Abstract
Objective
To evaluate if there are differences in the risks of obstetric outcomes in IVF/ICSI singleton pregnancies when compared fresh to frozen-thawed embryo transfers (FET).
Methods
This was a systematic review and meta-analysis evaluating the obstetric outcomes in singleton pregnancies after FET and fresh embryo transfer. The outcomes included in this study were pregnancy-induced hypertension (PIH), pre-eclampsia, placenta previa, and placenta accreta.
Results
The search yielded 654 papers, 6 of which met the inclusion criteria and reported on obstetric outcomes. When comparing pregnancies that arose from FET or fresh embryo transfer, there was an increase in the risk of obstetric complications in pregnancies resulting from FET when compared to those emerging from fresh embryo transfers in PIH (aOR 1.82; 95% CI 1.24-2.68), pre-eclampsia (aOR 1.32, 95% CI 1.07, 1.63), and placenta accreta (aOR 3.51, 95% CI 2.04-6.05). There were no significant differences in the risk between the FET and fresh embryo transfer groups when evaluating placenta previa (aOR 0.70; 95% CI 0.46-1.08).
Conclusion
The obstetric outcomes observed in pregnancies arising from ART may differ among fresh and FET cycles. Thus, when evaluating to perform a fresh embryo transfer or a freeze-all cycle, these differences found in obstetric outcomes between fresh and FET should be taken into account. The adverse obstetric outcomes after FET found in this study emphasize that the freeze-all policy should not be offered to all the patients, but should be offered to those with a clear indication of the benefit of this strategy.
Keywords: Obstetric outcome, fresh embryo transfer, frozen-thawed embryo transfer, placenta
INTRODUCTION
Today, nearly one in six couples faces fertility issues, as they fail to achieve a clinical pregnancy even after regular copulation (Boivin et al., 2007; Zegers-Hochschild et al., 2009). Consequently, couples are turning to assisted reproductive technology (ART) to become pregnant, which will hopefully result in the birth of a healthy baby. In 1983, the first frozen-thawed embryo was transferred by Trounson, which resulted in a successful pregnancy (Trounson & Mohr, 1983). Since then, continuous advancements in cryopreservation techniques have been made and at present, the quality and potential for frozen-thawed embryo implantation is comparable to those of fresh embryos (Herrero et al., 2011; Shapiro et al., 2010).
Although fresh embryo transfer is still the norm in most in vitro fertilization (IVF) treatments, as it involves a shorter process that leads to pregnancy, this method is related to increased hormone levels due to controlled ovarian stimulation (COS). The supra-physiologic hormonal levels observed during COS results in a suboptimal uterine environment that may negatively impact embryo implantation and placentation, eventually culminating to untoward obstetrical and perinatal outcomes (Imudia et al., 2012; Kalra & Molinaro, 2008; Kalra et al., 2011; Mainigi et al., 2016; Roque et al., 2017). Conversely, FET cultivates better environmental conditions within the uterus during embryo transfer, leading to improved endometrial receptivity (Barnhart, 2014; Weinerman & Mainigi, 2014). This better uterine environment may be related with better placentation during a FET cycle, leading to improved obstetric outcomes when compared to fresh transfer cycles (Maheshwari et al., 2012; Roque et al., 2015b; Shapiro et al., 2013). However, some studies have also shown that FET may have possible adverse effects on obstetric outcomes (Sazonova et al., 2012; Spijkers et al., 2017). Births from singleton ART pregnancies following FET have been associated with high birth weights, although there was a lower risk of preterm births when compared to fresh transfer cycles (Maheshwari et al., 2012; Spijkers et al., 2017; Wennerholm et al., 2013), a finding that highlights the impact of the clinical procedure itself, and not maternal characteristics, on these outcomes (Pinborg et al., 2014). Recently published meta-analysis comparing obstetric outcomes in pregnancies after fresh and FET did not report major obstetric outcomes such as pregnancy-induced hypertension (PIH), pre-eclampsia, placenta previa, and placenta accreta (Maheshwari et al., 2012; Pinborg et al., 2013).
To further examine the obstetric outcomes in singleton ART pregnancies, we performed a systematic review and meta-analysis of the available literature to compare the effects of FET and fresh embryo transfer on some major obstetric complications after IVF cycles that have not been reported in previous meta-analyses.
MATERIALS AND METHODS
This systematic review and meta-analysis was carried out in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Approval from the institutional review board was not undertaken because all the data was gathered from previously published papers.
Inclusion and exclusion criteria
We performed a systematic review and meta-analysis of observational studies. We carried out an extensive literature search in PubMed, EMBASE, and Cochrane databases from its inception through October 2015. We included only English-language papers and excluded conference abstracts if the full articles of the same study were not available. We also excluded studies that were performed without a control group. We used different search terms for obstetric outcomes in singleton pregnancies (e.g., "Obstetric outcomes", "Obstetric complications", "Pregnancy-induced hypertension", "Pre-eclampsia", "Placenta previa", and "Placenta accreta", "fresh embryo transfer", "frozen-thawed embryo trasnfer") and included articles comparing fresh embryo transfer with FET. We also reviewed the references of the selected studies and reviews to explore additional references. Only studies that provided the adjusted odds ratio (aOR) were included. The selection criteria are described in Table 1.
