Abstract
Qingdai, a traditional Chinese medicine (TCM) used for the treatment of chronic myeloid leukemia (CML) with good efficacy, has been used in China for decades. However, due to the complexity of traditional Chinese medicinal compounds, the pharmacological mechanism of Qingdai needs further research. In this study, we investigated the pharmacological mechanisms of Qingdai in the treatment of CML using network pharmacology approaches.
First, components in Qingdai that were selected by pharmacokinetic profiles and biological activity predicted putative targets based on a combination of 2D and 3D similarity measures with known ligands. Then, an interaction network of Qingdai putative targets and known therapeutic targets for the treatment of chronic myeloid leukemia was constructed. By calculating the 4 topological features (degree, betweenness, closeness, and coreness) of each node in the network, we identified the candidate Qingdai targets according to their network topological importance. The composite compounds of Qingdai and the corresponding candidate major targets were further validated by a molecular docking simulation.
Seven components in Qingdai were selected and 32 candidate Qingdai targets were identified; these were more frequently involved in cytokine-cytokine receptor interaction, cell cycle, p53 signaling pathway, MAPK signaling pathway, and immune system-related pathways, which all play important roles in the progression of CML. Finally, the molecular docking simulation showed that 23 pairs of chemical components and candidate Qingdai targets had effective binding.
This network-based pharmacology study suggests that Qingdai acts through the regulation of candidate targets to interfere with CML and thus regulates the occurrence and development of CML.
MeSH Keywords: Leukemia, Myelogenous, Chronic, BCR-ABL Positive; Medicine, Chinese Traditional; Molecular Mechanisms of Pharmacological Action; Protein Interaction Maps
Background
Chronic myeloid leukemia (CML) is a clonal hematopoietic stem cell proliferation-induced myeloproliferative disease [1]. It has high heterogeneity and distinct molecular genetic features – the unique cytogenetic features of CML are Philadelphia chromosome t (9; 22) (q34; q11) – in which the c-ABL protooncogene on the long arm of chromosome 9 translocates to the BCR of the long arm of chromosome 22, forming an BCR-ABL fusion gene [2,3], and it has become an important topic of research. Imatinib mesylate and the newer BCR-ABL tyrosine kinase inhibitors are the standard therapy for CML [4], which greatly improves the survival of patients with chronic myeloid leukemia; however, drug resistance and adverse effects remain a problem [5]. Therefore, looking for new strategies to improve the treatment of chronic myeloid leukemia treatment has important clinical significance.
Chinese herbal medicine is a unique medicine used in Chinese medicine to prevent and treat diseases. With the development of medicine around the world, China’s ancient Chinese medicine system is receiving the attention of the world. However, it is the most important and difficult task for Chinese traditional medicine to elucidate the interaction between the complex chemical systems of traditional Chinese medicine and the complex systems of diseases and syndromes. Qingdai is prepared as clumps of dry powder, obtained by machining the leaves or stems of Strobilanthes cusia, Polygonum tinctorium Ait, and Isatis indigotica Fort (Pharmacopoeia of the People’s Republic of China, 2010). Qingdai is one herb in Qing Huang San, which has been recorded in the “Jing Yue Quan Shu,” “Shi Yi De Xiao Fang,” “Qi Xiao Liang Fang,” and so on, and is Professor Zhou Aixiang’s classical prescription of CML treatment [6]. As confirmed by research, indirubin, a component of Qingdai, is indeed effective in the treatment of chronic myeloid leukemia [7]. Dai et al. treated K562 cells with different concentrations of Qingdai compound (2.5, 5, 7.5, 10, and 20 ug/ml) and harvested them at 24 h, reporting that the Qingdai compound inhibited proliferation and promoted apoptosis in K562 cells. Then, the expression of bcr/abl and JWA was detected by semi-quantitative RT-PCR, and concentration-dependent decreases were found in bcr-abl and JWA expression of K562 cells. It was proved that the Qingdai compound can partially promote the apoptosis of K562 cells by inhibiting the expression of bcr/abl and JWA in K562 cells [8]; however, its specific mechanism needs further study. Therefore, it is necessary to develop a novel strategy to understand the biological processes of the interactions among drugs, genes, and proteins at a systems level in order to discover the molecular mechanisms related to the therapeutic efficacy of TCM.
In recent years, with the continuous innovation and development of systems biology, network pharmacology and molecular docking provide feasible research strategies for exploring the intrinsic principles of effective intervention of traditional Chinese medicine (TCM) components and building multi-target precise treatment modes for TCM [9,10]. It has been successfully applied to the molecular network level understanding of the pharmacological mechanism of TCM. For example, in the treatment of diabetes mellitus, Huangqi and Huanglian showed the synergistic mechanism [11], and through these research strategies we demonstrated the important pharmacological mechanism of Yin huang Qing fei capsule in treating chronic bronchitis [12].
We based the present study on network pharmacology strategies to decipher the pharmacological mechanisms of Qingdai acting on CML. We offer a systems strategy: (1) We collected the chemical components of Qingdai and downloaded structure and screening index data; (2) We predicted putative targets of Qingdai and analyzed putative targets by Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis; (3) We collected the known therapeutic targets of drugs in the treatment of CML; (4) We analyzed and investigated the network between putative targets of Qingdai and known therapeutic targets of CML, which provide a strategy for the further study of the pharmacological mechanism of Qingdai on CML; (5) We performed molecular docking between the molecular compounds of Qingdai and the major targets to validate our findings using a computer-aided drug design method. We expected to achieve our experimental goals with this series of experimental methods.
Material and Methods
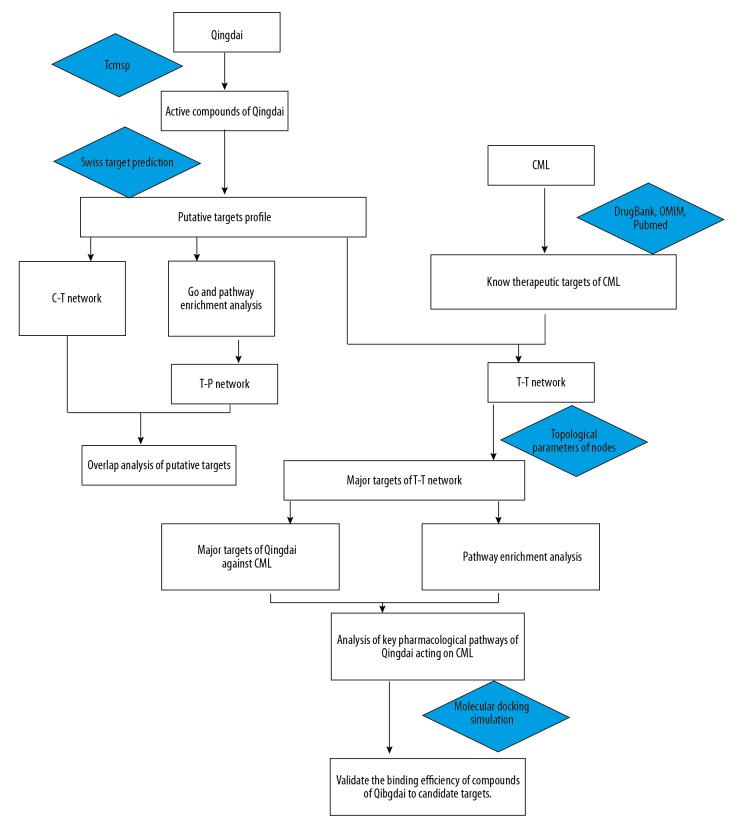
The technical strategy of this research is shown in Figure 1.
Figure 1.
The technical strategy of this research based on network pharmacology for deciphering Key pharmacological pathways of Qingdai acting on CML.
Data preparation
Active compounds of Qingdai
Compositive compounds of Qingdai were obtained from TCMSP Database and Literature database. TCMSP (http://ibts.hkbu.edu.hk/LSP/tcmsp.php), updated in 2014-05-31) [13], which is based on the framework of systems pharmacology for herbal medicines, consists of all the 499 Chinese herbs registered in the Chinese pharmacopoeia with 29 384 ingredients and 12 important ADME-related properties are provided for drug screening and evaluation. Then, through literature mining to prevent omissions, we set the criteria of OB greater than 30%, DL greater than 0.18, and Caco-2 greater than −0.14. When they met these criteria, these components were used as candidate compounds for further analysis. We collected information on 7 compounds and obtained the name of the molecule and its chemical structure. We obtained the molecular Smiles format through the PubChem (https://pubchem.ncbi.nlm.nih.gov/) database.
Known therapeutic targets of drugs in the treatment of chronic myelocytic leukemia
The known therapeutic targets of drugs in the treatment of chronic myeloid leukemia were obtained in 3 ways: PubMed (https://www.ncbi.nlm.nih.gov/pubmed,2017-7-31), DrugBank20 (http://www.drugbank.ca/, version 5.0.10, released 2017-11-14), and the Online Mendelian Inheritance in Man (OMIM) database (http://www.omim.org/, released on 2017-12-20) [14]. In the PubMed database, “chronic myeloid leukemia” was retrieved, and the restriction was “gene” and “Homo sapiens.” We verified the accuracy of the genes by consulting the literature related to these genes. In total, 252 known therapeutic targets of CML were chosen. In DrugBank, in order to improve accuracy, only the drugs that are approved by the Food and Drug Administration (FDA) and whose targets are human genes/proteins were selected, then we chose 265 targets for treating CML. In addition, when searching the OMIM database for “chronic myeloid leukemia” as a keyword, we collected 274 known therapeutic targets. After combining the data from these 3 databases and removing the duplicates, a total of 729 known targets for CML treatment were used for the next analysis. Supplementary Table 1 provides detailed information on these known therapeutic targets. We converted different types of ID proteins to UniProt IDs. To elucidate the signaling pathways involved in known therapeutic targets of CML, we used DAVID (Database Visualization and Integrated Discovery software, http://david.abcc.ncifcrf.gov version 6.7) and KEGG (Kyoto Encyclopedia of Genes and Genomes database, EGG, http://www.genome.jp/kegg/, updated on April 18, 2016) to perform enrichment pathways. The top 10 significant pathway terms were pathways in cancer, MAPK signaling pathway, natural killer cell-mediated cytotoxicity, Jak-STAT signaling pathway, cytokine-cytokine receptor interaction, chronic myeloid leukemia, prostate cancer, focal adhesion, ErbB signaling pathway, and neurotrophin signaling pathway.
Prediction of targets of Qingdai
Obtaining the target of Qingdai through experiments requires a great deal of manpower, material, and financial resources. To accurately predict the targets of bioactive molecules based on a combination of 2D and 3D similarity measures with known ligands, we used the web server Swiss Target Prediction (http://www.swisstargetprediction.ch/) to predict the putative targets of the active compounds of Qingdai. Predictions can be carried out in 5 different organisms, and mapping predictions by homology within and between different species is enabled for close paralogs and orthologs [15]. The “smiles” formats of 7 active compounds were imported into Swiss Target Prediction to predict their putative targets of action. It is noteworthy that the predicted putative target is limited to Homo sapiens, and to improve the reliability of predictions goal, only a high probability of target selected. A total of 112 therapeutic putative targets were obtained. All putative targets obtained were sent to Therapeutic Target Database (TTD) (http://bidd.nus.edu.sg/group/cjttd/, 2015-09-10), Comparative Toxicogenomics Database (CTD) (http://ctdbase.org/, 2017-12-05), and PharmGKB (https://www.pharmgkb.org/) to determine whether these putative targets have some connection to CML. To further understand the putative target of Qingdai, GO enrichment analysis and KEGG pathways analysis were performed.
