Abstract
Functional renal magnetic resonance imaging (MRI) has seen a number of recent advances, and techniques are now available that can generate quantitative imaging biomarkers with the potential to improve the management of kidney disease. Such biomarkers are sensitive to changes in renal blood flow, tissue perfusion, oxygenation and microstructure (including inflammation and fibrosis), processes that are important in a range of renal diseases including chronic kidney disease. However, several challenges remain to move these techniques towards clinical adoption, from technical validation through biological and clinical validation, to demonstration of cost-effectiveness and regulatory qualification. To address these challenges, the European Cooperation in Science and Technology Action PARENCHIMA was initiated in early 2017. PARENCHIMA is a multidisciplinary pan-European network with an overarching aim of eliminating the main barriers to the broader evaluation, commercial exploitation and clinical use of renal MRI biomarkers. This position paper lays out PARENCHIMA’s vision on key clinical questions that MRI must address to become more widely used in patients with kidney disease, first within research settings and ultimately in clinical practice. We then present a series of practical recommendations to accelerate the study and translation of these techniques.
Keywords: biomarker, chronic kidney disease, fibrosis, inflammation, MRI
INTRODUCTION
The global burden of kidney disease is significant, as >10% of the world’s adult population has chronic kidney disease (CKD) and in developed countries alone >2 million people sustain acute kidney injury (AKI) annually [1, 2]. Moreover, the incidences of both CKD and AKI are rising. Progression to end-stage kidney disease (ESKD) and cardiovascular complications impact significantly on patients’ outcomes, their quality of life and health care resource utilization. In the UK, 1.3% of National Health Service (NHS) spending (£1.45 billion per annum) has been attributed to CKD [3]. Landmark publications have consistently reinforced these messages and delineated the key areas in which progress is needed, specifically highlighting the need for better methods to diagnose kidney diseases [1, 4].
Current methods to assess CKD (e.g. serum creatinine, albuminuria and ultrasound) are insensitive to early kidney damage, do not usually provide insight into the aetiology of underlying kidney disease and do not reliably allow individual patient stratification in terms of prognosis or therapy decisions. Kidney biopsy is the only current method to assess renal microstructure, but it has several disadvantages, including its invasive nature and susceptibility to sampling bias. The clinical need for more specific diagnostic and prognostic tools is seen across the patient pathway and improved biomarkers that can determine the aetiology of kidney disease or characterize the dominant pathophysiological process in individual patients are urgently required.
Recent developments in functional and quantitative renal magnetic resonance imaging (MRI) techniques show great potential to address these challenges. There are now several techniques to generate MRI biomarkers, which can measure biophysical tissue properties that have been linked to fibrosis, inflammation, tissue oedema, perfusion, filtration and tissue oxygenation [5–15]. The most commonly encountered functional MRI techniques are summarized in Table 1. In contrast to conventional MRI, which is geared towards visual identification of gross anatomical changes, these new methods provide a means to characterize subtle tissue alterations caused by a range of kidney diseases. As such, MRI biomarkers may be able to pick up early signs of disease progression that have not yet led to a discernible effect on markers in blood and urine. In addition, MRI biomarkers are unique among diagnostic tools in that they characterize the entire kidney in high-spatial detail, are able to detect cortico-medullary and left-right changes separately, do not use ionizing radiation and can assess the degree of functional heterogeneity across the kidney. MRI is the only technique able to study the renal medulla in vivo, an area that may play an important role in the pathogenesis of CKD and AKI [32, 33].
Table 1.
Description of the most common MRI biomarkers currently available for assessment of kidney disease
| MRI technique | Description of MRI technique | Pathophysiological processes informed by MRI biomarker | Biomarker measured | Units of measurement |
|---|---|---|---|---|
| Volumetry [16, 17–19] | Gold standard technique. Volumes measured from T1- and/or T2-weighted structural images. | Kidney length and volume and their change over time are key measure in patients with ADPKD but may also be important in CKD progression, primary and secondary hyperfiltration in diabetic nephropathy, renal transplants, renal artery stenosis, vesicoureteric reflux. Cortical thickness may be more variable within a given kidney, limiting reproducibility. |
|
|
| Phase contrast MRI [20] | Measures blood flow in renal arteries. Exploits the different properties of moving versus static protons in a magnetic field. A moving proton will have a ‘phase shift’ proportional to its velocity, allowing calculation of flow. | Increased renal resistance to flow due to downstream microvascular obstruction, large-vessel arterial disease or changes in systemic haemodynamics. |
|
|
| ASL [14, 21–24] | ASL uses magnetically labelled water protons in blood that act as a diffusible tracer providing an internal endogenous contrast, following which labelled images are subtracted from control images to generate perfusion maps. | Cortical perfusion, which can be affected by a number of pathophysiological processes in acute and chronic renal disease. | Tissue blood flow | mL/min/100g |
| Diffusion weighted imaging (DWI) [12, 25] | Detects the displacement of water molecules within the architecture of tissues and quantifies this as the ADC. ADC may be affected by tubular flow and capillary perfusion, so true diffusion (D) can be measured using the IntraVoxel Incoherent Motion (IVIM) model, alongside pseudo-diffusion (tubular/vascular flow, D*) and flowing fraction (F). | Any changes in the renal microstructure, especially in the interstitium, for instance, renal fibrosis, cellular infiltration (inflammatory or tumorous) or oedema, changes in renal perfusion and in water handling in the tubular compartment. |
|
|
| Diffusion-tensor imaging (DTI) [12, 26] | Similar to DWI but also assesses directionality of diffusion (Brownian motion), which is quantified as a percentage of spatially oriented diffusion signal [fractional anisotropy (FA)]. Allows assessment of the degree of organization in space of oriented tissues. | Any changes in the microstructure that lead to a change in the preferred direction of water diffusion, for instance, tubular dilatation, tubular obstruction or a loss in the organization of medullary tubules. |
|
|
| BOLD MRI [13, 27] | Indirect assessment of oxygenation. Paramagnetic properties of deoxygenated haemoglobin act to shorten the transverse relaxation time constant (T2*). | Changes in renal oxygenation or changes in the microstructure of the capillary bed. Other factors such as hydration status, dietary sodium and susceptibility effects also alter T2*. |
|
|