Table 1.
PICOs - Population, Intervention, Comparison, and Outcomes of interest.
| Target population | Singleton pregnancies of women undergoing ART |
|---|---|
| Intervention | Fresh embryo transfer vs Frozen embryo transfer |
| Outcome measure | •Pregnancy-induced
hypertension •Pre-eclampsia •Placenta previa •Placenta accrete |
| Design | Cohorts or Case-control |
Eligibility criteria and data extraction
In a first screening, two independent authors (MR, MV) assessed all of the abstracts retrieved from the search, and then they obtained the full manuscripts of citations that fit the inclusion criteria. At first, the studies were screened based on the information available in the abstract and title. In the second phase, only those articles that were screened in the first phase were evaluated; at this point, they were assessed for their eligibility to be included in this study based on our aforementioned screening criteria. In the third phase, complete articles were assessed to define their eligibility for the meta-analysis. The authors considered study eligibility, assessed quality, and extracted data solving discrepancies by agreement, and if needed, reaching a consensus with a third author (SG). All authors critically analyzed the summarized results.
The original studies included here reported on the comparisons made between the outcomes of fresh embryo transfer and FET for singleton pregnancies following ART. Studies examining only frozen and donor oocytes were excluded.
Outcome measures
The outcomes were the development of pregnancy induced-hypertension (PIH), pre-eclampsia, placenta previa, and placenta accreta.
Risk of Bias assessment
To access the risk of bias of the studies included, we followed the ROBINS - I: the Risk Of Bias In Non-randomized studies of interventions (Sterne et al., 2016). The studies were evaluated on bias: due to confounding; in selection of participants; in classification of interventions; due to deviations from intended interventions; due to missing data; in measurements of outcomes; in selection of reported results. After that, an overall bias risk for each study was determined as low, moderate, serious, or critical.
Data extraction and analysis
To determine the pooled effect of each variable, we used a Mantel-Haenszel model and applied the fixed-effects model. The adjusted odds ratio (aOR) accompanied by the 95% confidence intervals (CIs) were calculated. Statistical significance was set at a p value <.05. We evaluated the degree of variation across studies attributable to heterogeneity with the I2 statistic. When the heterogeneity was greater than 50% (I2 > 50%), we applied the random-effects model (Higgins et al., 2003). We conducted a meta-analysis using Review Manager 5 Software (Cochrane Collaboration).
RESULTS
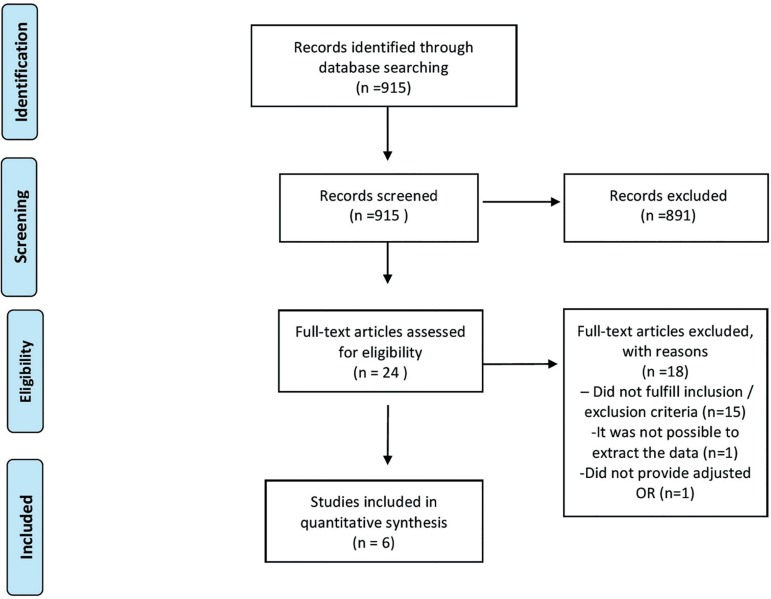
Our electronic search retrieved 915 articles but 891 were excluded at the title/abstract screening. One or both reviewers considered the remaining 24 studies eligible. Among these, eighteen articles were excluded because they did not fulfill the inclusion criteria, as they did not report the included outcomes in this review (Aytoz et al., 1999; Kalra & Molinaro, 2008; Wennerholm et al., 2013; Belva et al., 2008; Henningsen et al., 2011; Ishihara et al., 2014; Kato et al., 2012; Källén et al., 2005a, Li et al., 2014; Pelkonen et al., 2010; Pinborg et al., 2010; Schwarze et al., 2015; Shih et al., 2008; Wang et al., 2005; Wennerholm et al., 1997), did not provide the adjusted OR (Imudia et al., 2013), and the last excluded study because it did not present the comparison between fresh and FET cycles (Källén et al., 2005b). Six articles met inclusion criteria and were included in this review (Healy et al., 2010; Ishihara et al., 2014; Kaser et al., 2015; Opdahl et al., 2015; Rombauts et al., 2014; Sazonova et al., 2012) (Figure 1 - Flowchart). The characteristics of included articles are described in Table 2.
