Abstract
Objective
This study aimed to evaluate the effect of three days of GnRH antagonist pretreatment on the pregnancy outcomes of women with polycystic ovarian syndrome (PCOS) on GnRH antagonist protocols for IVF/ICSI.
Methods
Fifty women with PCOS in the control group received conventional antagonist protocols, starting on day 2 of the cycle. In the pretreatment group (n=38), a GnRH antagonist was administered from day 2 of the menstrual cycle for three days.
Results
Controlled ovarian stimulation (COS) duration and gonadotropin dosages were similar in both groups. The number of metaphase II (MII) oocytes, 2PN oocytes, embryos, along with implantation and clinical pregnancy rates, were higher in the pretreatment group when compared with controls, although the increment was not significant (P value ≥0.05). The chemical pregnancy rate was significantly higher in the pretreatment group. The rate of OHSS was significantly lower in the pretreatment than in the control group.
Conclusion
Women with PCOS offered early follicular phase GnRH antagonist pretreatment for three consecutive days had significantly fewer cases of OHSS and higher chemical pregnancy rates. There were trends toward greater numbers of MII oocytes, 2PN oocytes, and embryos, and higher clinical pregnancy rates in the pretreatment group.
Keywords: assisted reproductive technology, gonadotropins, polycystic ovarian syndrome
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a very common endocrine disorder. It affects 5-7% women of reproductive age and is the leading cause of anovulatory infertility in this age range (Singh et al., 2014; Kalem et al., 2017). Irregular menstruation, hirsutism, acne, and infertility are common clinical features. Forty percent of infertile women have anovulation/oligoovulation and PCOS accounts for 80% of these cases (Singh et al., 2014). Assisted reproductive technology (ART) protocols are indicated when infertile women with PCOS are unable to become pregnant through standard ovulation induction methods. Ovarian stimulation includes two methods to prevent premature LH surges, GnRH agonist and GnRH antagonist protocols (Toftager et al., 2016). However, issues such as increased risk of ovarian hyperstimulation syndrome (OHSS), increased immature oocyte rates, lower fertilization rates, lower embryo quality, and lower implantation rates are observed in the IVF cycles of women with PCOS when the two protocols are compared (Kalem et al., 2017).
GnRH antagonists have been extensively used in ART clinics during the past years and a variety of GnRH antagonist protocols have been suggested. In spite of GnRH agonist protocol cycles, GnRH antagonists cause immediate suppression of gonadotropin secretion, which results in shorter treatments and less patient distress (European and Middle East Orgalutran Study Group, 2001; Al-Inany et al., 2006; Devroey et al., 2009). Moreover, the use of GnRH antagonists has yielded significantly lower chances of hospitalization due to OHSS (Kolibianakis et al., 2006). Despite the benefits associated with GnRH antagonists, GnRH agonist protocols remain as the treatment of choice in controlled ovarian hyperstimulation (COH) in the majority of ART clinics. There are some reasons for this practice. First, some investigators have reported uncoordinated antral follicle growth during ovarian stimulation with GnRH antagonists, leading to an asynchrony of the follicular cohort (Fanchin et al., 2003), which in turn may raise concerns over the outcome of the treatment. The flexibility of GnRH agonist protocols also permits more controlled oocyte retrievals, significantly decreasing and even preventing the need to perform retrievals, whereas the initiation of ovarian stimulation in GnRH antagonist protocol relies on the random incidence of spontaneous menses (Guivarc'h-Levêque et al., 2010; Levy et al., 2009; Tremellen & Lane, 2010). Therefore, pretreatment with oral contraceptive pills (OCP) is frequently used in GnRH antagonist protocols to schedule the start of gonadotropin stimulation, although it considerably increases the consumption of gonadotropins and the duration of ovarian stimulation (Griesinger et al., 2008). A recent meta-analysis detected a considerable decrease in the ongoing pregnancy rate of patients prescribed pretreatment with OCP (Griesinger et al., 2010). Comparisons between a pituitary down-regulation protocol and a GnRH antagonist protocol at the beginning of ovarian stimulation found that women with PCOS in particular had higher serum gonadotropin and E2 levels. Consequently, in these women the unsuppressed level of FSH at the start of a GnRH antagonist cycle in contrast with a long GnRH agonist protocol allows the initial growth of a few leading follicles before the addition of exogenous rFSH (Blockeel et al., 2011a). We hypothesized that short pituitary suppression in the early follicular phase might mimic the pre-stimulation hormonal environment of long GnRH agonist protocols, challenging the idea that high endogenous FSH levels cause developmental asynchrony of early antral follicles while maintaining the benefits of short antagonist protocols.
