Abstract
Renal perfusion provides the driving pressure for glomerular filtration and delivers the oxygen and nutrients to fuel solute reabsorption. Renal ischaemia is a major mechanism in acute kidney injury and may promote the progression of chronic kidney disease. Thus, quantifying renal tissue perfusion is critically important for both clinicians and physiologists. Current reference techniques for assessing renal tissue perfusion have significant limitations. Arterial spin labelling (ASL) is a magnetic resonance imaging (MRI) technique that uses magnetic labelling of water in arterial blood as an endogenous tracer to generate maps of absolute regional perfusion without requiring exogenous contrast. The technique holds enormous potential for clinical use but remains restricted to research settings. This statement paper from the PARENCHIMA network briefly outlines the ASL technique and reviews renal perfusion data in 53 studies published in English through January 2018. Renal perfusion by ASL has been validated against reference methods and has good reproducibility. Renal perfusion by ASL reduces with age and excretory function. Technical advancements mean that a renal ASL study can acquire a whole kidney perfusion measurement in less than 5–10 min. The short acquisition time permits combination with other MRI techniques that might inform drug mechanisms and renal physiology. The flexibility of renal ASL has yielded several variants of the technique, but there are limited data comparing these approaches. We make recommendations for acquiring and reporting renal ASL data and outline the knowledge gaps that future research should address.
Keywords: arterial spin labelling, kidney, magnetic resonance imaging, renal perfusion, systematic review
INTRODUCTION
There is a complex interaction between renal perfusion, renal oxygen delivery, renal oxygen consumption and glomerular filtration. Interested readers are referred to detailed reviews [1, 2]. The kidney is unique because >80% of its oxygen consumption is used to power tubular sodium reabsorption. Consequently, the oxygen consumption of the kidney varies with glomerular filtration rate (GFR), and thus renal blood flow. In all other organs of the body, the direction of causation is reversed, with changes in tissue metabolic activity leading to changes in vascular tone, and thus perfusion. Another unique aspect of the renal circulation is the presence of separate cortical and medullary circulations. All blood flow to the kidney (∼25% of cardiac output at rest or ∼1200 mL/min or ∼400 mL/100 g/min in a 70 kg adult with a 300 g kidney) passes through the glomeruli of the renal cortices. The renal medullary circulation (the vasa recta) arises from the efferent arterioles of a subpopulation of glomeruli at the corticomedullary junction (the juxtamedullary glomeruli). Thus only ∼10% of renal blood flow perfuses the renal medulla, with evidence that cortical and medullary circulations are independently regulated [3]. There is evolving evidence that renal tissue ischaemia and associated hypoxia are critical factors in the initiation and progression of both acute kidney injury (AKI) and chronic kidney disease (CKD), irrespective of the underlying aetiology [4]. Thus to understand the physiological regulation of renal perfusion and the role of its dysregulation in kidney disease and injury, we require methods that allow quantification of renal perfusion both at the whole organ level and at the local tissue level. Traditional reference techniques for assessing renal tissue perfusion in animals and humans have significant limitations with no gold-standard technique available [5]. Arterial spin labelling (ASL) is a magnetic resonance imaging (MRI) technique that uses the magnetic labelling of water in arterial blood as an endogenous tracer to quantify regional perfusion. The purpose of this review is to outline the ASL technique and summarize all human non-cancer studies performed since the technique was first described. Current gaps in knowledge are identified and recommendations for future studies are made.
MATERIALS AND METHODS
A literature search was conducted in PubMed, Ovid MEDLINE and Ovid Embase on 4 January 2018. The search strategy is outlined in detail in the Supplementary data. In brief, it comprised the terms arterial spin label, kidney, renal circulation, renal blood flow and renal perfusion. Conference abstracts, animal studies and human studies of renal cancer were excluded. Studies not published in English were excluded. Study characteristics were abstracted and cross-validated by multiple reviewers.
RESULTS
Study characteristics and important MRI parameters are summarized in Supplementary data, Table S1. Studies were generally small (mean 25 ± 23 participants, range 4–98 participants) and predominantly described healthy volunteers. Hydration status was rarely reported. The time of day at which the scan was collected was described in only 7 of 53 studies, which might be pertinent due to circadian variations in renal haemodynamics. Renal cortical perfusion by ASL ranged from 139 to 427 mL/100 g/min in healthy volunteers and from 83 to 412 mL/100 g/min in a broad range of patient groups. The reproducibility of renal perfusion by ASL was reported in 17 of 53 studies. Several papers reported renal ASL perfusion values under physiological challenges. Renal ASL perfusion values were generally lower in CKD patients compared with healthy subjects and were correlated with estimated GFR (eGFR).