Figure 1.
Flowchart for the trial identification and selection process.
Table 2.
Characteristics of the included studies.
| Study | Study Design | Country | Period | Outcome included in the meta-analysis | FET vs Fresh aOR (95% CI) |
|---|---|---|---|---|---|
| Healy et al., 2010 | Retrospective cohort (population-based registry - Victoria) | Australia | 1992-2004 | Placenta previa | 0.71 (0.51, 1.00) |
| Ishihara et al., 2014 | Retrospective cohort (nationwide registry) | Japan | 2008-2010 | Pregnancy induced hypertension Placenta previa Placenta accreta |
1.58 (1.35, 1.86) 0.91 (0.70, 1.19) 3.16 (1.71, 6.23) |
| Kaser et al., 2015 | Case-control study (single-center analysis) | United States | 2005-2011 | Placenta accreta | 4.54 (1.65, 12.47) |
| Opdahl et al., 2015 | Retrospective cohort (nationwide registry) | Denmark, Norway and Sweden | 1988-2007 (Sweden and Norway) 1997-2007 (Denmark) |
Pregnancy induced hypertension | 2.39 (1.48, 3.86) |
| Rombauts et al., 2014 | Retrospective cohort (single-center analysis) | Australia | 2006-2012 | Placenta previa | 1.13 (0.61, 2.10) |
| Sazonova et al., 2012 | Retrospective cohort (nationwide registry) | Sweden | 2002-2006 | Pre-eclampsia Placenta previa |
1.32 (1.07, 1.63) 0.32 (0.19, 0.54) |
Pregnancy-induced hypertension
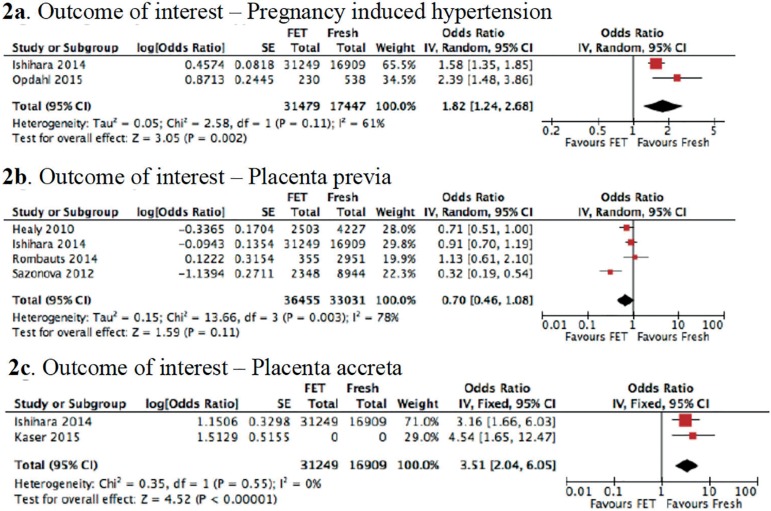
Two studies were included in this analysis (Ishihara et al., 2014; Opdahl et al., 2015). A total of 48,926 cases were eligible for inclusion in the analysis of PIH in singleton pregnancies after fresh embryo transfer and FET. Of these cases, 31,479 singleton pregnancies resulted from the transfer of frozen-thawed embryos, and 17,447 singleton pregnancies occurred after the transfer of fresh embryos. We found that the risk of developing PIH increased in the FET group compared to the fresh embryo transfer group (aOR: 1.82; 95% CI: 1.24-2.68; I2 = 61%; p=0.002) (Figure 2a).
Figure 2.
Summary of a meta-analysis (presenting the adjusted odds ratios [aOR] and 95% confidence intervals [CI]) examining the secondary obstetric outcomes in singleton ART pregnancies after FET and fresh embryo transfer. a: Summary of a meta-analysis of two studies (presenting the adjusted odds ratios [aOR] and 95% confidence intervals [CI]) examining PIH as an obstetric outcome in singleton ART pregnancies after FET and fresh embryo transfer. b: Summary of a meta-analysis of four studies (presenting the adjusted odds ratios [aOR] and 95% confidence interval [CI]) assessing placenta previa as an obstetric outcome in singleton ART pregnancies after FET and fresh embryo transfer. c: Summary of a meta-analysis of two studies (presenting the adjusted odds ratios [aOR] and 95% confidence intervals [CI]) evaluating placenta accreta as an obstetric outcome in singleton ART pregnancies after FET and fresh embryo transfer.