Furthermore, stable and early suppression of LH levels during the entire period of stimulation may be advantageous for implantation and pregnancy outcomes (Blockeel et al., 2011a). Occasional elevated baseline progesterone levels at the beginning of ART cycles and their association with reduced pregnancy outcomes is another problem in GnRH antagonist protocols (Kolibianakis et al., 2004a; Blockeel et al., 2011b). Based on these data, administration of GnRH antagonists for three consecutive days before the start of COS may normalize raised progesterone levels.
In agreement with the above findings, we posited that pretreatment with GnRH antagonists might allow follicular cohort synchronization and scheduling of ART treatment in women with PCOS. The purpose of this prospective randomized trial was to evaluate the effects of a 3-day course of GnRH antagonist pretreatment before the initiation of ovarian stimulation with gonadotropins on pregnancy outcomes.
MATERIALS AND METHODS
Study Design
This randomized clinical trial enrolled 88 women with PCOS based on the Rotterdam criteria, participating in an ART program at the Yazd Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, from March 2015 to March 2016. The Ethics Committee of the university approved the study. Informed written consent was obtained from all participating couples.
Patients with at least two of the following findings were included in the study: oligoovulation or anovulation; clinical or biochemical hyperandrogenism; and polycystic ovaries on ultrasound examination. Women aged 40 years or older, presence of severe male factor or systemic disease, use of hormone medication other than OCP, individuals on systemic drug therapy or with recurring IVF failure, recurrent pregnancy loss or uterine anomalies were excluded.
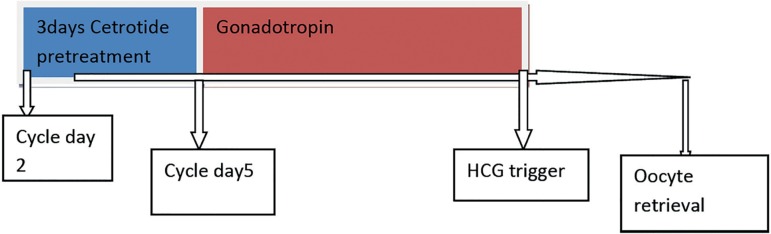
Two GnRH antagonist protocols for ovarian stimulation were compared. The patients were randomly (Random Digit Software) allocated to two groups. The 50 individuals assigned to the control group were prescribed a standard GnRH antagonist protocol. Controls were administered Gonal-f 150 IU (SA Merck Serono, Geneva, Switzerland) on cycle day 2 subcutaneously, and later 0.25 mg of cetrorelix (Cetrotide; Asta Medica, Frankfurt, Germany) daily when the leading follicle reached 14 mm in diameter until the HCG injection. The 38 women allocated in the pretreatment group were offered a modified protocol with antagonist administration for three days (starting on day 2 of the cycle) before the start of recombinant FSH (rFSH) therapy (Figure 1). Final oocyte maturation was triggered with HCG 10,000 IU (Pregnyl; Schering Plough) when the three larger follicles reached a mean diameter of 17 mm. Serum estrogen (E2) and endometrial thickness (ET) were measured on triggering day. Ultrasound-guided transvaginal oocyte retrieval was performed 36 hours later. Follicles measuring ≥14 mm were aspirated and the physicians performing follicular aspiration were blinded to the stimulation protocol. The IVF and ICSI procedures were performed, and the embryos were transferred on the third day after retrieval with a catheter (Labotect, Gotting, Germany). Embryo quality was assessed based on the morphology criteria described by Dokras et al.; cleavage-stage embryos were given grades A, B, C, or D. Embryos graded as D were not transferred. Grade A included embryos with no fragmentation and equal size homogenous blastomeres; grade B included embryos with fragmentation <20% and equal size homogenous blastomeres; grade C included embryos with fragmentation ranging from 20% to 50% and unequal size blastomeres; grade D included embryos with fragmentation >50% and unequal size blastomeres (Dokras et al., 1993).
Figure 1.
Schematic view of cetrotide pretreatment protocol.
