Abstract
Spinach production in soilless cultivation systems, mainly in substrate, transplanted with soil blocks and drip-irrigation is increasing worldwide. However, spinach establishment with soil blocks, with several seedlings per block compared with traditional planting methods, may affect light interception by plant canopy, wetting and salt patterns in root medium and therefore the plant growth and functional value of spinach. The effects of soil block number (plant density) and emitters spacing on plant growth, nitrate, proline and total phenols content and antioxidant activity were evaluated in spinach (Spinacia oleracea L). Five seedlings per soil block were transplanted to Styrofoam boxes filled with the substrate and grown during winter in an unheated greenhouse. Four treatments were carried out with two soil block numbers [8 (160 plants/m2) and 14 (280 plants/m2) soil blocks per Styrofoam box] and two emitter spacing [emitters spaced every 25 and 12.5 cm, respectively, with 4 and 8 emitters per Styrofoam box]. Neither plant density nor emitter spacing had any effect on shoot dry weight. Fresh yield increased as planting density and the number of emitters per Styrofoam box increased. The yield in Styrofoam boxes with 160 plants/m2 and 8 emitters (3.85 kg m−2) was ≈31 % lower than that obtained in treatment with 280 plants/m2 and 8 emitters (5.09 kg m−2). However, the leaf blade of the latter treatment presented a higher content of phenols and proline and greater antioxidant activity (FRAP and DPPH) as well as lower content of nitrate and lesser PDH activity. Decrease of space between emitters reduced the leaf blade nitrate concentration of spinach grown in a greenhouse during the winter. Leaf blade antioxidant activity (FRAP) decreased as planting density increased.
Keywords: Plant biology, Agriculture, Environmental science
1. Introduction
Spinach plays an important role in the Portuguese diet since is consumed in great quantities all year around. Moreover, it is low in calories and fat but is rich in minerals, fiber, vitamins, and phytochemicals (Alvino and Barbieri, 2016) and has high antioxidant activity (Ou et al., 2002; Ismail et al., 2004). Bioactive phytochemicals, ubiquitously distributed in nature, may constitute pigments that give a colorful appearance to foods, or secondary metabolism products that protect plant species from environmental attacks (Ismail et al., 2010) and increase their functional and nutritional value (Sucupira et al., 2012). However, phytochemical accumulation and antioxidant activity, are influenced by cultivation techniques (Ou et al., 2002), which in spinach production are changing. Spinach production in soilless cultivation systems, mainly in substrate, using soil blocks in plant establishment and drip-irrigation is increasing. The use of soil blocks, with several seedlings per block compared with traditional planting methods, greatly changes plant spacing which may affect photosynthetic photon flux density (PPFD) interception by plant canopy. Light is one of the primary factors that affect plant growth, yield, and the accumulation of phytochemicals such as phenols (Ghasemzadeh et al., 2010; Karimi et al., 2013; Bian et al., 2014; Chua et al., 2015; Kałużewicz et al., 2017; Jaganath and Zainal, 2016; Ilic et al., 2018). The concentration of NO3 in plant tissues increases due to low-level light conditions (Colla et al., 2018; Marschner, 2012; Santamaria et al., 1999; Santamaria, 2006). High levels of light exposure could induce the synthesis of phenolic compounds and, consequently, antioxidant activity in a number of vegetables (Oh et al., 2009; Samuoliené et al., 2012; Pérez-Lopez et al., 2018). However, the use of soil blocks affects correct emitter spacing, since it may also influence substrate covering, wetting and salt patterns in the root medium and water and nutrient uptake by plants (Valdés et al., 2015) which may lead to abiotic stress such as water deficit and/or salinity. Abiotic stress may influence yield, phytochemical accumulation, and antioxidant activity. For example, salt stress was found to increase polyphenols content in spinach (Shimomachi et al., 2008). However, the response in peas (Miljus-Djukic et al., 2013) and beans (Telesiñski et al., 2008) was influenced by the level of osmotic stress. In beans, moderate salinity stress decreased phenols content (Telesiñski et al., 2008). Proline (Pro) accumulation is also related to abiotic stress (Wang et al., 2009, 2011; Per et al., 2017) such as salinity and water deficit (Hayat et al., 2012; Reddy et al., 2015; Ashraf et al., 2018). In tomato grown hydroponically, proline was found to be a reliable indicator of the environmental stress imposed on plants (Claussen, 2005). Pro degradation is suppressed during saline or drought stress (Verbruggen and Hermans, 2008) by a pathway which involves Pro dehydrogenase (PDH, EC 1.5.5.2) a key enzyme of Pro catabolism (Per et al., 2017).
Despite increasing in the use soil blocks, for plant establishment in substrate drip-irrigated, there is currently very little information available concerning the influence of soil block number and emitter spacing on plant growth, and the functional value of spinach grown in substrate. Therefore, the aim of this study was to evaluate the effect of soil block number and emitter spacing on plant growth, nitrate, total phenols and proline concentration, FRAP and DPPH antioxidant activity and PDH enzyme activity in spinach grown in a substrate in an unheated greenhouse during the winter.
