Abstract
We report an Elizabethkingia anophelis case cluster associated with contaminated aerators and tap water in a children’s intensive care unit in Singapore in 2017. We demonstrate a likely transmission route for E. anophelis to patients through acquisition of the bacteria on hands of healthcare workers via handwashing.
Keywords: Elizabethkingia, Elizabethkingia anophelis, pediatrics, intensive care unit, ICU, aerator, water, alcohol handrub, handwashing, outbreak, bacteria, Singapore, tap water
Elizabethkingia anophelis is an emergent pathogen first described from midgut specimens of the Anopheles gambiae mosquito (1). To date, there have been 2 reported confirmed E. anophelis outbreaks in humans. One occurred in an adult critical care unit in Singapore; the second was a large community outbreak in the United States (Wisconsin, Michigan, and Illinois) (2–5). Water sources have been identified to harbor members of the genus Elizabethkingia, but the source of the community outbreak in the United States remains unknown (3,6). Effective interventions for outbreak control and transmission routes of E. anophelis remain unclear (3).
KK Women’s and Children’s Hospital (KKH) is the single largest public tertiary-care specialist women’s and children’s hospital in Singapore. The Children’s Intensive Care Unit (CICU) is a 16-bed unit that provides advanced monitoring and therapeutic technologies for critical pediatric cases. On May 30, 2017, an alert was triggered due to the detection of 3 patients with Elizabethkingia spp. within 13 days in the unit. The incidence rate of the cluster, 2.87/1,000 bed-days, was ≈4 times higher than the average rate in the previous 5 years, 0.63/1,000 bed-days (2012 through 2016). Initially, the strains were reported as E. meningoseptica, but subsequent testing confirmed the cluster to be associated with E. anophelis. We conducted an epidemiologic investigation to identify the source of the cluster. We also conducted a pragmatic experiment to test our hypothesis that E. anophelis could be transmitted by healthcare workers during handwashing with water contaminated with E. anophelis.
The Study
We collated clinical and epidemiologic data using a standardized spreadsheet for all patients testing positive for Elizabethkingia species in the KKH CICU in 2017. We also performed environmental sampling on all tap outlets and sinks in the clinical areas. For each tap, we swabbed the aerator and collected a water sample for culture. The water source of KKH has no supplemental treatments and meets WHO guidelines for drinking-water quality (7). To test our transmission hypothesis, we had 2 volunteer nurses place their hands on agar plates at 3 stages: before handwashing; after handwashing with chlorhexidine soap (4% wt/vol chlorhexidine gluconate; Microshield 4 Chlorhexidine Surgical Handwash, Schülke; Norderstedt, Germany) and tap water from the tap outlet in CICU known to be positive for E. anopheles; and finally after hand hygiene using alcohol-based hand rub (ABHR) (70% vol/vol ethanol and 0.5% wt/vol chlorhexidine gluconate; Microshield Handrub; Schülke).
We tested samples using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (VITEK MS; bioMérieux, Marcy-l’Étoile, France). We retested all samples positive for Elizabethkingia spp. by using 16S rDNA PCR: we extracted bacterial DNA and amplified 16S rDNA using primers 27f and 1492r (8). We performed sequencing using standard protocols and used BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) for comparison with database sequences.
The 3 cluster cases were the only patients positive for Elizabethkingia species in the CICU in 2017. All were detected from blind bronchial sampling (BBS) via endotracheal tube (ETT). (Table 1) Patient 3’s isolate was confirmed as E. anophelis. Unfortunately, the samples from the first 2 cases were not available for follow-up confirmatory testing. The patients were 2.8 months, 4.9 months, and 4.8 years of age, and all had significant underlying medical conditions. The average number of days in CICU before detection of Elizabethkingia species was 36 (range 11–66). None of the patients had been moved since admission, and 2 were cared for in single rooms.
Table 1. Characteristics of Elizabethkingia cases in children admitted to the Children’s Intensive Care Unit, KK Women’s and Children’s Hospital, Singapore, May 2017*.
| Category | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Sample date | 2017 May 15 | 2017 May 22 | 2017 May 28 |
| Sample type | ETT, BBS | ETT, BBS | ETT, BBS |
| Bacterial identification | |||
| MALDI-TOF mass spectrometry | E. meningoseptica | E. meningoseptica | E. meningoseptica |
| 16S rDNA | Isolate not available | Isolate not available | E. anophelis |
| Sex | M | F | F |
| Age, mo | 4.9 | 2.8 | 57.9 |
| Preterm birth | No | No | No |
| Underlying clinical condition | Duodenal atresia; small atrial septal heart defect | Pulmonary atresia; Large ventral septal heart defect; large patent ductus arteriosus | Thoracic tumor |
| Outcome | Discharged | Discharged | Deceased |
| Days in hospital | 11 | 83 | 33 |
| CICU bed type | Single room | 4-bed cubicle | Single room |
| Other beds used | No | No | No |
| Antimicrobial drug treatment within 72 h before detection | Piperacillin/tazobactam, ceftriaxone | Clindamycin | Piperacillin/tazobactam |
| History of immunosuppressive medication | No | No | Yes (chemotherapy) |
| On ECMO at time of detection | Yes | No | Yes |
*ETT, endotracheal tube; CICU, Children’s Intensive Care Unit; ECMO, extracorporeal membrane oxygenation; ETT, endotracheal tube; MALDI-TOF, matrix-assisted laser desorption/ionization time-of-flight.