| T1 mapping [5, 15] | Provides a quantitative map over the whole kidney for T1 values. T1 is a tissue-specific time variable that can distinguish different tissues. | Changes in the molecular environment, for example, water content, viscosity, temperature, fibrosis (due to the association of collagen with supersaturated hydrogel) and inflammation (interstitial oedema, cellular swelling). |
|
ms |
| T2 mapping [15, 28] | As with T1 mapping, provides quantification of T2 as a tissue-specific time parameter. Changes with tissue water content. | Changes in the molecular environment but assumed to be more sensitive to the effects of oedema and/or inflammation. Limited experience in human kidney disease to date. |
|
ms |
| MR renography [26] [sometimes referred to as dynamic contrast enhanced (DCE) MRI] | Uses gadolinium-based contrast agents to change the T1 relaxation time of water in tissues. Allows measurement of perfusion and GFR. Concerns exist when using gadolinium for research in advanced CKD (hence not discussed in this paper). | Perfusion and filtration per unit tissue, vascularity and tubular transit times. |
|
|
| Magnetization transfer (MT) [29] |
|
The fraction of large macromolecules or immobilized cell membranes in tissue; in the kidney, shown to correlate with fibrosis. | MT ratio | % |
| Emerging techniques [9, 30, 31] | A number of additional functional MRI techniques are also described, which currently require a larger amount of technical validation and are less widely available than other methods described in this table. These include (but are not limited to) elastography, hyperpolarization, and 23-sodium MRI. | Technique dependent | Technique dependent | Technique dependent |
In early 2017, the Cooperation in Science and Technology (COST) Action PARENCHIMA was formed to increase the standardization and availability of renal MRI biomarkers and drive a broader clinical uptake (www.renalmri.org). This position paper is the output of expert meetings of PARENCHIMA Working Group 3 (Bergamo, 13 July 2017; Berlin, 13 October 2017; Utrecht, 23 April 2018) and has two separate aims: (i) identify the key clinical questions related to CKD where MRI biomarkers can provide added value and improve patient outcomes and (ii) propose strategic recommendations for future clinical research studies involving functional renal MRI.
THE POTENTIAL CLINICAL UTILITY OF MRI BIOMARKERS
In this section, the possible clinical applications of MRI are discussed using the biomarker classification scheme proposed by the Biomarker Working Group of the US Food and Drug Administration (FDA) and the National Institutes of Health [34].
Diagnostic biomarkers: diagnosis and classification of disease
CKD is defined in the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines as abnormalities of kidney structure or function present for >3 months that have implications for health. The criteria include a reduced glomerular filtration rate (GFR), the presence of albuminuria or abnormalities of kidney structure [35]. Currently the aetiology of CKD remains unknown or at least uncertain in as many as 50% of patients [36]. In addition, early diagnosis of progressive conditions before the onset of reduced GFR or albuminuria may be of clinical benefit but is not possible at present [37]. CKD progression and maladaptive repair after AKI are characterized by a similar combination of mechanisms, including tissue injury, inflammation, haemodynamic alterations (glomerular capillary hyperfiltration and hypertension), vascular rarefaction, hypoxia and fibrosis [38, 39]. Probably the best predictor of declining renal function identified in clinical studies is interstitial fibrosis [38, 39]. While fibrosis is regarded as the final common pathway, CKD progression is a dynamic process in which these multiple mechanisms occur in a mutually stimulating fashion. CKD is therefore a heterogeneous syndrome with cause and outcomes varying significantly between individuals.
Currently, renal biopsy is the only tool in clinical practice that allows assessment of renal microstructure, pathology or the degree of fibrosis. Only a proportion of patients with CKD undergo renal biopsy. This is usually those with greater degrees of non-diabetic proteinuria and/or progressively declining estimated GFR (eGFR), and is mostly performed in the earlier stages of CKD. In patients with a renal transplant, biopsy is performed in response to graft dysfunction, although some centres also perform protocol biopsies to screen for subclinical rejection or chronic allograft nephropathy. However, renal biopsy has disadvantages: it is invasive (causing discomfort and risking complications), it is susceptible to sampling bias (examining only ∼0.002% of the total glomeruli of one kidney) and it is difficult to perform repeatedly to assess serial changes. Moreover, biopsy is problematic in specific patient categories (e.g. patients on anticoagulation and smaller kidney size), contraindicated in others (e.g. cystic kidney disease) and is limited in that it does not routinely sample the medulla, an area uniquely sensitive to hypoxic injury. Therefore significant challenges remain in current clinical practice to characterize the dominant pathophysiological processes in an individual.
There are now a number of techniques within the armamentarium of renal MRI that offer the unique opportunity to assess the different pathological processes in-vivo, on a whole-organ basis and without the need for intravenous contrast agents [40]. Establishing the biological validity of these measures is an important first step in the biomarker pathway. This refers to the determination of which biological processes relevant to CKD can be specifically and reliably detected and quantified by these methods and which are the optimal MRI measures or combination of measures to do so. To date, however, very few current studies have thoroughly characterized the specificity of MRI biomarkers for discrete pathological processes, creating a situation where individual magnetic resonance (MR) techniques may be confounded by a number of clinical, physiological and pathological variables. Furthermore, the majority of MRI biomarkers have been assessed in isolation, making it difficult to determine which are the optimal MRI measures or combinations of measures for a given biological process. This is important as combinations of MRI biomarkers are likely to be more effective at understanding and describing specific pathophysiological processes. Similarly, there may be added value from the integration of renal MRI measures with circulating or urinary biomarkers of GFR or tubular injury.
Prognostic biomarkers: predicting disease progression
In addition to the question of whether MRI biomarkers can identify or measure aspects of biology that are relevant to renal disease, it is also important to establish whether they are able to predict important clinical outcomes (clinical validity). Adverse clinical outcomes include CKD progression, increased mortality, cardiovascular events and increased risk of AKI. While hard clinical endpoints such as progression to ESKD are widely accepted (including by regulatory bodies), the challenge for clinical studies is that prolonged follow-up periods, large sample sizes or the study of advanced disease stages are often necessary to adequately test these endpoints. This has led to extensive debate in the medical literature about the definition of surrogate endpoints of CKD progression [41], a variety of which have been proposed, including the trajectory of eGFR decline, percentage change in eGFR over time, threshold values of eGFR cut-offs, reductions in albuminuria and combinations of these [42, 43].