2. Materials and methods
2.1. Growth conditions and substrates
The experiment was conducted between december 2015 and february 2016 in a greenhouse located at the “Herdade Experimental da Mitra” (38°31′52″N; 8°01′05″W), University of Évora, Portugal. The greenhouse was covered with polycarbonate and had no supplementary lighting. Air temperatures inside the greenhouse ranged from 6 to 27 °C, and solar radiation ranged from 21.9 to 151.1 W m−2·d−1. The experiment comprised four treatments: two plant densities (PD), low plant density (LPD) (8 soil blocks per Styrofoam box - 160 plants/m2) and high plant density (HPD) (14 soil blocks per Styrofoam box – 280 plants/m2) and two emitter spacing (NE) [emitter spaced every 25.0 (NE4) and 12.5 cm (NE8), respectively 4 and 8 emitters per Styrofoam box].
Substrate [composted forest residues, husks, and white peat (2:1:1 v/v)], physicochemical properties were the following: organic matter = 63.0 % (w/w), pH 5.5–6.5, total nitrogen = 200–400 mg L−1, potassium = 150–300 mg L−1, phosphorus = 100–200 mg L−1, EC = 1–3 mS cm−1, C/N = <20, bulk density = 0.27 g cm−3, mass wetness = 2.4 g water per g substrate, moisture content = 71 %. Soil-blocked spinach (Spinacia oleracea L. cv. Tapir) seedlings (five seedlings per block) were transplanted (on 22 december) at 18 days after emergence in Styrofoam planting boxes (100-cm long × 25 cm wide × 10-cm high) filled with 14 L of substrate. Soil blocks with five spinach seedlings were spaced at 25-cm intervals in 160 plants/m2 treatment (8 soil blocks per Styrofoam box) and 14.5-cm intervals in 280 plants/m2 treatment (14 soil blocks per Styrofoam box), planted in two rows per box, 10 cm apart. Treatments were arranged in a randomized complete block design with five replicate boxes per treatment.
Each planting box was irrigated using 4 L h−1 pressure-compensating and anti-drain emitters, attached to 4 fine tubes (70-cm in length and with a diameter of 5 mm), inserted into substrate through a straight plastic arrow, 25.0 cm and 12.5 cm apart, along the center the line of the Styrofoam box, in the treatments with 4 and 8 emitters per Styrofoam box, respectively. Irrigation was scheduled three to seven times per day using a timer and averaged 20–30% drainage (leaching fraction) at Styrofoam box control (NE4 HPD) for each application. The duration of each irrigation event in the treatments with 8 emitters was half that of the treatments with 4 emitters.
Nutrient solution was applied daily by fertigation, from two days after transplanting to the day before harvesting. The solution was made from fresh tap water [electrical conductivity (EC) of 0.4–0.48 dS m−1; pH 7–7.4; 0.10–0.30 mmol L−1 NO3, 1 mmol L−1 Ca, 1 mmol L−1 Mg, 2.1 mmol L−1 Cl−, 0.7 mmol L−1 Na, 0.53 μ mol·L−1 Fe and 0.16 μmol L−1 Mn] and initially contained 9.84 mmol L−1 NO3, 3.14 mmol L−1 NH4, 0.76 mmol L−1 P, 4.24 mmol L−1 K, 3.19 mmol L−1 Ca, 1.08 mmol L−1 Mg, 1.25 mmol L−1 S, 46 μmol L−1 B; 3 μmol L−1 Cu, 16 μmol L−1 Fe, 19 μmol L−1 Mn, 1 μmol L−1 Mo, and 2 μmol L−1 Zn and 2.1 Cl and 0.7 mmol L−1 Na. The concentration was adjusted (N-nitrate concentration in the nutrient solution was reduced in order to avoid high nitrate accumulation in spinach tissues) at 29 days after transplanting (DAT) to 4.24 mmol L−1 NO3, 4.10 mmol L−1 NH4, 0.62 mmol L−1 P, 3.00 mmol L−1 K, 2.12 mmol L−1 Ca, 0.88 mmol L−1 Mg, 0.87 mmol L−1 S, 46 μmol L−1 B, 7.86 μmol L−1 Cu, 8.95 μmol L−1 Fe, 18.3 μmol L−1 Mn, 1 μmol L−1 Mo, 1 μmol L−1 Zn, 2.1 mmol L−1 Cl and 0.7 mmol L−1 Na. The final pH of both solutions ranged from 5.9 to 6.1.
2.2. Measurements
The EC and the concentration of NO3 in the drainage water from each box was measured twice a week using a conductivity meter (LF 330 WTW, Weilhein, Germany), and an ion-specific electrode and meter (Crison Instruments, Barcelona, Spain), respectively, following the procedures outlined by Prazeres (2005).
For yield evaluation, the plants of 4 soil blocks per box were harvested at 44 DAT. The plants (shoots) were cut off at 1 cm above the leaf base. Two representative plants (shoots) were also harvested at 30, 36 and 44 DAT from each box, were washed, oven-dried at 70 °C for 2–3 days, and weighed.
Additional leaf samples were stored at −80 °C for NO3 determination (Lastra, 2003). The samples were oven-dried at 65 °C for 48 h, weighed (0.1000 g), macerated in a mortar, homogenized in a test tube with 10 mL of distilled water, agitated in a vortex, and incubated for 1 h at 45 °C in a shaking water bath. Filtrated extract was then mixed with salicylic acid in 5% sulfuric acid (1:4), incubated for 20 min at room temperature, and mixed with 9.5 mL of 2 M sodium hydroxide. The concentration of NO3 in the solution was then determined reading the absorbance at 338 and 440 nm, using a calibration curve (NO3, n = 6 concentrations between 0 and 500 mg/L).