BASIC PRINCIPLES OF ASL
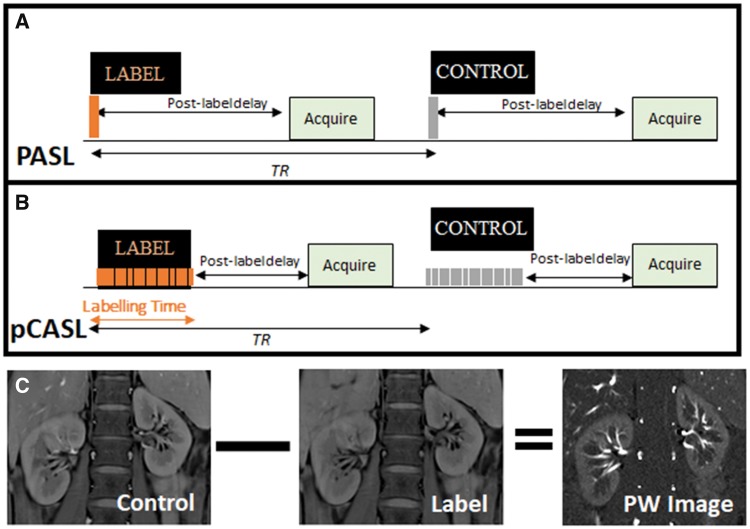
ASL uses blood water as a freely diffusible tracer to quantify renal perfusion in physiological units of mL/100 g/min. The basic ASL experiment collects two image types, referred to as the ‘label’ (or tag) and ‘control’ images. The label is applied using radiofrequency (RF) pulses to alter the longitudinal magnetization of protons in the arterial blood water before it enters the imaging plane, and an image is collected after a delay time. The control image is acquired at the same delay time without labelling the arterial blood protons. Provided that the inverted magnetization of the inflowing blood is the only difference between the control and label images, a simple subtraction of the label from the control image yields a perfusion-weighted image in which signal intensity is proportional to perfusion (Figure 1).
FIGURE 1:
Schematic showing basic principles of ASL. (A) Pulsed ASL (PASL) showing a single radiofrequency pulse for labelling followed by a post-label delay before the image is acquired within one TR (repetition time), with a label-control ASL pair shown. This sequence is then repeated to collect multiple ASL pairs. (B) Pseudocontinous ASL (pCASL) showing a train of radiofrequency pulses for labelling followed by a post-labelling delay. (C) Example control and label images and the resulting perfusion-weighted (PW) image created by subtraction of the label from the control image.
The signal in each voxel of the perfusion-weighted image is entered into a kinetic model to quantify the perfusion-weighted signal and thus generate a quantitative perfusion map (Supplementary data). ASL schemes can be classified into three major groups: pulsed ASL (PASL), continuous ASL (CASL) and pseudo-continuous ASL (pCASL) schemes, with PASL and pCASL being the most widely used schemes for renal ASL. The flow-sensitive alternating inversion recovery (FAIR) variant is the PASL scheme most widely used in renal imaging (Supplementary data, Table S1). It uses an RF pulse centred on the imaging plane to invert a large volume of blood (label) and an adiabatic RF pulse to invert a spatially selective region (control). In CASL, a single long-duration RF pulse is applied to invert the arterial magnetization as blood flows through the labelling plane while a gradient is applied in the direction of arterial flow. In pCASL, a long series of short RF pulses are used to mimic the action of CASL but limit the energy deposition (specific absorption rate). pCASL is more compatible with modern MRI scanners, thus making it more feasible for clinical use (Figure 1) than original CASL implementations. In all cases, the label decays with the longitudinal relaxation time (T1) for tissue or blood, which is a variable dependent on magnetic field strength. Each label or control image is acquired after a delay time, with the image readout taking one of several schemes (Supplementary data). ASL techniques have intrinsically low signal:noise ratio, with a typical renal cortex perfusion-weighted signal intensity of 5% of the control image signal. To improve signal:noise ratio, multiple ASL control-label pairs are collected and the perfusion-weighted difference signals are averaged. Renal ASL is inherently susceptible to respiratory-induced motion of the kidney between label and control images, although averaging acts to suppress to some extent such motion-related artefacts. Limiting motion artefacts during image acquisition is a critical issue in renal ASL [6]. Several alternative strategies have been employed to limit the effects of motion (Supplementary data).