The number of transferred embryos depended on embryo quality, patient age, and risk of OHSS. Women at moderate or severe risk of OHSS had their embryos frozen as described in Table 1.
Table 1.
Classification of OHSS.
| Grade | Symptom |
|---|---|
| Mild OHSS | Abdominal bloating |
| Mild abdominal pain | |
| Ovarian size usually <8 cm | |
| Moderate OHSS | Moderate abdominal pain |
| Nausea ± vomiting | |
| Ultrasound evidence of ascites | |
| Ovarian size usually 8 to 12 cm | |
| Severe OHSS | Clinical ascites (occasionally pleural effusion) |
| Oliguria | |
| Hematocrit (>45%) | |
| Hypoproteinemia | |
| Ovarian size usually >12 cm |
All patients were given 400 mg progesterone suppositories (Cox Pharmaceuticals, Barnstaple, UK) twice a day for luteal support, initiated on the day of oocyte retrieval. Serum B-hCG was checked 14 days after embryo transfer. If the patient became pregnant, then progesterone was continued until the 10th week of pregnancy. The implantation rate was calculated as the ratio between the number of gestational sacs and transferred embryos; chemical pregnancy was defined by serum B-hCG levels ≥50 IU/L 14 days after embryo transfer; clinical pregnancy was established as the presence of a gestational sac with fetal heartbeat identified by ultrasound examination five weeks after embryo transfer; miscarriages were defined as clinically recognized pregnancy losses before 20 weeks of gestation; and ongoing pregnancies were defined as pregnancies continued after 20 weeks of gestation.
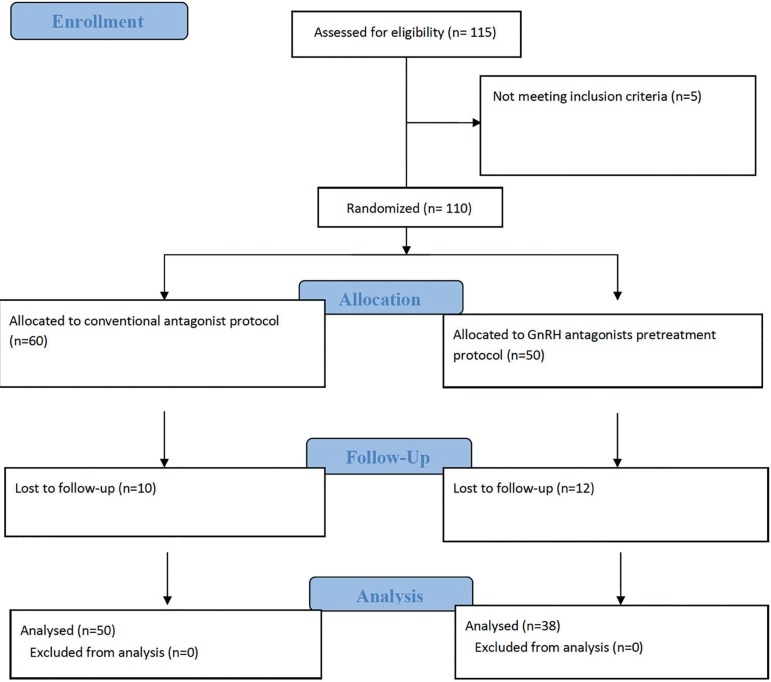
The two groups had different sizes on account of patients lost to follow-up (Figure 2).
Figure 2.
Consort flowchart.
Outcome Measures
The primary endpoints in this study were the number of cumulus oocyte complexes (COCs), metaphase II oocytes (MII), and 2-pronuclei (2PN) oocytes in each group. Secondary endpoints included fertilization, implantation, and pregnancy rates in each treatment group. Tertiary endpoints were risk of OHSS and miscarriage rates in each group. Demographic and clinical characteristics such as age, baseline serum hormone levels, AMH, duration of stimulation, and total cumulative dose of rFSH were also collected.
Statistical Analysis
SPSS software (Statistical Package for the Social Sciences version 16.0, SPSS Inc., Chicago, IL, USA) was used for all statistical calculations. Student's t-test was used to compare quantitative variables. Statistical significance was attributed to differences with a p value<0.05.