A well-known example of a prognostic MRI biomarker is total kidney volume (TKV) in autosomal dominant polycystic kidney disease (ADPKD). TKV is most accurately measured by MRI and is approved by the FDA as a prognostic enrichment biomarker to select patients for interventional clinical trials who are at higher risk for a progressive decline in renal function [44]. In clinical practice, TKV is now used as a biomarker to help inform the decision to initiate therapy with tolvaptan [45]. More generally, there remains a clear clinical need to better differentiate the large variation that is seen in individual risk of CKD progression. Illustrating this, in a Norwegian health survey cohort only 1.2% of a primary care CKD population progressed to ESKD, in contrast to much higher progression rates when proteinuria, diabetes or hypertension coexist [46, 47]. In an attempt to improve the characterization of individual risk of CKD progression, several prediction models based on clinical data have been developed, of which the Kidney Failure Risk Equation (KFRE) is the most widely accepted [48]. The score has been validated in several international cohorts and represents the current standard in ESKD risk prediction, despite being applicable only to CKD Stages 3–5 and over a relatively short time frame (the subsequent 5 years) [49, 50]. An area of recent progress in the application of functional MRI to characterize the risk of CKD progression is the use of blood oxygenation level dependent (BOLD) MRI, which has been shown to identify patients at risk for ESKD or rapid renal function decline over the subsequent 3 years [51]. Emerging evidence in diabetic kidney disease has also shown that arterial spin labelling (ASL) measurement of cortical perfusion may detect early haemodynamic changes [52].
Biomarkers that can predict patients’ risk of subsequent CKD progression would be valuable on several fronts. This would allow for better targeting or intensified treatment of higher-risk groups. Enrichment of study populations for clinical trials would also be possible, increasing the proportion of recruited patients at increased risk of progression, thus improving the efficiency of the trial and its likelihood of success. Timely preparation for renal replacement therapy (RRT) could be better delivered in those with progression despite treatment. Conversely, in low-risk patients, unnecessary interventions could be avoided, benefiting patients and reducing health care costs.
However, there are some additional considerations that are relevant to the establishment of clinical utility. It will be important to determine whether MRI biomarkers associate with adverse outcomes and whether they add significant improvements in performance to our current ability to identify patients at high risk of progression. Furthermore, the high cost of MRI scans and elaborate image processing is a critical factor in their ultimate utility and solid health economic evaluation should therefore be integrated early in the MRI biomarker development process.
Predictive biomarkers: patient selection for treatment
A promising area of application for MRI biomarkers is in targeting treatment choices based on more precise knowledge of mechanisms of disease and/or the risk of adverse outcomes in an individual. If MRI is used to enrich the study population for a clinical trial, a positive outcome for the intervention studied could lead to translation of the MRI biomarker into clinical practice as a companion diagnostic to identify patients suitable for the novel treatment. As examples, the ability to differentiate between active inflammation and fibrosis in a patient with CKD secondary to vasculitis or in a renal transplant would inform therapeutic decisions around the intensity of immunosuppressive therapy, which carries associated risks as well as potential therapeutic benefits. Renal perfusion and blood flow measured by ASL and phase contrast MRI may characterize the haemodynamic and thus potentially reversible consequences of treatments known to affect renal blood flow, such as inhibitors of the renin–angiotensin system [21]. Also, MR-derived structure–function relationships may predict those patients with renovascular disease most likely to respond to renal artery stenting [53]. MRI biomarkers could also be used to identify those patients in whom disease is too advanced to benefit from specific treatments. Such patients could then be excluded from clinical trials and in clinical practice avoid unnecessary treatments and their side effects.
Monitoring biomarkers: response to treatment
Renal MRI can also provide an improved way of monitoring response to therapy. In a small number of centres, this is current practice in patients with ADPKD treated with tolvaptan. It is also required in patients with tuberous sclerosis and angiomyolipoma treated with everolimus (NHS England Clinical Commissioning policy B14X09). In other forms of CKD, the effectiveness of treatment is currently monitored by eGFR and proteinuria; the degree of albuminuria reduction after the introduction of renin–angiotensin–aldosterone system blockade associates with a lower risk of CKD progression in diabetic and non-diabetic CKD [47, 54–56]. However, methods to provide improved assessments of response to treatment would certainly be of value, more so because non-proteinuric rather than proteinuric renal diseases are the leading cause of ESKD [57]. MRI measures without the need for intravenous contrast agents and without the risks of ionizing radiation are well suited to serial application. They can be performed before and after initiation of treatment and repeated serially over a longer time course to inform whether a treatment is providing benefit or to determine whether treatment needs to be changed in case of lack of adequate response. This may allow the monitoring of response to renoprotective therapy without the need for serial renal biopsies [58] and would be particularly important for treatments that are high risk or expensive, so that treatment is continued when effective but stopped earlier than currently when ineffective. However, using MRI biomarkers to assess treatment response will require robust validation of variability of the MRI measures.
Safety biomarkers: detecting drug toxicity
The same paradigm could be used to monitor for renal toxicity, for example, in high-risk populations such as those undergoing specific types of chemotherapy, or to monitor the nephrotoxic effects of any new drugs. In the latter situation, this has wider relevance than drugs targeting renal disease and could be applied to phase I–II trials of promising new drugs for any indication in which a specific concern may exist around effects on renal haemodynamics or potential nephrotoxicity.