VALIDATION OF RENAL ASL TO MEASURE RENAL PERFUSION
Techniques for assessing renal tissue perfusion in animals and humans have significant limitations, with no available gold-standard technique. In animal experiments, microspheres can be used. However, due to the phenomenon of plasma skimming, these methods are inaccurate in the kidney [5]. Furthermore, as microspheres are trapped in the pre-glomerular and glomerular circulations, they are unsuitable for medullary perfusion. Analyses of microsphere concentration must be done post-mortem, so this method is unsuitable for use in humans. The plasma clearance of para-aminohippurate measures effective renal blood flow in humans but is limited by its incomplete and variable renal extraction, making it a ‘bronze standard’ at best [5]. It also provides no information about the relative perfusion of the renal cortex and medulla. Renal scintigraphy lacks spatial resolution and uses ionizing radiation, limiting repeatability. Computed tomography also uses ionizing radiation [7] and requires contrast agents that can injure the kidney [8]. Dynamic gadolinium contrast-enhanced MRI can measure renal perfusion, but this method is not well validated and its use is restricted in CKD due to protocols to minimize the risk of nephrogenic systemic fibrosis [9].
The lack of a gold-standard technique for renal perfusion means that studies that validate renal ASL against reference techniques must be cautiously interpreted. Renal cortical perfusion measured by PASL-FAIR was compared with blood flow measured by ultrasound flowmetry in a single isolated ex vivo swine kidney, with the greatest observed difference of 13% [10]. Artz et al. [11] compared renal perfusion acquired with a PASL-FAIR technique at 1.5 T in 11 swines. Both ASL and microspheres showed an increase in perfusion during an acetylcholine challenge and a decrease during administration of isoflurane. The two perfusion techniques showed a good correlation (r = 0.81, P < 0.0001) with a linear relationship in the physiologic range (microsphere perfusion <550 mL/min/100 g), but perfusion values measured by ASL were systematically lower than those measured by microspheres. This may have been due in part to assumptions around the kinetic model used to determine the ASL perfusion values, but may also reflect the poor reproducibility and accuracy of the microsphere technique itself.
Ritt et al. [12] measured perfusion by both renal ASL and para-aminohippurate clearance in 24 patients with metabolic syndrome and a Cockroft and Gault estimated creatinine clearance ≥60 mL/min. Renal perfusion by the two techniques correlated modestly (r = 0.575, P < 0.001). After the patients had 2 weeks of therapy with telmisartan, an 11% increase of renal plasma flow by para-aminohippurate clearance was associated with a 6% increase by PASL-FAIR. Shimizu et al. [13] described a modest correlation between cortical renal perfusion by a pCASL technique and 99mTc-MAG3 scintigraphy in 14 healthy volunteers. There is conflicting evidence with respect to the similarity of perfusion estimates by dynamic gadolinium contrast-enhanced MRI and ASL [14–16]. This is expected given the differences in the tracer kinetic properties of magnetically labelled water and gadolinium contrast agents.
REPRODUCIBILITY OF RENAL ASL
Renal perfusion measurements by ASL are influenced by several patient-specific and technical factors, as discussed in the Supplementary data. Comparisons of reproducibility measures are also constrained by the disparate ways in which perfusion and reproducibility values are presented. Within-subject reproducibility of perfusion by renal ASL in 17 studies is summarized in Table 1. The range of study participants include healthy volunteers, hypertensives [25], patients with lupus nephritis [29], and kidney transplant recipients [17, 28]. Overall, cortical perfusion had moderate to good short-term reproducibility in the same visit [intraclass correlation (ICC) 0.62–0.98; coefficient of variation (CV) 3–18%] and between different visits (ICC 0.85–0.97; CV 4–13%). Ensuring reproducibility of medullary perfusion measurements provides additional challenges. These include reduced contrast:noise ratio due to lower perfusion, renal medullary volumes prone to errors in accurate segmentation and signal loss from the longer transit time of the magnetically labelled bolus as it initially passes through the cortex. Therefore it is not surprising that medullary perfusion is reported as less reproducible both within visits (ICC 0.27–0.94; CV 3–43%) and between visits (ICC 0.13–0.96; 4–37%). Studies in which both intra- and intervisit reproducibility were measured suggest that intervisit reproducibility is greater [17, 22, 26]. At the time of this review, there are no published studies comparing the reproducibility of ASL at different magnetic field strengths or under different labelling approaches. Similarly, we found no studies of reproducibility between centres.
Table 1.