Pre-eclampsia
There was only one study (Sazonova et al., 2012) evaluating this outcome. A total 2,348 singleton pregnancies after FET and 8,944 after fresh cycles were evaluated in the study. There was a higher risk of pre-eclampsia (aOR 1.32, 95% CI 1.07, 1.63) in singleton pregnancies after FET than after fresh cycles.
Placenta previa
There were 4 studies included in this analysis (Healy et al., 2010; Ishihara et al., 2014; Rombauts et al., 2014; Sazonova et al., 2012). A total of 69,486 pregnancies from four studies were included. It was found that 36,455 singleton pregnancies were reported after the transfer of frozen-thawed embryos, and 33,031 emerged following the transfer of fresh embryos. There were no significant differences in the risk of placenta previa development between the fresh embryo transfer and FET groups (aOR: 0.70; 95% CI: 0.46-1.08; I2 = 78%; p = 0.11) (Figure 2b).
Placenta accreta
Two studies were included in this outcome (Ishihara et al., 2014; Kaser et al., 2015). We found that the risk of placenta accreta development increased significantly in the FET group compared to the fresh embryo transfer group (aOR: 3.51; 95% CI: 2.04-6.05; I2 = 0%; p < 0.00001) (Figure 2c).
DISCUSSION
In this study, we performed a systemic review and meta-analysis of the effect of FET and fresh embryo transfer on the risks of developing major obstetric complications in singleton pregnancies following the use of ART. To our knowledge, the current study is the first systematic review and meta-analysis comparing the adjusted data of PIH, pre-eclampsia, placenta previa and placenta accreta in singleton pregnancies after fresh and FET cycles.
Elucidating the IVF effects on obstetric outcomes in singleton pregnancies is of utmost importance in the field of reproductive medicine and mother-child health. Successful IVF depends not only on the quality of the embryo (Lee et al., 2017), but also on endometrial receptivity and the environmental conditions of the uterus during the pre-implantation period (Barnhart, 2014; Schoolcraft et al., 2017; Shapiro et al., 2014). With continuing research in this area, updated knowledge on endometrial-embryo interactions can help researchers and clinicians better understand the positive and negative outcomes of IVF. When selecting an ART method that employs fresh embryo transfer, the primary concern is the use of COS, which can damage the endometrial and uterine environment (Roque, 2015a). FET cycles are performed in a physiological uterine environment and this may be the reason that some studies observed better IVF outcomes following FET than after fresh embryo transfer (Shapiro et al., 2014, 2011a,b; Chen et al., 2016; Roque et al., 2015b).
The application of FET has continuously increased over the last few years (Pereira & Rosenwaks, 2016) by as much as 82.5% in 2006-2012 nationally in USA, while globally, its application has increased by 27.6% from 2008-2010 (Dyer et al., 2016). Recent studies highlighted that FET is associated with better safety and obstetric outcomes when compared to fresh transfer cycles (Shapiro et al., 2013; Maheshwari et al., 2013; 2016). However, some studies have also intimated about the fact that FET may have possible adverse effects on obstetric outcomes (Sazonova et al., 2012; Spijkers et al., 2017). In the present study, we found an increase in the risk of PIH, pre-eclampsia and also placenta accreta in singleton pregnancies after FET when comparing to fresh embryo transfers. There were no differences in the risk of placenta previa when comparing fresh to FET.
Pregnancies following FET had significantly higher odds for developing obstetric outcomes such as pregnancy-induced hypertension (PIH) and placenta accreta (Ishihara et al., 2014). Recent studies have revealed that the PIH risk is increased in singleton ART pregnancies when compared with spontaneously conceived singleton pregnancies (Jackson et al., 2004; Thomopoulos et al., 2013). Importantly, the higher risk of PIH in pregnancies that result from FET may not be entirely associated with maternal characteristics. A study on the risk of hypertensive disorders suggested that the risk of PIH development is higher in pregnancies following FET compared to fresh embryo transfer, even when the same mother is considered (Opdahl et al., 2015). The authors also wondered whether there were cases where women had more than one embryo transferred, as this might also contribute to the lowered risk of PIH. Similarly, a Japanese study conducted in 2008-2010 indicated that the risk of PIH was higher in pregnancies after FET than after fresh embryo transfer (Ishihara et al., 2014). During the present meta-analysis, we observed similar outcomes; we found that the risk of PIH in singleton ART pregnancies increased after FET when compared with fresh embryo transfer (aOR = 1.82; 95% CI 1.24-2.68; p = 0.002).