For the determination of total phenols, free proline content and antioxidant activity (FRAP, DPPH) 1,000 g of leaf blade and petiole sample was homogenized in 8 mL of methanol:water solution (80:20,v/v) for 1 min and then centrifuged at 5 °C at 6,440 g for 5 min.
Total phenols content was determined using Folin–Ciocalteau's phenol reagent as described earlier (Bouayed et al., 2011), by spectrophotometry UV/Vis, reading the absorbance at 760 nm. The total phenols content expressed as milligrams of gallic acid equivalents per 100 g of fresh weight (FW) was calculated using a calibration curve (gallic acid, n = 6 concentrations from 0 to 50 mg/L).
Free proline levels were determined using the acid ninhydrin reaction in accordance with the method of Bates et al. (1973), reading the absorbance of formed formazone at 546 nm. Proline concentration was calculated using a calibration curve (L-proline, n = 6 concentrations from 0 to 20 mg/L).
Ferric-reducing antioxidant activity (FRAP) was determined in accordance with the method of Bouayed et al. (2011). Briefly, the FRAP reagent was prepared freshly by mixing 300 mM acetate buffer pH 3.6, 10 mM TPTZ solution in 40 mM HCl and 20 mM iron(III) chloride solution (10:1:1, v/v/v) and warmed to 37 °C before use. Then 0.050 mL of the sample (suitably diluted extracts or standard) was mixed with 0.950 mL of FRAP reagent. Absorbance was determined at 593 nm after the incubation of the mixture during 4 min. Antioxidant activity reported as milligrams of Trolox equivalents per 100 g of FW was calculated using a calibration curve (Trolox solution, n = 8 concentrations from 0 to 1120 mg/L).
Scavenging antioxidant activity (DPPH) was determined by measuring the ability of plant extracts to capture the stable organic radical DPPH• (2, 2-diphenyl-1-picryl-hydrazyl). Aliquots of an extemporaneous metanolic solution of 0.03 g/L DPPH•, kept in the dark, were added to a known volume of sample or standard solution. The reduction of DPPH• to DPPH-H (diphenyl-picryl hydrazine) was followed by reading the absorbance at 515 nm, at 25 °C, for 180 s. Antioxidant activity reported as milligrams of gallic acid equivalents per 100 g of FW was calculated using a calibration curve (gallic acid solution, n = 8 concentrations from 0 to 200 mg/L) (Brand-Williams et al., 1995).
Samples of 0.25 g of spinach leaf blade or petiole were macerated in the presence of liquid N2 and homogenized in 50 mM phosphate buffer pH 7.0 for the extract preparation used in the determination of proline dehydrogenase (PDH) enzyme activity. The supernatant obtained by means of the centrifugation of this extract for 15 min at 15,000 g at 4 °C, was collected and stored in aliquots at −20 °C for further use in determining protein content and PDH activity.
PDH activity was measured as described by Costilow and Cooper (1978). Enzyme activity was assayed following the reduction of NAD+ at 340 nm at 30 °C during 180 s in a double-beam Hitachi-U2001 spectrophotometer with the control of temperature. The 2 cm3 assay mixture contained 100 mM Na2CO3-NaHCO3 buffer pH 10.3, 10 mM NAD+, 2 mM L-proline and the enzyme extract, in which proline was used to initiate the reaction. The control cuvette contained all the solutions except NAD+. Enzyme activity was calculated on the basis of the reaction curve slope, using the extinction coefficient of ε = 6.22 mM−1 cm−1. PDH activity was expressed in nmol min−1/mg protein.
The protein content of the extract was determined in accordance with the Lowry method (Lowry et al., 1951), using a calibration curve (bovine serum albumin, BSA; n = 6 concentrations from 0 to 200 μg/mL).
A Thermo Scientific, Genesys 10S spectrophotometer (Waltham, Massachusetts, USA) was used in all content determination for spectrophotometric analysis.
2.3. Data analysis
Data were processed by means of the analysis of variance (Sokal and Rohlf, 1997), using SPSS Statistics 24 software (Chicago, Illinois, USA), licensed to University of Évora. Means were separated at the 5% level using Duncan's new multiple range test. Pearson correlation coefficients (r) are from SPSS.
