Abstract
Objective
Spermatogenesis is a complex process controlled by a plethora of genes. Changes in expression and function of these genes may thus lead to spermatogenic deficiency and male infertility. TEX11, TEX12, TEX14 and TEX15 are germ cell-specific genes expressed in the testis. TEX11, involved in the initiation and maintenance of chromosome synapses in meiotic chromosomes, has been shown to be essential for meiosis and fertility in males. TEX14, a component of intercellular bridges in germ cells, is required for spermatogenesis and fertility. TEX12 and TEX15 are essential for correct assembly of the synaptonemal complex and thus meiosis progression.
Methods
In order to examine whether changes in expression of these genes is associated with impaired spermatogenesis, expression levels of these genes were quantified by RT-qPCR on samples retrieved from infertile patients submitted to diagnostic testicular biopsy at Royan institute. Samples were divided into two groups of 18 patients with non-obstructive azoospermia considered as case; nine patients with obstructive azoospermia were included in the control group.
Results
A significant down-regulation of these genes was observed in the SCOS group when compared to the control group.
Conclusion
This result suggests that regular expression of TEX11, TEX12, TEX14 and TEX15 is essential for the early stages of spermatogenesis.
Keywords: Male infertility, Gene expression, Non-Obstructive Azoospermia, Intercellular bridges, Synaptonemal complex
INTRODUCTION
Male-factor infertility apparently accounts for 40% to 50% of infertile complications and may be defined by environmental reasons, infections, immunological or hormonal insufficiencies, while many are regulated by genetic factors (Hirsh, 2003; Brugh & Lipshultz, 2004; Boyle et al., 1992; Ma et al., 2016). Spermatogenesis is a complex process controlled by thousands of genes, where any change in the expression or function of these genes may lead to spermatogenic failure and male infertility (Zhou et al., 2009; Westerveld, 2008). Identification of stage-specific genes controlling spermatogenesis is thus important.
Previous studies have revealed that TEX11, TEX12, TEX14 and TEX15 expression is restricted to germ cells and is not detectable in somatic tissues of humans and mice (Wang et al., 2001; Adelman & Petrini, 2008). Multiple studies have shown that X-linked germ cell-specific genes such as testis-expressed gene 11 (Tex11) have significant roles in regulating male fertility (Zheng et al., 2010; Matzuk & Lamb, 2008). Furthermore, TEX11 expression has been associated with the onset of spermatogenesis, although restricted to spermatocytes and round spermatids (Wang et al., 2005; Tang et al., 2011).
The synaptonemal complex (SC) is a large protein structure needed for synapsis and the successful completion of meiotic cell division. Incorrect assembly of this complex in mice results in maturation arrest and infertility (Bolcun-Filas et al., 2009; Hamer et al., 2008; Kouznetsova et al., 2011), and may similarly lead to infertility, recurrent miscarriage and aneuploidies such as Down syndrome in humans (de Vries et al., 2005; Gerton & Hawley, 2005; Page & Hawley, 2003). Tex11, Tex12 and Tex15 are needed in chromosomal synapsis and meiotic recombination. More specifically, Tex11 forms distinct foci on homologous chromosomes that synapse with each other and seem to be a new component of meiotic nodules needed for recombination. In the absence of this protein, chromosomal synapsis stops and crossover formation is reduced (Stouffs & Lissens, 2012; Yang et al., 2008b). Tex12 is a meiosis-specific protein essential for the progression of synapsis between homologous chromosomes in male and female germ cells (Hamer et al., 2008). Loss of Tex15 leads to meiotic recombination failure, since this protein is responsible for the transfer of DNA repair proteins onto double strand break (DSB) locations (Yang et al., 2008a). TEX12 is small with no known domains, but has orthologs in other mammals such as mice, cows, and dogs (Hamer et al., 2006; Wang et al., 2001). TEX15 has orthologs in mammals and zebra fish (Yang et al., 2008a). It is abundantly expressed in post-meiotic germ cells, spermatogonia, and early spermatocytes, showing that this gene plays a role in different stages of spermatogenesis (Yang et al., 2008a; Wang et al., 2005). Interestingly, it is expressed in both the testis and ovaries, as is its mouse ortholog (Wang et al., 2001).
Germ cell intercellular bridges are required for fertility in invertebrates (Brill et al., 2000; Robinson et al., 1994; Robinson & Cooley, 1996) and have been preserved from invertebrates to humans (Fawcett et al., 1959), indicating their importance in fertility (Greenbaum et al., 2006; 2009). Mammalian intercellular bridges connect hundreds of germ cells in syncytia originated from a single spermatogonial stem cell (Huckins, 1971; Greenbaum et al., 2006; 2009). In the absence of cell bridges in mammalians, syncytium is thus not formed and as a result spermatogenesis is impaired (Greenbaum et al., 2006; 2009). TEX14 is needed to generate the germ cell intercellular bridges from the midbody (Greenbaum et al., 2006). Human TEX14 is preferentially expressed in the testis with its highest levels observed in spermatocytes and early round spermatids (Wu et al., 2003). Greenbaum et al. (2006) showed that Tex14-/- mutant male mice do not form intercellular bridges, thus confirming its essentiality in mammalian spermatogenesis. It was later revealed that Tex14 is also essential for complete spermatogenesis in Finnish Yorkshire boars as in mice (Sironen et al., 2011). Chung et al. (2013) recognized new loci showing association with development of testicular germ cell cancer (TGCT), one of which (rs9905704) located in TEX14. It has also been shown that common genetic variations in TEX14, which is over-expressed in breast tumors, is associated with risk of breast cancer in Caucasians (Kelemen et al., 2009). Recent studies showed that although Tex11-/-, Tex14-/- and Tex15 -/- mutant male mice were sterile because of meiotic arrest and disturbance of spermatogenesis, mutant females were fertile. They suggested that the infertility observed in mutant male mice was caused by loss of function of these genes and that Tex11, Tex14 and Tex15 were needed for meiosis progression and fertility only in male mice (Yang et al., 2008a; Greenbaum et al., 2006; Sironen et al., 2011). However, TEX12 has identical localization patterns in oocytes and spermatocytes, revealing that this protein is a common part of the central element of the synaptonemal complex in mammals and is not sex-specific (Hamer et al., 2006). Tex12 -/- mutant male mice were azoospermic and therefore sterile because of failure in chromosomal synapsis progression, while Tex12 -/- female mice were sterile because of loss of ovarian follicles (Hamer et al., 2008).