Network construction
Three types of visual networks were built:
The compound-target network (C-T network) is an interaction network using the active compounds of Qingdai and its corresponding putative targets.
The target-pathway network (T-P network) is composed of the putative targets and corresponding pathways.
The target-target network (T-T network) was built using the relationship between the putative targets of Qingdai and known therapeutic targets of the CML.
Cytoscape 3.5.1 (http://www.cytoscape.org/) is an open software application for visualizing, integrating, modeling, and analyzing interactive networks. All the networks were built using it.
Analysis of the target-target network (Qingdai putative target-known therapeutic targets of the CML network).
Li et al. [16] suggested that “If the degree of a node is more than 2 times the median degree of all the nodes in a network, the node may function as a big hub.” The topological features of the target-target network are analyzed by several important topological properties, such as “degree” [17], “betweenness” [17], “closeness” [17], and “coreness” (an iterative process in which nodes are removed from the network with minimal connection order) [18]. The larger a protein’s degree/node betweenness/closeness centrality, the more important that protein is in the PPI network [19]. Subsequently, the targets were screened for topological importance. Then, the major hubs were screened. The DAVID webserver was used to perform KEGG pathway enrichment analysis of the main targets.
Molecular docking simulation
We used computer molecular docking simulation techniques to verify the credibility of the study. SystemsDOCK (http://systemsdock.unit.oist.jp/) was used for molecule docking [20]. SystemsDock is a web server for network pharmacology-based prediction and analysis that permits docking simulation and molecular pathway mapping for comprehensive characterization of ligand selectivity and interpretation of ligand action on a complex molecular network. All the compounds and 3D structures of Qingdai were directly downloaded from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/, 2017-11-26), and we obtained the 3D structures of target genes from Uniprot (http://www.uniprot.org/, 2017-11) and PDM databases (http://www.rcsb.org/pdb/home/home.do). Docking scores were used to assess the binding affinities of compounds to the respective candidate target.
Results
Active compounds in Qingdai
A single Chinese medicine contains a large number of compounds, so it is helpful to identify these active compounds by means of network pharmacological virtual screening. A total of 53 compounds in Qingdai were obtained. Then, 3 ADME (absorption, distribution, metabolism, and excretion)-related models, including OB, DL, and Caco-2, were used to screen most of the active compounds from Qingdai. Finally, we selected 7 compounds from Qingdai (Table 1), and after text mining, found that most of these compounds possess potent pharmacological activities, such as indirubin, the main active and characteristic compound in Qingdai. Research shows that indirubin and its derivatives can be used to treat chronic myelogenous leukemia by potently inhibiting the Signal Transducer and Activator of Transcription 5 (Stat5) protein in CML cells [21], and indirubin and its derivatives could have anti-angiogenic activity [22]. Studies on Qingdainone have shown anti-tumor and anti-inflammatory effects [23]. Quindoline can cause cell cycle arrest, resulting in inhibition of cell proliferation and causing cell apoptosis [24]. Bisindigotin was found to dose-dependently inhibit TCDD-induced ethoxyresorufin O-demethylase (EROD) activity to achieve an anti-tumor effect [25]. Isoindigo can mediate the cell proliferation pathway to promote apoptosis [26,27]. Beta-sitosterol could inhibit the growth of bacteria and was found to be anti-inflammatory [28]. Indirubin and Indigotin were determined to be the quality markers of Qingdai in the Chinese Pharmacopoeia (The State Pharmacopoeia Commission of China, 2015).
Table 1.
Active compounds and ADME parameters of Qingdai.
| No | Name | Structure | OB (%) | DL | Caco-2 |
|---|---|---|---|---|---|
| MOL011100 | Bisindigotin |
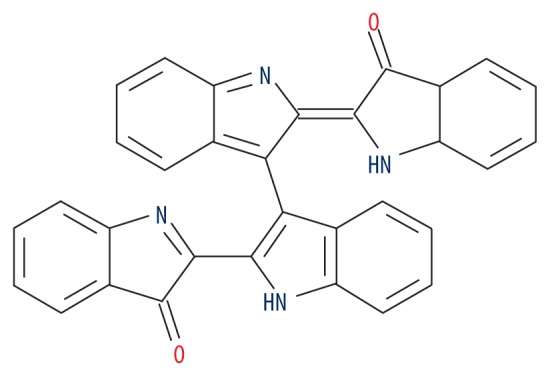
|
41.66 | 0.39 | 0.90 |
| MOL011332 | Quindoline |
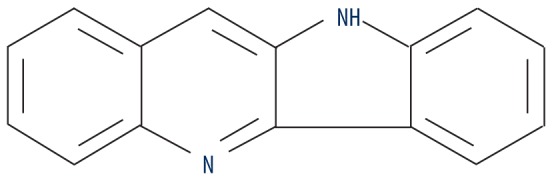
|
54.57 | 0.22 | 1.52 |
| MOL011335 | Isoindigo |
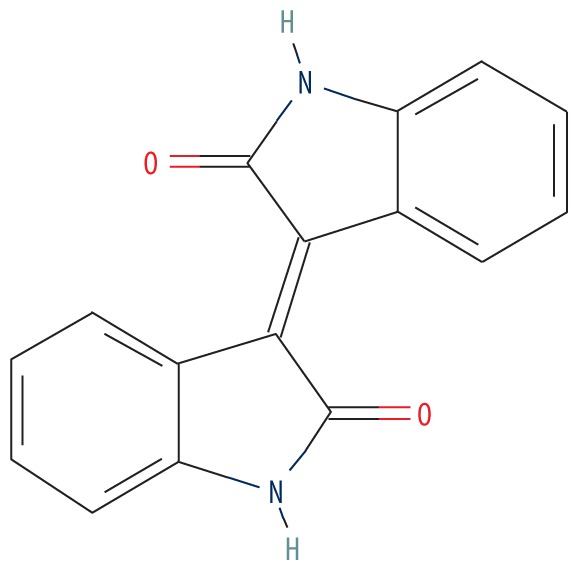
|
94.30 | 0.26 | 0.79 |
| MOL001781 | Indigotin |
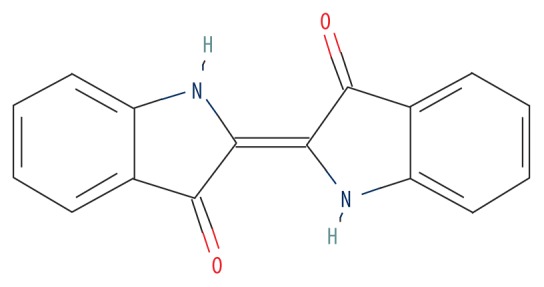
|
38.20 | 0.26 | 0.83 |
| MOL001810 | Qingdainone |
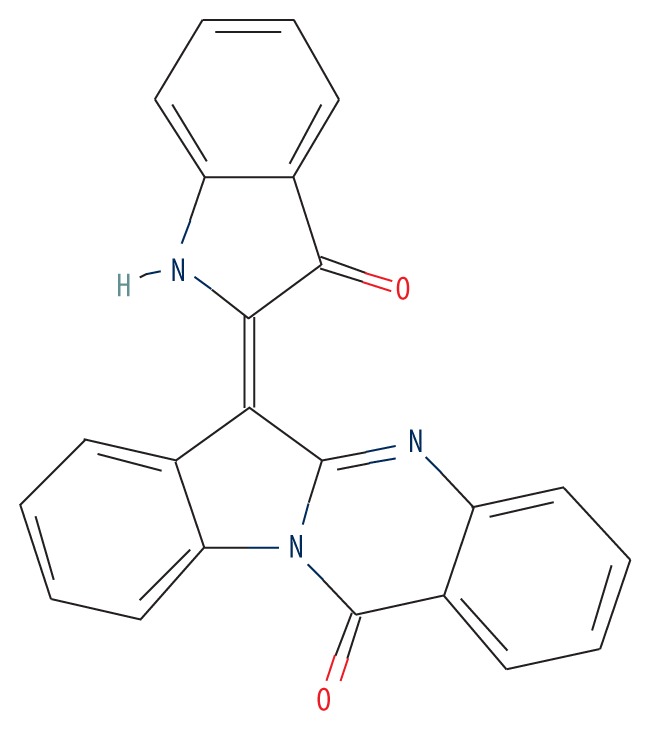
|
45.28 | 0.89 | 1.19 |
| MOL002309 | Indirubin |
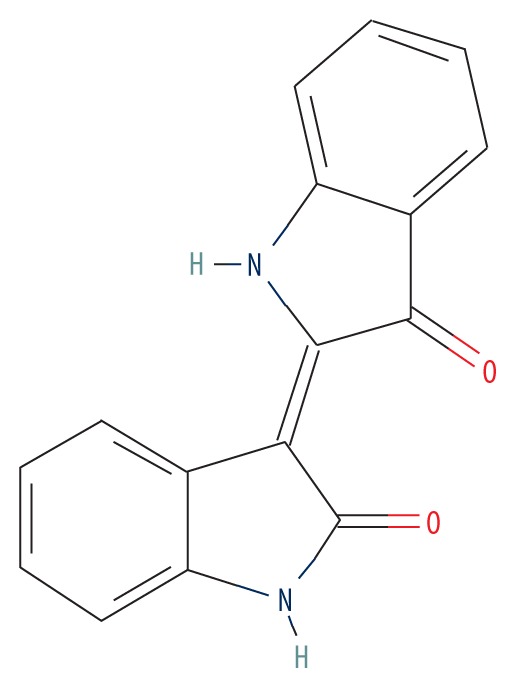
|
48.59 | 0.26 | 1.26 |
| MOL000358 | Beta-sitosterol |
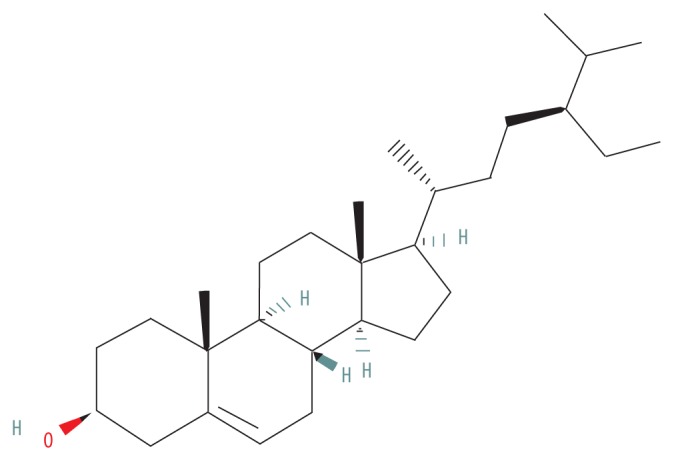
|
36.91 | 0.75 | 1.32 |
OB – oral bioavailability; DL – druglikeness; Caco-2 – Caco-2 permeability.
Putative targets of Qingdai
For Qingdai, through putative target prediction for the 7 components, a total of 112 targets were obtained. Cyclin-dependent kinases (CDKs) are involved in regulating both cell cycle and transcription. Indirubin inhibits CDK activity by K562 cell cycle arrest and promotes apoptosis [29,30]. With Quindoline, through prediction, MAPKs (mitogen-activated protein kinase) and CLKs were obtained. MAPKs play key roles in many cell proliferation-related signaling pathways [31]. Research by Ahmed K found in cancer cells that CLKs control the supply of full-length, functional mRNAs coding for a variety of proteins essential for cell growth and survival. Thus, inhibition of CLKs might become a novel anticancer strategy, leading to a selective depletion of cancer-related proteins after turnover [17]. β-sitosterol has antioxidant activity in a complex system [32]. Interestingly, 28 of the 112 putative target genes are common targets for one or more of these components, indicating that these components may be acting on some of the same biological processes or pathways, which reflects a synergistic effect between the individual components of TCM.
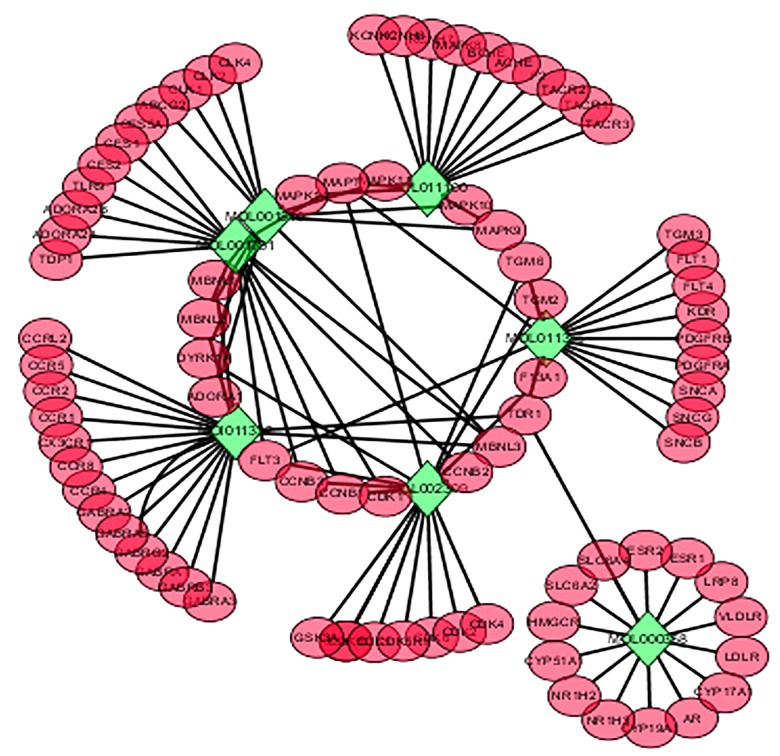
The C-T network was constructed to visualize and explain the complex relationship between the active compounds of Qingdai and its putative targets (Figure 2).
Figure 2.
Compound-Target network (C-T network). Network of 7 active compounds of Qingdai and 112 putative targets.
GO enrichment and KEGG pathway analysis of the putative targets
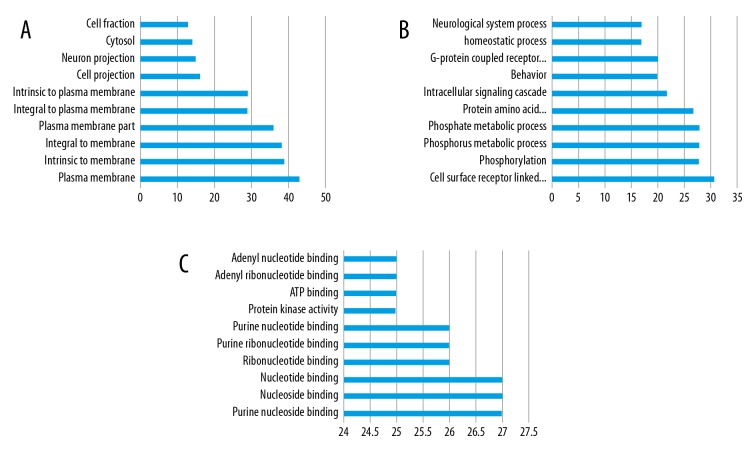
The GO and KEGG enrichment analysis were used to comment on the 112 putative targets of Qingdai. As shown in the results of the enrichment, a total of 433 GO enrichment results were obtained, including biological process (BP) (310 terms), molecular function (MF) (86 terms), and cellular component (CC) (38 terms). We set the level of statistical significance at P<0.05. Then, the top 10 significantly enriched terms were selected in the BP, MF, and CC categories listed in Figure 3. GO enrichment analysis showed that Qingdai can inhibit protein kinase phosphorylation and protein kinase to inhibit cell proliferation, block the cell signaling pathway to inhibit cell proliferation, and promote apoptosis. In addition, chemokines inhibit tumor growth and development by activating immunocompetent cytotoxic cells or inhibiting tumor-associated angiogenesis. In addition, Qingdai can be organized by cell division cycle of proliferation to inhibit cell proliferation or cell mitosis. In addition, it acts on GPCRs, which are closely related to biological behaviors such as the proliferation, invasion, and metastasis of tumors, involving the classical signal pathways such as ERK/MAPK [33]. In recent years, studies have shown that it can serve as a new target for anti-tumor drugs [34]. It is possible that the role of Qingdai on CML is through these molecular mechanisms.
Figure 3.
GO enrichment analysis of the putative targets of Qingdai. The top 10 significantly enriched terms in CC, BP, and MF categories. Cellular component (A), Biological process (B), Molecular function (C).
The putative targets of active compounds were mapped onto the 26 KEGG pathways (Figure 4). The neuroactive ligand-receptor interaction pathway showed the highest number of target connections (count=13), and cytokine-cytokine receptor interaction with 12 targets, pathways in cancer with 11, and included the focal adhesion, cell cycle, chemokine signaling pathway, MAPK signaling pathway, and p53 signaling pathway, respectively. These pathways have well-established roles in the inhibition of tumor cell growth and differentiation and promote tumor cell apoptosis. In addition, there are numerous signaling pathways involved in immunity and inflammation, such as Toll-like receptor signaling pathways, T cell receptor signaling pathway, and Fc epsilon RI signaling pathway. These pathways play an important role in the infection caused by chronic myeloid leukemia. These pathways of the targets show that Qingdai has a therapeutic effect for a variety of malignant tumors, endocrine disease, and inflammatory diseases. Details are provided in Table 2.
Figure 4.
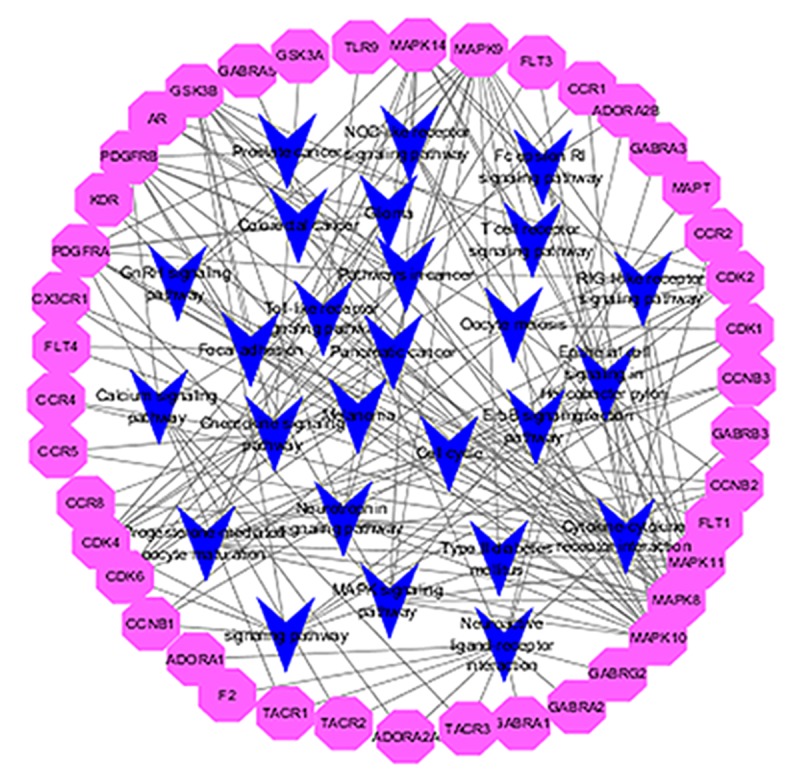
The network of putative targets of Qingdai and 26 KEGG pathways.
Table 2.
The 26 KEGG pathways associated with the putative targets of Qingdai.
| Term | Count | P-value |
|---|---|---|
| hsa04914: Progesterone-mediated oocyte maturation | 10 | 2.93E-07 |
| hsa04080: Neuroactive ligand-receptor interaction | 13 | 1.57E-05 |
| hsa04115: p53 signaling pathway | 7 | 8.78E-05 |
| hsa04060: Cytokine-cytokine receptor interaction | 12 | 1.03E-04 |
| hsa04110: Cell cycle | 8 | 3.91E-04 |
| hsa04510: Focal adhesion | 9 | 0.001437085 |
| hsa05210: Colorectal cancer | 6 | 0.002152457 |
| hsa05200: Pathways in cancer | 11 | 0.002692876 |
| hsa04062: Chemokine signaling pathway | 8 | 0.004097191 |
| hsa04621: NOD-like receptor signaling pathway | 5 | 0.004583166 |
| hsa04620: Toll-like receptor signaling pathway | 6 | 0.004793465 |
| hsa05120: Epithelial cell signaling in Helicobacter pylori infection | 5 | 0.006372769 |
| hsa04622: RIG-I-like receptor signaling pathway | 5 | 0.00742055 |
| hsa05212: Pancreatic cancer | 5 | 0.007793627 |
