancreas by endoscopic ultrasound with fine-needle aspiration. World J Gastroenterol. 2015;21:2367–73. doi: 10.3748/wjg.v21.i8.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polkowski M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy. 2005;37:635–45. doi: 10.1055/s-2005-861422. [DOI] [PubMed] [Google Scholar]
- 54.Matsushita M, Hajiro K, Okazaki K, et al. Gastric aberrant pancreas: EUS analysis in comparison with the histology. Gastrointest Endosc. 1999;49(4 Pt 1):493–7. doi: 10.1016/s0016-5107(99)70049-0. [DOI] [PubMed] [Google Scholar]
- 55.Kim JH, Lim JS, Lee YC, et al. Endosonographic features of gastric ectopic pancreases distinguishable from mesenchymal tumors. J Gastroenterol Hepatol. 2008;23:e301–7. doi: 10.1111/j.1440-1746.2008.05351.x. [DOI] [PubMed] [Google Scholar]
- 56.Chou JW, Cheng KS, Ting CF, et al. Endosonographic features of histologically proven gastric ectopic pancreas. Gastroenterol Res Pract 2014. 2014;160601 doi: 10.1155/2014/160601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiech T, Walch A, Werner M. Histopathological classification of nonneoplastic and neoplastic gastrointestinal submucosal lesions. Endoscopy. 2005;37:630–4. doi: 10.1055/s-2005-870127. [DOI] [PubMed] [Google Scholar]
- 58.Okanobu H, Hata J, Haruma K, et al. A classification system of echogenicity for gastrointestinal neoplasms. Digestion. 2005;72:8–12. doi: 10.1159/000087216. [DOI] [PubMed] [Google Scholar]
- 59.Gress F, Schmitt C, Savides T, et al. Interobserver agreement for EUS in the evaluation and diagnosis of submucosal masses. Gastrointest Endosc. 2001;53:71–6. doi: 10.1067/mge.2001.111384. [DOI] [PubMed] [Google Scholar]
- 60.Heinrich H. Ein Beitrag zur Histologie des so genannten akzessorischen Pankreas. Virchows Arch Pathol Anat Physiol. 1909;198:392–401. [Google Scholar]
- 61.Gaspar Fuentes A, Campos Terrech JM, Fernández Burgui JC, et al. [Pancreatic ectopias] Rev Esp Enferm Apar Dig. 1973;39:255–68. [PubMed] [Google Scholar]
- 62.Jochimsen PR, Shirazi SS, Lewis JW. Symptomatic ectopic pancreas relieved by surgical excision. Surg Gynecol Obstet. 1981;153:49–52. [PubMed] [Google Scholar]
- 63.Matsushita M, Takakuwa H, Nishio A. Endosonographic features of gastric adenomyoma, a type of ectopic pancreas. Endoscopy. 2003;35:621–2. doi: 10.1055/s-2003-40222. [DOI] [PubMed] [Google Scholar]
- 64.Ryu DY, Kim GH, Park DY, et al. Endoscopic removal of gastric ectopic pancreas: An initial experience with endoscopic submucosal dissection. World J Gastroenterol. 2010;16:4589–93. doi: 10.3748/wjg.v16.i36.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bain AJ, Owens DJ, Tang RS, et al. Pancreatic rest resection using band ligation snare polypectomy. Dig Dis Sci. 2011;56:1884–8. doi: 10.1007/s10620-011-1669-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu X, Wang G, Ge N, et al. Endoscopic removal of symptomatic gastric heterotopic pancreas: A report of nine cases. Surg Innov. 2013;20:NP40–6. doi: 10.1177/1553350613499453. [DOI] [PubMed] [Google Scholar]
- 67.Goto J, Ohashi S, Okamura S, et al. Heterotopic pancreas in the esophagus diagnosed by EUS-guided FNA. Gastrointest Endosc. 2005;62:812–4. doi: 10.1016/j.gie.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 68.Jang KY, Park HS, Moon WS, et al. Heterotopic pancreas in the stomach diagnosed by endoscopic ultrasound-guided fine needle aspiration cytology. Cytopathology. 2010;21:418–20. doi: 10.1111/j.1365-2303.2009.00727.x. [DOI] [PubMed] [Google Scholar]
- 69.Rocha HL, Bueno FK, Faraco J, et al. Heterotopic pancreas complicated by pseudocyst in the gastric wall diagnosed by endoscopic ultrasound-guided fine needle aspiration. Endosc Ultrasound. 2013;2:159–61. doi: 10.7178/eus.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akahoshi K, Oya M, Koga T, et al. Clinical usefulness of endoscopic ultrasound-guided fine needle aspiration for gastric subepithelial lesions smaller than 2 cm. J Gastrointestin Liver Dis. 2014;23:405–12. doi: 10.15403/jgld.2014.1121.234.eug. [DOI] [PubMed] [Google Scholar]
- 71.Attwell A, Sams S, Fukami N. Induction of acute ectopic pancreatitis by endoscopic ultrasound with fine-needle aspiration. Clin Gastroenterol Hepatol. 2014;12:1196–8. doi: 10.1016/j.cgh.2013.11.037. [DOI] [PubMed] [Google Scholar]
- 72.Kanayama K, Imai H, Yoneda M, et al. Cytological findings of an ectopic pancreas of the stomach obtained at endoscopic ultrasound-guided fine needle aspiration, differential diagnosis from acinar cell carcinoma: A case report. Cytopathology. 2016;27:379–81. doi: 10.1111/cyt.12302. [DOI] [PubMed] [Google Scholar]