3. Results and discussion
3.1. Drainage water
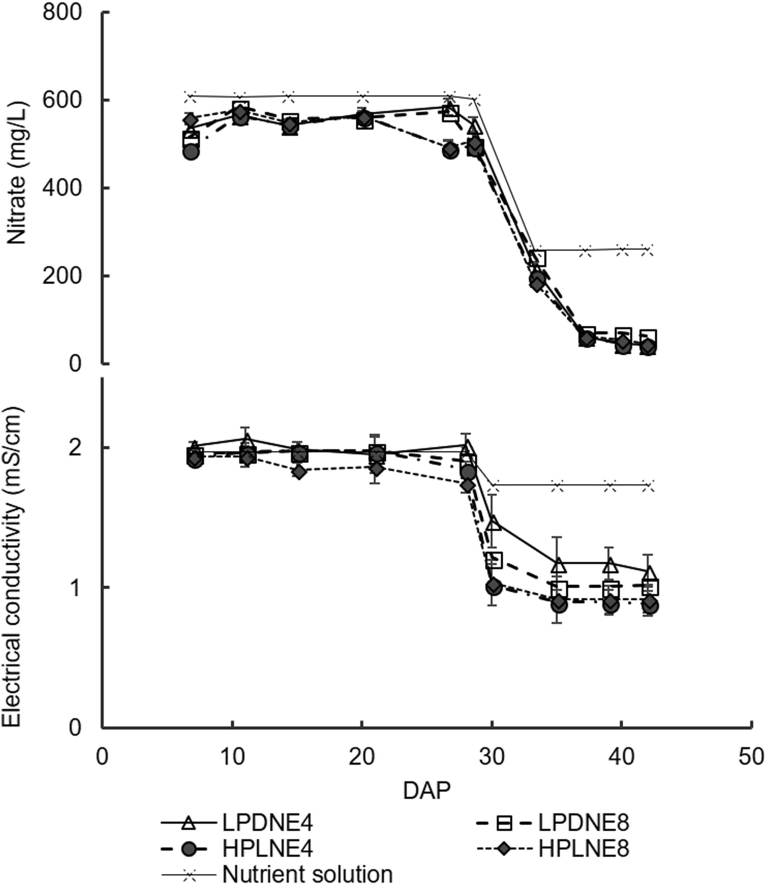
The concentration of NO3 and EC in drainage water was not significantly affected by interaction between plant density and emitter spacing. The concentration of NO3 in the drainage water collected after 30 DAT was greater in LPD than in HPD (Fig. 1). The concentration of NO3 in drainage water on the first six dates of measurement (Fig. 1) was slightly lower than the concentration applied in the nutrient solution (610 mg NO3 L−1) ranging from 488 to 587 mg L−1 (Fig. 1). The EC in drainage water after 30 DAT was lower in HPD than in LPD (Fig. 1). The NO3 concentration and the EC in the drainage water on the last four dates, as compared with the NO3 and the EC in the nutrient solution, decreased significantly (Fig. 1), due to the reduction of nitrate applied in the nutrient solution (262 mg NO3 L−1) and due to high nutrient uptake by spinach plants, as ≈85 % of total shoot dry weight was accumulated during this period (Fig. 3).
Fig. 1.
Effects of plant density (LPD – 160 plants/m2; HPD – 280 plants/m2) and emitter spacing (NE4 – emitters 25.0 cm apart; NE8 – emitters 12.5 cm apart) on the concentration of nitrate (NO3) and electrical conductivity (EC) of the drainage water. Each symbol represents the mean of five replicates, and the error bars represent ±1 SE.
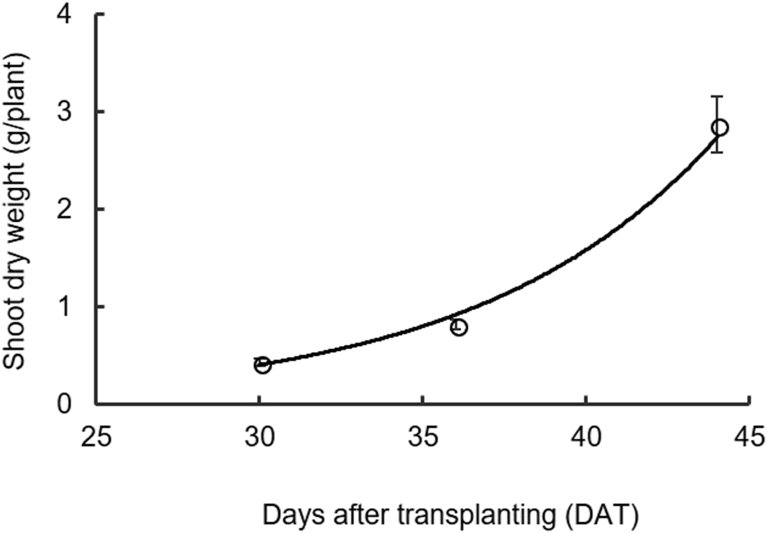
Fig. 3.
Relationship between shoot dry weight (g/plant) and days after transplanting (DAT). [Shoot dry weight (g/plant) = 0.0063e0.1365(DAT), R2 = 0.99, p < 0.001]. Each symbol represents the mean of five replicates averaged over four treatments, and the error bars represent ±1 SE.
3.2. Plant growth and yield
Shoot dry weight per plant was affected neither by planting density nor emitter spacing (Fig. 2) and it increased exponentially during the crop cycle (≈85% of total shoot dry weight was accumulated from 30 to 44 DAT) (Fig. 3). Plant growth in the first 30 DAT was minimal, thus, the influence of plant density on light interception in the first 30 days may have been low.