The homology of amino acid sequences and expression patterns of the human and murine TEX11, TEX12, TEX14 and TEX15 genes (Wang et al., 2001; Zheng et al., 2010; Tang et al., 2011; Stouffs et al., 2009; Greenbaum et al., 2006; Wu et al., 2003; Hamer et al., 2006 ; Yang et al., 2008b) suggests that these genes may play some a few in human spermatogenesis. Given that deficiency of these genes results in azoospermic mice (Zheng et al., 2010; Greenbaum et al., 2006; Wu et al., 2003; Hamer et al., 2006; Yang et al., 2008a), severe spermatogenic failure may thus be caused by their disruption. Therefore, this study aimed to replicate this finding in humans by examining the expression levels of TEX11, TEX12, TEX14, and TEX15 in the testis tissue of patients with non-obstructive azoospermia and comparing them against controls.
MATERIAL AND METHODS
Patients
Twenty-seven tissue samples were obtained from 18 patients with non-obstructive azoospermia (NOA) and nine subjects with obstructive azoospermia. All participating infertile men had undergone testicular sperm extraction (TESE) procedures at the Royan Institute. All patients gave written informed consent and the Ethical Review Board of the Royan Institute approved the study (reference number EC/90/ 1050).
Nine of the 18 patients with NOA were diagnosed with Sertoli cell-only syndromes (SCOS), while the other nine were diagnosed with maturation arrest at the spermatocyte stage. The control group consisted of nine patients with obstructive azoospermia. Patients with abnormal karyotypes and Y chromosome microdeletions were excluded from the study.
The age range of the patients at the time of diagnosis was 30 to 50 years and their mean age was 36.91±5.39 years. Hormonal levels were measured using a competitive ELISA kit (Monobind, CA, USA) according to the procedure described by Zangeneh et al. (2015). Cytogenetic analysis was performed based on standard methods (Asia et al., 2014).
Histological evaluation
After TESE, a portion of the testicular samples was submerged in Bouin's solution and sent for standard histopathological analysis (McLachlan et al., 2007). Residual tissues were collected by the Royan Tissue Bank. Testicular histopathology was categorized according to the most recognized pattern of spermatogenesis process: samples with complete spermatogenesis (obstructive azoospermia), samples with spermatogenic maturation arrest at the spermatocyte stage or SCOS.
RNA extraction
Total RNA from frozen testis tissue was extracted with TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Approximately 50-100 mg of testis tissue was pipetted into one mL of TRIzol. RNA was detached with chloroform, precipitated with isopropanol, washed with 75% ethanol, and finally dissolved in DEPC treated water (Li et al., 2006).
The concentration of RNA samples was determined spectrophotometrically by measuring absorption at 260 nm (Li et al., 2006), while DNA and protein contamination were checked by optical density (OD) measurements at 260/280 nm.
The integrity of total RNA was evaluated by measuring 260:280 nm absorption ratios and by gel electrophoresis on 1.2% agarose gel (Yu et al., 2007; Tang et al., 2006).
DNase treatment and cDNA synthesis
Before total RNA reverse transcription (RT), the samples were treated with DNase to eliminate DNA contamination using the DNase I (RNase free) kit (Fermentas, Life Sciences, UK). Total RNA (2 µg) was reverse-transcribed into cDNA in a reaction primed with random hexamer primers using the RevertAid H Minus First Strand cDNA Synthesis Kit (Fermentas, Life Sciences, UK) according to the manufacturer's recommendations. Polymerase chain reaction (PCR) primers for TEX11, TEX12, TEX14, and TEX15 were designed using PerlPrimer v1.1.20 (Table 1).
Table 1.
Oligonucleotide primer sequences
| Gene | Forward primer | Reverse primer | Amplification | Product size with | NCBIReference sequence |
|---|---|---|---|---|---|
| temperature (°C) | cDNA (bp) | gene | |||
| GAPDH | CTCATTTCCTGGTATGAC AACGA | CTTCCTCTTGTGCTCTTGCT | 60 | 121 | NG-007073.2 |
| TEX11 | GCCTGAATAGAGCCTTTGTGA | TAGATCAACTGCAACTGCCAT | 62 | 250 | NG-012574.1 |
| TEX12 | AGTCTCCAGTGCCAGATAGT | AGATTAATTTCCTTGCTCACATCA | 62 | 135 | NG-012574.1 |
| TEX14 | GGTTTATCCACCGCTCCCTC | CCTCTGTCCTCGCTTTCCAA | 62 | 198 | NG-012574.1 |
| TEX15 | AGGCAACATTCAAGCATCCA | AGTGAGCCAGGTAGTGATCTTT | 62 | 141 | NG-012574.1 |
After RNA extraction from testis tissue samples and cDNA synthesis, RT-PCR was performed with the related primers to confirm their expression in the samples of the three groups.