RESULTS
Eighty-eight patients were randomly assigned to either control (n=50) or pretreatment groups (n=38). A flowchart diagram with the phases of the trial is shown in Figure 2. Baseline characteristics of the study groups are presented in Supplemental Table 2. The groups did not significantly differ with regard to demographic and cycle parameters. Table 2 summarizes the outcome parameters of both treatment protocols. Embryos from 18 women in the control group and 15 in the pretreatment group were frozen due to risk of OHSS. The implantation rates in the pretreatment and control groups were 20.5%±27.3% and 11.8%±20.6%, respectively. The difference was not statistically significant. Chemical and clinical pregnancy rates in the pretreatment group were higher (p˂0.05) (Table 3). Ongoing pregnancy rates were higher in the pretreatment group, although not statistically different from the rates seen in the control group (Table 3).
Table 2.
Baseline and cycle characteristics of patients in both groups.
| Pretreatment group (n=38) | Control group (n=50) | p-value | |
|---|---|---|---|
| Age (years) | 28.07±3.85 | 29.04±4.76 | .313 |
| Duration of infertility (years) | 6.57±3.77 | 5.99±3.74 | .468 |
| AMH | 6.64±1.29 | 6.46±1.01 | .367 |
| End. Thickness at triggering day (mm) | 10.27±1.92 | 10.15±1.98 | .770 |
| Estradiol level at triggering day (ng/ml) | 3323±2670 | 2967±2149 | .491 |
| Gonadotropin Dose (IU) | 1598±1932 | 1515±1475 | .771 |
| Cycle duration (days) | 12.36±1.54 | 13.66±2.23 | .003 |
| COC number | 17.68±9.29 | 16.46±9.76 | .554 |
| Number of M2 oocytes | 14.65±8.30 | 14.10±8.79 | .764 |
| Number of 2PN oocytes | 8.84±6.67 | 7.40±6.41 | .308 |
| Total number of embryos | 7.94±6.16 | 6.94±6.06 | .446 |
| Number of embryos transferred | 2.80±0.7 | 2.69±0.6 | .676 |
| Number of embryos frozen | 3.56±2.10 | 4.13±2.01 | .454 |
Data are presented as mean value ± SD or number (%).
Table 3.
Pregnancy outcomes of patients in both groups.
| Pretreatment group (n=38) | Control group (n=50) | p-value | |
|---|---|---|---|
| Fertilization rate | 51.7% | 50.6% | 0.49 |
| Implantation rate | 20.5±27.3% | 11.8±20.6% | .091 |
| Chemical pregnancy rate (n, %)* | 9 (41%) | 3 (13%) | .035 |
| Clinical pregnancy rate (n, %)* | 7 (32%) | 2 (9%) | .050 |
| Ongoing pregnancy rate (n, %) | 6 (28%) | 2 (9%) | .103 |
| Moderate, severe risk of OHSS (n, %) | 15 (39%) | 18 (36%) | .739 |
| Miscarriage rate (n, %) | 3 (37%) | 1 (33%) | .898 |
Student’s t test.
DISCUSSION
The study showed that pretreatment with GnRH antagonists for three consecutive days before the start of ovarian stimulation tends to yield a greater number of COCs when compared with conventional GnRH antagonist protocols. Although not significant, a greater number of 2PN oocytes and higher pregnancy rates were also observed in the pretreatment group. Using a similar protocol with GnRH antagonist pretreatment, Younis et al. (2010) reported improved oocyte maturation and fertilization rates. Although our study included only 38 patients in pretreatment, potential improvements in clinical outcomes might be inferred.