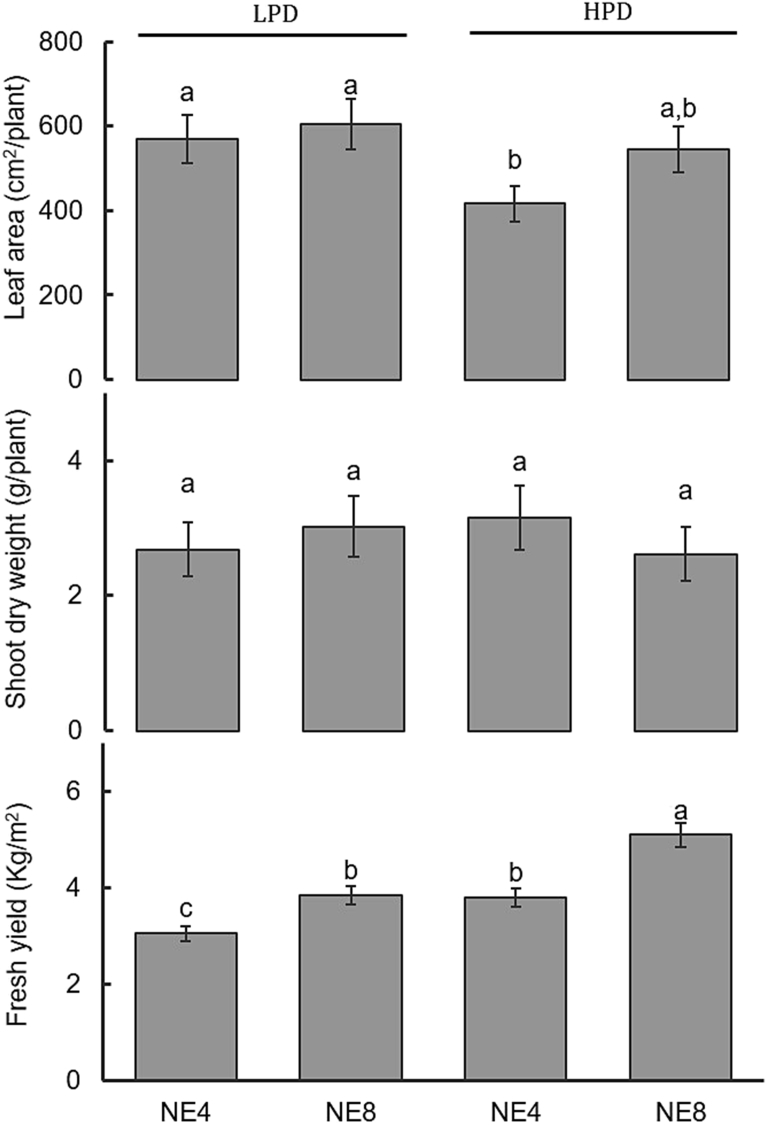
Fig. 2.
Effects of plant density (LPD – 160 plants/m2; HPD – 280 plants/m2) and emitter spacing (NE4 – emitters 25.0 cm apart; NE8 – emitters 12.5 cm apart) on leaf area, shoot dry weight and fresh yield of spinach. Means with different letters are significantly different at p < 0.05. Each bar represents the mean of five replicates, and the error bars represent ±1 SE.
Leaf area per plant decreased as plant density increased (Fig. 2). However, in high planting density treatment shading may be increased, since close planting usually causes mutual shading among individuals.
Yield (fresh weight) increased significantly as planting density and as the number of the emitters per Styrofoam box increased (Fig. 2). As shoot biomass was not affected by treatments, fresh yield increase due to an increase in emitter numbers, was probably due to an increase in leaf water content. This indicates that an increase in emitter numbers increased the rate of water uptake from the substrate. This may have a dilution effect on phytochemical content, which is highest in HPDNE8.
The yield in Styrofoam boxes with 280 plants/m2 and 8 emitters reached a maximum of 5.09 kg m−2 (≈31 % higher than in LPDNE8 and HPDNE4) (Fig. 2), and it was also higher than obtained when spinach was grown both in a floating system (Conesa et al., 2009) and in a substrate (Barcelos et al., 2016).
3.3. Phytochemical accumulation
3.3.1. Nitrate
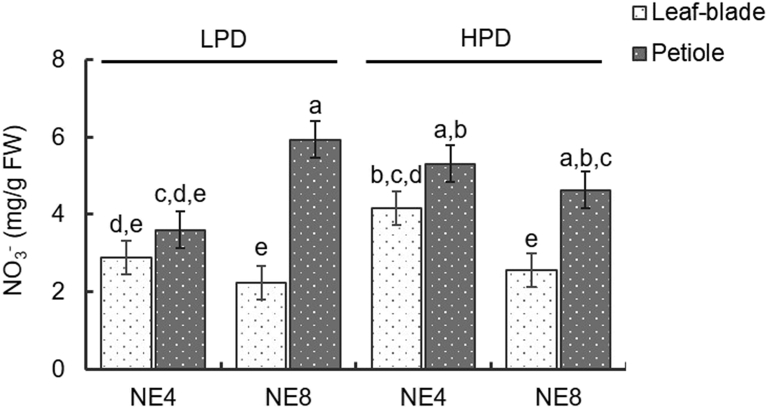
Leaf blade NO3 concentration was not significantly affected either by the interaction between treatments or by soil block number (Fig. 4). Therefore, an increase of leaf blade nitrate concentration due to higher leaf shading in treatments with 280 plants/m2 was not observed. Leaf blade nitrate concentration in baby spinach grown in a hydroponic system increased as plant density increased (Machuca et al., 2015), but planting densities (862, 1296 and 1728 plants/m2) were much higher than those in present study.
Fig. 4.
Effects of plant density (LPD – 160 plants/m2; HPD – 280 plants/m2) and emitter spacing (NE4 – emitters 25.0 cm apart; NE8 – emitters 12.5 cm apart) on leaf blade and petiole nitrate concentration. Means with different letters are significantly different at p < 0.05. Each bar represents the mean of five replicates, and the error bars represent ±1 SE.
Leaf blade NO3 concentration in HPD treatment decreased in plants irrigated with 8 emitters (Fig. 4), which could be related to higher leaf water content due to high water availability in the root medium. In leafy vegetables, Qiu et al. (2014) observed that nitrate accumulation decreased with increasing soil water content.