Reproducibility of renal perfusion by ASL
| References | Participants, n | B0 (T) | Intravisit |
Intervisit |
|||
|---|---|---|---|---|---|---|---|
| ICC | CV (%) | ICC | CV (%) | Interval (days)a | |||
| Artz et al. [17] | 10 (HV) + 14 (P) | 1.5 |
|
4.8–6.0 (C) 16.7–26.7 (M) | 0.89–0.94 (C) 0.13–0.63 (M) |
|
|
| Chowdhury et al. [18] | 12 (HV) | 1.5 | 3.3 (C) | ||||
| Chowdhury et al. [19] | 12 (HV) | 1.5 | 3.3 (C) | ||||
| Cox et al. [20] | 11 (HV) | 1.5/3 |
|
|
NR | ||
| Cutajar et al. [21] | 5 (HV) | 1.5 |
|
21 (8–174)a | |||
| Cutajar et al. [14] | 16 (HV) | 1.5 | 14–18 (W) | 7 (7–56)a | |||
| Gardener and Francis [22] | 4 (HV) | 1.5 | 10–20 (C) | NR | |||
| Getzin et al. [23] | 15 (HV) | 1.5 |
|
6.7 (C) 10 (M) | |||
| Gillis et al. [24] | 12 (HV) | 3 |
|
|
4–28 | ||
| Hammon et al. [25] | 5 (HV)+ 9 (P) | 1.5 |
|
|
|
|
14 |
| Karger et al. [26] | 3 (HV) | 1.5 | 4.1 (W) | 28 | |||
| Kim et al. [27] | 25 (HV) | 3 |
|
|
|||
| Lanzman et al. [28] | 3 (P) | 1.5 | 4.1 (C) | <35 | |||
| Rapacchi et al. [29] | 10 (HV) + 10(P) | 1.5 |
|
|
|||
| Robson et al. [30] | 4 (HV | 1.5 | 1.7–8 (W) | 7–23 (W) | 7 | ||
| Robson et al. [31] | 4 (HV) | 1.5 | 8.8 (W) | ||||
| Wu et al. [16] | 4 (HV) | 3 | 7.9 (C)7.2 (M) | ||||
Values are mean ± SD or median (range).
B0, magnetic field strength (Tesla); C, cortex; CV, coefficient of variation; HV, healthy volunteers; M, medulla; NR, not reported; P, patients; W, whole kidney.
RENAL ASL IN HEALTHY VOLUNTEERS
This review has highlighted several caveats in terms of both the ASL acquisition and perfusion quantification methods (Supplementary data). Supplementary data, Table S1 shows the range of reported renal cortical perfusion values ranging from (139–427 mL/100 g/min) in healthy volunteers. Authors described renal perfusion by ASL under physiological challenges including water loading [33], intravenous saline [19, 20] and injections of furosemide [34]. Reduced renal perfusion with age was found in two recent studies [13, 21].
RENAL ASL IN KIDNEY DISEASE
Renal ASL has been applied in the study of CKD [18, 21, 33, 35–41], AKI [42], lupus nephritis [30, 43], metabolic syndrome [12], diabetes [39, 44], hypertension [45, 46], heart failure [36] and renovascular disease [38]. Consistent findings from these studies are that renal cortical perfusion is reduced in CKD compared with healthy volunteers; renal perfusion reduces with increasing stage of CKD and correlates to eGFR (Table 2).
Table 2.
Significant correlations of renal perfusion by ASL with eGFR
| Reference | Setting | eGFR method | r-value |
|---|---|---|---|
| Breidthardt-2015 [35] | CKD | MDRD | 0.52 |
| Gillis-2016 [36] | CKD | CKD-EPI | 0.73 |
| Li-2017 [32] | CKD | CKD-EPI | 0.67 |
| Mora-Gutierrez-2017 [38] | CKD | MDRD | 0.62 |
| Artz-2011-MRI [48] | Healthy volunteer/Transplant | MDRD | 0.85/0.62 |
| Heusch-2014 [46] | Transplant | MDRD | 0.59 |
| Hueper-2015 [47] | Transplant | MDRD | 0.64 |
RENAL ASL IN RENAL TRANSPLANT RECIPIENTS AND LIVING KIDNEY DONORS
Renal ASL has been applied in kidney transplant studies. Prominent findings include that renal cortical ASL perfusion values differ between patients with early and delayed graft function and correlate with allograft function [29, 47–51]. In a group of 98 transplant recipients, perfusion by PASL-FAIR reduced with increasing stage of CKD [47]. Cutajar et al. [51] showed that renal ASL can be used to determine filtration fraction and could potentially act as a biomarker of renal functional reserve in potential living kidney donors.
LONGITUDINAL AND MULTIPARAMETRIC STUDIES
The non-invasive, short-lived nature of the tracer and rapid acquisition time make renal ASL inherently suited to repeated studies over a range of timescales. These allow repeated measures experiments that would not be possible with any other perfusion technique in humans and may provide novel insights into drug mechanisms and renal pathophysiology. Examples of longitudinal studies include two randomized double-blinded crossover studies by Chowdhury et al. [19, 20]. Distinct effects of commonly used intravenous fluids on renal perfusion were found by performing ASL six times over 2 h after an infusion of one intravenous fluid then repeating the experiment on a second day for a different intravenous fluid. Niles et al. [50] performed serial ASL imaging in 15 matched pairs of living kidney donors and recipients four times over 2 years. Significant differences were found in the 2-year profiles of cortical perfusion between the remaining kidney of donors compared with the transplanted kidney. Furthermore, a potential long-term protective effect of losartan on perfusion was seen between transplant recipients. The ability of renal ASL to track the fate of perfusion in the transplanted and remaining kidney elegantly demonstrates the potential of the technique. Longitudinal studies using renal ASL have also been described in the setting of renin–angiotensin system blockade [12, 24, 46], hyperglycaemia [44], renal denervation [45] and extracorporeal lithotripsy [53].