| hsa04664: Fc epsilon RI signaling pathway | 5 | 0.010293415 |
| hsa04722: Neurotrophin signaling pathway | 6 | 0.011255823 |
| hsa04020: Calcium signaling pathway | 7 | 0.012206329 |
| hsa05215: Prostate cancer | 5 | 0.016120746 |
| hsa04912: GnRH signaling pathway | 5 | 0.022181176 |
| hsa04010: MAPK signaling pathway | 8 | 0.026015669 |
| hsa04660: T cell receptor signaling pathway | 5 | 0.030365615 |
| hsa05214: Glioma | 4 | 0.03155056 |
| hsa04114: Oocyte meiosis | 5 | 0.032191066 |
| hsa05218: Melanoma | 4 | 0.042714992 |
| hsa04012: ErbB signaling pathway | 4 | 0.040141341 |
| hsa04930: Type II diabetes mellitus | 3 | 0.034045303 |
Pharmacological mechanisms of Qingdai acting on chronic myeloid leukemia
The link between traditional Chinese medicine and disease is complex. To illustrate the basic relationship between them, the T-T network was performed for analysis. T-T network consisted of 571 nodes and 10 169 edges. The major hubs in the hub interaction network were determined by calculating 4 features: “degree,” “node betweenness,” “closeness”, and “K value”. There were 195 major hubs, including 32 Qingdai targets (Table 3) and 168 known therapeutic targets of chronic myeloid leukemia. Interestingly, there were 11 targets that were common to both that were screened. Then, a network of major hubs based on their direct interactions was constructed (Figure 5).
Table 3.
The 32 major targets information of Qingdai.
| ID | Target | Uniprot ID | Gene name | PDB ID |
|---|---|---|---|---|
| MT-1 | Mitogen-activated protein kinase 8 | P45983 | MAPK8 | IUKH |
| MT-2 | Estrogen receptor | P03372 | ESR1 | 1A52 |
| MT-3 | Mitogen-activated protein kinase 14 | Q16539 | MAPK14 | 1A9U |
| MT-4 | Cyclin-dependent kinase 2 | P24941 | CDK2 | 1AQ1 |
| MT-5 | Vascular endothelial growth factor receptor 2 | P35968 | KDR | 1VR2 |
| MT-6 | Cyclin-dependent kinase 4 | P11802 | CDK4 | 2W96 |
| MT-7 | Androgen receptor | P10275 | AR | 1E3G |
| MT-8 | Prothrombin | P00734 | F2 | 1A2C |
| MT-9 | Cyclin-dependent kinase 1 | P06493 | CDK1 | 4Y72 |
| MT-10 | Glycogen synthase kinase-3 beta | P49841 | GSK3B | 1GNG |
| MT-11 | Platelet-derived growth factor receptor beta | P09619 | PDGFRB | 1GQ5 |
| MT-12 | Mitogen-activated protein kinase 9 | P45984 | MAPK9 | 3E7O |
| MT-13 | G2/mitotic-specific cyclin-B1 | P14635 | CCNB1 | 2B9R |
| MT-14 | Mitogen-activated protein kinase 11 | Q15759 | MAPK11 | 3GC8 |
| MT-15 | Receptor-type tyrosine-protein kinase FLT3 | P36888 | FLT3 | 1RJB |
| MT-16 | Cyclin-dependent kinase 6 | Q00534 | CDK6 | 1BI7 |
| MT-17 | Vascular endothelial growth factor receptor 1 | P17948 | FLT1 | 1FLT |
| MT-18 | Toll-like receptor 9 | Q9NR96 | TLR9 | 3WPB |
| MT-19 | Cyclin-dependent-like kinase 5 | Q00535 | CDK5 | 1H4L |
| MT-20 | C-C chemokine receptor type 5 | P51681 | CCR5 | 4MBS |
| MT-21 | Alpha-synuclein | P37840 | SNCA | 2X6M |
| MT-22 | Low-density lipoprotein receptor | P01130 | LDLR | 1AJJ |
| MT-23 | Estrogen receptor beta | Q92731 | ESR2 | 1L2J |
| MT-24 | Glycogen synthase kinase-3 alpha | P49840 | GSK3A | 2DFM |
| MT-25 | Aromatase | P11511 | CYP19A1 | 3EQM |
| MT-26 | Platelet-derived growth factor receptor alpha | P16234 | PDGFRA | 5GRN |
| MT-27 | Mitogen-activated protein kinase 10 | P53779 | MAPK10 | 1JNK |
| MT-28 | C-C chemokine receptor type 2 | P41597 | CCR2 | 5T1A |
| MT-29 | Cyclin-dependent kinase 3 | Q00526 | CDK3 | ILFN |
| MT-30 | Microtubule-associated protein tau | P10636 | MAPT | 2ON9 |
| MT-31 | ATP-binding cassette sub-family G member 2 | Q9UNQ0 | ABCG2 | 5NJ3 |
| MT-32 | Vascular endothelial growth factor receptor 3 | P35916 | FLT4 | 4BSJ |
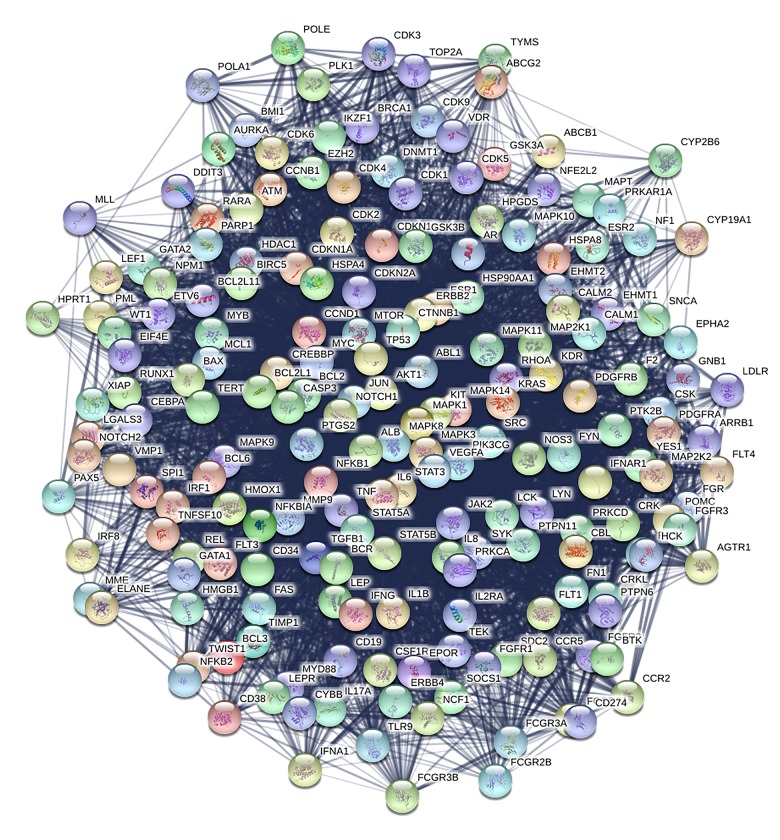
Figure 5.
The network of 195 major hubs based on their direct interactions, consisting of 195 nodes and 5943 edges. Nodes represent proteins. Colored nodes are query proteins and first shell of interactors. White nodes are second shell of interactors. Empty nodes are proteins of unknown 3D structure. Filled nodes have some 3D structure known or predicted. Edges represent protein-protein associations and line thickness indicates the strength of data support.
To further decipher the pharmacological mechanism by which Qingdai affects CML, pathway enrichment analysis was performed using the KEGG pathway database. We found that the major hubs were significantly related to various physiological processes, mainly concentrated in 5 annotation clusters, including epidermal growth factor receptor signaling pathways for cell growth, proliferation, differentiation and metabolism, malignant pathways, immune and inflammation-related pathways, and angiogenesis-related pathways. Chronic myeloid leukemia is a malignant proliferative disease of bone marrow hematopoietic cells and is closely related with ErbB receptor overexpression [35]. ErbB receptor signaling regulates cell proliferation, migration, differentiation, apoptosis, and cell migration through Akt, MAPK, and many other pathways. In many forms of malignancy in organs such as the breasts, ovaries, brain, and prostate gland [36], members of the ErbB family, as well as some of their ligands, are often overexpressed, amplified, or mutated, making them an important therapeutic target [37]. Immune and inflammatory signaling pathways include the Toll-like receptor signaling pathway, T cell receptor signaling pathway, B cell receptor signaling pathway, and Fc epsilon RI signaling pathway. TLR activation has been described to play a role in other leukemias, such as chronic lymphocytic leukemia [38]. T cell receptor (TCR) activation can promote many signal transduction cascades and ultimately determine cell fate by regulating cytokine production, cell survival, proliferation, and differentiation [39]. Regulatory T (Treg) cells can weaken anti-tumor immune responses, which could serve as a promising immuno-therapeutic approach for tumors [40]. The Fc epsilon RI receptor induces multiple signaling pathways that control the secretion of allergic mediators and induction of cytokine gene transcription, resulting in secretion of various molecules: IL-4, IL-5, IL-6, IL-10, IL-13, INF-gamma (interferon-gamma), and TNF-alpha (tumor necrosis factor alpha) [41]. We provide detailed information of the 20 most meaningful enrichment pathways in Table 4.
Table 4.
The Top 20 enrichment pathways of 195 major hubs.
| Term | Count | Value |
|---|---|---|
| hsa05200: Pathways in cancer | 70 | 2.22E-41 |
| hsa04010: MAPK signaling pathway | 34 | 5.88E-12 |
| hsa05220: Chronic myeloid leukemia | 29 | 9.85E-24 |
| hsa04062: Chemokine signaling pathway | 28 | 1.71E-11 |
| hsa04510: Focal adhesion | 28 | 9.51E-11 |
| hsa05215: Prostate cancer | 27 | 5.69E-19 |
| hsa04722: Neurotrophin signaling pathway | 27 | 4.59E-15 |
| hsa04012: ErbB signaling pathway | 26 | 4.46E-18 |
| hsa04060: Cytokine-cytokine receptor interaction | 26 | 5.61E-07 |
| hsa05210: Colorectal cancer | 23 | 4.84E-15 |
| hsa05221: Acute myeloid leukemia | 22 | 1.16E-17 |
| hsa04620: Toll-like receptor signaling pathway | 22 | 2.94E-12 |
| hsa04660: T cell receptor signaling pathway | 22 | 1.16E-11 |
| hsa04650: Natural killer cell mediated cytotoxicity | 22 | 7.02E-10 |
| hsa04630: Jak-STAT signaling pathway | 22 | 1.23E-08 |
| hsa05212: Pancreatic cancer | 20 | 3.49E-13 |
| hsa04664: Fc epsilon RI signaling pathway | 19 | 1.82E-11 |
| hsa04910: Insulin signaling pathway | 19 | 1.88E-07 |
| hsa05214: Glioma | 18 | 4.43E-12 |
| hsa04110: Cell cycle | 18 | 3.16E-07 |
Drug targets reported to be involved in CML pathogenesis for the treatment of CML are involved in cell cycle, growth inhibition, MAPK, ErBb, transforming growth factor beta, and p53 signaling pathways. Interestingly, the 32 Qingdai putative targets included in the major hubs of the T-T network were also included in these pathways. In addition, 32 putative targets were involved in immune and inflammation-related pathways, such as Toll-like receptor, NOD-like receptor, RIG-I-like receptor, and Fc epsilon RI T cell receptor signaling pathway.
To further explore the molecular mechanism of action of Qingdai on CML, we reviewed the literature on the role of Qingdai putative targets in these pathways. Qingdainone, bisindigotin, isoindigo, and indirubin all have target enrichment in the MAPK signaling pathway (MAPK14, MAPT, PDGFRA, PDGFRB, MAPK9, MAPK11, MAPK8, and MAPK10). CD Kang et al. showed that the inhibition of ERK/MAPK induced apoptosis in K562 cells [42]. PDGFRA/B are oncogenes involving tyrosine kinases [43]. Aberrant activity of PTK (protein tyrosine kinases) has been implicated in the stimulation of cancer growth and progression, the induction of drug resistance, tumor neovascularization, tissue invasion, extravasation, and the formation of metastases [44]. We speculate that isoindigo in Qingdai inhibits CML by acting on PDGFRA/B. In the ErbB pathway, GSK3B plays a pivotal role in preserving quiescent HSCs, which has now opened new therapeutic avenues for understanding leukemic stem cell function [45]. Through the cytokine-cytokine receptor interaction, cytokines act on the immune system and hematopoietic system and play an important regulatory role in cell–cell interactions, cell proliferation, differentiation, and effector functions [46]. The p53 protein network regulates important mechanisms in DNA damage repair, cell cycle regulation/checkpoints, and cell senescence and apoptosis, as demonstrated by its ability to positively regulate the expression of various pro-apoptotic genes [47]. In addition, research shows that p53 can stably induce CML cell apoptosis [48]. Cyclin-dependent kinases (CDKS) are a family of serine/threonine kinases that have been firmly established as key regulators of transcription processes underlying coordinated cell cycle entry and sequential progression in nearly all types of proliferative cells [49]. Infection with CML has important secondary symptoms. Enrichment pathways of Qingdai putative targets involve immune and inflammatory pathways, which activate the patient’s own immune system and enhance the defence against sources of external infection, such as phagocytosis of immune cells, which plays an essential role in host defence mechanisms by enveloping and destroying infectious pathogens [41].
