Most people are familiar with terpenes whether they know it or not. These are the compounds that give many of our foods wonderful aromas and tastes and many of our household cleaning agents their fresh scents. But these are just some of the obvious ways we have taken advantage of these compounds. Biologically, terpenes are immensely important compounds in plants, fungi, bacteria, and insects that provide unique means for these organisms to sense and interact with their environment as well as serve as hormones that orchestrate developmental programs. For example, plants emit a wide range of terpenes that attract beneficial insects (1). Some plant families are notable also for their ability to synthesize and secrete antimicrobial terpenes as a defense compound against fungal and bacterial challenges (2). Still other plants that occupy resource-limited habitats such as deserts are known to constitutively secrete terpenes into their rhizosphere as a means of inhibiting the growth of competitor plant species (3). Fungi and bacteria likewise are known to synthesize a plethora of terpenes (4). Gibberella fujikuroi produces the well characterized plant growth regulator gibberellic acid, a diterpene, when it infects rice (5). This infection process results in unusually tall rice plants that can lodge easily and plants having other developmental abnormalities. Fusarium species and other relatives probably represent an even greater agricultural problem, because these fungi produce particularly noxious terpenes known as mycotoxins when they infect wheat and other stored grains (6).
What makes terpenes so unique, however, is the chemical wizardry nature has mastered to produce these compounds. Of the tens of thousands of natural products identified to date, ≈¼–½ of these are estimated to be terpenes. At first glance, this preponderance of terpenes may not seem such a difficult problem. The more than 20,000 different terpene compounds identified to date are derived largely from three biosynthetically related, yet simple-looking precursors (Fig. 1). These precursors, GPP, FPP, and GGPP, are essentially polymers of two, three, or four isoprene groups (5-carbon alkene units) covalently tethered together and punctuated by a terminal diphosphate group. The magic begins when one starts to gaze at the amazing number of structural derivatives that can arise from each of these precursors and then considers how this diversity might arise by the action of a single biosynthetic step catalyzed by a terpene synthase. (Although terpene synthase is the proper term used in reference to these enzymes, many investigators prefer “cyclase” because of its descriptive value.) Somehow the terpene cyclases are capable of binding the allylic diphosphate substrates and initiating catalysis by cleaving off the diphosphate group, leaving behind a reactive allylic carbocation. Next, these same enzymes must protect the reactive carbocation(s) from being prematurely quenched or lost to the bulk solvent while simultaneously coaxing the reaction intermediates down one of an almost infinite number of reaction cascades involving two, three, or more partial reactions. Finally, the terpene cyclases must release these hydrophobic reaction products into an aqueous, hydrophilic environment. Although our understanding of these amazing catalysts has progressed nicely in the recent past, another important step forward in unraveling the secrets of the terpene cyclases is reported in this issue by Rynkiewicz et al. (7).
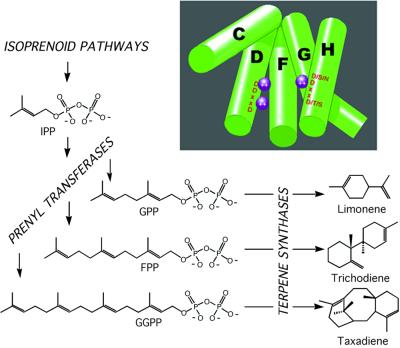
Figure 1.
All terpenes are derived from allylic diphosphates, which are polymers of repeating isopentyl units (IPP) put together by the action of prenyltransferases. Monoterpenes are derived from the C10 precursor geranyl diphosphate (GPP), sesquiterpenes from farnesyl diphosphate (FPP), and diterpenes from geranylgeranyldiphosphate (GGPP) by the action of terpene synthases or cyclases. The terpene synthases impose unique regio- and stereochemical configurations onto the reaction intermediates during catalysis that impart novel characteristics to the reaction products. (Insert) A depiction of the terpene synthase fold, five of the most highly conserved structural helices that make up the catalytic or active site of terpene synthases (7, 18).