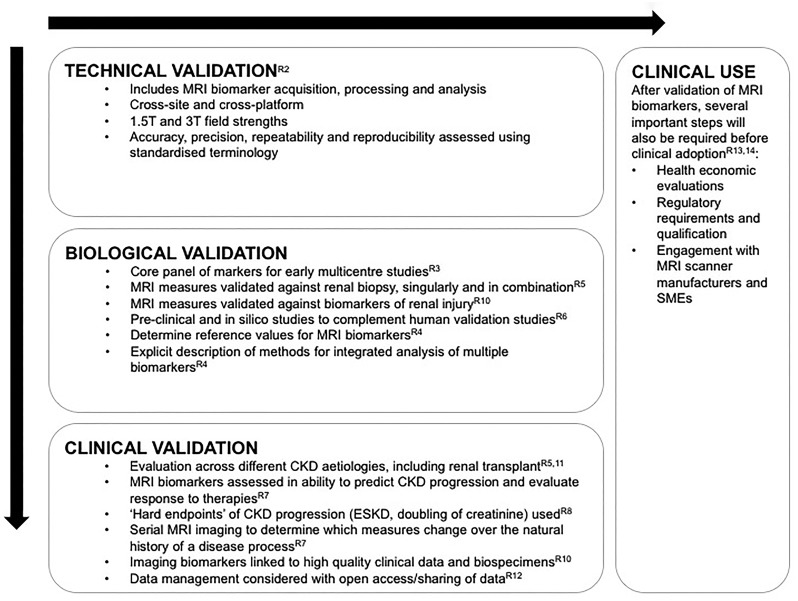
STRATEGIC RECOMMENDATIONS FOR FUTURE CLINICAL TRIALS INVOLVING RENAL MRI
Following the roadmap for imaging biomarkers in oncology, before a renal MRI biomarker can be widely used in clinical research or routine practice it must cross two ‘translational gaps’ [59]. First, the novel biomarker must be shown to be a valid tool for testing hypotheses in a research setting and second, it must be clinically useful and cost effective in the routine management of patients. In this section we will focus largely on the former, though there is some overlap. Note that a valid biomarker does not necessarily play an essential role in the pathway to target organ injury, for example, haemoglobinA1c is not pathogenic but is a good biomarker of diabetic control and predictive of clinical outcomes [60]. We adhere to the general principles described previously in the roadmap for translation of imaging biomarkers in cancer [59], but propose additional recommendations specific to the future study of multiparametric renal MRI biomarkers with the aim of increasing the speed of their translation. Recommendations are summarized in Figure 1.
FIGURE 1.
Summary of recommendations to progress renal MRI biomarkers. Technical validation should precede biological and clinical validation, although this process is likely to occur in parallel as well as sequentially; this bidirectional process is represented by the arrows. The labels with the prefix ‘R’ indicate the specific recommendation linked to each statement.
Recommendation 1: Funding applications, publications and published protocols describing renal MRI biomarkers should report full details of study design, patient selection, image acquisition, post-processing, image analysis and quality assurance processes [59]
Useful information is available from the FDA’s draft guidance on Clinical Trial Imaging Endpoint Process Standards [61]. To facilitate the comparison between protocols, the PARENCHIMA consensus-building initiatives will include the development of a standardized checklist or template onto which studies can map specifics of study design, patient selection, image acquisition, post-processing, image analysis and quality assurance processes. Harmonization of these fields with existing standards such as Digital Imaging and Communications in Medicine (DICOM) should also be considered to facilitate interfacing with existing imaging data management systems.
Recommendation 2: Technical validation should be achieved prior to multisite clinical studies in order to de-risk costly and time-consuming studies and to inform statistical analysis of the results [59]
Technical validation includes determining the accuracy, precision and repeatability of measurements, as well as cross-site and cross-vendor reproducibility. Technical validation can be approached in a study-specific manner, with the methods and protocols applied in the study tested prior to patient recruitment. This approach was followed by the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) study in ADPKD [16] and is suitable for studies using biomarkers or assays that have not yet been technically validated. As demonstrated in the landmark CRISP study, these methods have the potential to become reference standards for future studies and clinical translation, as well as a benchmark against which to evaluate future methods. Risks with study-specific technical validation are duplication of efforts and potential emergence of competing standards. Research coordination efforts combining technical validation with a strategy to upscale the validated methods and increase their availability are critical to avoiding these potential problems and in providing a sustainable solution. Researchers should adhere to standardized terminology and analysis approaches for quantitative imaging biomarkers as previously described [62, 63].
Recommendation 3: For multicentre studies, a core panel of MRI biomarkers should be defined that are standardized, comparable and deliverable at all sites
The term ‘multiparametric MRI’ refers to any combination of functional MRI measures alongside standard anatomical images. In the context of renal imaging, there are a range of functional MRI measures that differ in their complexity and availability. At present, candidates for this panel are likely to include TKV, renal artery blood flow, longitudinal relaxation time (T1), transverse relaxation time (T2) and the apparent diffusion coefficient (ADC). However, a core panel should not be a barrier that limits current expertise and future refinement of more advanced techniques, for example, for cortical perfusion or tissue stiffness. In some cases, substudies could allow efficient evaluation of novel biomarkers if these are ‘added on’ to the core panel of biomarkers in those centres with the necessary expertise. Statistical input to ensure appropriate study design and power of such substudies would be essential, and with future technological advances the core panel of biomarkers is likely to expand.
Recommendation 4: Studies using multiparametric MRI should explicitly describe their approach to the integrated analysis of multiple measures, and data that may inform future prioritization of biomarkers should be reported in full
Integration of multiple MRI biomarkers is potentially complex, with each measurement generating large amounts of data that can be analysed on a whole-kidney basis or by segmentation (e.g. cortex, medulla and corticomedullary difference). The degree of variation across the kidney may also be important. Due to the range of pathological processes involved in CKD progression, it is likely that multiple measures are required to differentiate between phenotypic groups. While some measures may be additive in combination, others may be redundant. This may differ depending on the clinical situation. Establishing reference values for MRI biomarkers will be important and complex MRI datasets (especially if multiparametric) will require detailed analysis plans with input from both imaging and biostatistics experts.
Recommendation 5: The biological validity of renal MRI needs to be determined through cross-sectional studies in which MRI biomarkers are compared against renal biopsy
In clearly defined patient cohorts (both CKD and renal transplantation), individual or combinations of MRI biomarkers should be compared against quantitative renal histology techniques that measure important biological processes such as fibrosis (glomerulosclerosis score, extent of interstitial fibrosis, collagen accumulation), inflammation (density of inflammatory cells and cell subtypes), peritubular capillary density and podocyte loss. Such studies will be greatly aided by the use of digital pathology techniques and computer-driven methods for quantification [64]. In addition, combining MRI and renal biopsies for the determination of cortical volume and glomerular density offers the potential to provide estimates of glomerular numbers and single nephron GFR.