Quantitative RT-PCR (RT-qPCR)
RT-qPCR was carried out to confirm and analyze the expression levels of target genes in the SCOS, MA, and control groups. The reactions were processed on a 7500 Real Time PCR machine (Applied Biosystems, Carlsbad, CA, USA) using the Power SYBR Green PCR master mix (Applied Biosystem, EU). The amplification solution contained 10 µl of Power SYBR Green PCR master mix, 50 ng of cDNA and 5 picomoles of each primer, yielding a final volume of 20 µl. Cycling conditions included an initial step of enzyme activation at 95°C for 10 minutes, followed by 40 cycles of denaturation at 95°C for 15 seconds, annealing at 62°C for 30 seconds, and extension at 72°C for 30 seconds. Transcript levels normalized to human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) showed minimum variation among individual samples. GAPDH was used as an internal positive control. Each sample was run in duplicate and the mean value was calculated. No primer-dimer formation was observed during PCR amplification.
Immunohistochemistry
The expression of TEX14 was analyzed in the SO/H (n=3), SCOS (n=3), and MA (n=3) groups by immunohistochemistry. First, testis tissue sections were deparaffinized in xylene washes and then rehydrated in descending concentrations of ethanol washes. Endogenous peroxidase activity was blocked for 1 h at 37°C with goat serum (Sigma-Aldrich, St Louis, MO, USA), and triton X 100 was used to permeabilize cell membranes. Primary (Tex14 Antibody, NBP1-85424 Novus, Biologicals USA, 1:100) and secondary antibodies (goat anti-rabbit HRP, ab97051, 1:100) were added and protein localization was visualized using a Dako Liquid DAB+ Substrate Chromogen System (Dako, Glostrup, Denmark). Finally, cells were imaged using an Olympus IX 71 microscope (Julaton & Pera, 2011). Negative controls were generated by the same method as the positive controls; however, the primary antibody was replaced with PBS solution.
Statistical analysis
Data on clinical characteristics were shown as mean ± SEM. The normality of variables was analyzed with the Kolmogorov test. Differences in the mean values among the three groups were analyzed by one-way analysis of variance (ANOVA). The tests cited above were performed on SPSS statistical software package (SPSS Inc, Chicago, IL, USA) version 22.0. Real-time data were processed and analyzed using a two-tailed t-test. Differences with p-values <0.05 were considered significant.