Many of the synthetic approaches to the building of organic molecules have attempted to capture the magic of the terpene synthases by mimicking various facets of these reactions. However, it is probably the regio- and stereochemical aspects of these enzymes that have drawn the greatest attention of those working in the field (Fig. 2). The regiochemistry reference simply means that there are a number of options for carbon–carbon bond formation during catalysis. The terpene cyclases are specialists at this, because they dictate which carbons form bonds with which and do it with extremely high fidelity. The reference to stereochemical control refers to the fact that some of the bonds formed during catalysis occur around chiral centers. This means there are two sides or faces to a typical terpene molecule and that the terpene cyclase enzymes have no difficulty in specifying bond formation to one side or the other but generally not a mixture of both.
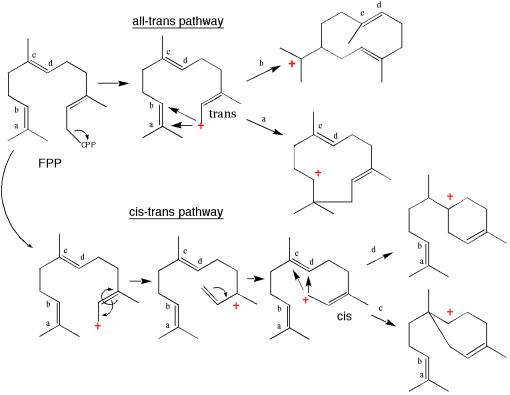
Figure 2.
Terpene synthases catalyze regio- and stereo-specific reactions that are illustrated here for sesquiterpene cyclases. Pentalenene synthase (16), aristolochene synthase (14), and epi-aristolochene synthase (15) catalyze all-trans pathway reactions, and trichodiene synthase (7) catalyzes a cis-trans-mediated reaction.
Significant progress has been made during the last decade in detailing the biochemical reactions of terpene synthases (8–10) and sesquiterpene synthases (11–13) in particular. For example, over 190 sesquiterpene cyclase gene sequences now are reported in GenBank, a significant number of which have been expressed functionally in heterologous hosts, and impressive progress in probing structure-function relationships with a combination of site-directed mutagenesis (8, 12, 13) and crystallographic studies (14–18) has been reported. However, much of that progress has been limited to a particular class of sesquiterpene cyclases. The cyclase enzymes such as pentalenene synthase (16) from a bacterium, aristolochene synthase (14) from a fungus, and epi-aristolochene synthase from a plant (15) all are functional in monomeric form, and each appears to have a couple of conserved metal binding motifs, the DDXXD motif, that was proposed originally as a coordination site for the binding of the diphosphate moiety of FPP to the enzymes (19). Equally important is that these enzymes channel substrate down a reaction pathway that we refer to as the all-trans pathway (Fig. 2). That is, these enzymes catalyze the ionization of FPP followed by the formation of a macrocyclic intermediate by bond formation between C1 with either C10 (aristolochene and epi-aristolochene synthase) or C11 (pentalenene synthase).
The report by Rynkiewicz et al. (7) takes our understanding of cyclases forward on three important fronts by determining the structure of trichodiene synthase, a sesquiterpene synthase that uses the cis-trans pathway of catalysis (Fig. 2). First, although there were earlier indications from conventional biochemical studies that trichodiene synthase and several other terpene synthases might form quaternary structures (20), the solved structure now provides unequivocal evidence for this assertion. A second important finding is that a major confirmation change occurs when pyrophosphate is bound to the trichodiene synthase. This induced change is analogous to that reported previously for epi-aristolochene synthase when a nonhydrolyzable FPP analog bound to the enzyme (15). Now Rynkiewicz et al. (7) show that binding of pyrophosphate alone is sufficient to cause this conformational change. That pyrophosphate binding alone induces this confirmation change is significant and supports previous speculation about how pyrophosphate binding could serve as a trigger to initiate catalysis (21).