In addition, some of the putative targets have a special role in CML. The FMS-like tyrosine kinase 3 (FLT3) gene encodes a class III receptor tyrosine kinase (RTK) that plays important roles in the proliferation, differentiation, and survival of hematopoietic stem and progenitor cells (HSPCs), and FLT3 is frequently mutated and overexpressed in hematologic malignancies [50]. The AGM130 compound is derived from indirubin, which is known as a CDK inhibitor. Research shows that the AGM130 compound efficiently decreased the viability of CML-derived K562 cells, which suggests that AGM130 is a strong candidate for treating Imatinib-resistant CML [51]. In addition, patients with ABCG2 diplotypes might be at higher risk for the rapid and severe development of CML and have a weaker response to treatments with imatinib [52]. We hypothesize that it binds to ABCG2 to enhance the efficacy and reduce the risk of imatinib resistance.
On this basis, the major putative targets of Qingdai that are significantly associated with these biological processes and pathways might play a role in the treatment of CML.
Molecular docking validation
Molecular docking is a rapid method to predict the binding force between traditional Chinese medicine components and the target. It is based on the docking of the ligand and the acceptor’s spatial structure. SystemsDock applies AutoDock VINA [53] to perform docking simulation based on the characterized binding interaction and molecular properties [19]. DocK-IN utilizes a machine learning algorithm (Random Forest) together with a series of characterized binding interactions and test compound molecular properties, usually ranging from 0 to 10 (from weak to strong binding) allowing a straightforward indication of binding strength [20]. The 7 compounds of Qingdai and the corresponding candidate major targets were further validated by a molecular docking simulation. As a result, 23 pairs of components of Qingdai and candidate targets had binding efficiencies. Detailed information about the results of molecular docking are described in Supplementary Table 2. These findings require further experimental verification.
Discussion
In the application of traditional Chinese medicine treatment of CML, Qingdai is given high priority for selection, and has been frequently used in TCM prescriptions. In vitro experiments clearly demonstrated that Qingdai has the ability to inhibit K562 cell proliferation and promote its apoptosis. We used modern network pharmacology and molecular docking technology to explain the effective substance basis and multi-targeting effect of Qingdai treatment of CML. The study of traditional Chinese medicine theory and value is based on the scientific methodology of systematic medicine and has the significance of integrating innovation. In our research, we screened 7 Qingdai active compounds and, from a total of 112 predicted targets of active compounds, obtained 32 major targets of Qingdai for treatment of chronic myeloid leukemia, and enriched 15 signaling pathways related to the treatment of CML. Then, we verified the results of our study by molecular docking. The present study shows the following:
By predicting the targets of 7 compounds in Qingdai, we constructed a C-T network and performed GO analysis and KEGG analysis of the putative targets to provide clues to the pharmacology research of Qingdai.
We constructed the Qingdai putative target-known therapeutic targets of the CML network, suggesting that Qingdai may affect the disease-related pathways of chronic myeloid leukemia by regulating its candidate targets, such as the cytokine-cytokine receptor interaction, cell cycle, p53 signaling pathway, MAPK signaling pathway, and immune system-related pathways.
According to the molecular docking simulation, 23 pairs of components of Qingdai and corresponding putative targets had strong binding efficiencies.
Concusions
Network pharmacology for the study of complex mechanisms of Chinese medicine intervention disease provides new ideas and new methods. This research explored the molecular mechanism of the effects of Qingdai on CML based on these ideas. Our study was based on bioinformatics analysis and computer simulation analysis. Further clinical application assessments and experimental validations for these predicted results are required.
Supplementary Tables
Supplementary Table 1.
known therapeutic targets of CML.
| ESR1 | MLL | IMD21 | QACR | SLC2 | LY75 | SFRP1 | PTK2B |
| TP53 | HRX | MME | ACRB | SLCO1B1 | CBY1 | LEF1 | ELANE |
| BCR | HTRX1 | CD10 | CNGA1 | SLC22A8 | ARHGAP26 | DDIT3 | |
| WDSTS | CALLA | KCNMA1 | POLB | ST3GAL1 | GSTA1 | PTCH1 | |
| ABCB1 | CBL2 | NEP | CLCN2 | CDA | MIR199B | MECOM | CD247 |
| MTHFR | NSLL | CMT2T | IL17A | NT5E | ST8SIA4 | SRSF1 | STAT5B |
| TNF | CLLS5 | SCA43 | ADRB1 | DCTD | ATG4B | CDKN1C | AICDA |
| JAK2 | FLI1 | MLF1 | TUBB1 | ABCC10 | MIRLET7I | MIR17 | IDH2 |
| IL6 | BDPLT21 | LPP | CYP2E1 | SLC29A1 | PTBP2 | CRK | AXL |
| TGFB1 | ETV6 | CHIC2 | VDR | 1KBKG | SPRED2 | GATA2 | CDK9 |
| AKT1 | TEL | BTL | MPL | PRKCA | OSBP2 | NOTCH2 | PMP22 |
| GSTM1 | THC5 | PBT | CHRM1 | IMPDH1 | DDX43 | PRTN3 | ADIPOR1 |
| CTNNB1 | KRAS2 | RAP1GDS1 | CNRM2 | IMPDH2 | P2RX5 | IL32 | IKZF1 |
| KRAS | RASK2 | TCS1 | BCHE | PB1 | MKNK2 | MIR223 | TET2 |
| GSTT1 | NS | BST2 | UREC | NT5C2 | UBASH3B | IL24 | MTHFD1L |
| NFKB1 | CFC2 | DKCA2 | MMP12 | ENPP1 | MIR30E | ARRB1 | NFKB2 |
| BRCA1 | RALD | DKCB4 | NS5B | PRKCD | ATP5F1 | PAX5 | ERCC5 |
| MMP9 | CMTS | PFBMFT1 | ADRB2 | MS4A1 | KIR2DL5B | WSB1 | DAPK1 |
| STAT3 | PTPN11 | CMM9 | PTAFR | HCK | GAS2 | CIP2A | POU2F1 |
| ABL1 | PTP2C | LIFR | HRH1 | CDK2 | FAM27E5 | KIF11 | SPI1 |
| PTGS2 | SHP2 | SWS | NS5A | MAP2K1 | ETNK1 | MIR31 | SLC22A1 |
| CDKN2A | NS1 | STWS | ADRA2A | MAP2K2 | MIR1301 | BIN1 | SEP9 |
| IL1B | JMML | SJS2 | ADRA2B | MAP3K2 | ST8SIA6 | SET | |
| MYC | METCDS | ACSL6 | CHD1 | 1FNAR2 | LOC107126288 | JUP | |
| GSTP1 | CLLS2 | FACL6 | DRD2 | POLA1 | LOC107126281 | REL | |
| CXCL8 | D13S25 | ACS2 | DRD1 | SLC28A3 | MIR564 | HOXA9 | |
| LEP | DBM | IRF1 | HTR2A | PARP1 | MIR2278 | CRKL | |
| TERT | FLVCR2 | MAR | OPRM1 | PARP2 | MIR4701 | CSF3R | |
| BCL2 | C14orf58 | GRAF | PDE4B | PARP3 | ABL | SLCO1B3 | |
| IFNG | CCT | PDGFR | PANX1 | CD22 | GF1R | MTHFD1 | |
| MTOR | PVHH | IBGC4 | CYP3N4 | POLE | SPC | IFNAR1 | |
| XRCC1 | EPV | IMF1 | CASR | POLE2 | EPHA2 | EHMT2 | |
| FAS | SERPINA1 | PENTT | KCND | POLE3 | ICK | CDC6 | |
| CCND1 | PI | KOGS | HTR7 | POLE4 | YES1 | ADIPOR2 | |
| BIRC5 | AAT | NSD1 | ORM2 | PNP | KIT | PER3 | |
| GSK3B | TCL1B | ARA267 | SLC6A2 | C3 | FYN | KIR2DS2 | |
| MAPK14 | TML1 | STO | SMPD1 | C4A | BTK | EPHB4 | |
| ATM | TCL1A | SOTOS1 | HTR1A | C4B | NR4A3 | MEF2C | |
| MIR21 | TCL1 | CLLS4 | NOMO1 | C5 | CSK | ASXL1 | |
| HMGB1 | MYL | DEK | MAP2 | AOX1 | EPHA5 | HES1 | |
| CYP1A1 | CREBBP | D6S231E | PHD | DNMT1 | FGR | KIR2DL2 | |
| NOTCH1 | CBP | CTEPH1 | CA2 | SLC01A2 | FRK | PRAME | |
| KDR | RSTS1 | HLA-B | NR1I2 | HSD11B1 | HSPA8 | SMO | |
| NFE2L2 | MYH11 | SPDA1 | POMC | ALB | ZAK | ORM1 | |
| HLA-G | AAT4 | HLA-DPB1 | CALM1 | RARA | PPAT | MSR1 | |
| CASP3 | FAA4 | TREM2 | YARS | RARB | CYP3A4 | NUMB | |
| ABCG2 | FUS | MYB | TAT | 1KBKB | CYP1A2 | CAMK2G | |
| EZH2 | TLS | ALL2 | SLC16A2 | TXNRD1 | CYP1B1 | CCDC170 | |
| CDKN1B | ALS6 | MLSM7 | TFPI | MAPK3 | FM03 | GPX3 | |
| JUN | ETM4 | DEL7q | GNRHR | MAPK1 | RET | MIR451A | |
| ERCC2 | CBFB | C7DELq | KCNQ2 | CDKN1A | NTPK1 | CDK7 | |
| TNFSF10 | PEBP2B | NCF1 | KCNQ3 | HDAC1 | CSF1R | MIR101-1 | |
| RHOA | DIA4 | PRSS2 | HTR3A | PML | DDR1 | IRF8 | |
| WT1 | NMOR1 | TRY2 | GRIA1 | ADA | CYP3A7 | KIR2DS4 | |
| PDCD1 | CYBA | SCLL | GNB1 | CD38 | CYP2C9 | ID4 | |
| LGALS3 | NF1 | NSD3 | MRD42 | CD19 | CYP2D6 | CD33 | |
| CYP3A5 | VRNF | WHSC1L1 | CTRC | RXRA | CYP2C19 | KIR2DS1 | |
| CD274 | WSS | SLC20A2 | CLCR | RXRG | PTGS1 | HMMR | |
| KCNH2 | NFNS | MLVAR | IMD22 | 1GFBP3 | SLC22A2 | SOCS2 | |
| LCN2 | ERBB2 | GLVR2 | TPOR | PSG5 | ABCA3 | BLK | |
| AURKA | NGL | IBGC1 | MPLV | CSF2RA | CYP2C8 | RMND1 | |
| RUNX1 | NEU | NBN | THCYT2 | 1L3RA | UGT1A1 | MIR224 | |
| LEPR | HER2 | NBS1 | TAL1 | SDC2 | GSTA2 | RANGAP1 | |
| HSP90AA1 | MSF | THCYT3 | TCL5 | PRG2 | MGST2 | FUT1 | |
| NPM1 | MSF1 | LALL | SCL | EPOR | FLT3 | PCM1 | |
| AKAP12 | NAPB | BSAP | BCL10 | GPRC5A | RPI2 | MIR130A | |
| BCL2L1 | SH3GL1 | ALL3 | IMD37 | NROB1 | RPL3 | MIR7-1 | |
| MCL1 | EEN | TAL2 | GFI1 | ALDH1A2 | TEK | AHI1 | |
| IL2RA | LYL1 | CLLS3 | ZNF163 | RARRES1 | FGFR1 | CDKN2C | |
| PLK1 | CEBPA | CHDSKM | SCN2 | LCN1 | FGFR2 | ZBTB2 | |
| PTPN22 | CEBP | NUP214 | RBM15 | OBP2A | FGFR3 | PLCD1 | |
| XIAP | BCL3 | D9S46E | SPEN | RBP4 | FGFR4 | MTSS1 | |
| BCL2L11 | BAX | CAN | OTT | PDK4 | LCK | MSI2 | |
| DPP4 | TAM | CAIN | IGFR2 | CYP26A1 | SRC | FERMT3 | |
| SYK | MST | AF10 | CD32 | HPGDS | ABCB11 | PIWIL1 | |
| PDGFRA | CBFA2 | ALL1 | PBX1 | ATP1A1 | FCGR3B | RAPGEF1 | |
| MIR155 | AML1 | MBL2 | CAKUHED | DGUOK | C1R | MKNK1 | |
| TWIST1 | CML | MBL | ABL2 | 1TGAL | C1QA | EHMT1 | |
| CBL | PHL | MBP1 | ABLL | TOP2A | C1QB | USF2 | |
| STAT5A | ALL | MBL2D | ARG | PDLA1 | C1QC | MIR10A | |
| BMI1 | HMOX1 | MBPD | NCF2 | CBR1 | FCGR3A | IL1RAP | |
| HSPA4 | HMOX1D | LMO1 | FLVCR1 | AKR1A1 | C1S | MIR320A | |
| TGM2 | NCF4 | RBTN1 | AXPC1 | AKR1 | FCGR1A | LTB4R2 | |
| NFKBIA | P40PHOX | RHOM1 | PCARP | NQO1 | FCGR2A | SETBP1 | |
| CD34 | CGD3 | LMO2 | COPD | NOS3 | FCGR2B | ULBP2 | |
| CALR | MKL1 | RBTNL1 | TCL4 | NDUFS3 | FCGR2C | BTG1 | |
| MIR34A | AMKL | RHOM2 | ERBB4 | NDUFS7 | RRM2 | KDM5A | |
| XRCC3 | MAL | TTG2 | HER4 | POR | DCK | MLLT3 | |
| PDGFRB | XK | CLLS1 | ALS19 | ABCC3 | PRKAR2A | PHF6 | |
| SOCS1 | MCLDS | SMAR | SGOL1 | HPRT1 | PRKAR1A | CD7 | |
| ABCC1 | CYBB | NUMA1 | SGO | TUBB | PDE3A | PTPRG | |
| IFNA1 | CGD | PICALM | SGO1 | TUBA4A | 1FNAR1 | FCER1G | |
| PIK3CG | AMCBX2 | CALM | CAID | DHFR | FNTB | HULC | |
| CYP2B6 | IMD34 | CLTH | THRB | FPGS | PDGFD | MIR196B | |
| EIF4E | GATA1 | LAP | ERBA2 | TYMS | FLT1 | MR1 | |
| XPC | GF1 | ATA | THR1 | ATIC | FLT4 | RIN1 | |
| AURKB | ERYF1 | AT1 | PRTH | GGH | UGT1A3 | ST3GAL4 | |
| PTPN6 | NFE1 | ZBTB16 | MYD88 | FOLR1 | UGT1A4 | FIP1L1 | |
| DNMT3A | XLTDA | ZNF145 | MYD88D | IFNAR2 | UGT1A9 | MIR148B | |
| BCL6 | XLTT | PLZF | DCML | NS3 | UGT2B7 | MIR326 | |
| ALOX5 | XLANP | KMT2A | MONOMAC | 4A | UGT2B15 | MIR486-1 |
Supplementary Table 2.
Molecular docking between the 7 compounds of Qingdai and the corresponding candidate major targets.
| Compounds and targets | Protein-ligand interactions of the docking pose | Score |
|---|---|---|
| Qingdainone, MAPK9 |
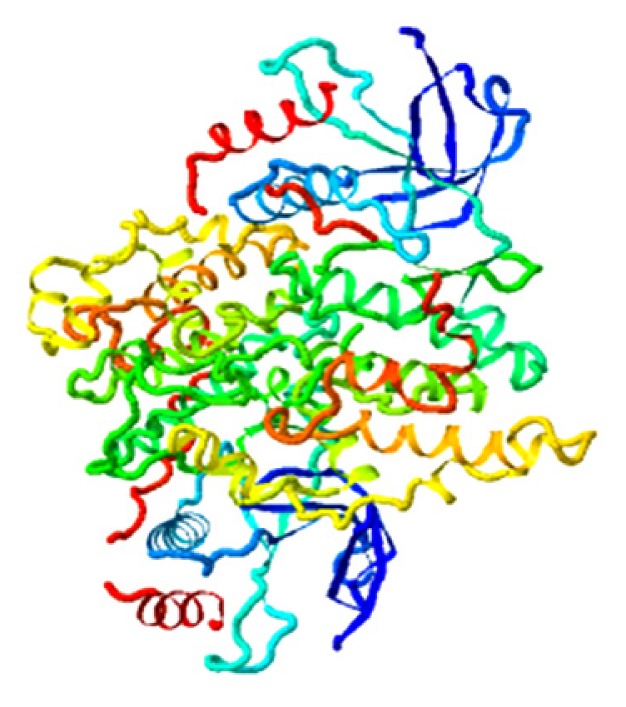
|
4.306 |
| Qingdainone, MAPK10 |
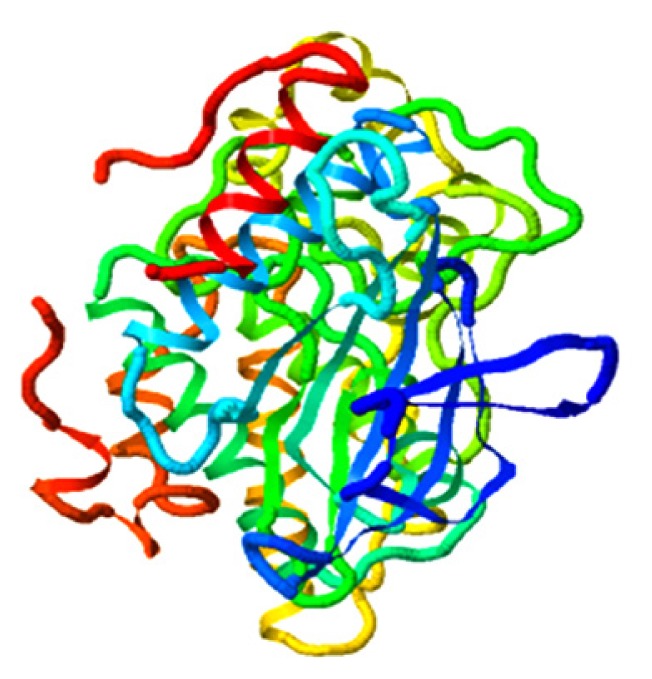
|
4.684 |
| Qingdainone, MAPK11 |
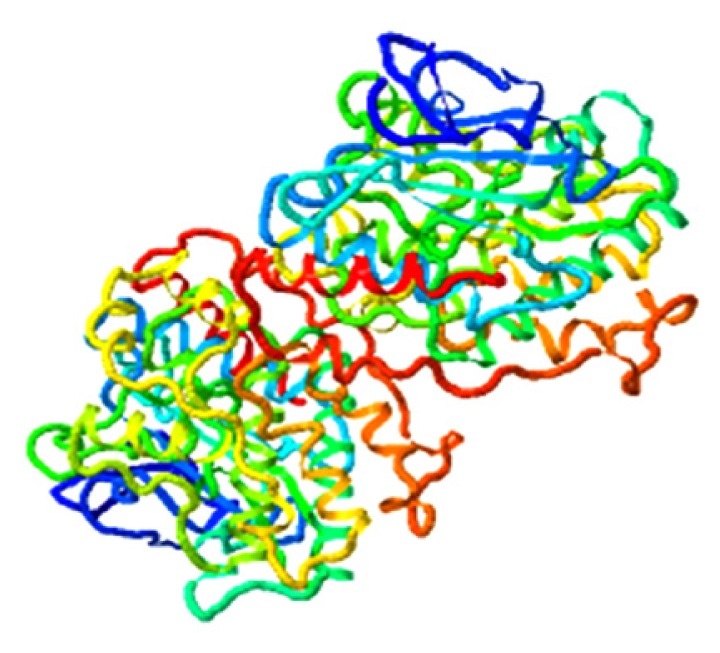
|
4.435 |
| Qingdainone, MAPK11 |
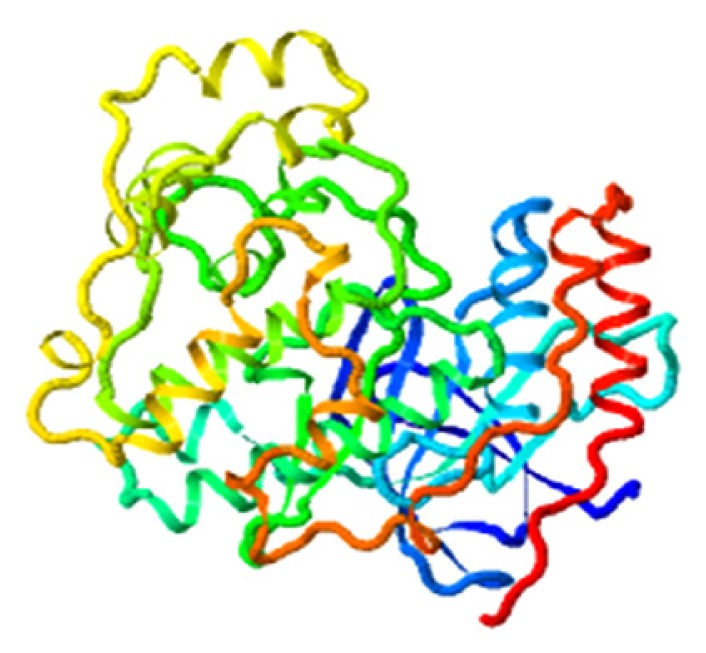
|
4.844 |
| Bisindigotin, MAPK9 |
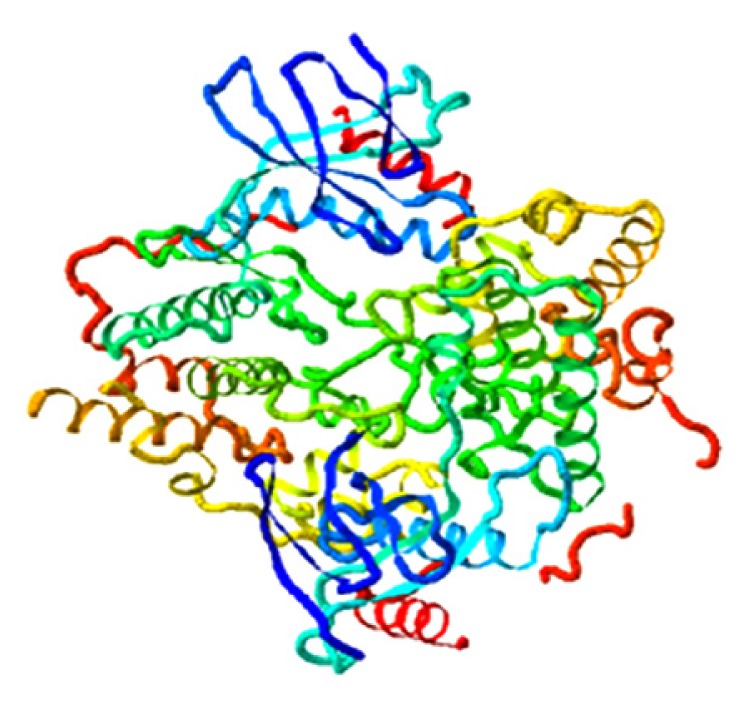
|
4.053 |
| Bisindigotin, MAPK10 |
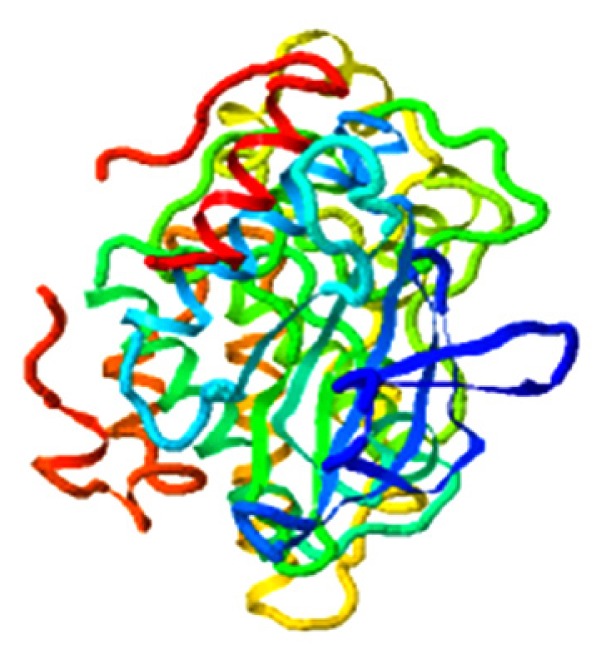
|
3.562 |
| Bisindigotin, MAPK11 |