RECOMMENDATIONS AND CONCLUSION
Renal ASL is a powerful tool for non-invasive measurement of regional renal perfusion in humans, with a typical in plane resolution of 2–3 mm. Renal perfusion lacks a gold standard but the ASL technique has been validated against microspheres in animals and para-aminohippurate clearance in humans. Renal ASL yielded reproducible measurements of renal cortical and medullary perfusion in the studies that have been performed to date. Reproducibility is poorer in the medulla than the cortex, as expected. Renal ASL is highly suited to repeated measurements and longitudinal studies demonstrating the potential prognostic ability of renal ASL are anticipated. The technique shows great promise as part of multiparametric studies of renal structure and function that might inform drug development and be clinically informative. At the time of this review, there are insufficient data comparing several renal ASL variants to make definitive recommendations on the best practice for ASL data acquisition in terms of the optimal ASL labelling scheme, image readout strategy and motion compensation method. Furthermore, there is conflicting evidence and no direct comparisons to resolve whether background suppression improves ASL quantification. Multidelay acquisitions to model arterial transit time cannot be currently universally recommended as these are more technically challenging; however, they should be considered where desired. The most historically popular technique is a pulsed ASL scheme with a single-slice balanced steady-state free precession readout and motion correction. However, there are well-supported rationales for more recently described alternative approaches that suit whole kidney perfusion measurements. There is also variation in how ASL images are analysed to quantify perfusion values in terms of assumptions in the kinetic model and how renal cortex and medulla regions are defined. The lack of a harmonized approach to image acquisition, analysis and reporting might hinder progress in the clinical validation and use of the technique. Thus we have made some recommendations in Table 3. It is important to emphasize that in longitudinal studies the precise way in which ASL is applied is likely to matter less than ensuring consistency of experimental conditions for repeated measures to maintain reproducibility. The priority areas in which more studies are urgently needed include animal studies to validate ASL against reference renal perfusion techniques such as transit time ultrasound flowmetry; human longitudinal multiparametric MRI studies that combine ASL perfusion with other MRI measures outlined in this issue [53–55]; clinical studies that use measured rather than eGFR and studies that validate renal perfusion against a clinical outcome, such as a change in GFR, fibrosis or graft survival. In conclusion, renal ASL is almost ready for clinical use. Collaborative projects such as the COST action PARENCHIMA will accelerate clinical validation of renal ASL as part of a multiparametric assessment of renal perfusion, oxygenation, oedema and fibrosis.
Table 3.
Minimum recommendations for reporting renal ASL studies
| Outline patient preparation (e.g. hydration) |
| Outline subject characteristics |
| Measure systemic haemodynamics ( e.g. blood pressure) |
| Report current medications (especially those targeting the renin– angio-tensin system) |
| Outline the labelling scheme |
| Report the duration of the post-label delay |
| Outline the readout scheme |
| Explicitly outline how motion compensation was handled |
| Explicitly state whether background suppression was applied |
| Acquire a T1 map to use in the kinetic model |
| Describe how the region of interest for ASL analysis is selected |
| Report cortical and medullary values separately |
| Report within-subject reproducibility for your site preferably both within and between visits |
| Measure other renal MRI parameters if possible (e.g. BOLD, diffusion) |
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
This article is based upon work from COST Action Magnetic Resonance Imaging Biomarkers for Chronic Kidney Disease (PARENCHIMA), funded by COST (European Cooperation in Science and Technology). www.cost.eu. For additional information please visit PARENCHIMA project website: www.renalmri.org
FUNDING
A.O. is funded by a National Institute for Health Research (NIHR) Clinical Lectureship (CL-2014-06-003) and an Academy of Medical Sciences grant (SGL015\1019). F.N. acknowledges funding from the Great Ormond Street Hospital Children's Charity (V0318), Kidney Research UK (ST1/2013) and support from the NIHR Great Ormond Street Hospital Biomedical Research Centre. A.A.H. acknowledges funding from the Netherlands Organization for Scientific Research (14951). S.T.F. and C.E.B. acknowledge funding from Kidney Research UK (IN_011_20170303). M.A.F. acknowledges funding from the Spanish Ministry of Economy and Competitiveness (IEDI-2017-00826). The literature search support of Elham Aalai of Manchester University NHS Foundation Trust is acknowledged.