Huirne et al. (2007) suggested that early suppression of endogenous FSH results in improved follicular development. In a GnRH antagonist protocol, higher serum gonadotropin and estradiol levels are found at the beginning of ovarian stimulation, when compared with long GnRH agonist protocols (Albano et al., 2000; Borm & Mannaerts, 2000). As a result, the unsuppressed FSH level at the beginning of a GnRH antagonist cycle allows the initial growth of a few leading follicles before the initiation of exogenous rFSH, in contrast with a long GnRH agonist protocol. Pretreatment with estrogen or OCP in GnRH antagonist regimens offers a simple alternative to achieve endogenous gonadotropin suppression during the early follicular phase (de Ziegler, 1995; van Heusden & Fauser, 1999). In order to challenge the idea that elevated endogenous FSH levels cause developmental asynchrony of early antral follicles, Fanchin et al. (2003) posited that "luteal phase E2 pretreatment and premenstrual administration of GnRH antagonist can reduce the size and improve the homogeneity of early antral follicles". We propose that short pituitary suppression in the early follicular phase might mimic the hormonal environment of long GnRH agonist protocols, while protecting the benefits of short antagonist protocols. Additionally, stable and early suppression of LH levels during the period of stimulation may be advantageous for implantation and pregnancy outcomes (Kolibianakis et al., 2003). Kolibianakis et al. (2003; 2004b) demonstrated that "high exposure of the genital tract to LH and E2 in the early follicular phase is associated with a lower chance of pregnancy". As a corollary to this study, the same researchers concluded that giving a GnRH antagonist from day 1 of stimulation is an effective protocol that leads to effective results (implantation rate of 26.5%; ongoing pregnancy rate of 39.7% per started cycle and of 42.4% per ET).
On the day of hCG administration, significantly higher E2 levels were found in the pretreatment group, which may be explained by the increased recruitment of oocytes in this group. Although supraphysiological serum E2 levels might lead to adverse effects on oocyte/embryo quality and to impaired endometrial receptivity, higher E2 levels on the day of hCG administration have no impact on pregnancy rates in GnRH antagonist protocols (Kyrou et al., 2009). Similarly, progesterone levels were significantly elevated on the last day of stimulation in the pretreatment group, but not to the extent that it might have a detrimental effect on the implantation potential of a good-quality cleavage-stage embryo (Bosch et al., 2010).
The clinical potential of this modified short patient-friendly protocol is associated with a small financial cost per cycle. A possible problem of this pretreatment protocol is the addition of three SC injections. Although this trial suggests promising results in that a trend toward higher pregnancy rates was detected in the GnRH antagonist pretreatment group, statistical power analysis might be helpful to define the sample size needed to add weight to this observation. This approach might also be accepted for the added convenience of enabling scheduled GnRH antagonist cycles and for optimizing the organization of ART centers through the administration of GnRH antagonist cycles for a varying number of days (2, 3 or 4 days), based on the planned start of ovarian stimulation. This protocol might be used with oocyte donors to help synchronization with recipients. The additional advantage of triggering final oocyte maturation with a GnRH agonist is that it allows patients to avoid OHSS.
CONCLUSION
In conclusion, pretreatment with GnRH antagonists for women with PCOS for three consecutive days before the beginning of ovarian stimulation was associated with improved pregnancy results. Further investigation is needed to find whether GnRH antagonist pretreatment leads to better coordination of multifollicular development in high responders.
Acknowledgments
The authors would like to thank the Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran, for the financial support provided to the study.
Funding Statement
Funding: This study was funded by the Yazd research and clinical center for infertility Shahid Sadoughi University of Medical Sciences (grant number 3906)
Footnotes
Compliance with Ethical Standards
Funding: This study was funded by the Yazd research and clinical center for infertility Shahid Sadoughi University of Medical Sciences (grant number 3906)
Ethical approval: The authors did not perform studies with animals for this article.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors of this article have no conflicts of interest to declare.