Recommendation 6: To overcome the limitations of biopsy, biological validation also requires studies assessing MRI biomarkers in animal models of renal disease or in human nephrectomy specimens, as well as in silico studies linking biology to measurement
There are potential weaknesses of relying only on a routine renal biopsy as the reference standard against which to evaluate MRI measures. These include the tiny sample volume of renal biopsy compared with the whole-kidney coverage of MRI data, the ‘patchy’ nature of kidney damage that may not be reflected in a small biopsy sample, that patients undergoing renal biopsy for clinical reasons are not necessarily representative of the full spectrum of CKD and that renal biopsy does not always allow validation of medullary biomarkers. To date, whole-kidney MRI results have been compared with biopsy without matching to the sampled site, resulting unsurprisingly in only modest correlations between whole-kidney MRI measures and quantification of fibrosis on biopsy [9].
Validation studies in animal models or nephrectomy specimens can bridge these gaps. Preclinical models may allow comprehensive and detailed validation of biomarkers against well-characterized pathological states and the effect of interventions. However, even these studies are limited because many biological properties change at the same time during disease progression. Hence observing a correlation between, for instance, cortical relaxation time and a histopathological measure of fibrosis does not necessarily demonstrate a causal relation between the two if specific interventions are not used. Therefore it is important that experimental work is further complemented by thorough in silico modelling trying to provide a direct mechanistic link between biological properties and MRI biomarkers and generating testable hypotheses.
Recommendation 7: MRI biomarkers should be tested prospectively in longitudinal patient cohort studies with adequate sample size and suitable length of follow-up
Demonstrating a close and biologically plausible association between renal MRI biomarkers and clinically important outcome measures is vital and is the criterion used by regulatory bodies to assess biomarkers and surrogate endpoints. A recent study of BOLD MRI by Pruijm et al. [51] is a rare example of a study addressing these questions directly. Within this broad concept, there are several points to consider. While demonstration of biological and clinical validity is separate, there are practical and efficiency reasons to link their study, so we recommend that well-characterized participants in cross-sectional studies are also recruited for longitudinal follow-up with assessment of clinical outcomes, with or without repeat MRI scanning. Serial imaging should establish which MRI parameters change over time in association with or predating CKD progression or regression. These studies would also be important to establish the optimal frequency of serial renal imaging. A further requirement for biological and clinical validity is demonstrating that MRI biomarkers change in response to treatment.
Recommendation 8: Studies should be performed that assess MRI biomarkers against hard clinical endpoints of CKD progression
Hard clinical endpoints such as the development of ESKD (eGFR <15 mL/min/1.73m2 or initiation of RRT) or substantial reductions in GFR of 30, 40 or >50% (doubling of serum creatinine) are the only measures of CKD progression currently accepted by the FDA. MRI biomarkers should be evaluated in terms of their ability to identify patients at higher risk of progressing to these endpoints. Alternative definitions of CKD progression (e.g. percentage reductions in eGFR, eGFR trajectory) should be recorded as secondary endpoints. This approach has the obvious drawback of requiring larger numbers of participants and longer study duration, which will be more challenging in terms of feasibility and cost. Evaluation of MRI biomarkers in populations defined as high risk of progression based on clinical features may permit more feasible sample sizes and shorter study length. Alternatively, efficient methods of tracking long-term outcomes may be utilized, for example, through the use of registry data for long-term tracking of RRT initiation data.
Recommendation 9: In both cross-sectional and longitudinal studies, careful consideration must be given to the choice of method for assessing renal function
Assessing GFR is a critical element to any clinical study of CKD, although it should be noted that cross-sectional comparisons against GFR alone will not be adequate to establish the utility of MRI biomarkers. The gold standard method for GFR measurement is inulin clearance, but this is invasive and expensive and inulin is difficult to dissolve and maintain in solution. Measurement of GFR using other exogenous filtration markers (e.g. iohexol) is the reference method in clinical research and some clinical scenarios and would be optimal for cross-sectional studies [65]. eGFR is easier to obtain and theoretically could be more suitable for longitudinal studies tracking changes in renal function over time. However, eGFR lacks precision, especially at higher GFR levels, and is not only dependent on GFR, but also on non-GFR determinants included in the equation [65], although in longitudinal studies non-GFR determinants of serum creatinine may become less relevant since they will not vary considerably in the same individual. The accuracy of eGFR can be improved by using combined creatinine and cystatin-based estimating equations, but the benefit is marginal [66]. Given the weight of evidence that albuminuria is a marker of kidney damage and an independent predictor of adverse outcomes associated with CKD [67], studies should correlate MRI measures with the magnitude of albuminuria and changes in albuminuria over time. In cross-sectional studies, fasting and hydration should be documented, as these are known to modulate MRI biomarkers and estimates of renal function.
Recommendation 10: Biosample storage, data storage and matched image banking should be built into prospective MRI studies to generate linked clinical data, biosamples and imaging biomarkers
Imaging biobanks will permit assessments of combinations of genetic, molecular and imaging biomarkers, the use of additional filtration markers that may help improve the accuracy of eGFR equations or the use of a panel of urine and blood injury markers to provide an additional way of establishing biological validity (e.g. neutrophil gelatinase-associated lipocalin, kidney injury molecule-1) [68]. A similar strategy holds for biomarkers of renal fibrosis (e.g. matrix metalloproteinase 9 and bone morphogenic protein 7), inflammation biomarkers (e.g. monocyte chemoattractant protein 1, tumour necrosis factor receptor 1 and 2) [69] or combined proteomic panels (e.g. CKD 273) [70].
Recommendation 11: Careful consideration of aetiology of CKD should be incorporated into the study design
It may be challenging to strike a balance between a more generalizable CKD population that is heterogeneous in terms of the clinical and pathophysiological characteristics versus a tightly defined patient cohort with greater homogeneity but from which findings may not be generalizable and who may be more difficult to recruit. Studies focused on specific disease aetiologies may be important to identify MRI measures that are relevant to a particular disease. The importance of renal size and cyst volume in polycystic kidney disease is one example of this [71]. Where study design includes one or more disease-specific subgroup analyses, it is important to ensure that each subgroup analysis is adequately powered to detect the proposed outcomes. In addition, care should be taken in assigning aetiology and if this is unclear, it should be recognized as such to limit uncertainty introduced by misdiagnosis. The presence of comorbidities and their potential effect on MRI biomarkers should also be considered.