CONFLICT OF INTEREST STATEMENT
None declared. This paper has not been published previously in whole or part.
REFERENCES
- 1. Evans RG, Gardiner BS, Smith DW. et al. Intrarenal oxygenation: unique challenges and the biophysical basis of homeostasis. Am J Physiol Renal Physiol 2008; 295: F1259–F1270 [DOI] [PubMed] [Google Scholar]
- 2. Evans RG, Ince C, Joles JA. et al. Haemodynamic influences on kidney oxygenation: clinical implications of integrative physiology. Clin Exp Pharmacol Physiol 2013; 40: 106–122 [DOI] [PubMed] [Google Scholar]
- 3. Pallone TL, Edwards A, Mattson DL.. Renal medullary circulation. Compr Physiol 2012; 2: 97–140 [DOI] [PubMed] [Google Scholar]
- 4. Ow CPC, Ngo JP, Ullah MM. et al. Renal hypoxia in kidney disease: cause or consequence? Acta Physiol (Oxf) 2018; 222: e12999. [DOI] [PubMed] [Google Scholar]
- 5. Beierwaltes WH, Harrison-Bernard LM, Sullivan JC. et al. Assessment of renal function; clearance, the renal microcirculation, renal blood flow, and metabolic balance. Compr Physiol 2013; 3: 165–200 [DOI] [PubMed] [Google Scholar]
- 6. Nery F, Gordon I, Thomas D.. Non-invasive renal perfusion imaging using arterial spin labeling MRI: challenges and opportunities. Diagnostics 2018; 8: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lerman LO, Taler SJ, Textor SC. et al. Computed tomography-derived intrarenal blood flow in renovascular and essential hypertension. Kidney Int 1996; 49: 846–854 [DOI] [PubMed] [Google Scholar]
- 8. Maioli M, Toso A, Leoncini M. et al. Persistent renal damage after contrast-induced acute kidney injury: incidence, evolution, risk factors, and prognosis. Circulation 2012; 125: 3099–3107 [DOI] [PubMed] [Google Scholar]
- 9. Fraum TJ, Ludwig DR, Bashir MR. et al. Gadolinium-based contrast agents: A comprehensive risk assessment. J Magn Reson Imaging 2017; 46: 338–353 [DOI] [PubMed] [Google Scholar]
- 10. Warmuth C, Nagel S, Hegemann O. et al. Accuracy of blood flow values determined by arterial spin labeling: a validation study in isolated porcine kidneys. J Magn Reson Imaging 2007; 26: 353–358 [DOI] [PubMed] [Google Scholar]
- 11. Artz NS, Wentland AL, Sadowski EA. et al. Comparing kidney perfusion using noncontrast arterial spin labeling MRI and microsphere methods in an interventional swine model. Invest Radiol 2011; 46: 124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ritt M, Janka R, Schneider MP. et al. Measurement of kidney perfusion by magnetic resonance imaging: comparison of MRI with arterial spin labeling to para-aminohippuric acid plasma clearance in male subjects with metabolic syndrome. Nephrol Dial Transplant 2010; 25: 1126–1133 [DOI] [PubMed] [Google Scholar]
- 13. Shimizu K, Kosaka N, Fujiwara Y. et al. Arterial transit time-corrected renal blood flow measurement with pulsed continuous arterial spin labeling MR imaging. Magn Reson Med Sci 2017; 16: 38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cutajar M, Thomas DL, Hales PW. et al. Comparison of ASL and DCE MRI for the non-invasive measurement of renal blood flow: quantification and reproducibility. Eur Radiol 2014; 24: 1300–1308 [DOI] [PubMed] [Google Scholar]
- 15. Conlin CC, Oesingmann N, Bolster B Jr.. et al. Renal plasma flow (RPF) measured with multiple-inversion-time arterial spin labeling (ASL) and tracer kinetic analysis: validation against a dynamic contrast-enhancement method. Magn Reson Imaging 2017; 37: 51–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu WC, Su MY, Chang CC. et al. Renal perfusion 3-T MR imaging: a comparative study of arterial spin labeling and dynamic contrast-enhanced techniques. Radiology 2011; 261: 845–853 [DOI] [PubMed] [Google Scholar]
- 17. Artz NS, Sadowski EA, Wentland AL. et al. Reproducibility of renal perfusion MR imaging in native and transplanted kidneys using non-contrast arterial spin labeling. J Magn Reson Imaging 2011; 33: 1414–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chowdhury AH, Cox EF, Francis ST. et al. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte(R) 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg 2012; 256: 18–24 [DOI] [PubMed] [Google Scholar]