|
3.787 |
| Bisindigotin, MAPK14 |
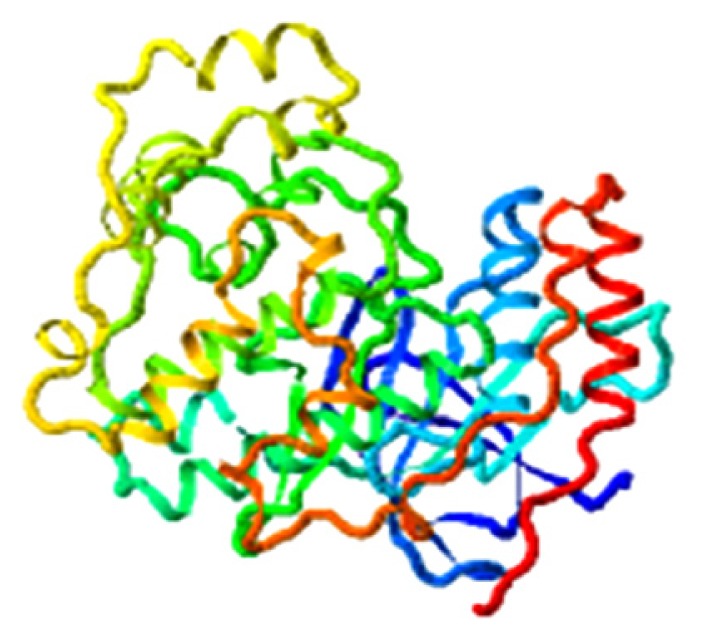
|
4.039 |
| Bisindigotin, F2 |
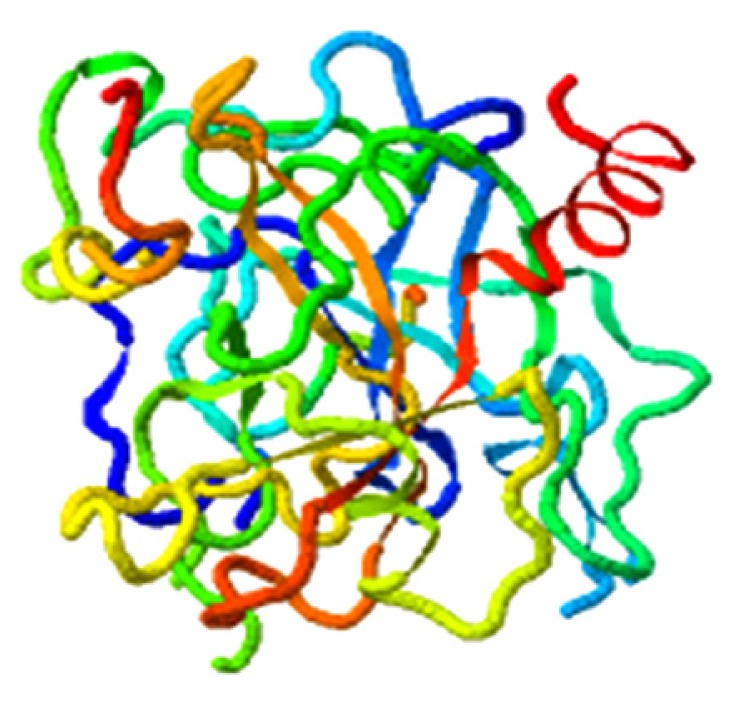
|
4.598 |
| Isoindigo, FLT4 |
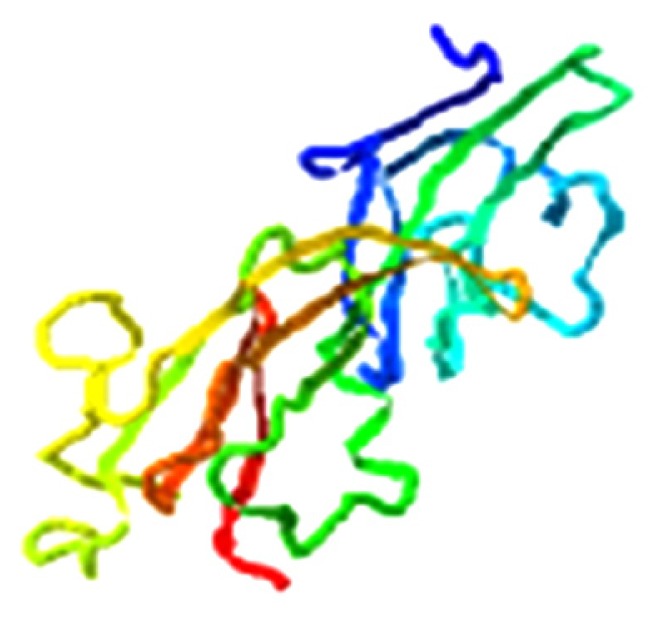
|
7.117 |
| Isoindigo, PDGFRB |
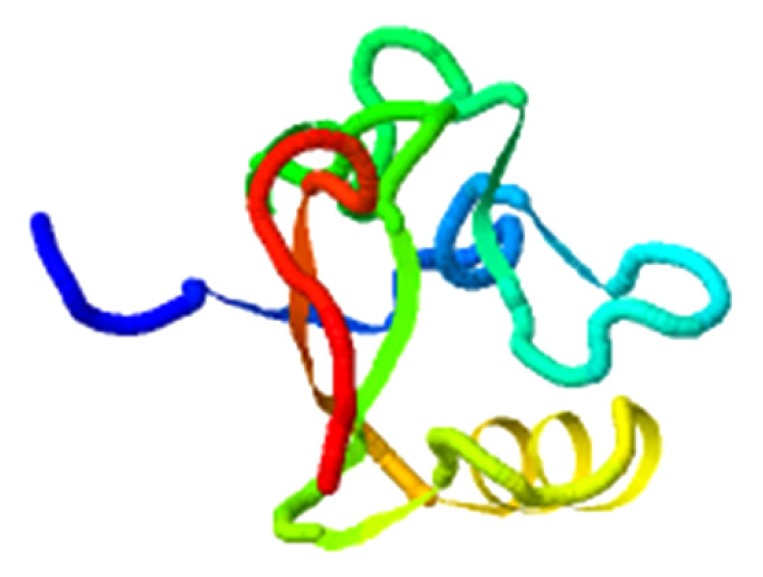
|
5.516 |
| Isoindigo, FLT3 |
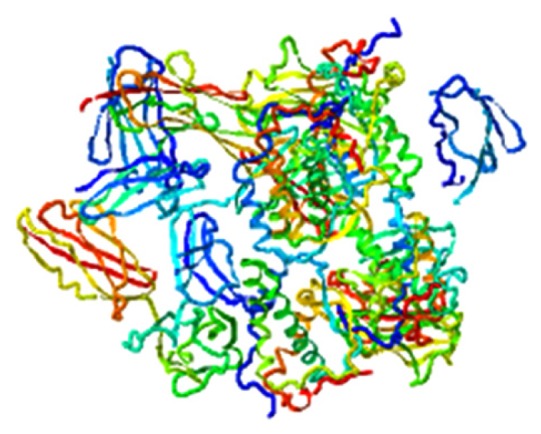
|
7.780 |
| Indigotin, CDK1 |
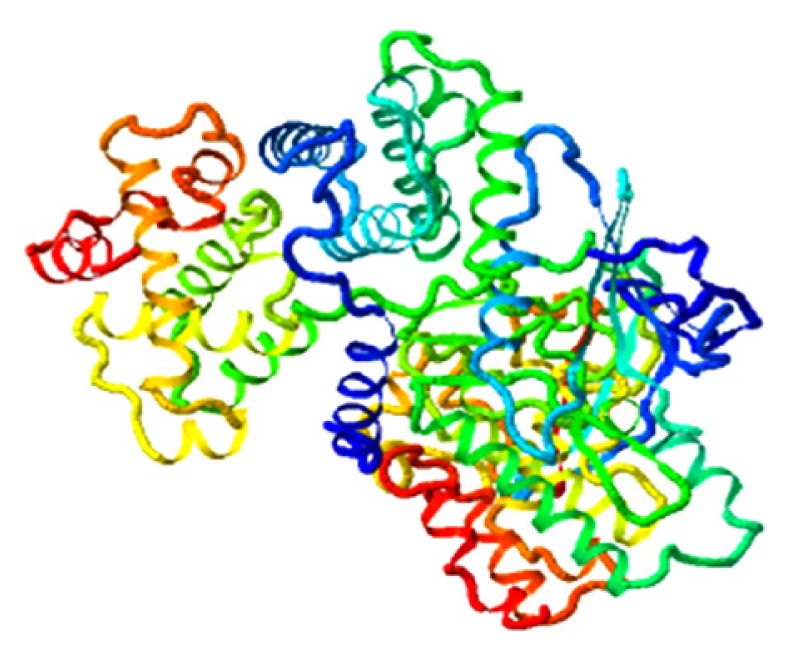
|
6.860 |
| Indirubin, CDK1 |
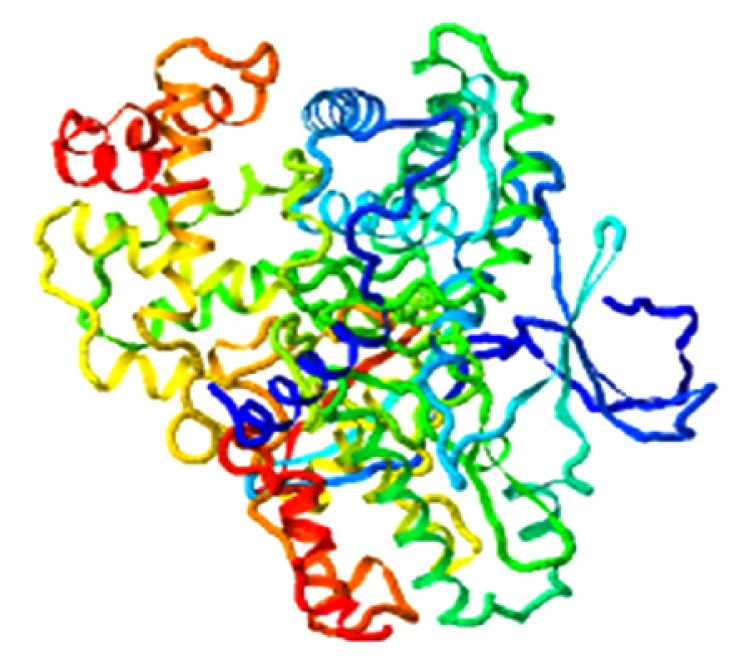
|
2.633 |
| Indirubin, CDK4 |
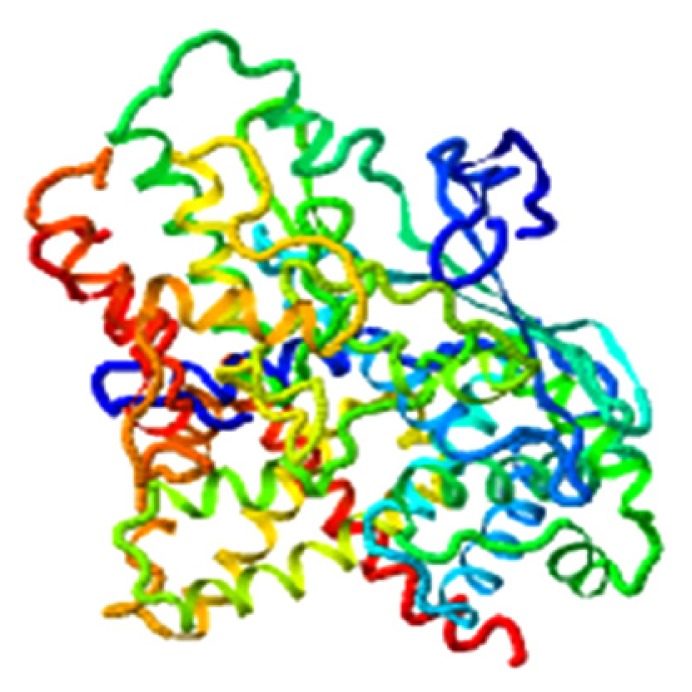
|
2.051 |
| Indirubin, CDK2 |
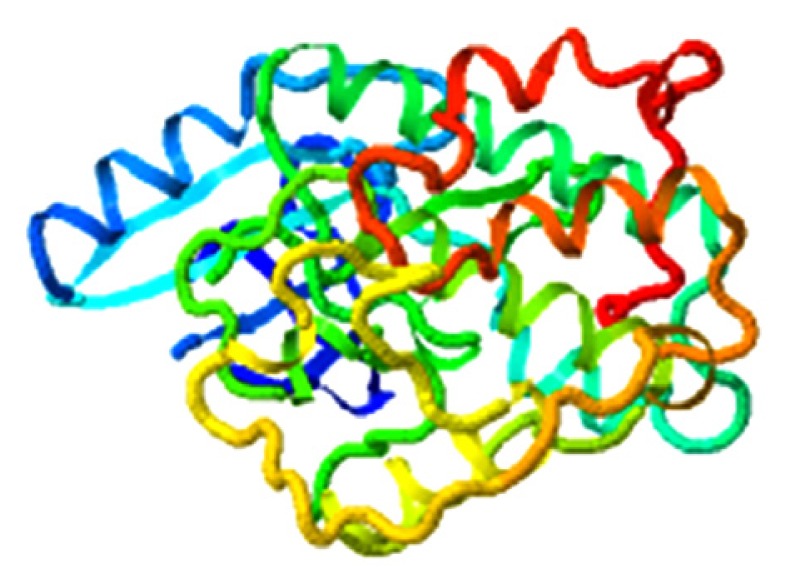
|
2.583 |
| Indirubin, FLT3 |
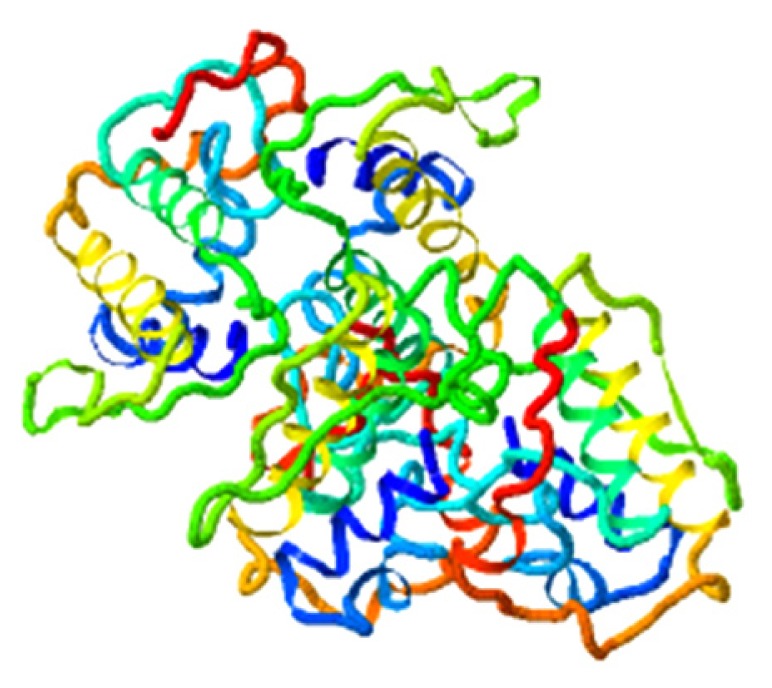
|
3.217 |
| Indirubin, GSK3B |
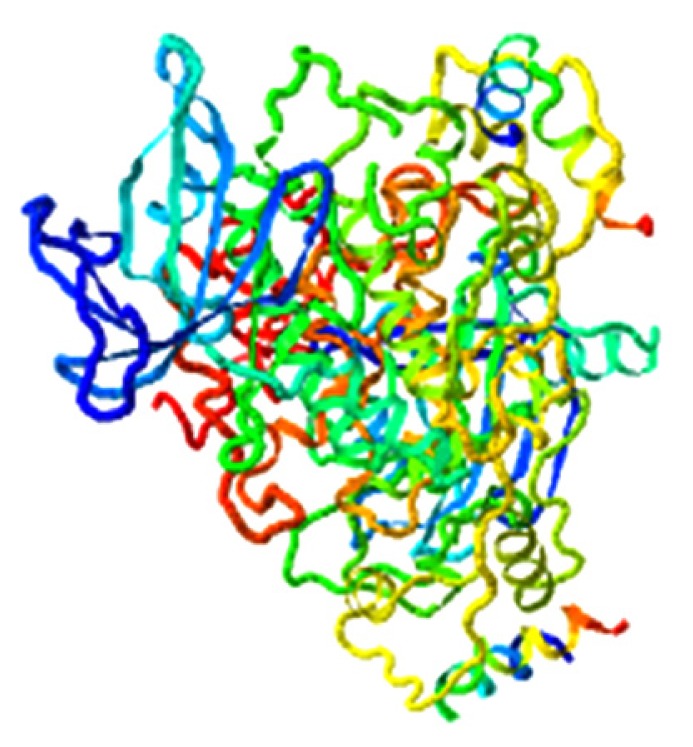
|
1.963 |
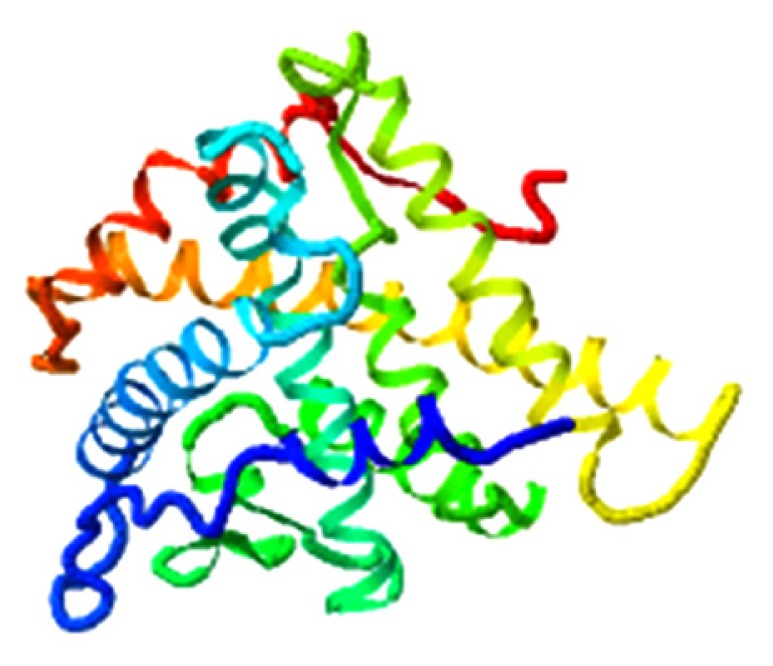
| Beta-sitosterol,AR |
|
8.365 |
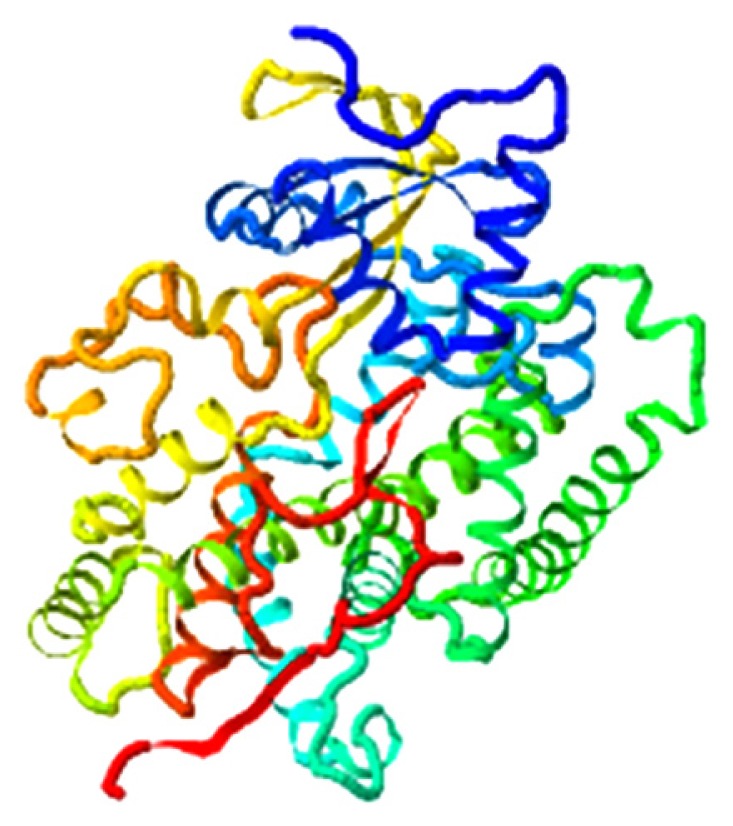
| Beta-sitosterol, CYP19A1 |
|
8.335 |
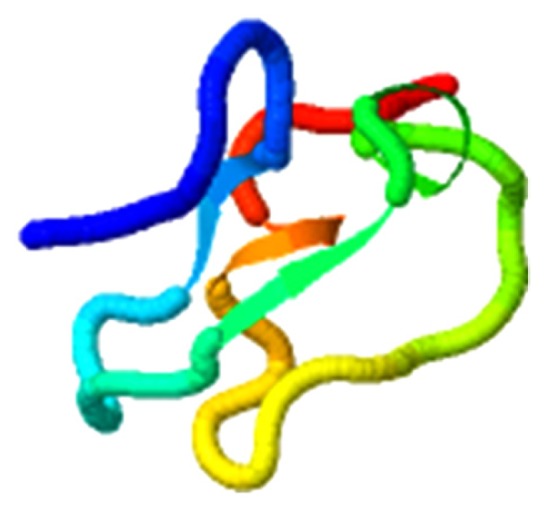
| Beta-sitosterol, LDLR |
|
4.981 |
| Beta-sitosterol, ESR1 |
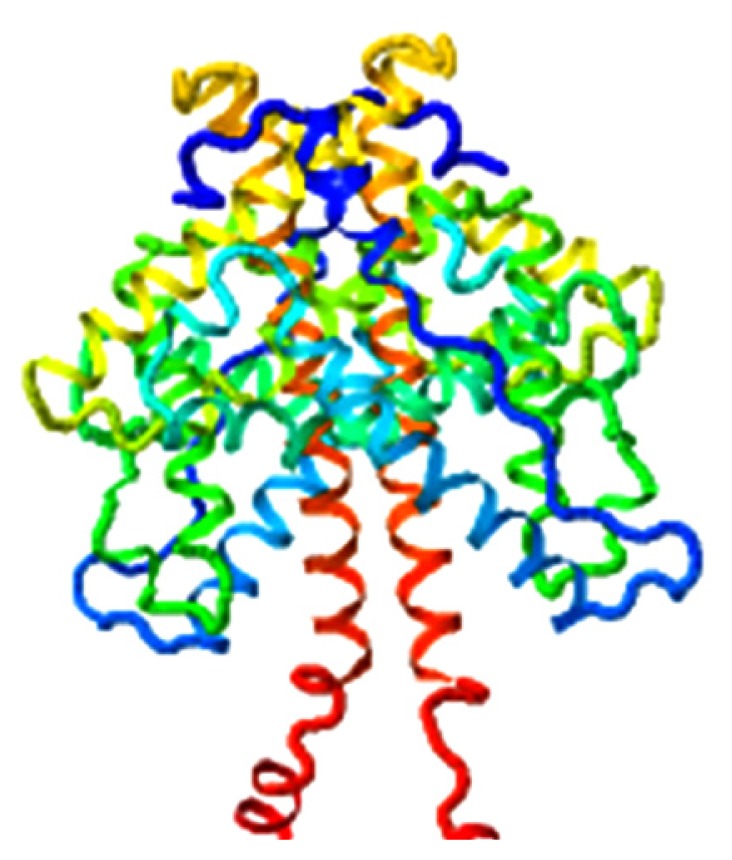
|
8.372 |
| Beta-sitosterol, ESR2 |
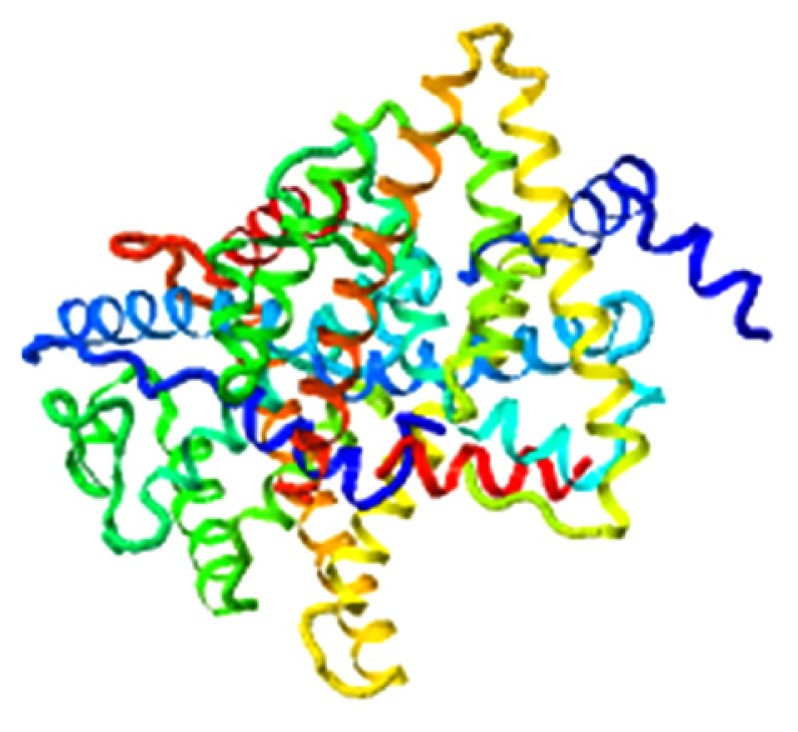
|
8.321 |
Footnotes
Source of support: This work was supported by grants from the National Natural Science Foundation of China (No. 81673799) and the National Natural Science Foundation of China Youth Fund (No. 81703915)
References
- 1.Sawyers CL. Chronic myeloid leukemia. N Engl J. 1999;340:1330–40. doi: 10.1056/NEJM199904293401706. [DOI] [PubMed] [Google Scholar]
- 2.Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–93. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 3.Kidan N, Khamaisie H, Ruimi N, et al. Ectopic expression of Snail and Twist in Ph+ leukemia cells upregulates CD44 expression and alters their differentiation potential. J Cancer. 2017;8:3952–68. doi: 10.7150/jca.19633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osorio S, Escudero-Vilaplana V, Gómez-Centurión I, et al. Inadequate response to imatinib treatment in chronic myeloid leukemia due to a drug interaction with phenytoin. J Oncol Pharm Pract. 2017 doi: 10.1177/1078155217743565. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Huang R, Liu H, Chen Y, et al. EPS8 regulates proliferation, apoptosis and chemosensitivity in BCR-ABL positive cells via the BCR-ABL/PI3K/AKT/mTOR pathway. Oncol Rep. 2018;39:119–28. doi: 10.3892/or.2017.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu C, Liu LJ, Zhou C, et al. [Analysis of mechanism of indigo naturalis in treating chronic myelocytic leukemia based on three-dimentional model of protein-protein interaction network-molecular docking technique – in vitro experiment]. Chinese Journal of Experimental Traditional Medical Formulae. 2017;23:206–11. [in Chinese] [Google Scholar]
- 7.Gaboriaud-Kolar N, Myrianthopoulos V, Vougogiannopoulou K, et al. Natural-based indirubins display potent cytotoxicity toward wild-type and T315I-resistant leukemia cell lines. J Nat Prod. 2016;79:2464–71. doi: 10.1021/acs.jnatprod.6b00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai HP, Shen Q, Zhou JW, et al. [Influence of Qingdai compound on expression of bcr/abl and JWA in K562 cells]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2005;13:809–11. [in Chinese] [PubMed] [Google Scholar]
- 9.Hopkins AL. Network pharmacology: The next paradigm in drug discovery. Nat Chem Biol. 2008;4:682–90. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- 10.Liang X, Li H, Li S. A novel network pharmacology approach to analyse traditional herbal formulae: The Liu-Wei-Di-Huang pill as a case study. Mol Biosyst. 2014;10:1014–22. doi: 10.1039/c3mb70507b. [DOI] [PubMed] [Google Scholar]
- 11.Yue SJ, Liu J, Feng WW, et al. System pharmacology-basedissection of the synergistic mechanism of Huangqi and Huanglian for diabetes mellitus. Front Pharmacol. 2017;8:694. doi: 10.3389/fphar.2017.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu GH, Zhang YQ, Ren WQ, et al. Network pharmacology-based identification okey pharmacological pathways of Yin-Huang-Qing-Fei capsule acting on chronic bronchitis. Int J Chron Obstruct Pulmon Dis. 2016;12:85–94. doi: 10.2147/COPD.S121079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ru JL, Li P, Wang JN, et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wishart DS, Knox C, Guo AC, et al. DrugBank: A knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008;36:901–6. doi: 10.1093/nar/gkm958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.David G, Aurelien G, Matthias W, et al. SwissTargetPrediction: A web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014;42:32–38. doi: 10.1093/nar/gku293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S, Zhang ZQ, Wu LJ, et al. Understanding ZHENG in traditional Chinese medicine in the context of neuro-endocrine-immune network. IET Syst Biol. 2007;1:51–60. doi: 10.1049/iet-syb:20060032. [DOI] [PubMed] [Google Scholar]
- 17.ElHady AK, Abdel-Halim M, Abadi AH, Engel M. Development of selective Clk1 and -4 inhibitors for cellular depletion of cancer-relevant proteins. J Med Chem. 2017;60:5377–91. doi: 10.1021/acs.jmedchem.6b01915. [DOI] [PubMed] [Google Scholar]
- 18.Wuchty S, Almaas E. Evolutionary cores of domain co-occurrence networks. BMC Evol Biol. 2005;5:24. doi: 10.1186/1471-2148-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Liu Z, Li C, et al. Drug target prediction based on the herbs components: The study on the multitargets pharmacological mechanism of Qishenkeli acting on the coronary heart disease. Evid Based Complement Alternat Med. 2012;2012:698531. doi: 10.1155/2012/698531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsin KY, Matsuoka Y, Asai Y, et al. SystemsDock: A web server for network pharmacology-based prediction and analysis. Nucleic Acids Res. 2016;44:507–13. doi: 10.1093/nar/gkw335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tittikpina NK, Nana F, Fontanay S, et al. Antibacterial activity and cytotoxicity of Pterocarpus erinaceus Poir extracts, fractions and isolated compounds. J Ethnopharmacol. 2018;212:200–7. doi: 10.1016/j.jep.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 22.Nam S, Scuto A, Yang F, et al. Indirubin derivatives induce apoptosis of chronic myelogenous leukemia cells involving inhibition of Stat5 signaling. Mol Oncol. 2012;6:276–83. doi: 10.1016/j.molonc.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye HZ, Huang H, Huang JF, et al. Establishment and application of UPLC fingerprint for indigo naturalis. Fu Jian Fen Xi Ce Shi. 2016;25:6–13. [Google Scholar]