RESULTS
Patient clinical characteristics
The characteristics of patients including age and LH, FSH, and testosterone levels are listed in Table 2. There was no significant difference in age, LH or testosterone serum levels between the three groups; however, the FSH serum levels between these groups were significantly different (p=0.01).
Table 2.
Clinical characteristics of patient groups. Values are expressed as mean±SEM
| Patients groups | Age(years) | FSH(mIU/mL) | LH(mIU/mL) | Testosterone (ng/mL) |
|---|---|---|---|---|
| Maturation arrest (MA) | 37.28±3.28(30-50) | 5.62±1.51 | 4.32±1.26 | 5.28±0.84 |
| Sertoli-cell-only syndrome (SCOS) | 37.33±1.20(33-42) | 18.57±3.90 | 3.73±1.82 | 3.27±0.59 |
| Control | 36.33±1.24(30-42) | 8.37±1.73 | 3.70±0.87 | 5.61±3.57 |
| p-value | 0.924 | 0.010* | 0.941 | 0.237 |
Values are expressed as mean±SEM.
Significant difference based on ANOVA. Normal range of FSH: 1.5-12.4 mIU/mL Normal range of LH: 1.0-10.0 mIU/mL Normal range of Testosterone: 2.0-8.0ng/mL
Gene expression analyses
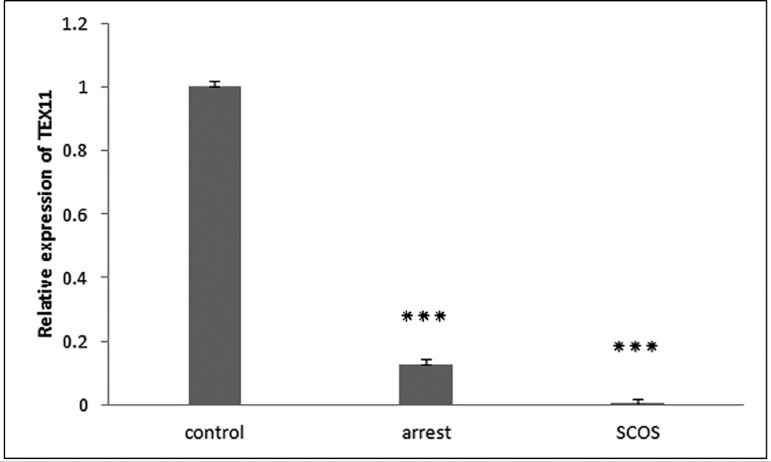
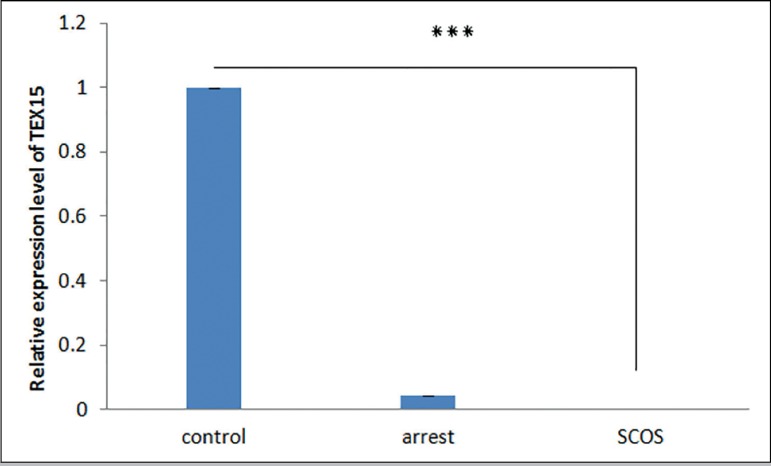
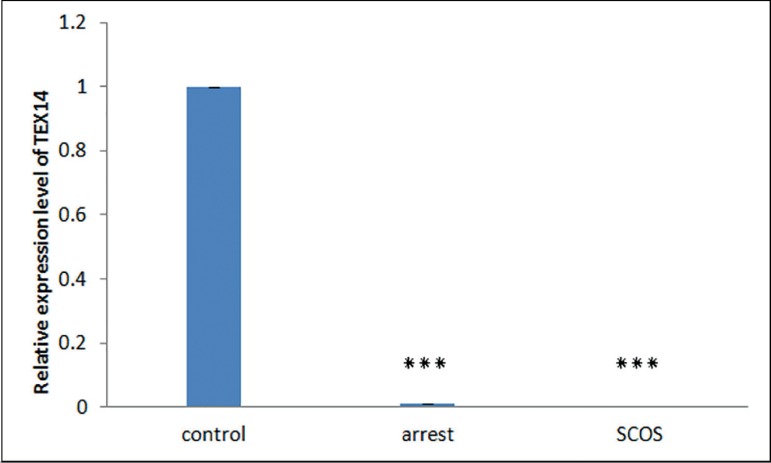
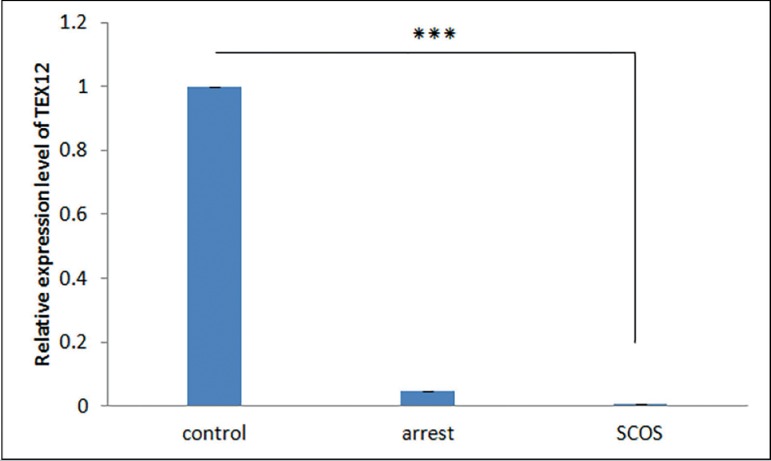
The RT-qPCR results demonstrated that transcripts of all four TEX genes existed in all samples of the SCOS, MA, and control groups. Differential expression analysis showed that expression of TEX genes is significantly lower in SCOS testes samples when compared with controls (p<0.01) (Figures 1-4). The expression of TEX11 and TEX14 was also significantly lower in MA samples compared with controls (p≤0.038) (Figures 1,3); however, expression of TEX12 and TEX15 was not significantly different (TEX12,15 p>0.05) (Figures 2,4). Expression levels of TEX12, TEX14 and TEX15 in SCOS and MA samples were not significantly different (TEX12, 14,15 p>0.05) (Figures 2,3, and 4); however, TEX11 expression was significantly lower in SCOS samples (p=0.003) (Figure 1).
Figure 1.
Comparison of the expression levels of TEX11 between MA, SCOS, and control patients. *** p<0.05
Figure 4.
Comparison of the expression levels of TEX15 between MA, SCOS, and control patients. *** p<0.05
Figure 3.
Comparison of the expression levels of TEX14 between MA, SCOS, and control patients. *** p<0.05
Figure 2.