- 19. Chowdhury AH, Cox EF, Francis ST. et al. A randomized, controlled, double-blind crossover study on the effects of 1-L infusions of 6% hydroxyethyl starch suspended in 0.9% saline (voluven) and a balanced solution (Plasma Volume Redibag) on blood volume, renal blood flow velocity, and renal cortical tissue perfusion in healthy volunteers. Ann Surg 2014; 259: 881–887 [DOI] [PubMed] [Google Scholar]
- 20. Cox EF, Buchanan CE, Bradley CR. et al. Multiparametric renal magnetic resonance imaging: validation, interventions, and alterations in chronic kidney disease. Front Physiol 2017; 8: 696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cutajar M, Thomas DL, Banks T. et al. Repeatability of renal arterial spin labelling MRI in healthy subjects. Magma 2012; 25: 145–153 [DOI] [PubMed] [Google Scholar]
- 22. Gardener AG, Francis ST.. Multislice perfusion of the kidneys using parallel imaging: image acquisition and analysis strategies. Magn Reson Med 2010; 63: 1627–1636 [DOI] [PubMed] [Google Scholar]
- 23. Getzin T, May M, Schmidbauer M. et al. Usability of functional MRI in clinical studies for fast and reliable assessment of renal perfusion and quantification of hemodynamic effects on the kidney. J Clin Pharmacol 2018; 58: 466–473 [DOI] [PubMed] [Google Scholar]
- 24. Gillis KA, McComb C, Foster JE. et al. Inter-study reproducibility of arterial spin labelling magnetic resonance imaging for measurement of renal perfusion in healthy volunteers at 3 Tesla. BMC Nephrol 2014; 15: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hammon M, Janka R, Siegl C. et al. Reproducibility of kidney perfusion measurements with arterial spin labeling at 1.5 Tesla MRI combined with semiautomatic segmentation for differential cortical and medullary assessment. Medicine (Baltimore) 2016; 95: e3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karger N, Biederer J, Lusse S. et al. Quantitation of renal perfusion using arterial spin labeling with FAIR-ULFARE. Magn Reson Imaging 2000; 18: 641–647 [DOI] [PubMed] [Google Scholar]
- 27. Kim DW, Shim WH, Yoon SK. et al. Measurement of arterial transit time and renal blood flow using pseudocontinuous ASL MRI with multiple post-labeling delays: feasibility, reproducibility, and variation. J Magn Reson Imaging 2017; 46: 813–819 [DOI] [PubMed] [Google Scholar]
- 28. Lanzman RS, Wittsack HJ, Martirosian P. et al. Quantification of renal allograft perfusion using arterial spin labeling MRI: initial results. Eur Radiol 2010; 20: 1485–1491 [DOI] [PubMed] [Google Scholar]
- 29. Rapacchi S, Smith RX, Wang Y. et al. Towards the identification of multi-parametric quantitative MRI biomarkers in lupus nephritis. Magn Reson Imaging 2015; 33: 1066–1074 [DOI] [PubMed] [Google Scholar]
- 30. Robson PM, Madhuranthakam AJ, Dai W. et al. Strategies for reducing respiratory motion artifacts in renal perfusion imaging with arterial spin labeling. Magn Reson Med 2009; 61: 1374–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robson PM, Madhuranthakam AJ, Smith MP. et al. Volumetric arterial spin-labeled perfusion imaging of the kidneys with a three-dimensional fast spin echo acquisition. Acad Radiol 2016; 23: 144–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li LP, Tan H, Thacker JM. et al. Evaluation of renal blood flow in chronic kidney disease using arterial spin labeling perfusion magnetic resonance imaging. Kidney Int Rep 2017; 2: 36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang J, Zhang Y, Yang X. et al. Hemodynamic effects of furosemide on renal perfusion as evaluated by ASL-MRI. Acad Radiol 2012; 19: 1194–1200 [DOI] [PubMed] [Google Scholar]
- 34. Boss A, Martirosian P, Graf H. et al. High resolution MR perfusion imaging of the kidneys at 3 Tesla without administration of contrast media. Rofo 2005; 177: 1625–1630 [DOI] [PubMed] [Google Scholar]
- 35. Breidthardt T, Cox EF, Squire I. et al. The pathophysiology of the chronic cardiorenal syndrome: a magnetic resonance imaging study. Eur Radiol 2015; 25: 1684–1691 [DOI] [PubMed] [Google Scholar]
- 36. Gillis KA, McComb C, Patel RK. et al. Non-contrast renal magnetic resonance imaging to assess perfusion and corticomedullary differentiation in health and chronic kidney disease. Nephron 2016; 133: 183–192 [DOI] [PubMed] [Google Scholar]
- 37. Michaely HJ, Schoenberg SO, Ittrich C. et al. Renal disease: value of functional magnetic resonance imaging with flow and perfusion measurements. Invest Radiol 2004; 39: 698–705 [DOI] [PubMed] [Google Scholar]
