Abstract
We sequenced the virus genomes from 3 pregnant women in Thailand with Zika virus diagnoses. All had infections with the Asian lineage. The woman infected at gestational week 9, and not those infected at weeks 20 and 24, had a fetus with microcephaly. Asian lineage Zika viruses can cause microcephaly.
Keywords: Zika virus, genome sequence, congenital Zika virus infection with microcephaly, pregnancy, viruses, vector-borne infections, Thailand, birth defects, congenital infection, microcephaly, Asian lineage
Although Zika virus has circulated in Asia longer than in the Americas, only 3 confirmed cases of congenital Zika virus infection with microcephaly have been reported in Asia (2 Thailand, 1 Vietnam) (1). As of June 2018, the genomic sequences of the viruses from these 3 cases have not been reported; thus, whether these cases were caused by an Asian lineage or an imported American lineage is unknown.
Several mechanisms involving virus genome sequences have been proposed to explain how Zika virus might cause microcephaly (2). Liang et al. (3) showed in vitro that replication of both the African (strains MR766 and IbH30656) and American (strain H/PF/2013) lineage viruses suppress Akt phosphorylation; this suppression is caused by an accumulation of mutations in the Zika virus genome that increase the number of phosphorylation sites on virus proteins that compete with host proteins for phosphorylation. Yuan et al. proposed that a serine to asparagine substitution (S17N) in the premembrane protein (stably conserved in the American lineage but not in the Asian) contributes to the onset of microcephaly (4). An increased frequency of retinoic acid response elements in the American lineage genome versus the Asian lineage genome has also been observed (2). We question these explanations because we report a confirmed case of congenital Zika virus infection with microcephaly in Thailand caused by an Asian lineage virus.
We sequenced 7 Zika virus genomes obtained from 5 patients, including 3 pregnant women (PW1–3), in 2016 and 2017. PW1 had fever, maculopapular rash, and mild conjunctivitis at 24 weeks of gestation. Her urine sample was positive for Zika virus (BKK05, GenBank accession no. MG807647), and she gave birth to an infant without birth defects at full term. PW2 had a suspected Zika virus infection at 9 weeks’ gestation with high fever, maculopapular rash, and mild conjunctivitis. At 16 weeks, a sample of the amniotic fluid was positive for Zika virus (BKK03, GenBank accession no. MG548660). The pregnancy was terminated at 17 weeks. Autopsy of the fetus demonstrated a head circumference of 12.5 cm (less than the third percentile for this gestational age); Zika virus was detected in the brain (BKK02, GenBank accession no. MF996804) and placenta (BKK04, GenBank accession no. MG548661). No other etiologic agents associated with birth defects (cytomegalovirus, herpes simplex virus types 1 and 2, rubella virus, syphilis virus, Toxoplasma gondii, Treponema pallidum) were detectable by real-time PCR. PW2 had detectable hepatitis B viral surface antigen but no concurrent medical conditions. These findings suggest that Zika virus was the causative agent of this case of microcephaly. PW3 had a maculopapular rash without fever or conjunctivitis and received a Zika virus diagnosis at 20 weeks’ gestation. Her urine sample was positive for Zika virus (BKK07, GenBank accession no. MH013290), and she gave birth to a healthy infant at full term. The last 2 samples were from a 6-year-old child with mild fever and maculopapular rash (BKK06, GenBank accession no. MG807647) and a 64-year-old man with fever and maculopapular rash (BKK01, GenBank accession no. KY272987).
We retrieved 121 nonredundant Zika virus genomes (444 viruses, 99.9% nucleic acid identity cutoff) from GenBank to compare these isolates by phylogenetic analysis. All 7 BKK Zika virus isolates grouped within the Asian lineage (Figure). Virus from the amniotic fluid (BKK03), fetal brain (BKK02), and placenta (BKK04) of PW2 closely resembled each other (5 mismatches in BKK04 and 6 in BKK03, overall 99.898% identity). These 3 isolates were separated on the tree from their closest neighbor, a 2016 isolate from Singapore, by 40 mismatches. The number of retinoic acid response elements and predicted phosphorylation sites in BKK01–BKK07 was the same as the number in other Asian lineage Zika viruses (2). Also, the S17N substitution in premembrane was absent in all 7 isolates. Thus, all 3 proposed mechanisms failed to explain the case of congenital Zika virus infection with microcephaly in PW2. This case clinically resembled that of a woman in Finland infected during week 11 of pregnancy while traveling in Mexico, Guatemala, and Belize (5); in that case, Zika virus was detected in the brain of the aborted fetus at week 21.
Figure.
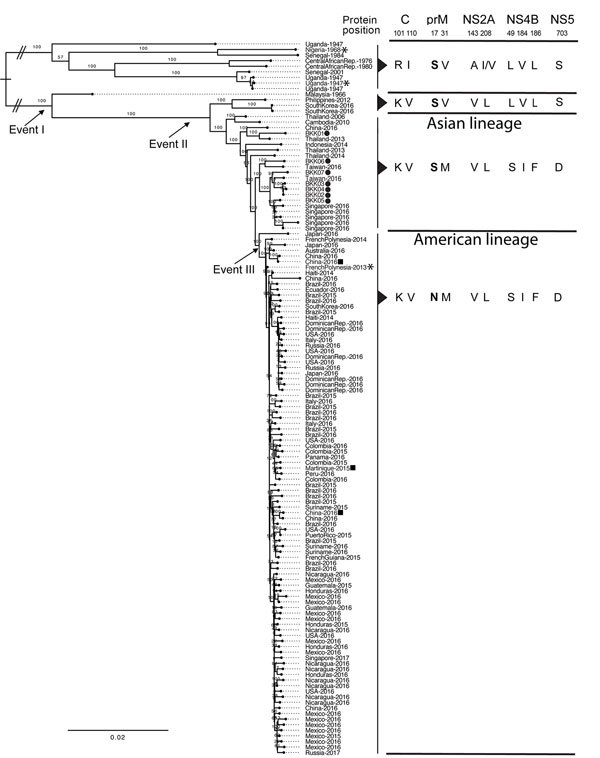
Maximum-likelihood phylogenetic analysis of nonredundant Zika virus genomes including 7 isolates from patients in Thailand, 2016–2017, and amino acid changes corresponding with 3 evolutionary events (2). Circles indicate the Zika virus isolates from this report; the Zika virus strains used by Liang et al. (3) are indicated by asterisks and Yuan et al. (4) by squares. The key amino acid residue changes corresponding with the 3 evolutionary events (2) are shown, and the conserved amino acid substitution S17N, present in the American lineage but not in the other lineages, is in bold. The amino acid residues of the 7 isolates from this report are identical to those of the other Asian lineage isolates. C, capsid; prM, premembrane; NS, nonstructural protein. Scale bar indicates nucleotide changes per basepair.