Comparison of the expression levels of TEX12 between MA, SCOS, and control patients. *** p<0.05
Immunohistochemical analysis of TEX14 expression
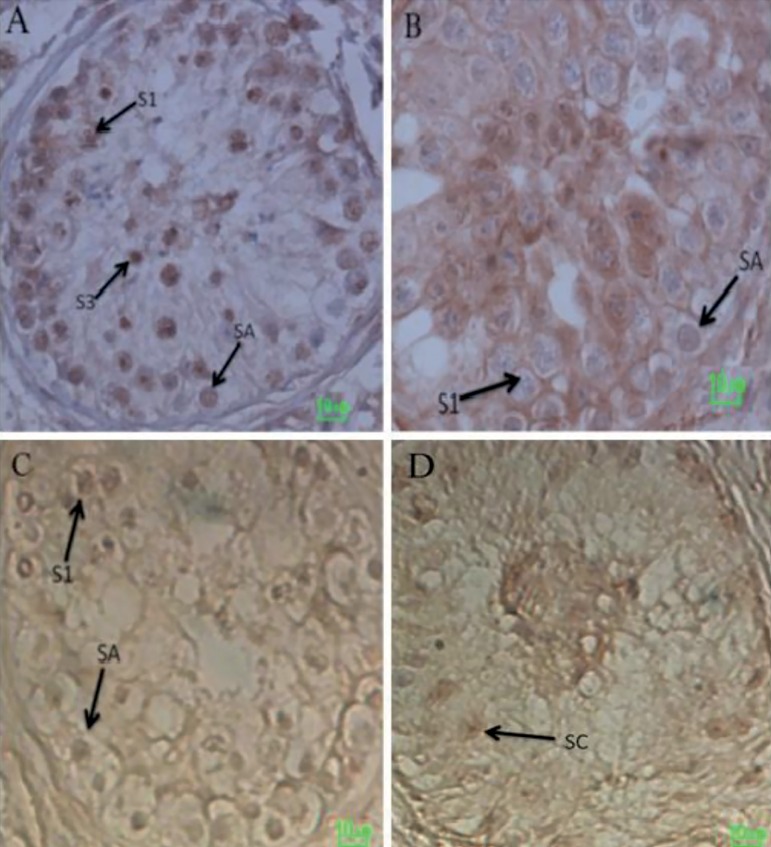
Immunohistochemical analysis with TEX14 antibody indicated absence of Leydig cell staining in several testis samples in all three groups. TEX14 was however detected in a small number of Sertoli cells, averaging 1-2 positive cells per tubule, consistent with the low level of RNA detected by RT-qPCR. The intensity of staining in germ cells of control tissues was higher than in MA tissues (Figure 5). TEX14 was expressed specifically by germ cells at varying stages of spermatogenesis. Type A spermatogonia (SA), primary spermatocytes (S1), and early round spermatids (S3) were positively stained for TEX14. No positive signal was detected in SCOS tissue samples.
Figure 5.
(A-D) Immunohistochemical analysis of adult testis sections with TEX14 antibody. (A) TEX14 is expressed in human testis cross-sections in obstructive azoospermia tissue samples. TEX14 is expressed specifically by germ cells at varying stages of spermatogenesis. Type A spermatogonia (SA), primary spermatocytes (S1) and early round spermatids (S3) stain positively for TEX14. (B) TEX14 is expressed in human testis cross-sections in MA tissue samples. (C) Negative control of testis cross-sections in MA tissue samples. (D) No positive signal is detected in adult human testis of SCOS tissue samples. The scale bar represents 10µm
DISCUSSION
The same expression pattern of TEX11, TEX12, TEX14 and TEX15 genes in mice and humans indicates that these conserved genes may have an important role in the early stages of mammalian spermatogenesis (Zheng et al., 2010; Tang et al., 2011; Stouffs et al., 2009; Greenbaum et al., 2006; Wu et al., 2003; Hamer et al., 2006; Yang et al., 2008a). Although the function of these genes in humans is not well-known, their roles in the progression of mouse spermatogenesis (Zheng et al., 2010; Greenbaum et al., 2006; Wu et al., 2003; Hamer et al., 2006; Yang et al., 2008b) led us to hypothesize that they were likely to have the same roles in humans. To test this hypothesis, we compared the expression levels of these genes in testicular tissue samples of infertile men with two distinct histological patterns of spermatogenic failure (MA and SCOS) - our case group - to the levels observed in a control group including men with complete spermatogenesis (obstructive azoospermia).
Given that there was no germ cell in the testicular tissue samples of SCOS patients and all four TEX genes are germ cell-specific, down-regulation of these genes was expected. This observation is in line with the findings in mice, in which the lack of these genes disrupts the development of meiosis, resulting in male infertility (Zheng et al., 2010; Greenbaum et al., 2006; Wu et al., 2003; Hamer et al., 2006; Yang et al., 2008). The similar expression pattern and sequence conservation of TEX11 seen in mice, humans, and pigs indicate that this gene is highly conserved and may have an important role in mammalian testicular function, including spermatogenesis (Zheng et al., 2010; Tang et al., 2011; Stouffs et al., 2009). TEX11 expression in testis is correlated with the onset of spermatogenesis and is restricted to spermatocytes and round spermatids (Tang et al., 2011). Stage-specific expression of TEX11 in porcine meiotic germ cells is in agreement with findings in mice, where the lack of Tex11 disturbs the progression of meiosis and thus results in infertility. The loss of function of Tex11 in Tex11-null mice resulted in meiotic arrest and deletion of spermatocytes (Yang et al., 2008b). In summary, the expression pattern of Tex11 is highly preserved in rodents and higher mammals such as pigs and humans (Tang et al., 2011).
Yang et al. (2015) demonstrated that the frequency of rare TEX11 mutations is significantly higher in azoospermic men, suggesting that TEX11 is essential for human spermatogenesis and mutations in this single X-linked gene is the cause of infertility in ~1% of azoospermic men. The authors also reported that the level of TEX11 protein must be above a critical threshold for meiosis to progress, and that low-expressing TEX11 alleles may thus result in human male infertility. Yatsenko et al. (2015) hypothesized that mutations in human TEX11 disrupt the formation and function of the synaptonemal complex, resulting in disturbance of pachytene synapsis, meiotic arrest, and azoospermia. The authors reported that hemizygous TEX11 mutations were a common cause of meiotic arrest and azoospermia in infertile men.
Our results showed that TEX11 expression was significantly decreased in MA patients when compared to controls. As dysfunction of TEX11 results in meiotic arrest and sterility in mice (Adelman & Petrini, 2008; Yang et al., 2008b; Stouffs et al., 2009), impairment of spermatogenesis and maturation arrest in this group may be linked to TEX11 down-regulation. This down-regulation was significantly more pronounced in SCOS patients than in patients with MA. Our results confirmed the involvement of TEX11 in human spermatogenesis. Furthermore, our findings indicated that expression of this gene is required for the completion of spermatogenesis.