Recommendation 12: Data and original images should be made available for secondary research and/or educational or commercial purposes when appropriate
Collecting patient data, especially MR images, is extremely costly. Sharing these data makes the most of public research funding, in some cases reducing the need for rescanning patients, and promotes scientific rigour by enabling independent verification of study results. For MRI methods research, the availability of large well-organized image banks has major value as a benchmark for new methods, and the use of common reference data can also be a significant driver for more standardized approaches. In order to enable efficient data sharing, studies should establish detailed a priori data management plans and conform to good data management practice, the use of standards such as DICOM to improve interoperability and dedicated software designed for managing and sharing medical images in a multicentre setting. Patient consent forms should account for broad future data sharing where appropriate.
Recommendation 13: Health economic evaluations must be factored into clinical research studies where possible
Measurement of MRI biomarker panels is expensive compared with blood and urine biomarkers. Consequently, financial concerns may prevent the uptake of MRI biomarkers, even when clinically validated. Robust health economic studies will form an important element of future research to determine whether the higher initial cost is offset by savings in health care–related costs from improved patient treatment pathways or outcomes that are enabled by the imaging biomarker(s).
Recommendation 14: Regulatory requirements and the needs of industry and small and medium-sized enterprises should be considered when designing studies aimed at the validation of renal MRI biomarkers
Obtaining regulatory approval is a lengthy process, especially in slowly progressive chronic diseases such as CKD. This was seen in the 12-year process of qualification of TKV in ADPKD. Hence it is critical to engage with this process early and ensure that data are collected according to the appropriate standards. In addition, the requirements of the pharmaceutical industry for drug development should be considered. Collaboration with MRI scanner manufacturers will be essential to support the scalability of the validated imaging biomarkers, which can ultimately be achieved only if vendors agree to distribute them on their platforms; this process should also lead to reductions in scan acquisition times for multiparametric protocols. Small and medium-sized enterprises will likely play an important role in the scalability of the biomarkers by providing software and services for centralized or local image processing and quality control. Involving them early in the setup and running of studies will ensure that imaging biomarkers are collected in a manner that is compatible with real-world conditions, shortening the route to clinical translation.
SUMMARY
Renal MRI has enormous potential to allow assessment of pathophysiological changes in the kidney that may improve diagnosis and prognosis and guide treatment in patients with kidney disease. By issuing this position paper, we hope to support and align the growth of a body of complementary technical, preclinical and clinical research and provide a structure by which multicentre studies in this field can be accelerated, with the ultimate aim of improving clinical outcomes in patients with CKD.
ACKNOWLEDGEMENTS
This article is based upon work from COST Action Magnetic Resonance Imaging Biomarkers for Chronic Kidney Disease (PARENCHIMA), funded by COST (European Cooperation in Science and Technology). www.cost.eu. For additional information please visit PARENCHIMA project website: www.renalmri.org.
FUNDING
P.B. is supported by the Deutsche Forschungsgemeinschaft (DFG; BO 3755/6-1, SFB/TRR57, SFB/TRR219) and by the German Ministry of Education and Research (BMBF Consortium STOP-FSGS number 01GM1518A). Alberto Ortiz is supported by Instituto de Salud Carlos III (PI16/2057, RETIC REDINREN RD16/0009), FEDER funds and Comunidad de Madrid B2017/BMD-3686 CIFRA2-CM. Aghogho Odudu is supported by an Academy of Medical Sciences starter grant (SGL015/1019) and National Insitute for Health Research Lectureship (CL-2014-06-003). S.S. is partly funded by the BEAt-DKD project through the IMI 2 JU under grant agreement 115974 (www.beat-dkd.eu). J.P.V. and H.C.T. are partially supported by Swiss National Foundation grants FNS 32003B_159714/1 (ME10250) and IZCOZ0_177140/1. M.W. is supported by the Austrian Science Fund (FWF) project P28867.
CONFLICT OF INTEREST STATEMENT
N.G. reports being a member of the advisory board of Supersonic Imagine and receiving personal fees from Guerbet Group. P.D.H. is employed by Antaros Medical.