In plants irrigated with 8 emitters leaf blade NO3 concentration values ranged from 2.23 to 2.56 mg g−1 fresh weight (FW). These values were lower than those allowed by European Commission Regulation no. 1258/2011 for fresh spinach (3.5 mg g−1 fresh weight). Therefore, the use of spacing emitters could provide a way to reduce nitrate concentration in the fresh weight of leaf blade of spinach grown in a greenhouse during the winter.
Petiole NO3 concentration was affected by the interaction between the factors (p < 0.001) and it was greater in the treatment with 160 plants/m2 and 8 emitters per Styrofoam box (5.9 mg·NO3 g−1 FW). Petiole NO3 concentrations were higher than those in the leaf blade, as reported by others authors (Marschner, 2012), and higher than the value allowed by the European Commission (3.5 mg g−1 FW) (Fig. 4).
3.3.2. Total phenols content
The interaction between plant density and the number of emitters per Styrofoam box on leaf blade total phenols content (TPC) was significant (p < 0.001), which indicates that the response of leaf blade TPC to plant density differed as a function of the number of emitters per Styrofoam box (Fig. 5).
Fig. 5.
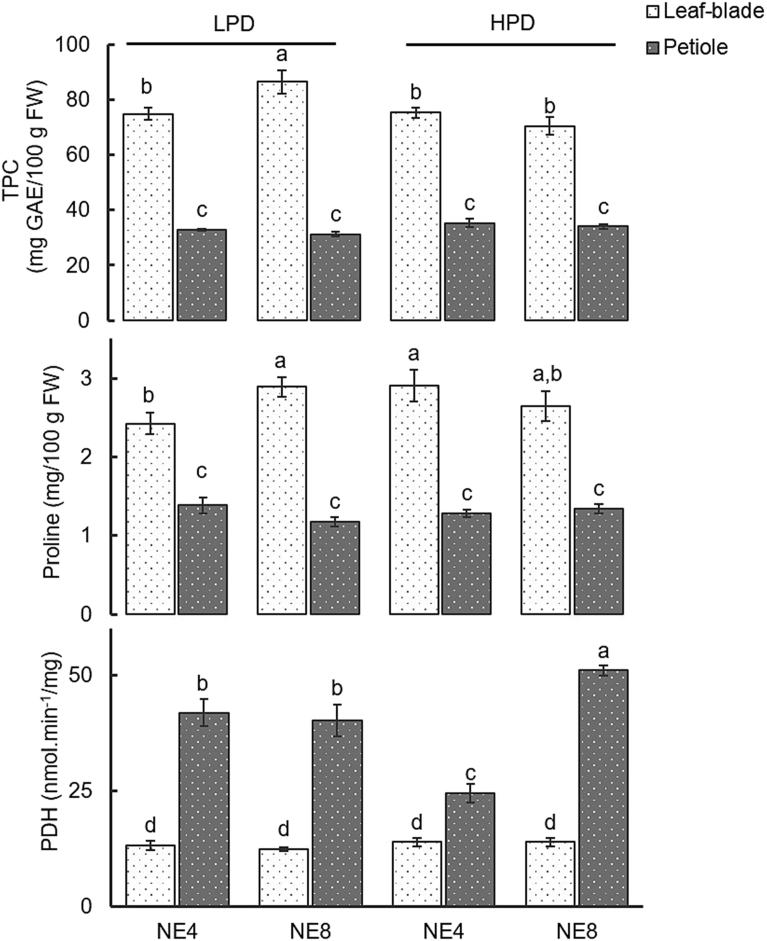
Effects of plant density (LPD – 160 plants/m2; HPD – 280 plants/m2) and emitter spacing (NE4 – emitters 25.0 cm apart; NE8 – emitters 12.5 cm apart) on total phenols content, proline content and PDH enzyme activity in the leaf blade and petiole. Means with different letters are significantly different at p < 0.05, FW-fresh weight. Each bar represents the mean of five replicates, and the error bars represent ±1 SE.
Leaf blade TPC was highest in the LPDNE8 treatment, reaching a maximum of 86.5 mg GAE/100 g FW. Leaf blade TPC in the HPD treatment was not affected by spacing emitters, although fresh yield in NE8 was 30 % higher than in NE4 (Fig. 5), therefore the high leaf water content of the leaves of the plants grown in HPDN8 did not lead to a decrease in TPC concentration in leaves. As leaf shading may have increased when plant density increased (Fig. 2), a light-dependent induction of phenylpropanoid biosynthesis may have occurred in spinach of LPDNE8 treatment, accounting in part, for the highest value of TPC (Pérez-Lopez et al., 2013, 2015, 2018; Crozier et al., 2007; Leyva et al., 1995).
Leaf blade TPCs ranged from an average 79–86.5 mg GAE/100 g FW. These values were similar to those measured in spinach by Proteggente et al. (2002) (72.1 mg GAE/100 g FW) and Chu et al. (2002) (90 mg GAE/100 g FW) and lower than the levels reported by Machado et al. (unpublished results) (106–130 mg GAE/100 g FW), Bayili et al. (2011) (182.5 mg GAE/100 g FW) and Mazzucotelli et al. (2017) (104.7 mg GAE/100 g FW). The variation in TPC values may be due to many factors, such as spinach variety, maturity at harvest and growth conditions (Howard et al., 2002). In general, total phenols content increases as abiotic stress (light conditions, salinity, etc.) increases (D'Souza and Devaraj, 2010; Karimi et al., 2013; Miljus-Djukic et al., 2013; Bian et al., 2014; Klados and Tzortzakis, 2014; Alam et al., 2015; Kyriacou and Rouphael, 2018) though in beans, moderate salinity stress led to a decrease in phenols content (Telesiñski et al., 2008).