Tex12 expression is limited to meiotic cell division and its promoter, like many other germ cell-specific genes, is quenched in somatic cells by transcription factor E2F6 (Pohlers et al., 2005). Previous studies showed that TEX12 localizes to the central element of the synaptonemal complex. Their results also revealed that without TEX12, the synaptonemal complex lacks a correct central element structure, synapsis cannot complete, and meiotic recombination and crossing-over do not occur (Hamer et al., 2006). Since loss of TEX12 results in the inaccurate assembly of the synaptonemal complex and non-chromosomal synapsis (Hamer et al., 2006), reduced expression of this gene in the MA group in relation to the control group was expected; however, our results did not reveal a significant difference in expression between these two groups.
Previous studies of Tex15 in mice showed this gene is necessary for chromosomal synapsis, DSB repair, and meiotic recombination during meiosis; therefore, TEX15 is thought of as a possible factor in spermatogenic failure risk (Yang et al., 2008a). Okutman et al. (2015) showed that a nonsense mutation in TEX15 is the cause of spermatogenic defect in familial cases of teratozoospermia and infertility. Immunohistochemistry confirmed these findings showing high levels of expression in germ cells and low levels of expression in Sertoli cells. The tests also revealed that Tex15 knock-out produced spermatogenesis meiotic arrest and severe reduction in testicular size. Therefore, we expected to observe down-regulation of TEX15 in the MA group when compared to controls. However, our results did not show any significant difference. This may possibly be due to high levels of expression in spermatogonia and early spermatocytes in the testicular tissue of MA patients. Previous studies have shown spermatogenic disruption in Tex14 knockout mice before the first meiotic division due to loss of intercellular bridges (Bolcun-Filas et al., 2009; Greenbaum et al., 2006; 2009). Moreover, a study on Tex14 mutant pigs showed that Tex14 is also essential for the completion of spermatogenesis in pigs (Sironen et al., 2011). Although TEX14 localizes to intercellular bridges of both female and male mice germ cells, disruption of this gene leads to sterility only in males (Brill et al., 2000; Greenbaum et al., 2006). The comparison of TEX14 expression between control and MA groups showed that TEX14 expression had a significant decrease in MA samples. As dysfunction of TEX14 results in meiotic arrest and sterility in mice (Greenbaum et al., 2006; 2009; Wu et al., 2003), impairment of spermatogenesis and maturation arrest may be linked to the down-regulation of TEX14. These results were confirmed by immunohistochemistry, with TEX14 expressed in type A spermatogonia, primary spermatocytes, and early round spermatids in control samples. Conversely, this protein was absent or hardly visible in type A spermatogonia, primary spermatocytes, and early round spermatids of MA samples. These results support the possibility that TEX14 under-expression in MA patients is etiologic, suggesting that the low expression of this protein in these patients is unlikely to be caused by the lack of germ cells.
CONCLUSION
This study demonstrated that TEX11, TEX12, TEX14 and TEX15 are essential for the completion of spermatogenesis. Down-regulation of these genes, especially TEX14 and TEX11, may lead to impaired spermatogenesis in infertile men. Studies on other independent sample sets are required to confirm this association.
ACKNOWLEDGMENT
This study was funded by a grant from the Royan Institute, Tehran, Iran. The authors would like to thank to Dr. A.H. Shahverdi, Dr. A. Vosough, and Mrs. N. Fatemi for their invaluable support.
Footnotes
Congresses where the study was presented: Middle East Fertility Society (MEFS) congress 2012, 2013 Royan International Twin Congress 2013, 2014
CONFLICTS OF INTEREST
None to declare
REFERENCES
- Adelman CA, Petrini JH. ZIP4H (TEX11) deficiency in the mouse impairs meiotic double strand break repair and the regulation of crossing over. PLoS Genet. 2008;4:e1000042. doi: 10.1371/journal.pgen.1000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asia S, Vaziri Nasab H, Sabbaghian M, Kalantari H, Zari Moradi S, Gourabi H, Mohseni Meybodi A. A Rare De novo Complex Chromosomal Rearrangement (CCR) Involving Four Chromosomes in An Oligo-asthenosperm Infertile Man. Cell J. 2014;16:377–382. [PMC free article] [PubMed] [Google Scholar]
- Bolcun-Filas E, Speed R, Taggart M, Grey C, de Massy B, Benavente R, Cooke HJ. Mutation of the mouse Syce1 gene disrupts synapsis and suggests a link between synaptonemal complex structural components and DNA repair. PLoS Genet. 2009;5:e1000393. doi: 10.1371/journal.pgen.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle CA, Khoury MJ, Katz DF, Annest JL, Kresnow MJ, DeStefano F, Schrader SM. The relation of computer-based measures of sperm morphology and motility to male infertility. Epidemiology. 1992;3:239–246. doi: 10.1097/00001648-199205000-00009. [DOI] [PubMed] [Google Scholar]