- 24.Zhang Y, Zhang DY, Cao JJ, et al. Interaction of Quindoline derivative with telomeric repeat – containing RNA induces telomeric DNA-damage response in cancer cells through inhibition of telomeric repeat factor. Biochim Biophys Acta. 2017;1861:3246–56. doi: 10.1016/j.bbagen.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Wei XY, Leung CY, Wong CK, et al. Bisindigotin, a TCDD Antagonist from the Chinese Medicinal Herb Isatis indigotica. J Nat Prod. 2005;68:427–29. doi: 10.1021/np049662i. [DOI] [PubMed] [Google Scholar]
- 26.Mathieu S, Fadoua B, Samir M, et al. Synthesis and antiproliferative activities of diversely substituted glycosyl-isoindigo derivatives. Eur J Med Chem. 2006;41:88–100. doi: 10.1016/j.ejmech.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Nishiumi S, Yamamoto N, Kodoi R, et al. Antagonistic and agonistic effects of indigoids on the transformation of an aryl hydrocarbon receptor. Arch Biochem Biophys. 2008;470:187–99. doi: 10.1016/j.abb.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 28.Alex D, Lam IK, Lin Z, Lee SM. Indirubin shows anti-angiogenic activity in an in vivo zebrafish model and an in vitro HUVEC model. J Ethnopharmacol. 2010;131:242–47. doi: 10.1016/j.jep.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 29.Zhen Y, Sørensen V, Jin Y, et al. Indirubin-30-monoxime inhibits autophosphorylation of FGFR1and stimulates ERK1/2 activity via p38 MAPK. Oncogene. 2007;26:6372–85. doi: 10.1038/sj.onc.1210473. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki K, Adachi R, Hirayama A, et al. Indirubin, a Chinese anti-leukemia drug, promotes neutrophilic differentiation of human myelocytic leukemia HL-60 cells. Br J Haematol. 2005;130:681–90. doi: 10.1111/j.1365-2141.2005.05655.x. [DOI] [PubMed] [Google Scholar]
- 31.Xiliang C. Biochemistry. People’s Medical Publishing House; China: 2010. p. 375. [Google Scholar]
- 32.Zuo Y, Zhu RT, Feng LX, et al. [Antioxidant activities of β-sitosterol in complicated systems]. Journal of the Chinese Cereals and Oils Association. 2017;32 [in Chinese] [Google Scholar]
- 33.Xu YJ, Wang S, Liang QM. [Relation between G protein-coupled receptor and development of tumour]. Chinese Journal of Cancer Prevention and Treatment. 2013;20:712–16. [in Chinese] [Google Scholar]
- 34.Chen LY, Yang Y, An S, et al. [Cross-talk of GPCRs and RTKs and its effects on oncotherapy]. Chinese Pharmacological Bulletin. 2017;33:454–60. [in Chinese] [Google Scholar]
- 35.Zhang J, Zhao A, Sun L, et al. Selective surface marker and miRNA profiles of CD34+ blast-derived microvesicles in chronic myelogenous leukemia. Oncol Lett. 2017;14:1866–74. doi: 10.3892/ol.2017.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holbro T, Civenni G, Hynes NE. The ErbB receptors and their role in cancer progression. Exp Cell Res. 2003;284:99–110. doi: 10.1016/s0014-4827(02)00099-x. [DOI] [PubMed] [Google Scholar]
- 37.L’Allemain G. HER-ErbB family of receptors and their ligands: Mechanisms of activation, signals and deregulation in cancer. Bull Cancer. 2003;90:179–85. [PubMed] [Google Scholar]
- 38.Lin Y, Zhang L, Cai AX, et al. Effective posttransplant antitumor immunity is associated with TLR-stimulating nucleic acid-immunoglobulin complexes in humans. J Clin Invest. 2011;121:1574–84. doi: 10.1172/JCI44581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burbach BJ, Medeiros RB, Mueller KL, Shimizu Y. T-cell receptor signaling to integrins. Immunol Rev. 2007;218:65–81. doi: 10.1111/j.1600-065X.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- 40.Pedros C, Canonigo-Balancio AJ, Kong KF, Altman A. Requirement of Treg-intrinsic CTLA4/PKCη signaling pathway for suppressing tumor immunity. JCI Insight. 2017 doi: 10.1172/jci.insight.95692. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki R. The emerging picture of mast cell activation: The complex regulatory network of high-affinity receptor for immunoglobulin E signaling. Biol Pharm Bull. 2017;40:1828–32. doi: 10.1248/bpb.b17-00465. [DOI] [PubMed] [Google Scholar]
- 42.Kang CD, Yoo SD, Hwang BW, et al. The inhibition of ERK/MAPK not the activation of JNK/SAPK is primarily required to induce apoptosis in chronic myelogenous leukemic K562 cells. Leuk Res. 2000;24:527–34. doi: 10.1016/s0145-2126(00)00010-2. [DOI] [PubMed] [Google Scholar]
- 43.Klener P, Klener P. ABL1, SRC and other non-receptor protein tyrosine kinases as new targets for specific anticancer therapy. Klin Onkol. 2010;23:203–9. [PubMed] [Google Scholar]
- 44.Kim M, Baek M, Kim DJ. Protein tyrosine signaling and its potential therapeutic implications in carcinogenesis. Curr Pharm Des. 2017;23:4226–46. doi: 10.2174/1381612823666170616082125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saponaro C, Maffia M, Di Renzo N, Coluccia AM. Is going for cure in CML targeting aberrant glycogen synthase kinase 3β? Curr Drug Targets. 2017;18:396–404. doi: 10.2174/1389450117666160817151723. [DOI] [PubMed] [Google Scholar]
- 46.Yang JG, Wang LL, Ma DC. Effects of vascular endothelial growth factors and their receptors on megakaryocytes and platelets and related diseases. Br J Haematol. 2018;180(3):321–34. doi: 10.1111/bjh.15000. [DOI] [PubMed] [Google Scholar]
- 47.Smiles WJ, Camera DM. The guardian of the genome p53 regulates exercise-induced mitochondrial plasticity beyond organelle biogenesis. Acta Physiol (Oxf) 2018;222(3) doi: 10.1111/apha.13004. [DOI] [PubMed] [Google Scholar]
- 48.Cheng Y, Hao Y, Zhang A, et al. Persistent STAT5-mediated ROS production and involvement of aberrant p53 apoptotic signaling in the resistance of chronic myeloid leukemia to imatinib. Int J Mol Med. 2018;41:455–63. doi: 10.3892/ijmm.2017.3205. [DOI] [PubMed] [Google Scholar]
- 49.He G, Yang X, Wang G, et al. Cdk7 is required for activity-dependent neuronal gene expression, long-lasting synaptic plasticity and long-term memory. Front Mol Neurosci. 2017;10:365. doi: 10.3389/fnmol.2017.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ayuna H, Daniel MS, Kannan N, Ito T. RNA binding protein MSI2 positively regulates FLT3 expression in myeloid leukemia. Leuk Res. 2017;54:47–54. doi: 10.1016/j.leukres.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim WS, Lee MJ, Kim DH, et al. 5′-OH-5-nitro-Indirubin oxime (AGM130), an Indirubin derivative, induces apoptosis of Imatinib-resistant chronic myeloid leukemia cells. Leuk Res. 2013;37:427–33. doi: 10.1016/j.leukres.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 52.da Cunha Vasconcelos F, Mauricio Scheiner MA, Moellman-Coelho A, et al. Low ABCB1 and high OCT1 levels play a favorable role in the molecular response to imatinib in CML patients in the community clinical practice. Leuk Res. 2016;51:3–10. doi: 10.1016/j.leukres.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 53.Trott O, Olson AJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem. 2010;31:455–61. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