Placenta accreta is a very rare complication in pregnancies that result from ART. This type of placental development can lead to serious maternal outcomes and subsequent hysterectomy. One study reported that there were higher odds of developing placenta accreta following FET (Ishihara et al., 2014). Further, a multivariate analysis exploring FET as a risk factor for placenta accreta was carried out, and the authors found that FET is a strong independent risk factor for placenta accreta, even after controlling for those conditions that are known risk factors for this condition and other possible complications unique to ART (Kaser et al., 2015). The authors further confirmed that the increased risk of placenta accreta is directly associated with factors related to FET and not with patient characteristics. They proposed that the possible mechanisms underlying the increased risk of this pregnancy complication might include lower serum E2 levels and a thinner endometrial lining in FET cycles, which both contribute to uncontrolled growth of the extravillous trophoblast into the myometrium (Kaser et al., 2015). Similar to the previous studies, we also found that there are increased outcomes of placenta accreta in singleton pregnancies after FET than after fresh embryo transfer (aOR 3.51; 95% CI 2.04-6.05; p<0.001). It is noteworthy that higher serum E2 levels are associated with the risk of fetal growth restriction and pre-eclampsia. Hence, it is necessary to manipulate the level of serum E2 for ART cycles, and further studies are required in this direction.
Various reports have suggested that there is a higher rate of placenta previa in ART singleton pregnancies when compared with spontaneous pregnancies (Healy et al., 2010; Källén et al., 2005a,b; Jackson et al., 2004; Romundstad et al., 2006; Schieve et al., 2007). Few studies have also performed comparisons of the risk of placenta previa in cryopreservation and fresh cycles (Wikland et al., 2010; Healy et al., 2010; Pelkonen et al., 2010). A lower rate of placenta previa was found in singleton pregnancies following cryopreservation cycles than in fresh cycles (Sazonova et al., 2012). Conversely, some studies indicated that there were no associations between the risk of placenta previa and the type of embryo transfer method used (Healy et al., 2010; Ishihara et al., 2014; Rombauts et al., 2014). In our meta-analysis, we found that there was no significant variations in the risk of placenta previa in singleton pregnancies after FET and fresh cycles (aOR = 0.70; 95% CI 0.46-1.08; p = 0.11).
Detailed studies are needed to better understand the effects of COS and cryopreservation on the health of mothers and their offspring. Our study is based on observational studies, making it subject to biases. Moreover, in this study it is not possible to evaluate between the different types of cryopreservation protocols (slow freezing or vitrification) and also the embryo developmental stage (cleavage or blastocyst). The findings of our study should be considered with caution as the overall quality of evidence is low to moderate. Although this study included few papers, it raises concerns about the risk of some major obstetric complications after FET. These findings are important to be taken into account when evaluating to perform a fresh embryo transfer or freeze-all cycle.
In conclusion, the obstetric outcomes observed in pregnancies arising from ART may differ among fresh and FET cycles. Thus, when evaluating to perform a fresh embryo transfer or a freeze-all cycle, these differences observed in obstetric outcomes between fresh and FET should be taken into account. The adverse obstetric outcomes after FET observed in this study emphasize that the freeze-all policy should not be offered to all the patients, but should be offered to those with a clear indication of the benefits of such strategy.
REFERENCES
- Aytoz A, Van den Abbeel E, Bonduelle M, Camus M, Joris H, Van Steirteghem A, Devroey P. Obstetric outcome of pregnancies after the transfer of cryopreserved and fresh embryos obtained by conventional in-vitro fertilization and intracytoplasmic sperm injection. Hum Reprod. 1999;14:2619–2624. doi: 10.1093/humrep/14.10.2619. [DOI] [PubMed] [Google Scholar]
- Barnhart KT. Introduction: are we ready to eliminate the transfer of fresh embryos in in vitro fertilization? Fertil Steril. 2014;102:1–2. doi: 10.1016/j.fertnstert.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belva F, Henriet S, Van den Abbeel E, Camus M, Devroey P, Van der Elst J, Liebaers I, Haentjens P, Bonduelle M. Neonatal outcome of 937 children born after transfer of cryopreserved embryos obtaned by ICSI and IVF and comparison with outcome data of fresh ICSI and IVF cycles. Hum Reprod. 2008;23:2227–2238. doi: 10.1093/humrep/den254. [DOI] [PubMed] [Google Scholar]
- Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506–1512. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, Yang J, Liu J, Wei D, Weng N, Tian L, Hao C, Yang D, Zhou F, Shi J, Xu Y, Li J, Yan J, Qin Y, Zhao H, Zhang H, Legro RS. Fresh versus Frozen Embryos for Infertility in the Polycystic Ovary Syndrome. N Engl J Med. 2016;375:523–533. doi: 10.1056/NEJMoa1513873. [DOI] [PubMed] [Google Scholar]
- Dyer S, Chambers GM, de Mouzon J, Nygren KG, Zegers-Hochschild F, Mansour R, Ishihara O, Banker M, Adamson GD. International Committee for Monitoring Assisted Reproductive Technologies world report: Assisted Reproductive Technology 2008, 2009 and 2010. Hum Reprod. 2016;31:1588–1609. doi: 10.1093/humrep/dew082. [DOI] [PubMed] [Google Scholar]
- Healy DL, Breheny S, Halliday J, Jaques A, Rushford D, Garrett C, Talbot JM, Baker HW. Prevalence and risk factors for obstetric haemorrhage in 6730 singleton births after assisted reproductive technology in Victoria Australia. Hum Reprod. 2010;25:265–274. doi: 10.1093/humrep/dep376. [DOI] [PubMed] [Google Scholar]
- Henningsen AK, Pinborg A, Lidegaard Ø, Vestergaard C, Forman JL, Andersen AN. Perinatal outcome of singleton siblings born after assisted reproductive technology and spontaneous conception: Danish national sibling-cohort study. Fertil Steril. 2011;95:959–963. doi: 10.1016/j.fertnstert.2010.07.1075. [DOI] [PubMed] [Google Scholar]
- Herrero L, Martinez M, Garcia-Velasco JA. Current status of human oocyte and embryo cryopreservation. Curr Opin Obstet Gynecol. 2011;23:245–250. doi: 10.1097/GCO.0b013e32834874e2. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altmann DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imudia AN, Awonuga AO, Doyle JO, Kaimal AJ, Wright DL, Toth TL, Styer AK. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil Steril. 2012;97:1374–1379. doi: 10.1016/j.fertnstert.2012.03.028. [DOI] [PubMed] [Google Scholar]
- Imudia AN, Awonuga AO, Kaimal AJ, Wright DL, Styer AK, Toth TL. Elective cryopreservation of all embryos with subsequent cryothaw embryo transfer in patients at risk for ovarian hyperstimulation syndrome reduces the risk of adverse obsttric outcomes: a preliminary study. Fertil Steril. 2013;99:168–173. doi: 10.1016/j.fertnstert.2012.08.060. [DOI] [PubMed] [Google Scholar]
- Ishihara O, Araki R, Kuwahara A, Itakura A, Saito H, Adamson GD. Impact of frozen-thawed single-blastocyst transfer on maternal and neonatal outcome: an analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan. Fertil Steril. 2014;101:128–133. doi: 10.1016/j.fertnstert.2013.09.025. [DOI] [PubMed] [Google Scholar]
- Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol. 2004;103:551–563. doi: 10.1097/01.AOG.0000114989.84822.51. [DOI] [PubMed] [Google Scholar]
- Källén B, Finnström O, Nygren KG, Olausson PO. In vitro fertilization (IVF) in Sweden: infant outcome after different IVF fertilization method. Fertil Steril. 2005a;84:611–617. doi: 10.1016/j.fertnstert.2005.02.038. [DOI] [PubMed] [Google Scholar]
- Källén B, Finnström O, Nygren KG, Otterblad Olausson P, Wennerholm UB. In vitro fertilisation in Sweden: obstetric characteristics, maternal morbidity and mortality. BJOG. 2005b;112:1529–1535. doi: 10.1111/j.1471-0528.2005.00745.x. [DOI] [PubMed] [Google Scholar]
- Kalra SK, Molinaro TA. The association of in vitro fertilization and perinatal morbidity. Semin Reprod Med. 2008;26:423–435. doi: 10.1055/s-0028-1087108. [DOI] [PubMed] [Google Scholar]
- Kalra SK, Ratcliffe SJ, Coutifaris C, Molinaro T, Barnhart KT. Ovarian stimulation and low birth weight in newborns conceived through in vitro fertilization. Obstet Gynecol. 2011;118:863–871. doi: 10.1097/AOG.0b013e31822be65f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaser DJ, Melamed A, Bormann CL, Myers DE, Missmer SA, Walsh BW, Racowsky C, Carusi DA. Cryopreserved embryo transfer is an independent risk factor for placenta accreta. Fertil Steril. 2015;103:1176–1184. doi: 10.1016/j.fertnstert.2015.01.021. [DOI] [PubMed] [Google Scholar]