- Brill JA, Hime GR, Scharer-Schuksz M, Fuller MT. A phospholipid kinase regulates actin organization and intercellular bridge formation during germline cytokinesis. Development. 2000;127:3855–3864. doi: 10.1242/dev.127.17.3855. [DOI] [PubMed] [Google Scholar]
- VM 3rd Brugh, Lipshultz LI. Male factor infertility: evaluation and management. Med Clin North Am. 2004;88:367–385. doi: 10.1016/S0025-7125(03)00150-0. [DOI] [PubMed] [Google Scholar]
- Chung CC, Kanetsky PA, Wang Z, Hildebrandt MA, Koster R, Skotheim RI, Kratz CP, Turnbull C, Cortessis VK, Bakken AC, Bishop DT, Cook MB, Erickson RL, Fosså SD, Jacobs KB, Korde LA, Kraggerud SM, Lothe RA, Loud JT, Rahman N, Skinner EC, Thomas DC, Wu X, Yeager M, Schumacher FR, Greene MH, Schwartz SM, McGlynn KA, Chanock SJ, Nathanson KL. Meta-analysis identifies four new loci associated with testicular germ cell tumor. Nat Genet. 2013;45:680–685. doi: 10.1038/ng.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries FAT, de Boer E, van den Bosch M, Baarends WM, Ooms M, Yuan L, Liu JG, van Zeeland AA, Heyting C, Pastink A. Mouse Sycp1 functions in synaptonemal complex assembly, meiotic recombination, and XY body formation. Genes Dev. 2005;19:1376–1389. doi: 10.1101/gad.329705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett DW, Ito S, Slautterback D. The occurrence of intercellular bridges in groups of cells exhibiting synchronous differentiation. J Biophys Biochem Cytol. 1959;5:453–460. doi: 10.1083/jcb.5.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerton JL, Hawley RS. Homologous chromosome interactions in meiosis: diversity amidst conservation. Nat Rev Genet. 2005;6:477–487. doi: 10.1038/nrg1614. [DOI] [PubMed] [Google Scholar]
- Greenbaum MP, Iwamori N, Agno JE, Matzuk MM. Mouse TEX14 is required for embryonic germ cell intercellular bridges but not female fertility. Biol Reprod. 2009;80:449–457. doi: 10.1095/biolreprod.108.070649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum MP, Yan W, Wu MH, Lin YN, Agno JE, Sharma M, Braun RE, Rajkovic A, Matzuk MM. TEX14 is essential for intercellular bridges and fertility in male mice. Proc Natl Acad Sci U S A. 2006;103:4982–4987. doi: 10.1073/pnas.0505123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer G, Gell K, Kouznetsova A, Novak I, Benavente R, Höög C. Characterization of a novel meiosis-specific protein within the central element of the synaptonemal complex. J Cell Sci. 2006. 119:4025–4032. doi: 10.1242/jcs.03182. [DOI] [PubMed] [Google Scholar]
- Hamer G, Wang H, Bolcun-Filas E, Cooke HJ, Benavente R, Höög C. Progression of meiotic recombination requires structural maturation of the central element of the synaptonemal complex. J Cell Sci. 2008;121:2445–2451. doi: 10.1242/jcs.033233. [DOI] [PubMed] [Google Scholar]
- Hirsh A. Male subfertility. BMJ. 2003;327:669–672. doi: 10.1136/bmj.327.7416.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckins C. The spermatogonial stem cell population in adult rats. I. Their morphology, proliferation and maturation. Anat Rec. 1971;169:533–557. doi: 10.1002/ar.1091690306. [DOI] [PubMed] [Google Scholar]
- Julaton VT, Reijo Pera RA. NANOS3 function in human germ cell development. Hum Mol Genet. 2011;20:2238–2250. doi: 10.1093/hmg/ddr114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelemen LE, Wang X, Fredericksen ZS, Pankratz VS, Pharoah PD, Ahmed S, Dunning AM, Easton DF, Vierkant RA, Cerhan JR, Goode EL, Olson JE, Couch FJ. Genetic variation in the chromosome 17q23 amplicon and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18:1864–1868. doi: 10.1158/1055-9965.EPI-08-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouznetsova A, Benavente R, Pastink A, Höög C. Meiosis in mice without a synaptonemal complex. PLoS One. 2011;6:e28255. doi: 10.1371/journal.pone.0028255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HG, Liao AH, Ding XF, Zhou H, Xiong CL. The expression and significance of CATSPER1 in human testis and ejaculated spermatozoa. Asian J Androl. 2006;8:301–306. doi: 10.1111/j.1745-7262.2006.00132.x. [DOI] [PubMed] [Google Scholar]
- Ma TJ, Zhang XJ, Ding XP, Chen HH, Zhang YW, Ding M. Association of single nucleotide polymorphisms in UBR2 gene with idiopathic aspermia or oligospermia in Sichuan, China. Andrologia. 2016;48:1253–1260. doi: 10.1111/and.12569. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14:1197–1213. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan RI, Rajpert-De Meyts E, Hoei-Hansen CE, de Kretser DM, Skakkebaek NE. Histological evaluation of the human testis--approaches to optimizing the clinical value of the assessment: mini review. Hum Reprod. 2007;22:2–16. doi: 10.1093/humrep/del279. [DOI] [PubMed] [Google Scholar]
- Okutman O, Muller J, Baert Y, Serdarogullari M, Gultomruk M, Piton A, Rombaut C, Benkhalifa M, Teletin M, Skory V. Exome sequencing reveals a nonsense mutation in TEX15 causing spermatogenic failure in a Turkish family. Hum Mol Genet. 2015;24:5581–5588. doi: 10.1093/hmg/ddv290. [DOI] [PubMed] [Google Scholar]