Petiole TPCs were not influenced by treatments, while they were ≈2.3 times lower than in leaf blade (p < 0.05) (Fig. 5). Different content of total phenols in different plant parts was also determined in spinach by Machado et al., (unpublished results), and by Kollia et al. (2017) in artichoke.
3.3.3. Proline
Leaf blade proline content was lower in plants in the LPD treatment irrigated with four emitters (2.42 mg/100 g FW) (Fig. 5). Leaf blade proline content ranged from an average 2.42–2.91 mg/100 g FW. These values are lower than those obtained by Yoon et al. (2017) (148.8 mg/100 g DW) and Aslan et al. (2016) (5.4–7.2 mg/100 g FW) in spinach grown in the greenhouse and in the open air, respectively. The lower values of Pro found in the present study may indicate that spinach plants were not subject to high levels of abiotic stress, since its accumulation is closely related to salinity and water deficit (Claussen, 2005; Pérez-López et al., 2018).
Petiole proline content was not affected by the treatments (p < 0.05) (Fig. 5) and ranged from an average 1.26–1.34 mg/100 g FW. These values were lower than those found in the leaf blade.
3.3.4. PDH enzyme activity
Leaf blade PDH enzyme activity was not significantly affected by treatments and ranged from 12.36 to 13.94 nmol min−1/mg prot, these values being higher than those reported in maize by Li et al. (2013) (14.5 nmol min−1/g FW), but lower than those described by Tian et al. (2016) in millet cultivar seeds (35 U min−1/mg prot).
Petiole PDH enzyme activity was affected by treatments (p < 0.01). The highest value for this enzyme activity was found in plants in the HPD treatment irrigated with 8 emitters. However, proline petiole values were not affected (Fig. 5).
PDH activity found in petioles was higher than that found in the leaf blade, which may explain the low levels of proline found in the petiole (Fig. 5), because, as previously mentioned, PDH is one of the enzymes involved in Pro catabolism (Kiyosue et al., 1996; Per et al., 2017). Leaf blade and petiole PDH enzyme activity did not correlate with the respective proline levels. However, in mulberry, proline dehydrogenase activity was found to decreases progressively as proline increased owing to water stress (Chaitanya et al., 2009).
3.3.5. Antioxidant activities
Leaf blade FRAP decreased as plant density increased (Fig. 6) (p < 0.01). This could be related to an increase in leaf shading caused by plant number in the HPD treatment. Light conditions, as previously mentioned, influence phytochemical accumulation, and antioxidant activity (Bian et al., 2014; Karimi et al., 2013; Kyriacou and Rouphael, 2018). Leaf blade FRAP antioxidant activity was not affected by spacing emitters. Thus the high leaf water content of the leaves irrigated with 8 emitters did not lead to a decrease in FRAP.
Fig. 6.
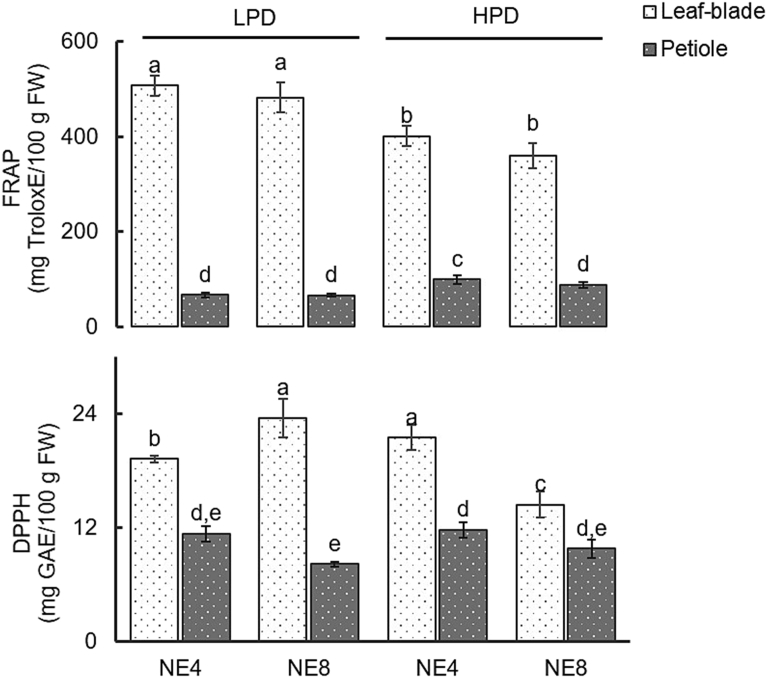
Effects of plant density (LPD – 160 plants/m2; HPD – 280 plants/m2) and emitter spacing (NE4 – emitters 25.0 cm apart; NE8 – emitters 12.5 cm apart) on FRAP and DPPH in the leaf blade and petiole. Means with different letters are significantly different at p < 0.05, FW- fresh weight. Each bar represents the mean of five replicates, and the error bars represent ±1 SE.