known therapeutic targets of CML.
| ESR1 | MLL | IMD21 | QACR | SLC2 | LY75 | SFRP1 | PTK2B |
| TP53 | HRX | MME | ACRB | SLCO1B1 | CBY1 | LEF1 | ELANE |
| BCR | HTRX1 | CD10 | CNGA1 | SLC22A8 | ARHGAP26 | DDIT3 | |
| WDSTS | CALLA | KCNMA1 | POLB | ST3GAL1 | GSTA1 | PTCH1 | |
| ABCB1 | CBL2 | NEP | CLCN2 | CDA | MIR199B | MECOM | CD247 |
| MTHFR | NSLL | CMT2T | IL17A | NT5E | ST8SIA4 | SRSF1 | STAT5B |
| TNF | CLLS5 | SCA43 | ADRB1 | DCTD | ATG4B | CDKN1C | AICDA |
| JAK2 | FLI1 | MLF1 | TUBB1 | ABCC10 | MIRLET7I | MIR17 | IDH2 |
| IL6 | BDPLT21 | LPP | CYP2E1 | SLC29A1 | PTBP2 | CRK | AXL |
| TGFB1 | ETV6 | CHIC2 | VDR | 1KBKG | SPRED2 | GATA2 | CDK9 |
| AKT1 | TEL | BTL | MPL | PRKCA | OSBP2 | NOTCH2 | PMP22 |
| GSTM1 | THC5 | PBT | CHRM1 | IMPDH1 | DDX43 | PRTN3 | ADIPOR1 |
| CTNNB1 | KRAS2 | RAP1GDS1 | CNRM2 | IMPDH2 | P2RX5 | IL32 | IKZF1 |
| KRAS | RASK2 | TCS1 | BCHE | PB1 | MKNK2 | MIR223 | TET2 |
| GSTT1 | NS | BST2 | UREC | NT5C2 | UBASH3B | IL24 | MTHFD1L |
| NFKB1 | CFC2 | DKCA2 | MMP12 | ENPP1 | MIR30E | ARRB1 | NFKB2 |
| BRCA1 | RALD | DKCB4 | NS5B | PRKCD | ATP5F1 | PAX5 | ERCC5 |
| MMP9 | CMTS | PFBMFT1 | ADRB2 | MS4A1 | KIR2DL5B | WSB1 | DAPK1 |
| STAT3 | PTPN11 | CMM9 | PTAFR | HCK | GAS2 | CIP2A | POU2F1 |
| ABL1 | PTP2C | LIFR | HRH1 | CDK2 | FAM27E5 | KIF11 | SPI1 |
| PTGS2 | SHP2 | SWS | NS5A | MAP2K1 | ETNK1 | MIR31 | SLC22A1 |
| CDKN2A | NS1 | STWS | ADRA2A | MAP2K2 | MIR1301 | BIN1 | SEP9 |
| IL1B | JMML | SJS2 | ADRA2B | MAP3K2 | ST8SIA6 | SET | |
| MYC | METCDS | ACSL6 | CHD1 | 1FNAR2 | LOC107126288 | JUP | |
| GSTP1 | CLLS2 | FACL6 | DRD2 | POLA1 | LOC107126281 | REL | |
| CXCL8 | D13S25 | ACS2 | DRD1 | SLC28A3 | MIR564 | HOXA9 | |
| LEP | DBM | IRF1 | HTR2A | PARP1 | MIR2278 | CRKL | |
| TERT | FLVCR2 | MAR | OPRM1 | PARP2 | MIR4701 | CSF3R | |
| BCL2 | C14orf58 | GRAF | PDE4B | PARP3 | ABL | SLCO1B3 | |
| IFNG | CCT | PDGFR | PANX1 | CD22 | GF1R | MTHFD1 | |
| MTOR | PVHH | IBGC4 | CYP3N4 | POLE | SPC | IFNAR1 | |
| XRCC1 | EPV | IMF1 | CASR | POLE2 | EPHA2 | EHMT2 | |
| FAS | SERPINA1 | PENTT | KCND | POLE3 | ICK | CDC6 | |
| CCND1 | PI | KOGS | HTR7 | POLE4 | YES1 | ADIPOR2 | |
| BIRC5 | AAT | NSD1 | ORM2 | PNP | KIT | PER3 | |
| GSK3B | TCL1B | ARA267 | SLC6A2 | C3 | FYN | KIR2DS2 | |
| MAPK14 | TML1 | STO | SMPD1 | C4A | BTK | EPHB4 | |
| ATM | TCL1A | SOTOS1 | HTR1A | C4B | NR4A3 | MEF2C | |
| MIR21 | TCL1 | CLLS4 | NOMO1 | C5 | CSK | ASXL1 | |
| HMGB1 | MYL | DEK | MAP2 | AOX1 | EPHA5 | HES1 | |
| CYP1A1 | CREBBP | D6S231E | PHD | DNMT1 | FGR | KIR2DL2 | |
| NOTCH1 | CBP | CTEPH1 | CA2 | SLC01A2 | FRK | PRAME | |
| KDR | RSTS1 | HLA-B | NR1I2 | HSD11B1 | HSPA8 | SMO | |
| NFE2L2 | MYH11 | SPDA1 | POMC | ALB | ZAK | ORM1 | |
| HLA-G | AAT4 | HLA-DPB1 | CALM1 | RARA | PPAT | MSR1 | |
| CASP3 | FAA4 | TREM2 | YARS | RARB | CYP3A4 | NUMB | |
| ABCG2 | FUS | MYB | TAT | 1KBKB | CYP1A2 | CAMK2G | |
| EZH2 | TLS | ALL2 | SLC16A2 | TXNRD1 | CYP1B1 | CCDC170 | |
| CDKN1B | ALS6 | MLSM7 | TFPI | MAPK3 | FM03 | GPX3 | |
| JUN | ETM4 | DEL7q | GNRHR | MAPK1 | RET | MIR451A | |
| ERCC2 | CBFB | C7DELq | KCNQ2 | CDKN1A | NTPK1 | CDK7 | |
| TNFSF10 | PEBP2B | NCF1 | KCNQ3 | HDAC1 | CSF1R | MIR101-1 | |
| RHOA | DIA4 | PRSS2 | HTR3A | PML | DDR1 | IRF8 | |
| WT1 | NMOR1 | TRY2 | GRIA1 | ADA | CYP3A7 | KIR2DS4 | |
| PDCD1 | CYBA | SCLL | GNB1 | CD38 | CYP2C9 | ID4 | |
| LGALS3 | NF1 | NSD3 | MRD42 | CD19 | CYP2D6 | CD33 | |
| CYP3A5 | VRNF | WHSC1L1 | CTRC | RXRA | CYP2C19 | KIR2DS1 | |
| CD274 | WSS | SLC20A2 | CLCR | RXRG | PTGS1 | HMMR | |
| KCNH2 | NFNS | MLVAR | IMD22 | 1GFBP3 | SLC22A2 | SOCS2 | |
| LCN2 | ERBB2 | GLVR2 | TPOR | PSG5 | ABCA3 | BLK | |
| AURKA | NGL | IBGC1 | MPLV | CSF2RA | CYP2C8 | RMND1 | |
| RUNX1 | NEU | NBN | THCYT2 | 1L3RA | UGT1A1 | MIR224 | |
| LEPR | HER2 | NBS1 | TAL1 | SDC2 | GSTA2 | RANGAP1 | |
| HSP90AA1 | MSF | THCYT3 | TCL5 | PRG2 | MGST2 | FUT1 | |
| NPM1 | MSF1 | LALL | SCL | EPOR | FLT3 | PCM1 | |
| AKAP12 | NAPB | BSAP | BCL10 | GPRC5A | RPI2 | MIR130A | |
| BCL2L1 | SH3GL1 | ALL3 | IMD37 | NROB1 | RPL3 | MIR7-1 | |
| MCL1 | EEN | TAL2 | GFI1 | ALDH1A2 | TEK | AHI1 | |
| IL2RA | LYL1 | CLLS3 | ZNF163 | RARRES1 | FGFR1 | CDKN2C | |
| PLK1 | CEBPA | CHDSKM | SCN2 | LCN1 | FGFR2 | ZBTB2 | |
| PTPN22 | CEBP | NUP214 | RBM15 | OBP2A | FGFR3 | PLCD1 | |
| XIAP | BCL3 | D9S46E | SPEN | RBP4 | FGFR4 | MTSS1 | |
| BCL2L11 | BAX | CAN | OTT | PDK4 | LCK | MSI2 | |
| DPP4 | TAM | CAIN | IGFR2 | CYP26A1 | SRC | FERMT3 | |
| SYK | MST | AF10 | CD32 | HPGDS | ABCB11 | PIWIL1 | |
| PDGFRA | CBFA2 | ALL1 | PBX1 | ATP1A1 | FCGR3B | RAPGEF1 | |
| MIR155 | AML1 | MBL2 | CAKUHED | DGUOK | C1R | MKNK1 | |
| TWIST1 | CML | MBL | ABL2 | 1TGAL | C1QA | EHMT1 | |
| CBL | PHL | MBP1 | ABLL | TOP2A | C1QB | USF2 | |
| STAT5A | ALL | MBL2D | ARG | PDLA1 | C1QC | MIR10A | |
| BMI1 | HMOX1 | MBPD | NCF2 | CBR1 | FCGR3A | IL1RAP | |
| HSPA4 | HMOX1D | LMO1 | FLVCR1 | AKR1A1 | C1S | MIR320A | |
| TGM2 | NCF4 | RBTN1 | AXPC1 | AKR1 | FCGR1A | LTB4R2 | |
| NFKBIA | P40PHOX | RHOM1 | PCARP | NQO1 | FCGR2A | SETBP1 | |
| CD34 | CGD3 | LMO2 | COPD | NOS3 | FCGR2B | ULBP2 | |
| CALR | MKL1 | RBTNL1 | TCL4 | NDUFS3 | FCGR2C | BTG1 | |
| MIR34A | AMKL | RHOM2 | ERBB4 | NDUFS7 | RRM2 | KDM5A | |
| XRCC3 | MAL | TTG2 | HER4 | POR | DCK | MLLT3 | |
| PDGFRB | XK | CLLS1 | ALS19 | ABCC3 | PRKAR2A | PHF6 | |
| SOCS1 | MCLDS | SMAR | SGOL1 | HPRT1 | PRKAR1A | CD7 | |
| ABCC1 | CYBB | NUMA1 | SGO | TUBB | PDE3A | PTPRG | |
| IFNA1 | CGD | PICALM | SGO1 | TUBA4A | 1FNAR1 | FCER1G | |
| PIK3CG | AMCBX2 | CALM | CAID | DHFR | FNTB | HULC | |
| CYP2B6 | IMD34 | CLTH | THRB | FPGS | PDGFD | MIR196B | |
| EIF4E | GATA1 | LAP | ERBA2 | TYMS | FLT1 | MR1 | |
| XPC | GF1 | ATA | THR1 | ATIC | FLT4 | RIN1 | |
| AURKB | ERYF1 | AT1 | PRTH | GGH | UGT1A3 | ST3GAL4 | |
| PTPN6 | NFE1 | ZBTB16 | MYD88 | FOLR1 | UGT1A4 | FIP1L1 | |
| DNMT3A | XLTDA | ZNF145 | MYD88D | IFNAR2 | UGT1A9 | MIR148B | |
| BCL6 | XLTT | PLZF | DCML | NS3 | UGT2B7 | MIR326 | |
| ALOX5 | XLANP | KMT2A | MONOMAC | 4A | UGT2B15 | MIR486-1 |
Supplementary Table 2.
Molecular docking between the 7 compounds of Qingdai and the corresponding candidate major targets.
| Compounds and targets | Protein-ligand interactions of the docking pose | Score |
|---|---|---|
| Qingdainone, MAPK9 |