- Kato O, Kawasaki N, Bodri D, Kuroda T, Kawachiya S, Kato K, Takehara Y. Neonatal outcome and birth defects in 6623 singletons born following minimal ovarian stimulation and vitrified fresh single embryo transfer. Eur J Obstet Gynecol Reprod Biol. 2012;161:46–50. doi: 10.1016/j.ejogrb.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Lee SH, Lee JH, Park YS, Yang KM, Lim CK. Comparison of clinical outcomes between in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) in IVF-ICSI split insemination cycles. Clin Exp Reprod Med. 2017;44:96–104. doi: 10.5653/cerm.2017.44.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wang YA, Ledger W, Edgar DH, Sullivan EA. Clinical outcomes following cryopreservation of blastocysts by vitrification or slow freezing: a population-based cohot study. Hum Reprod. 2014;29:2794–2801. doi: 10.1093/humrep/deu246. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Pandey S, Shetty A, Hamilton M, Bhattacharya S. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril. 2012;98:368–377. doi: 10.1093/humupd/dms018. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Kalampokas T, Davidson J, Bhattacharya S. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of blastocyst-stage versus cleavage-stage embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril. 2013;100:1615–1621. doi: 10.1016/j.fertnstert.2013.08.044. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Raja EA, Bhattacharya S. Obstetric and perinatal outcomes after either fresh or thawed frozen embryo transfer: an analysis of 112,432 singleton pregnancies recorded in the Human Fertilisation and Embriology Authority anonymized dataset. Fertil Steril. 2016;106:1703–1708. doi: 10.1016/j.fertnstert.2016.08.047. [DOI] [PubMed] [Google Scholar]
- Mainigi M, Rosenzweig JM, Lei J, Mensah V, Thomaier L, Talbot CC Jr, Olalere D, Ord T, Rozzah R, Johnston MV, Burd I. Peri-Implantation Hormonal Milieu: Elucidating Mechanisms of Adverse Neurodevelopmental Outcomes. Reprod Sci. 2016;23:785–794. doi: 10.1177/1933719115618280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdahl S, Henningsen AA, Tiitinen A, Bergh C, Pinborg A, Romundstad PR, Wennerholm UB, Gissler M, Skjaerven R, Romundstad LB. Risk of hypertensive disorders in pregnancies following assisted reproductive technology: a cohort study from the CoNARTaS group. Hum Reprod. 2015;30:1724–1731. doi: 10.1093/humrep/dev090. [DOI] [PubMed] [Google Scholar]
- Pelkonen S, Koivunen R, Gissler M, Nuojua-Huttunen S, Suikkari AM, Hydén-Granskog C, Martikainen H, Tiitinen A, Hartikainen AL. Perinatal outcome of children born after frozen and fresh embryo transfer: the Finnish cohort study 1995-2006. Hum Reprod. 2010;25:914–923. doi: 10.1093/humrep/dep477. [DOI] [PubMed] [Google Scholar]
- Pereira N, Rosenwaks Z. A fresh(er) perspective on frozen embryo transfers. Fertil Steril. 2016;106:257–258. doi: 10.1016/j.fertnstert.2016.06.028. [DOI] [PubMed] [Google Scholar]
- Pinborg A, Loft A, Aaris Henningsen AK, Rasmussen S, Andersen AN. Infant outcome of 957 singletons born after frozen embryo replacement: the Danish National Cohort Study 1995-2006. Fertil Steril. 2010;94:1320–1327. doi: 10.1016/j.fertnstert.2009.05.091. [DOI] [PubMed] [Google Scholar]
- Pinborg A, Wennerholm UB, Romundstad LB, Loft A, Aittomaki K, Söderström-Anttila V, Nygren KG, Hazekamp J, Bergh C. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update. 2013;19:87–104. doi: 10.1093/humupd/dms044. [DOI] [PubMed] [Google Scholar]
- Pinborg A, Henningsen AA, Loft A, Malchau SS, Forman J, Andersen AN. Large baby syndrome in singletons born after frozen embryo transfer (FET): is it due to maternal factors or the cryotechnique? Hum Reprod. 2014;29:618–627. doi: 10.1093/humrep/det440. [DOI] [PubMed] [Google Scholar]
- Rombauts L, Motteram C, Berkowitz E, Fernando S. Risk of placenta praevia is linked to endometrial thickness in a retrospective cohort study of 4537 singleton assisted reproduction technology births. Hum Reprod. 2014;29:2787–2793. doi: 10.1093/humrep/deu240. [DOI] [PubMed] [Google Scholar]
- Romundstad LB, Romundstad PR, Sunde A, von Düring V, Skjaerven R, Vatten LJ. Increased risk of placenta previa in pregnancies following IVF/ICSI; a comparison of ART and non-ART pregnancies in the same mother. Hum Reprod. 2006;21:2353–2358. doi: 10.1093/humrep/del153. [DOI] [PubMed] [Google Scholar]
- Roque M. Freeze-all policy: is it time for that? J Assist Reprod Genet. 2015a;32:171–176. doi: 10.1007/s10815-014-0391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque M, Valle M, Guimarães F, Sampaio M, Geber S. Freeze-all policy: fresh vs. frozen-thawed embryo transfer. Fertil Steril. 2015b;103:1190–1193. doi: 10.1016/j.fertnstert.2015.01.045. [DOI] [PubMed] [Google Scholar]
- Roque M, Valle M, Kostolias A, Sampaio M, Geber S. Freeze-all cycle in reproductive medicine: current perspectives. JBRA Assist Reprod. 2017;21:49–53. doi: 10.5935/1518-0557.20170012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazonova A, Källen K, Thurin-Kjellberg A, Wennerholm UB, Bergh C. Obstetric outcome in singletons after in vitro fertilization with cryopreserved/thawed embryos. Hum Reprod. 2012;27:1343–1350. doi: 10.1093/humrep/des036. [DOI] [PubMed] [Google Scholar]
- Schieve LA, Cohen B, Nannini A, Ferre C, Reynolds MA, Zhang Z, Jeng G, Macaluso M, Wright VC, Massachusetts Consortium for Assisted Reproductive Technology Epidemiologic Research (MCARTER) A population-based study of maternal and perinatal outcomes associated with assisted reproductive technology in Massachusetts. Matern Child Health J. 2007;11:517–525. doi: 10.1007/s10995-007-0202-7. [DOI] [PubMed] [Google Scholar]