REFERENCES
- Albano C, Felberbaum RE, Smitz J, Riethmüller-Winzen H, Engel J, Diedrich K, Devroey P. Hum Reprod. Vol. 15. European Cetrorelix Study Group; 2000. Ovarian stimulationwith HMG: results of a prospective randomized phase III European study comparing the luteinizing hormone-releasing hormone (LHRH)-antagonist cetrorelix and the LHRH-agonist buserelin; pp. 526–531. [DOI] [PubMed] [Google Scholar]
- Al-Inany HG, Abou-Setta AM, Aboulghar M. Gonadotrophin-releasing hormone antagonists for assisted conception. Cochrane Database Syst Rev. 2006;3:CD001750. doi: 10.1002/14651858.CD001750.pub2. [DOI] [PubMed] [Google Scholar]
- Blockeel C, Riva A, De Vos M, Haentjens P, Devroey P. Administration of a gonadotropin-releasing hormone antagonist during the 3 days before the initiation of the in vitro fertilization/intracytoplasmic sperm injection treatment cycle: impact on ovarian stimulation. A pilot study. Fertil Steril. 2011a;95:1714-9.e1-2. doi: 10.1016/j.fertnstert.2011.01.028. [DOI] [PubMed] [Google Scholar]
- Blockeel C, Baumgarten M, De Vos M, Verheyen G, Devroey P. Administration of GnRH antagonists in case of elevated progesterone at initiation of the cycle: a prospective cohort study. Curr Pharm Biotechnol. 2011b;12:423–428. doi: 10.2174/138920111794480633. [DOI] [PubMed] [Google Scholar]
- Borm G, Mannaerts B. Hum Reprod. Vol. 15. The European Orgalutran Study Group; 2000. Treatment with the gonadotrophin-releasing hormone antagonist ganirelix in women undergoing ovarian stimulation with recombinant follicle stimulating hormone is effective, safe and convenient: results of a controlled, randomized, multicenter trial; pp. 1490–1498. [DOI] [PubMed] [Google Scholar]
- Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Jenkins J, Pellicer A. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod. 2010;25:2092–2100. doi: 10.1093/humrep/deq125. [DOI] [PubMed] [Google Scholar]
- de Ziegler D. Hormonal control of endometrial receptivity. Hum Reprod. 1995;10:4–7. doi: 10.1093/humrep/10.1.4. [DOI] [PubMed] [Google Scholar]
- Devroey P, Aboulghar M, Garcia-Velasco J, Griesinger G, Humaidan P, Kolibianakis E, Ledger W, Tomás C, Fauser BC. Improving the patient's experience of IVF/ICSI: a proposal for an ovarian stimulation protocol with GnRH antagonist co-treatment. Hum Reprod. 2009;24:764–774. doi: 10.1093/humrep/den468. [DOI] [PubMed] [Google Scholar]
- Dokras A, Sargent IL, Barlow DH. Fertilization and early embryology: an indicator of developmental potential? Hum Reprod. 1993;8:2119–2127. doi: 10.1093/oxfordjournals.humrep.a137993. [DOI] [PubMed] [Google Scholar]
- European and Middle East Orgalutran Study Group Comparable clinical outcome using the GnRH antagonist ganirelix or a long protocol of the GnRH agonist triptorelin for the prevention of premature LH surges in women undergoing ovarian stimulation. Hum Reprod. 2001;16:644–651. doi: 10.1093/humrep/16.4.644. [DOI] [PubMed] [Google Scholar]
- Fanchin R, Salomon L, Castelo-Branco A, Olivennes F, Frydman N, Frydman R. Luteal estradiol pretreatment coordinates follicular growth during controlled ovarian hyperstimulation with GnRH antagonists. Hum Reprod. 2003;18:2698–2703. doi: 10.1093/humrep/deg516. [DOI] [PubMed] [Google Scholar]
- Griesinger G, Venetis CA, Marx T, Diedrich K, Tarlatzis BC, Kolibianakis EM. Oral contraceptive pill pretreatment in ovarian stimulation with GnRH antagonists for IVF: a systematic review and meta analysis. Fertil Steril. 2008;90:1055–1063. doi: 10.1016/j.fertnstert.2007.07.1354. [DOI] [PubMed] [Google Scholar]
- Griesinger G, Kolibianakis EM, Venetis C, Diedrich K, Tarlatzis B. Oral contraceptive pretreatment significantly reduces ongoing pregnancy likelihood in gonadotropin-releasing hormone antagonist cycles: an updated meta-analysis. Fertil Steril. 2010;94:2382–2384. doi: 10.1016/j.fertnstert.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Guivarc'h-Levêque A, Arvis P, Bouchet JL, Broux PL, Moy L, Priou G, Vialard J, Colleu D. Efficiency of antagonist IVF cycle programming by estrogens. Gynecol Obstet Fertil. 2010;38:18–22. doi: 10.1016/j.gyobfe.2009.04.028. In French. [DOI] [PubMed] [Google Scholar]