The 3 cases in pregnant women described here support the hypothesis that the timing of Zika virus infection during pregnancy might be a key contributor to the development of microcephaly during congenital Zika virus infection. PW2 was infected around week 9 of gestation, during embryonic neurulation and cortical neurogenesis, which lay the foundation for the developing brain. Infection during week 20 (for PW3) and 24 (for PW1) of gestation did not led to microcephaly. Our observations are in agreement with reports involving American lineage Zika viruses that show a high risk for microcephaly when infection occurs before week 21 (6), during weeks 7–14 (7), or during the first trimester (8–10). Our findings show that Zika viruses circulating in Asia can cause microcephaly, just like American strains.
Acknowledgments
This work was funded in part by Siriraj Research (grant no. RO16034012), the Helen Adams & Arkansas Research Alliance Endowed Chair, and the National Institute of General Medical Sciences of the National Institutes of Health (award no. P20GM125503).
Biography
Dr. Wongsurawat is a postdoctoral research fellow at the Arkansas Center for Genomic Epidemiology & Medicine in Little Rock, Arkansas, USA. Her primary research interests are applying next-generation and third-generation sequencing technologies to perform DNA and RNA viral genome and metagenome sequencing.
Footnotes
Suggested citation for this article: Wongsurawat T, Athipanyasilp N, Jenjaroenpun P, Jun S-R, Kaewnapan B, Wassenaar TM, et al. Case of microcephaly after congenital infection with Asian lineage Zika virus, Thailand. Emerg Infect Dis. 2018 Sep [date cited]. https://doi.org/10.3201/eid2409.180416
These first authors contributed equally to this article.
These senior authors contributed equally to this article.
References
- 1.Lim SK, Lim JK, Yoon IK. An update on Zika virus in Asia. Infect Chemother. 2017;49:91–100. 10.3947/ic.2017.49.2.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jun SR, Wassenaar TM, Wanchai V, Patumcharoenpol P, Nookaew I, Ussery DW. Suggested mechanisms for Zika virus causing microcephaly: what do the genomes tell us? BMC Bioinformatics. 2017;18(Suppl 14):471. 10.1186/s12859-017-1894-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang Q, Luo Z, Zeng J, Chen W, Foo SS, Lee SA, et al. Zika virus NS4A and NS4B proteins deregulate akt-mTOR signaling in human fetal neural stem cells to inhibit neurogenesis and induce autophagy. Cell Stem Cell. 2016;19:663–71. 10.1016/j.stem.2016.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan L, Huang XY, Liu ZY, Zhang F, Zhu XL, Yu JY, et al. A single mutation in the prM protein of Zika virus contributes to fetal microcephaly. Science. 2017;358:933–6. 10.1126/science.aam7120 [DOI] [PubMed] [Google Scholar]
- 5.Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jääskeläinen AJ, Smura T, et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med. 2016;374:2142–51. 10.1056/NEJMoa1601824 [DOI] [PubMed] [Google Scholar]
- 6.Sohan K, Cyrus CA. Ultrasonographic observations of the fetal brain in the first 100 pregnant women with Zika virus infection in Trinidad and Tobago. Int J Gynaecol Obstet. 2017;139:278–83. 10.1002/ijgo.12313 [DOI] [PubMed] [Google Scholar]
- 7.Parra-Saavedra M, Reefhuis J, Piraquive JP, Gilboa SM, Badell ML, Moore CA, et al. Serial head and brain imaging of 17 fetuses with confirmed Zika virus infection in Colombia, South America. Obstet Gynecol. 2017;130:207–12. 10.1097/AOG.0000000000002105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleber de Oliveira W, Cortez-Escalante J, De Oliveira WT, do Carmo GM, Henriques CM, Coelho GE, et al. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:242–7. 10.15585/mmwr.mm6509e2 [DOI] [PubMed] [Google Scholar]
- 9.Cuevas EL, Tong VT, Rozo N, Valencia D, Pacheco O, Gilboa SM, et al. Preliminary report of microcephaly potentially associated with Zika virus infection during pregnancy—Colombia, January–November 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1409–13. 10.15585/mmwr.mm6549e1 [DOI] [PubMed] [Google Scholar]
- 10.Reynolds MR, Jones AM, Petersen EE, Lee EH, Rice ME, Bingham A, et al. ; U.S. Zika Pregnancy Registry Collaboration. Vital Signs: Update on Zika virus–associated birth defects and evaluation of all U.S. infants with congenital Zika virus exposure—U.S. Zika Pregnancy Registry, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:366–73. 10.15585/mmwr.mm6613e1 [DOI] [PMC free article] [PubMed] [Google Scholar]