- 38. Mora-Gutierrez JM, Garcia-Fernandez N, Slon Roblero MF. et al. Arterial spin labeling MRI is able to detect early hemodynamic changes in diabetic nephropathy. J Magn Reson Imaging 2017; 46: 1810–1817 [DOI] [PubMed] [Google Scholar]
- 39. Rossi C, Artunc F, Martirosian P. et al. Histogram analysis of renal arterial spin labeling perfusion data reveals differences between volunteers and patients with mild chronic kidney disease. Invest Radiol 2012; 47: 490–496 [DOI] [PubMed] [Google Scholar]
- 40. Tan H, Koktzoglou I, Prasad PV.. Renal perfusion imaging with two-dimensional navigator gated arterial spin labeling. Magn Reson Med 2014; 71: 570–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dong J, Yang L, Su T. et al. Quantitative assessment of acute kidney injury by noninvasive arterial spin labeling perfusion MRI: a pilot study. Sci China Life Sci 2013; 56: 745–750 [DOI] [PubMed] [Google Scholar]
- 42. Skeoch S, Hubbard Cristinacce PL, Dobbs M. et al. Evaluation of non-contrast MRI biomarkers in lupus nephritis. Clin Exp Rheumatol 2017; 35: 954–958 [PubMed] [Google Scholar]
- 43. Hirshberg B, Qiu M, Cali AM. et al. Pancreatic perfusion of healthy individuals and type 1 diabetic patients as assessed by magnetic resonance perfusion imaging. Diabetologia 2009; 52: 1561–1565 [DOI] [PubMed] [Google Scholar]
- 44. Ott C, Janka R, Schmid A. et al. Vascular and renal hemodynamic changes after renal denervation. Clin J Am Soc Nephrol 2013; 8: 1195–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schneider MP, Janka R, Ziegler T. et al. Reversibility of the effects of aliskiren in the renal versus systemic circulation. Clin J Am Soc Nephrol 2012; 7: 258–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Heusch P, Wittsack HJ, Blondin D. et al. Functional evaluation of transplanted kidneys using arterial spin labeling MRI. J Magn Reson Imaging 2014; 40: 84–89 [DOI] [PubMed] [Google Scholar]
- 47. Hueper K, Gueler F, Brasen JH. et al. Functional MRI detects perfusion impairment in renal allografts with delayed graft function. Am J Physiol Renal Physiol 2015; 308: F1444–F1451 [DOI] [PubMed] [Google Scholar]
- 48. Artz NS, Sadowski EA, Wentland AL. et al. Arterial spin labeling MRI for assessment of perfusion in native and transplanted kidneys. Magn Reson Imaging 2011; 29: 74–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ren T, Wen CL, Chen LH. et al. Evaluation of renal allografts function early after transplantation using intravoxel incoherent motion and arterial spin labeling MRI. Magn Reson Imaging 2016; 34: 908–914 [DOI] [PubMed] [Google Scholar]
- 50. Niles DJ, Artz NS, Djamali A. et al. Longitudinal assessment of renal perfusion and oxygenation in transplant donor-recipient pairs using arterial spin labeling and blood oxygen level-dependent magnetic resonance imaging. Invest Radiol 2016; 51: 113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cutajar M, Hilton R, Olsburgh J. et al. Renal blood flow using arterial spin labelling MRI and calculated filtration fraction in healthy adult kidney donors pre-nephrectomy and post-nephrectomy. Eur Radiol 2015; 25: 2390–2396 [DOI] [PubMed] [Google Scholar]
- 52. Abd Ellah M, Kremser C, Pallwein L. et al. Changes of renal blood flow after ESWL: assessment by ASL MR imaging, contrast enhanced MR imaging, and renal resistive index. Eur J Radiol 2010; 76: 124–128 [DOI] [PubMed] [Google Scholar]
- 53. Pruijm M, Mendichovszky IA, Liss P. et al. Renal blood oxygenation level-dependent magnetic resonance imaging to measure renal tissue oxygenation: a statement paper and systematic review. Nephrol Dial Transplant 2018; 33 (Suppl 2): ii22–ii28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Caroli A, Schneider M, Friedli I. et al. Diffusion-weighted magnetic resonance imaging to assess diffuse renal pathology: a systematic review and statement paper. Nephrol Dial Transplant 2018; 33 (Suppl 2): ii29–ii40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wolf M, de Boer A, Sharma K. et al. Magnetic resonance imaging T1- and T2-mapping to assess renal structure and function: a systematic review and statement paper. Nephrol Dial Transplant 2018; 33 (Suppl 2): ii41–ii50 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.