REFERENCES
- 1. Levin A, Tonelli M, Bonventre J. et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet 2017; 390: 1888–1917 [DOI] [PubMed] [Google Scholar]
- 2. Goldstein SL, Jaber BL, Faubel S. et al. AKI transition of care: a potential opportunity to detect and prevent CKD. Clin J Am Soc Nephrol 2013; 8: 476–483 [DOI] [PubMed] [Google Scholar]
- 3. Kerr M, Bray B, Medcalf J. et al. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant 2012; 27 (Suppl 3): iii73–iii80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lancet Editorial Board. The global issue of kidney disease. Lancet 2013; 382: 101. [DOI] [PubMed] [Google Scholar]
- 5. Friedli I, Crowe LA, Berchtold L. et al. New magnetic resonance imaging index for renal fibrosis assessment: a comparison between diffusion-weighted imaging and T1 mapping with histological validation. Sci Rep 2016; 6: 30088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hueper K, Khalifa AA, Brasen JH. et al. Diffusion-weighted imaging and diffusion tensor imaging detect delayed graft function and correlate with allograft fibrosis in patients early after kidney transplantation. J Magn Reson Imaging 2016; 44: 112–121 [DOI] [PubMed] [Google Scholar]
- 7. Kaimori JY, Isaka Y, Hatanaka M. et al. Visualization of kidney fibrosis in diabetic nephropathy by long diffusion tensor imaging MRI with spin-echo sequence. Sci Rep 2017; 7: 5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kirpalani A, Hashim E, Leung G. et al. Magnetic resonance elastography to assess fibrosis in kidney allografts. Clin J Am Soc Nephrol 2017; 12: 1671–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leung G, Kirpalani A, Szeto SG. et al. Could MRI be used to image kidney fibrosis? A review of recent advances and remaining barriers. Clin J Am Soc Nephrol 2017; 12: 1019–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morrell GR, Zhang JL, Lee VS.. Magnetic resonance imaging of the fibrotic kidney. J Am Soc Nephrol 2017; 28: 2564–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grenier N, Merville P, Combe C.. Radiologic imaging of the renal parenchyma structure and function. Nat Rev Nephrol 2016; 12: 348–359 [DOI] [PubMed] [Google Scholar]
- 12. Caroli A, Schneider M, Friedli I. et al. Diffusion-weighted magnetic resonance imaging to assess diffuse renal pathology: a systematic review and statement paper. Nephrol Dial Transplant 2018; 33 (Suppl 2): ii29–ii40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pruijm M, Mendichovszky IA, Liss P. et al. Renal blood oxygenation level-dependent magnetic resonance imaging to measure renal tissue oxygenation: a statement paper and systematic review. Nephrol Dial Transplant 2018; 33 (Suppl 2): ii22–ii28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Odudu A, Nery F, Harteveld AA. et al. Arterial spin labelling MRI to measure renal perfusion: a systematic review and statement paper. Nephrol Dial Transplant 2018; 33 (Suppl 2): ii15–ii21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wolf M, de Boer A, Sharma K. et al. Magnetic resonance imaging T1- and T2-mapping to assess renal structure and function: a systematic review and statement paper. Nephrol Dial Transplant 2018; 33 (Suppl 2): ii41–ii50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bae KT, Commean PK, Lee J.. Volumetric measurement of renal cysts and parenchyma using MRI: phantoms and patients with polycystic kidney disease. J Comput Assist Tomogr 2000; 24: 614–619 [DOI] [PubMed] [Google Scholar]
- 17. Bae K, Park B, Sun H. et al. Segmentation of individual renal cysts from MR images in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2013; 8: 1089–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brosnahan GM. Volume progression in polycystic kidney disease. N Engl J Med 2006; 355: 733. [DOI] [PubMed] [Google Scholar]
- 19. Irazabal MV, Rangel LJ, Bergstralh EJ. et al. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol 2015; 26: 160–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dambreville S, Chapman AB, Torres VE. et al. Renal arterial blood flow measurement by breath-held MRI: accuracy in phantom scans and reproducibility in healthy subjects. Magn Reson Med 2010; 63: 940–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Niles DJ, Artz NS, Djamali A. et al. Longitudinal assessment of renal perfusion and oxygenation in transplant donor-recipient pairs using arterial spin labeling and blood oxygen level-dependent magnetic resonance imaging. Invest Radiol 2016; 51: 113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gardener AG, Francis ST.. Multislice perfusion of the kidneys using parallel imaging: image acquisition and analysis strategies. Magn Reson Med 2010; 63: 1627–1636 [DOI] [PubMed] [Google Scholar]
- 23. Ritt M, Janka R, Schneider MP. et al. Measurement of kidney perfusion by magnetic resonance imaging: comparison of MRI with arterial spin labeling to para-aminohippuric acid plasma clearance in male subjects with metabolic syndrome. Nephrol Dial Transplant 2010; 25: 1126–1133 [DOI] [PubMed] [Google Scholar]
- 24. Tan H, Koktzoglou I, Prasad PV.. Renal perfusion imaging with two-dimensional navigator gated arterial spin labeling. Magn Reson Med 2014; 71: 570–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Le Bihan D, Breton E, Lallemand D. et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988; 168: 497–505 [DOI] [PubMed] [Google Scholar]
- 26. Notohamiprodjo M, Reiser MF, Sourbron SP.. Diffusion and perfusion of the kidney. Eur J Radiol 2010; 76: 337–347 [DOI] [PubMed] [Google Scholar]
- 27. Pruijm M, Milani B, Burnier M.. Blood oxygenation level-dependent MRI to assess renal oxygenation in renal diseases: progresses and challenges. Front Physiol 2017; 7: 667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Bazelaire CM, Duhamel GD, Rofsky NM. et al. MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology 2004; 230: 652–659 [DOI] [PubMed] [Google Scholar]
- 29. Adler J, Swanson SD, Schmiedlin-Ren P. et al. Magnetization transfer helps detect intestinal fibrosis in an animal model of Crohn disease. Radiology 2011; 259: 127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Francis S, Buchanan CE, Prestwich B. et al. Sodium MRI: a new frontier in imaging in nephrology. Curr Opin Nephrol Hypertens 2017; 26: 435–441 [DOI] [PubMed] [Google Scholar]
- 31. Schroeder M, Laustsen C.. Imaging oxygen metabolism with hyperpolarized magnetic resonance: a novel approach for the examination of cardiac and renal function. Biosci Rep 2017; 37: BSR20160186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dugbartey GJ. The smell of renal protection against chronic kidney disease: Hydrogen sulfide offers a potential stinky remedy. Pharmacol Rep 2018; 70: 196–205 [DOI] [PubMed] [Google Scholar]
- 33. Hueper K, Gutberlet M, Brasen JH. et al. Multiparametric functional MRI: non-invasive imaging of inflammation and edema formation after kidney transplantation in mice. PLoS One 2016; 11: e0162705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource Silver Spring, MD: US Food and Drug Administration and Bethesda, MD: National Institutes of Health. 2016. https://www.ncbi.nlm.nih.gov/books/NBK326791/ [PubMed]
- 35. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [Google Scholar]