|
4.306 |
| Qingdainone, MAPK10 |

|
4.684 |
| Qingdainone, MAPK11 |

|
4.435 |
| Qingdainone, MAPK11 |

|
4.844 |
| Bisindigotin, MAPK9 |

|
4.053 |
| Bisindigotin, MAPK10 |

|
3.562 |
| Bisindigotin, MAPK11 |

|
3.787 |
| Bisindigotin, MAPK14 |

|
4.039 |
| Bisindigotin, F2 |

|
4.598 |
| Isoindigo, FLT4 |

|
7.117 |
| Isoindigo, PDGFRB |

|
5.516 |
| Isoindigo, FLT3 |

|
7.780 |
| Indigotin, CDK1 |

|
6.860 |
| Indirubin, CDK1 |

|
2.633 |
| Indirubin, CDK4 |

|
2.051 |
| Indirubin, CDK2 |

|
2.583 |
| Indirubin, FLT3 |

|
3.217 |
| Indirubin, GSK3B |

|
1.963 |
| Beta-sitosterol,AR |

|
8.365 |
| Beta-sitosterol, CYP19A1 |

|
8.335 |
| Beta-sitosterol, LDLR |

|
4.981 |
| Beta-sitosterol, ESR1 |

|
8.372 |
| Beta-sitosterol, ESR2 |

|
8.321 |