Of the 27 environmental samples collected from 9 tap outlets or sinks in the unit, 10 samples were positive for E. anophelis and 1 positive for E. meningoseptica. Only 1 room (single bed) in the unit was negative for Elizabethkingia bacteria. All 3 Elizabethkingia case-patients’ rooms or cubicles were confirmed positive for E. anophelis from their respective tap outlets (aerator or water or both). The tap outlet from 1 cubicle not associated with any of the cases was positive for both Elizabethkingia species, E. meningoseptica in water and E. anophelis in the aerator. The Figure illustrates the spatial distribution of Elizabethkingia bacteria detected in tap outlets stratified by aerator, water, or sinks in the unit.
Figure.
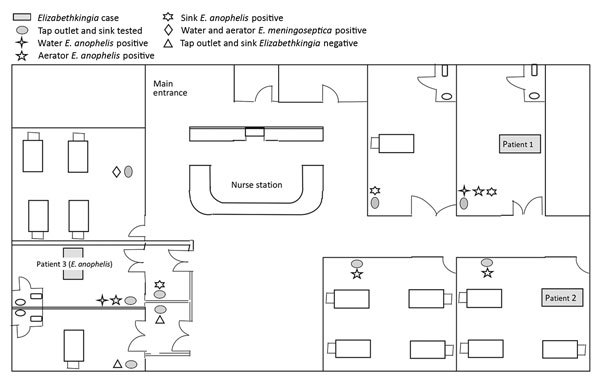
Spatial distribution of Elizabethkingia isolates by location (patients, tap water, aerators, and sinks) in children's intensive care unit, KK Women’s and Children’s Hospital, Singapore, May 2017.
Our transmission experiment found that 1 staff member (staff B) acquired E. anophelis on her hands after handwashing (Table 2). After hand hygiene using ABHR, both staff members had no detectable microbial growth on their hands.
Table 2. Potential transmission route of E. anophelis via handwashing for 2 hospital staff, Children’s Intensive Care Unit, KK Women’s and Children’s Hospital, Singapore, May 2017.
| Procedure |
Hands culture result |
|
| Staff A |
Staff B |
|
| Before handwashing | Coagulase-negative Staphylococcus sp. | Coagulase-negative Staphylococcus sp. |
| After handwashing with chlorhexidine soap | Coagulase-negative Staphylococcus sp. | E. anophelis |
| After use of alcohol-based hand rub | No growth | No growth |
Upon detection of the case cluster, we reinforced standard precautions, specifically hand hygiene compliance, and implemented environmental and patient-care equipment cleaning. We had all aerators permanently removed from the tap outlets in the CICU following confirmation of Elizabethkingia bacteria. The water from all 5 tap outlets previously found to be positive for Elizabethkingia bacteria in aerator or water was negative upon repeat testing after the intervention. We also recommended prioritizing hand hygiene using ABHR over handwashing unless hands were visibly soiled. All staff were reminded not to dispose of body fluids from patients into sinks used for handwashing because this was previously identified to be associated with Elizabethkingia tap colonization (2). In addition, we ended the use of tap water for patient care and allowed only sterile water. After these interventions, no additional cases of Elizabethkingia occurred in the unit for >4 months.
Conclusions
We report a confirmed E. anophelis case cluster affecting infants and children in the CICU of a pediatric hospital. Our investigation identified the likely source of E. anophelis to be tap outlets with aerators. We confirmed that removal of the aerators was effective in eliminating E. anophelis from tap water sources. We also demonstrated a likely transmission route for E. anophelis to patients through acquisition of the bacteria on hands of healthcare workers via handwashing. Subsequent use of ABHR was effective in eliminating the acquired E. anophelis from workers’ hands.
Although 2 patients’ isolates were not available for confirmatory testing, we detected E. anophelis in the tap outlets where they were cared for, suggesting that the Elizabethkingia species detected in their samples was highly likely to be E. anophelis. Isolates were initially misidentified as E. meningoseptica by MALDI-TOF mass spectrometry because E. anophelis was not represented in our routine database and only present in research databases of MALDI-TOF mass spectrometry systems (9). This discrepancy means that E. anophelis is probably overlooked in most diagnostic microbiology laboratories. There is a clinical need to differentiate these species in light of observations that E. anophelis infections tend to be more severe and associated with more deaths than are E. meningoseptica infections (10).