The third and perhaps most intriguing observation from this work is the overall conservation of a three-dimensional structure known as the terpene fold in trichodiene synthase (Fig. 1). This fold was observed earlier in pentalenene synthase (16), aristolochene synthase (14), and epi-aristolochene synthase (15), all enzymes that catalyze the cyclization of FPP down the all-trans pathway (Fig. 2). The terpene fold is present also in two other terpene biosynthetic enzymes, farnesyl diphosphate synthase (17) and squalene synthase (18). Both of these enzymes use similar reaction mechanisms as the terpene cyclases except that FPP synthase condenses two allylic diphosphates in a head-to-tail fashion to generate FPP, whereas squalene synthase catalyzes the head-to-head condensation of two FPP moieties to create the 30-carbon intermediate squalene. Trichodiene synthase catalyzes the cyclization of FPP down a very different reaction cascade, the cis-trans pathway (Fig. 2), in comparison to the cyclases, the structures of which were determined previously. Hence, there was at least some anticipation that the trichodiene synthase structure might reveal a new spatial motif capable of directing different chemical magic.
Overall, the conservation of the terpene fold in all these very different terpene biosynthetic enzymes is and is not surprising. Conservation of the fold is surprising, because there is very little primary sequence similarity between the fungal sesquiterpene synthases and any of the other enzymes except for the rudiments of the aspartate-rich domains that play a role in metal binding. However, conservation of the terpene fold perhaps is not surprising when one considers the similarities observed in plant terpene synthases (2, 22, 23). For the several hundred mono-, sesqui-, and diterpene synthases now isolated and sequenced from a wide range of plant species including monocots and dicots, a surprising degree of sequence similarity is observed. For example, even though monocots and dicots are thought to have split from one another 90 million years ago (24), ≈600 million years after the split of true fungi from plants (25), maize sesquiterpene synthases are upwards of 40% similar to the tobacco terpene synthases (23). Perhaps more telling is the conservation of intron placement within the plant terpene synthases, which seems extremely well preserved (22). Certainly it seems as if all the plant terpene synthases diverged from a common ancestral gene. Whether this ancestral gene is common to that giving rise to the fungal terpene synthase genes is a bit more obtuse and difficult to ascertain. With the report by Rynkiewicz et al. (7), a fairly strong case for divergent evolution from a common ancestral gene for the fungal and plant terpene synthases, FPP synthase, and squalene synthase can be made by using some standard criteria (26). One, all these enzymes share similar catalytic mechanisms. Two, the substrates appear to bind to the enzymes similarly (via the diphosphate substituent). Three, the functional domains or segments of the proteins comprising the active site are arrayed in a similar linear order across the various primary sequences for these proteins. Four, there is excellent conservation of the terpene synthase fold across the board.
The suggestion that the terpene synthases evolved by a divergent mechanism rather than a convergent mechanism has interesting implications for future experimental work. Because the terpene synthase fold defines the active site scaffold for all these enzymes, the divergent model predicts that it is simply the way in which the various amino acid side chains are arrayed around the active site that distinguishes one terpene synthase activity from another. This does not mean we should stop isolating terpene cyclase genes and characterizing the encoded enzymes. Quite the contrary, it suggests that such comparative studies should help correlate specific synthase functions (i.e., all-trans versus cis-trans catalytic pathways) with the primary sequences found within the domains making up the terpene synthase fold. Another prediction arising from the divergent evolution model for terpene synthases is that it should be possible to capture all the known and unknown catalytic functions of terpene synthases by simply substituting the various active site residues around one terpene synthase fold or scaffold. In short, we seem well poised to gain an understanding rather than just to discover the chemical wizardry that has made the terpene synthases so enigmatic.
Footnotes
See companion article on page 13543.