- Page SL, Hawley RS. Chromosome choreography: the meiotic ballet. Science. 2003;301:785–789. doi: 10.1126/science.1086605. [DOI] [PubMed] [Google Scholar]
- Pohlers M, Truss M, Frede U, Scholz A, Strehle M, Kuban RJ, Hoffmann B, Morkel M, Birchmeier C, Hagemeier C. A role for E2F6 in the restriction of male-germ-cell-specific gene expression. Curr Biol. 2005;15:1051–1057. doi: 10.1016/j.cub.2005.04.060. [DOI] [PubMed] [Google Scholar]
- Robinson DN, Cant K, Cooley L. Morphogenesis of Drosophila ovarian ring canals. Development. 1994;120:2015–2025. doi: 10.1242/dev.120.7.2015. [DOI] [PubMed] [Google Scholar]
- Robinson DN, Cooley L. Stable intercellular bridges in development: the cytoskeleton lining the tunnel. Trends Cell Biol. 1996;6:474–479. doi: 10.1016/0962-8924(96)84945-2. [DOI] [PubMed] [Google Scholar]
- Sironen A, Uimari P, Venhoranta H, Andersson M, Vilkki J. An exonic insertion within Tex14 gene causes spermatogenic arrest in pigs. BMC Genomics. 2011;12:591–591. doi: 10.1186/1471-2164-12-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouffs K, Lissens W. X chromosomal mutations and spermatogenic failure. Biochim Biophys Acta. 2012;1822:1864–1872. doi: 10.1016/j.bbadis.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Stouffs K, Tournaye H, Liebaers I, Lissens W. Male infertility and the involvement of the X chromosome. Hum Reprod Update. 2009;15:623–637. doi: 10.1093/humupd/dmp023. [DOI] [PubMed] [Google Scholar]
- Tang A, Yu Z, Gui Y, Guo X, Long Y, Cai Z. Identification and characteristics of a novel testis-specific gene, Tsc24, in human and mice. Biol Pharm Bull. 2006;29:2187–2191. doi: 10.1248/bpb.29.2187. [DOI] [PubMed] [Google Scholar]
- Tang L, Zeng W, Clark RK, Dobrinski I. Characterization of the porcine testis-expressed gene 11 (Tex11) Spermatogenesis. 2011;1:147–151. doi: 10.4161/spmg.1.2.16680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PJ, McCarrey JR, Yang F, Page DC. An abundance of X-linked genes expressed in spermatogonia. Nat Genet. 2001;27:422–426. doi: 10.1038/86927. [DOI] [PubMed] [Google Scholar]
- Wang PJ, Page DC, McCarrey JR. Differential expression of sex-linked and autosomal germ-cell-specific genes during spermatogenesis in the mouse. Hum Mol Genet. 2005;14:2911–2918. doi: 10.1093/hmg/ddi322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerveld GH. Unraveling the genetics of spermatogenic failure. Amsterdam: University of Amsterdam; 2008. p. 128. Thesis. [Google Scholar]
- Wu MH, Rajkovic A, Burns KH, Yan W, Lin YN, Matzuk MM. Sequence and expression of testis-expressed gene 14 (Tex14): a gene encoding a protein kinase preferentially expressed during spermatogenesis. Gene Expr Patterns. 2003;3:231–236. doi: 10.1016/S1567-133X(03)00036-X. [DOI] [PubMed] [Google Scholar]
- Yang F, Eckardt S, Leu NA, Mclaughlin KJ, Wang PJ. Mouse TEX15 is essential for DNA double-strand break repair and chromosomal synapsis during male meiosis. J Cell Biol. 2008a;180:673–679. doi: 10.1083/jcb.200709057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Gell K, van der Heijden GW, Eckardt S, Leu NA, Page DC, Benavente R, Her C, Höög C, McLaughlin KJ, Wang PJ. Meiotic failure in male mice lacking an X-linked factor. Genes Dev. 2008b;22:682–691. doi: 10.1101/gad.1613608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Silber S, Leu NA, Oates RD, Marszalek JD, Skaletsky H, Brown LG, Rozen S, Page DC, Wang PJ. TEX11 is mutated in infertile men with azoospermia and regulates genome‐wide recombination rates in mouse. EMBO Mol Med. 2015;7:1198–1210. doi: 10.15252/emmm.201404967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsenko AN, Georgiadis AP, Röpke A, Berman AJ, Jaffe T, Olszewska M, Westernströer B, Sanfilippo J, Kurpisz M, Rajkovic A, Yatsenko SA, Kliesch S, Schlatt S, Tüttelmann F. X-linked TEX11 mutations, meiotic arrest, and azoospermia in infertile men. N Engl J Med. 2015;372:2097–2107. doi: 10.1056/NEJMoa1406192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Tang A, Gui Y, Guo X, Zhu H, Long Y, Li Z, Cai Z. Identification and characteristics of a novel testis-specific gene, Tsc21, in mice and human. Mol Biol Rep. 2007;34:127–134. doi: 10.1007/s11033-006-9026-6. [DOI] [PubMed] [Google Scholar]
- Zangeneh F, Yazdi RS, Naghizadeh MM, Abedinia N. Effect of Ramadan Fasting on Stress Neurohormones in Women with Polycystic Ovary Syndrome. J Family Reprod Health. 2015;9:51–57. [PMC free article] [PubMed] [Google Scholar]
- Zheng K, Yang F, Wang PJ. Regulation of male fertility by X‐linked genes. J Androl. 2010;31:79–85. doi: 10.2164/jandrol.109.008193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Qin D, Tang A, Zhou D, Qin J, Yan B, Diao R, Jiang Z, Cai Z, Gui Y. Developmental expression pattern of a novel gene, TSG23/Tsg23, suggests a role in spermatogenesis. Mol Hum Reprod. 2009;15:223–230. doi: 10.1093/molehr/gap015. [DOI] [PubMed] [Google Scholar]