- Schoolcraft W, Meseguer M, The Global Fertility Alliance Paving the way for a gold standard of care for infertility treatment: improving outcomes through standardization of laboratory procedures. Reprod Biomed Online. 2017;35:391–399. doi: 10.1016/j.rbmo.2017.06.023. [DOI] [PubMed] [Google Scholar]
- Schwarze JE, Crosby JA, Zegers-Hochschild F. Effect of embryo freezing on perinatal outcome after assisted reproduction techniques: lessons from Latin American Registry of Assisted Reproduction. Reprod Biomed Online. 2015;31:39–43. doi: 10.1016/j.rbmo.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Similar ongoing pregnancy rates after blastocyst transfer in fresh donor cycles and autologous cycles using cryopreserved bipronuclear oocytes suggest similar viability of transferred blastocysts. Fertil Steril. 2010;93:319–321. doi: 10.1016/j.fertnstert.2009.07.966. [DOI] [PubMed] [Google Scholar]
- Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011a;96:344–348. doi: 10.1016/j.fertnstert.2011.05.050. [DOI] [PubMed] [Google Scholar]
- Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in high responders. Fertil Steril. 2011b;96:516–518. doi: 10.1016/j.fertnstert.2011.02.059. [DOI] [PubMed] [Google Scholar]
- Shapiro BS, Daneshmand ST, Restrepo H, Garner FC, Aguirre M, Hudson C. Matched-cohort comparison of single-embryo transfers in fresh and frozen-thawed embryo transfer cycles. Fertil Steril. 2013;99:389–392. doi: 10.1016/j.fertnstert.2012.09.044. [DOI] [PubMed] [Google Scholar]
- Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C. Clinical rationale for cryopreservation of entire embryo cohorts in lieu of fresh transfer. Fertil Steril. 2014;102:3–9. doi: 10.1016/j.fertnstert.2014.04.018. [DOI] [PubMed] [Google Scholar]
- Shih W, Rushford DD, Bourne H, Garrett C, McBain JC, Healy DL, Baker HW. Factors affecting low birthweight after assisted reproduction technology: difference between transfer of fresh and cryopreserved embryos suggests an adverse effect of oocyte collection. Hum Reprod. 2008;23:1644–1653. doi: 10.1093/humrep/den150. [DOI] [PubMed] [Google Scholar]
- Spijkers S, Lens JW, Schats R, Lambalk CB. Fresh and Frozen-Thawed Embryo Transfer Compared to Natural Conception: Differences in Perinatal Outcome. Gynecol Obstet Invest. 2017;82:538–546. doi: 10.1159/000468935. h10.1159/000468935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JA, Hernán MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies if interventions. BMJ. 2016;355:i4919–i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos C, Tsioufis C, Michalopoulou H, Makris T, Papademetriou V, Stefanadis C. Assisted reproductive technology and pregnancy-related hypertensive complications: a systematic review. J Hum Hypertens. 2013;27:148–157. doi: 10.1038/jhh.2012.13. [DOI] [PubMed] [Google Scholar]
- Trounson A, Mohr L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature. 1983;305:707–709. doi: 10.1038/305707a0. [DOI] [PubMed] [Google Scholar]
- Wang YA, Sulliven EA, Black D, Dean J, Bryant J, Chapman M. Preterm birth and low birth weight after assisted reproductive technology-related pregnancy in Australia between 1996 and 2000. Fertil Steril. 2005;83:1650–1658. doi: 10.1016/j.fertnstert.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Weinerman R, Mainigi M. Why we should transfer frozen instead of fresh embryos: the translational rationale. Fertil Steril. 2014;102:10–18. doi: 10.1016/j.fertnstert.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerholm UB, Hamberger L, Nilsson L, Wennergren M, Wikland M, Bergh C. Obstetric and perinatal outcome of children conceived from cryopreserved embryos. Hum Reprod. 1997;12:1819–1825. doi: 10.1093/humrep/12.8.1819. [DOI] [PubMed] [Google Scholar]
- Wennerholm UB, Henningsen AK, Romundstad LB, Bergh C, Pinborg A, Skjaerven R, Forman J, Gissler M, Nygren KG, Tiitinen A. Perinatal outcomes of children born after frozen-thawed embryo transfer: a Nordic cohort study from the CoNARTaS group. Hum Reprod. 2013;28:2545–2553. doi: 10.1093/humrep/det272. [DOI] [PubMed] [Google Scholar]
- Wikland M, Hardarson T, Hillensjö T, Westin C, Westlander G, Wood M, Wennerholm UB. Obstetric outcomes after transfer of vitrified blastocysts. Hum Reprod. 2010;25:1699–1707. doi: 10.1093/humrep/deq117. [DOI] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, Sullivan E, Vanderpoel S, International Committee for Monitoring Assisted Reproductive Technology. World Health Organization International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92:1520–1524. doi: 10.1016/j.fertnstert.2009.09.009. [DOI] [PubMed] [Google Scholar]