References
- 1.Kessler A, Baldwin I T. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- 2.Chappell J. Plant Physiol. 1995;107:1–6. doi: 10.1104/pp.107.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens K L. Isopentenoids in Plants. New York: Dekker; 1984. pp. 63–80. [Google Scholar]
- 4.Connolly J D, Hill R A. Dictionary of Terpenoids. New York: Chapman & Hall; 1992. [Google Scholar]
- 5.MacMillan J. Nat Prod Rep. 1997;14:221–243. [Google Scholar]
- 6.Desjardins A E, Hohn T M. Mol Plant–Microbe Interact. 1997;10:147–152. doi: 10.1094/mpmi-5-249. [DOI] [PubMed] [Google Scholar]
- 7.Rynkiewicz M J, Cane D E, Christianson D W. Proc Natl Acad Sci USA. 2001;98:13543–13548. doi: 10.1073/pnas.231313098. . (First Published November 6, 2001; 10.1073/pnas.231313098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams D C, McGarvey D J, Katahira E J, Croteau R. Biochemistry. 1998;37:12213–12220. doi: 10.1021/bi980854k. [DOI] [PubMed] [Google Scholar]
- 9.Ravn M M, Coates R M, Flory J E, Peters R J, Croteau R. Org Lett. 2000;2:573–576. doi: 10.1021/ol991230p. [DOI] [PubMed] [Google Scholar]
- 10.Schwab W, Williams D C, Davis E M, Croteau R. Arch Biochem Biophys. 2001;392:123–136. doi: 10.1006/abbi.2001.2442. [DOI] [PubMed] [Google Scholar]
- 11.Cane D E, Tsantrizos Y S. J Am Chem Soc. 1996;118:10037–10040. [Google Scholar]
- 12.Rising K A, Starks C M, Noel J P, Chappell J. J Am Chem Soc. 2000;122:1861–1866. [Google Scholar]
- 13.Cane D E, Xue Q, Van Epp J, Tsantrizos Y S. J Am Chem Soc. 1996;118:8499–8500. [Google Scholar]
- 14.Caruthers J M, Kang I, Rynkiewicz M J, Cane D E, Christianson D W. J Biol Chem. 2000;275:25533–25539. doi: 10.1074/jbc.M000433200. [DOI] [PubMed] [Google Scholar]
- 15.Starks C M, Back K, Chappell J, Noel J P. Science. 1997;277:1815–1819. doi: 10.1126/science.277.5333.1815. [DOI] [PubMed] [Google Scholar]
- 16.Lesburg C A, Zhai G, Cane D E, Christianson D W. Science. 1997;277:1820–1824. doi: 10.1126/science.277.5333.1820. [DOI] [PubMed] [Google Scholar]
- 17.Tarshis L C, Yan M, Poulter C D, Sacchettini J C. Biochemistry. 1994;33:10871–10877. doi: 10.1021/bi00202a004. [DOI] [PubMed] [Google Scholar]
- 18.Pandit J, Danley D E, Schulte G K, Mazzalupo S, Pauly TA, Hayward C M, Humanaka E S, Thompson J F, Harwood H J. J Biol Chem. 2000;275:30610–30617. doi: 10.1074/jbc.M004132200. [DOI] [PubMed] [Google Scholar]
- 19.Song L, Poulter D C. Proc Natl Acad Sci. 1994;91:3044–3048. doi: 10.1073/pnas.91.8.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cane D E, Wu Z, Oliver J S, Hohn T M. Arch Biochem Biophys. 1993;300:416–422. doi: 10.1006/abbi.1993.1056. [DOI] [PubMed] [Google Scholar]
- 21.Lesburg C A, Caruthers J M, Paschall CM, Christianson D W. Curr Opin Struct Biol. 1998;8:695–703. doi: 10.1016/s0959-440x(98)80088-2. [DOI] [PubMed] [Google Scholar]
- 22.Trapp S C, Croteau R B. Genetics. 2001;158:811–832. doi: 10.1093/genetics/158.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen B, Zheng Z, Dooner H K. Proc Natl Acad Sci USA. 2000;97:14807–14812. doi: 10.1073/pnas.240284097. . (First Published December 5, 2000; 10.1073/pnas.240284097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gandolfo M A, Nixon K C, Crepet W L, Stevenson D W, Friis E M. Nature (London) 1998;394:532–533. [Google Scholar]
- 25.Stewart W N, Rothwell G W. Paleobotany and the Evolution of Plants. 2nd Ed. Cambridge, U.K.: Cambridge Univ. Press; 1993. p. 57. [Google Scholar]
- 26.Fersht A. Enzyme Structure and Mechanism. New York: Freeman; 1984. pp. 22–23. [Google Scholar]