We showed how handwashing, despite the use of chlorhexidine soap, is a possible vehicle of transmission for E. anophelis from an affected tap outlet via the hands of healthcare workers to patients. Perinatal transmission of E. anophelis was previously documented to have occurred from a mother with chorioamnionitis to her neonate (11). We confirmed that hand hygiene using ABHR was effective in removing E. anophelis from hands of healthcare workers, which has implications for infection control. Although current hand hygiene guidelines prioritize ABHR over handwashing when hands are not visibly soiled, there is no requirement to perform ABHR in addition to handwashing (12). Therefore, most staff consider handwashing as complying with hand hygiene requirements. Our findings support using ABHR as the primary hand-hygiene method in clinical care, especially in critical care units and in outbreak situations involving waterborne organisms such as E. anophelis.
Acknowledgments
We thank hospital colleagues and staff, especially those from the CICU, Infection Control Unit, Facilities Management, and Environmental Services for their support and dedication in controlling the cluster and ensuring safe care for patients.
Biography
Dr. Yung is a consultant with the Infectious Disease Service, KK Women’s and Children’s Hospital, Singapore. He has a keen interest in infectious diseases, vaccines, outbreak control, epidemiology, and public health.
Footnotes
Suggested citation for this article: Yung CF, Maiwald M, Loo LH, Soong HY, Tan CB, Lim PK, et al. Elizabethkingia anophelis and association with tap water and handwashing, Singapore. Emerg Infect Dis. 2018 Sep [date cited]. https://doi.org/10.3201/eid2409.171843
References
- 1.Kämpfer P, Matthews H, Glaeser SP, Martin K, Lodders N, Faye I. Elizabethkingia anophelis sp. nov., isolated from the midgut of the mosquito Anopheles gambiae. Int J Syst Evol Microbiol. 2011;61:2670–5. 10.1099/ijs.0.026393-0 [DOI] [PubMed] [Google Scholar]
- 2.Balm MN, Salmon S, Jureen R, Teo C, Mahdi R, Seetoh T, et al. Bad design, bad practices, bad bugs: frustrations in controlling an outbreak of Elizabethkingia meningoseptica in intensive care units. J Hosp Infect. 2013;85:134–40. 10.1016/j.jhin.2013.05.012 [DOI] [PubMed] [Google Scholar]
- 3.CDC. Multi-state cluster of Elizabethkingia anophelis in Wisconsin, Michigan, and Illinois. 2016. [cited 2017 Sep 28]. https://www.cdc.gov/elizabethkingia/outbreaks/index.html
- 4.Perrin A, Larsonneur E, Nicholson AC, Edwards DJ, Gundlach KM, Whitney AM, et al. Evolutionary dynamics and genomic features of the Elizabethkingia anophelis 2015 to 2016 Wisconsin outbreak strain. Nat Commun. 2017;8:15483. 10.1038/ncomms15483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teo J, Tan SYY, Liu Y, Tay M, Ding Y, Li Y, et al. Comparative genomic analysis of malaria mosquito vector-associated novel pathogen Elizabethkingia anophelis. Genome Biol Evol. 2014;6:1158–65. 10.1093/gbe/evu094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore LSP, Owens DS, Jepson A, Turton JF, Ashworth S, Donaldson H, et al. Waterborne Elizabethkingia meningoseptica in adult critical care. Emerg Infect Dis. 2016;22:9–17. 10.3201/eid2201.150139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Guidelines for drinking-water quality (GDWQ). 2017. [cited 2018 Jul 9]. http://www.who.int/water_sanitation_health/water-quality/guidelines/en/
- 8.Maiwald M. Broad-range PCR for detection and identification of bacteria. In: Persing DH, Tenover FC, Tang YW, Nolte FS, Hayden RT, van Belkum A, editors. Molecular microbiology: diagnostic principles and practice, 2nd ed. Washington: American Society for Microbiology; 2011. p. 491–505. [Google Scholar]
- 9.Han MSKH, Kim H, Lee Y, Kim M, Ku NS, Choi JY, et al. Relative prevalence and antimicrobial susceptibility of clinical isolates of Elizabethkingia species based on 16S rRNA gene sequencing. J Clin Microbiol. 2016;55:274–80. 10.1128/JCM.01637-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau SK, Chow WN, Foo CH, Curreem SO, Lo GC, Teng JL, et al. Elizabethkingia anophelis bacteremia is associated with clinically significant infections and high mortality. Sci Rep. 2016;6:26045. 10.1038/srep26045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau SKP, Wu AKL, Teng JLL, Tse H, Curreem SO, Tsui SK, et al. Evidence for Elizabethkingia anophelis transmission from mother to infant, Hong Kong. Emerg Infect Dis. 2015;21:232–41. 10.3201/eid2102.140623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. WHO guidelines on hand hygiene in health care. Geneva: The Organization; 2009. [Google Scholar]