- Huirne JA, Homburg R, Lambalk CB. Are GnRH antagonists comparable to agonists for use in IVF? Hum Reprod. 2007;22:2805–2813. doi: 10.1093/humrep/dem270. [DOI] [PubMed] [Google Scholar]
- Kalem MN, Kalem Z, Gurgan T. Effect of metformin and oral contraceptives on polycystic ovary syndrome and IVF cycles. J Endocrinol Invest. 2017;40:745–752. doi: 10.1007/s40618-017-0634-x. [DOI] [PubMed] [Google Scholar]
- Kolibianakis EM, Albano C, Kahn J, Camus M, Tournaye H, Van Steirteghem AC, Devroey P. Exposure to high levels of luteinizing hormone and estradiol in the early follicular phase of gonadotropin-releasing hormone antagonist cycles is associated with a reduced chance of pregnancy. Fertil Steril. 2003;79:873–880. doi: 10.1016/S0015-0282(02)04920-8. [DOI] [PubMed] [Google Scholar]
- Kolibianakis EM, Zikopoulos K, Smitz J, Camus M, Tournaye H, Van Steirteghem AC, Devroey P. Elevated progesterone at initiation of stimulation is associated with a lower ongoing pregnancy rate after IVF using GnRH antagonists. Hum Reprod. 2004a;19:1525–1529. doi: 10.1093/humrep/deh272. [DOI] [PubMed] [Google Scholar]
- Kolibianakis EM, Zikopoulos K, Smitz J, Camus M, Tournaye H, Van Steirteghem AC, Devroey P. Administration of gonadotropin-releasing hormone antagonist from day 1 of stimulation in in vitro fertilization. Fertil Steril. 2004b;82:223–226. doi: 10.1016/j.fertnstert.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Kolibianakis EM, Collins J, Tarlatzis BC, Devroey P, Diedrich K, Griesinger G. Among patients treated for IVF with gonadotropins and GnRH analogues, is the probability of live birth dependent on the type of analogue used? A systematic review and meta-analysis. Hum Reprod Update. 2006;12:651–671. doi: 10.1093/humupd/dml038. [DOI] [PubMed] [Google Scholar]
- Kyrou D, Popovic-Todorovic B, Fatemi HM, Bourgain C, Haentjens P, Van Landuyt L, Devroey P. Does the estradiol level on the day of human chorionic gonadotrophin administration have an impact on pregnancy rates in patients treated with rec-FSH/GnRH antagonist? Hum Reprod. 2009;24:2902–2909. doi: 10.1093/humrep/dep290. [DOI] [PubMed] [Google Scholar]
- Levy MJ, Gordon K, Ijzerman-Boon PC. Reducing the incidence of weekend oocyte retrievals in GnRH antagonist protocols by selective initiation of COS on cycle day 1 through 4. Fertil Steril. 2009;26:S167–S168. doi: 10.1016/j.fertnstert.2009.07.1317. [DOI] [PubMed] [Google Scholar]
- Singh N, Naha M, Malhotra N, Lata K, Vanamail P, Tiwari A. Comparison of gonadotropin-releasing hormone agonist with GnRH antagonist in polycystic ovary syndrome patients undergoing in vitro fertilization cycle: Retrospective analysis from a tertiary center and review of literature. J Hum Reprod Sci. 2014;7:52–57. doi: 10.4103/0974-1208.130852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toftager M, Bogstad J, Bryndorf T, Løssl K, Roskær J, Holland T, Prætorius L, Zedeler A, Nilas L, Pinborg A. Risk of severe ovarian hyperstimulation syndrome in GnRH antagonist versus GnRH agonist protocol: RCT including 1050 first IVF/ICSI cycles. Hum Reprod. 2016;31:1253–1264. doi: 10.1093/humrep/dew051. [DOI] [PubMed] [Google Scholar]
- Tremellen KP, Lane M. Avoidance of weekend oocyte retrievals during GnRH antagonist treatment by simple advancement or delay of hCG administration does not adversely affect IVF live birth outcomes. Hum Reprod. 2010;25:1219–1224. doi: 10.1093/humrep/deq059. [DOI] [PubMed] [Google Scholar]
- van Heusden AM, Fauser BC. Activity of the pituitary-ovarian axis in the pill-free interval during use of low-dose combined oral contraceptives. Contraception. 1999;59:237–243. doi: 10.1016/S0010-7824(99)00025-6. [DOI] [PubMed] [Google Scholar]
- Younis JS, Soltsman S, Izhaki I, Radin O, Bar-Ami S, Ben-Ami M. Early and short follicular gonadotropin releasing hormone antagonist supplementation improves the meiotic status and competence of retrieved oocytes in in vitro fertilization-embryo transfer cycles. Fertil Steril. 2010;94:1350–1355. doi: 10.1016/j.fertnstert.2009.08.033. [DOI] [PubMed] [Google Scholar]