- 36. Titze S, Schmid M, Kottgen A. et al. Disease burden and risk profile in referred patients with moderate chronic kidney disease: composition of the German Chronic Kidney Disease (GCKD) cohort. Nephrol Dial Transplant 2015; 30: 441–451 [DOI] [PubMed] [Google Scholar]
- 37. Sanchez-Nino MD, Sanz AB, Ramos AM. et al. Clinical proteomics in kidney disease as an exponential technology: heading towards the disruptive phase. Clin Kidney J 2017; 10: 188–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Venkatachalam MA, Griffin KA, Lan R. et al. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 2010; 298: F1078–F1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Taal MW. Adaptation to nephron loss and mechanisms of progression in chronic kidney disease In: Skorecki K, Chertow GM, Marsden PA. et al. , eds. Brenner & Rector's The Kidney, 10th edn. Philadelphia: Elsevier, 2016 [Google Scholar]
- 40. Cox EF, Buchanan CE, Bradley CR. et al. Multiparametric renal magnetic resonance imaging: validation, interventions, and alterations in chronic kidney disease. Front Physiol 2017; 8: 696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Levey AS, Inker LA, Matsushita K. et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 2014; 64: 821–835 [DOI] [PubMed] [Google Scholar]
- 42. Lambers Heerspink HJ, Weldegiorgis M, Inker LA. et al. Estimated GFR decline as a surrogate end point for kidney failure: a post hoc analysis from the Reduction of End Points in Non-Insulin-Dependent Diabetes with the Angiotensin II Antagonist Losartan (RENAAL) study and Irbesartan Diabetic Nephropathy Trial (IDNT). Am J Kidney Dis 2014; 63: 244–250 [DOI] [PubMed] [Google Scholar]
- 43. Coresh J, Turin TC, Matsushita K. et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014; 311: 2518–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. US Food and Drug Administration. Qualification of Biomarker—Total Kidney Volume in Studies for Treatment of Autosomal Dominant Polycystic Kidney Disease. 2015. https://www.fda.gov/downloads/Drugs/Guidances/UCM458483.pdf
- 45. Gansevoort RT, Arici M, Benzing T. et al. Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: a position statement on behalf of the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice. Nephrol Dial Transplant 2016; 31: 337–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hallan SI, Dahl K, Oien CM. et al. Screening strategies for chronic kidney disease in the general population: follow-up of cross sectional health survey. BMJ 2006; 333: 1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gisen G. Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 1997; 349: 1857–1863 [PubMed] [Google Scholar]
- 48. Tangri N, Stevens LA, Griffith J. et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA 2011; 305: 1553–1559 [DOI] [PubMed] [Google Scholar]
- 49. Peeters MJ, van Zuilen AD, van den Brand JA. et al. Validation of the kidney failure risk equation in European CKD patients. Nephrol Dial Transplant 2013; 28: 1773–1779 [DOI] [PubMed] [Google Scholar]
- 50. Tangri N, Grams ME, Levey AS. et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA 2016; 315: 164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pruijm M, Milani B, Pivin E. et al. Reduced cortical oxygenation predicts a progressive decline of renal function in patients with chronic kidney disease. Kidney Int 2018; 93: 932–940 [DOI] [PubMed] [Google Scholar]
- 52. Mora-Gutierrez JM, Garcia-Fernandez N, Slon Roblero MF. et al. Arterial spin labeling MRI is able to detect early hemodynamic changes in diabetic nephropathy. J Magn Reson Imaging 2017; 46: 1810–1817 [DOI] [PubMed] [Google Scholar]
- 53. Chrysochou C, Green D, Ritchie J. et al. Kidney volume to GFR ratio predicts functional improvement after revascularization in atheromatous renal artery stenosis. PLoS One 2017; 12: e0177178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brenner BM, Cooper ME, de Zeeuw D. et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869 [DOI] [PubMed] [Google Scholar]
- 55. Lambers Heerspink HJ, Gansevoort RT.. Albuminuria is an appropriate therapeutic target in patients with CKD: the pro view. Clin J Am Soc Nephrol 2015; 10: 1079–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hou FF, Xie D, Zhang X. et al. Renoprotection of Optimal Antiproteinuric Doses (ROAD) study: a randomized controlled study of benazepril and losartan in chronic renal insufficiency. J Am Soc Nephrol 2007; 18: 1889–1898 [DOI] [PubMed] [Google Scholar]
- 57. Bolignano D, Zoccali C.. Non-proteinuric rather than proteinuric renal diseases are the leading cause of end-stage kidney disease. Nephrol Dial Transplant 2017; 32: ii194–ii199 [DOI] [PubMed] [Google Scholar]
- 58. Ruggenenti P, Perticucci E, Cravedi P. et al. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19: 1213–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. O'Connor JPB, Aboagye EO, Adams JE. et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol 2017; 14: 169–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ghezzi P, Davies K, Delaney A. et al. Theory of signs and statistical approach to big data in assessing the relevance of clinical biomarkers of inflammation and oxidative stress. Proc Natl Acad Sci USA 2018; 115: 2473–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. US Food and Drug Administration. Clinical Trial Imaging Endpoint Process Standards Guidance for Industry: U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER). 2015. https://www.fda.gov/downloads/drugs/guidances/ucm268555.pdf
- 62. Sullivan DC, Obuchowski NA, Kessler LG. et al. Metrology standards for quantitative imaging biomarkers. Radiology 2015; 277: 813–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Huang EP, Wang XF, Choudhury KR. et al. Meta-analysis of the technical performance of an imaging procedure: guidelines and statistical methodology. Stat Methods Med Res 2015; 24: 141–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Barisoni L, Hodgin JB.. Digital pathology in nephrology clinical trials, research, and pathology practice. Curr Opin Nephrol Hypertens 2017; 26: 450–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Delanaye P, Melsom T, Ebert N. et al. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 2: Why to measure glomerular filtration rate with iohexol? Clin Kidney J 2016; 9: 700–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Inker LA, Schmid CH, Tighiouart H. et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367: 20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gansevoort RT, Matsushita K, van der Velde M. et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011; 80: 93–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Koyner JL, Parikh CR.. Clinical utility of biomarkers of AKI in cardiac surgery and critical illness. Clin J Am Soc Nephrol 2013; 8: 1034–1042 [DOI] [PubMed] [Google Scholar]
- 69. Greenberg JH, Kakajiwala A, Parikh CR. et al. Emerging biomarkers of chronic kidney disease in children. Pediatr Nephrol 2018; 33: 925–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pontillo C, Mischak H.. Urinary peptide-based classifier CKD273: towards clinical application in chronic kidney disease. Clin Kidney J 2017; 10: 192–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Grantham JJ, Torres VE.. The importance of total kidney volume in evaluating progression of polycystic kidney disease. Nat Rev Nephrol 2016; 12: 667–677 [DOI] [PMC free article] [PubMed] [Google Scholar]