Leaf blade FRAP values ranged from an average of 464.5–507 Trolox mg/100 g FW and from 374 to 401 Trolox mg/100 g FW in the LPD and HPD treatments, respectively. These values were higher than those reported for spinach by Apak et al. (2007) (267 Trolox mg/100 g FW) but much lower than the values observed by Sreeramulu et al. (2013) (1380.6 Trolox mg/100 g FW) and Machado et al. (unpublished results) (700–800 Trolox mg/100 g FW). The variations between the findings of different studies may be related to methodological differences such as extraction technique, and the factors that affected antioxidant activity in vegetables such as genotype, location, harvest season, cultural techniques (Ou et al., 2002) and abiotic stress (Per et al., 2017).
Petiole FRAP values in the treatments ranged from an average 65.78 to 99.08 Trolox mg/100 g FW. These values were similar to those observed in spinach grown in cocopeat (Machado et al., unpublished results). The FRAP of the leaf blade was 4.5–7.0 times higher than in petioles. This behavior was also reported by Yosefi et al. (2010) in spinach and by Petropoulos et al. (2017) in Greek artichoke.
There was no overall effect of either number emitters or plant density on leaf blade ROS scavenging antioxidant activity (DPPH) because the interaction between the factors was significant (p < 0.001). Leaf blade DPPH in the LPD treatment with 8 emitters was 1.6 times greater than in the HPD treatment with 8 emitters (Fig. 6).
Petiole DPPH decreased as the number of emitters increased (Fig. 6). Petioles DPPH ranged from an average 8.1–11.8 mg/100 FW, and these values were 1.47–2.88 times lower than in leaf blade.
Leaf blade total phenols content was positively and linearly correlated with leaf blade DPPH antioxidant activity (r = 0.862, p < 0.01), but not with FRAP. The same correlation was also observed in some other crops while it was not observed in others (Stratil et al., 2006; Salandanan et al., 2009). These differing results can be explained by the fact that antioxidant activity depends not only on total phenols but also on other antioxidants (Salandanan et al., 2009), which are influenced by a large range of factors (Alvino and Barbieri, 2016).
Leaf blade DPPH and FRAP values were not related linearly with fresh yield. The lowest value of the DPPH was observed in the treatment with highest fresh yield (Figs. 2 and 6). Leaf blade FRAP values in the HPD treatment were similar, despite the fresh yield in the plants irrigated with 8 emitters having increased by thirty per cent.
4. Conclusions
Plant density and emitter number per Styrofoam had no effect on shoot dry weight but led to an increased in fresh yield. The decrease in the spacing of emitters led to a decrease in leaf blade NO3 concentration. Leaf blade ferric reduction (FRAP) activity declined as plant density decreased. The interaction between plant density and the number of emitters affected proline, total phenols, and antioxidant scavenging activity (DPPH). The leaf blade of spinach grown in low plant density treatment (8 soil blocks) with 8 emitters per Styrofoam box presented the highest levels of phenols content, proline content and antioxidant activity (FRAP and DPPH) and the lowest levels of PDH activity and nitrate content. Plant density and emitter spacing had no influence on the accumulation of proline, total phenols or the antioxidant activity of petiole.
Declarations
Author contribution statement
Rui Machado: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Isabel Alves-Pereira, Rui Fereira; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by domestic funding provided the FCT - Foundation for Science and Technology as part of Project UID/AGR/00115/2013.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
This work received domestic funding provided the FCT - Foundation for Science and Technology as part of Project UID/AGR/00115/2013.
References
- Alam M.A., Juraimi A.S., Rafii M.Y., Hamid A.A., Aslani F., Alam M.Z. Effects of salinity and salinity-induced augmented bioactive compounds in purslane (Portulaca oleracea L.) for possible economical use. Food Chem. 2015;169:439–447. doi: 10.1016/j.foodchem.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Alvino A., Barbieri B. Vegetables of temperate climates: leafy vegetables. In: Caballero B., Finglas P., Toldrá F., editors. The Encyclopedia of Food and Health. Academic Press; Oxford: 2016. pp. 393–400. [Google Scholar]
- Apak R., Güçlü K., Demirata B., Ӧzyürek M., Çelik S., Bektaşoğlu B., Berker K., Ӧzyurt D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules. 2007;12(7):1496–1547. doi: 10.3390/12071496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M.A., Iqbal M., Rasheed R., Hussain I., Riaz M., Arif M.S. Environmental stress and secondary metabolites in plants: an overview. In: Ahmad P., Ahanger M.A., Singh V.P., Tripathi D.K., Alam P., Alyemeni M.N., editors. Plant Metabolites and Regulation Under Environmental Stress. Academic Press; London: 2018. pp. 153–167. [Google Scholar]
- Aslan M., Sultana B., Anwar F., Munir H. Foliar spray of selected plant growth regulators affected the biochemical and antioxidant attributes of spinach in a field experiment. Turk. J. Agric. For. 2016;40:136–145. [Google Scholar]
- Barcelos C., Machado R.M.A., Alves-Pereira I., Ferreira R., Bryla D.R. Effects of substrate type on plant growth and nitrogen and nitrate concentration in spinach. Int. J. Plant Biol. 2016;7(1):44–47. [Google Scholar]
- Bates L.S., Waldren R.P., Teare I.D. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39(1):205–207. [Google Scholar]
- Bayili R.G., Abdoul-Latif F., Kone O.H., Diao M., Bassole I.H., Dicko M.H. Phenolic compounds and antioxidant activities in some fruits and vegetables from Burkina Faso. Afr. J. Biotechnol. 2011;10(62):13543–13547. [Google Scholar]
- Bian Z.H., Yang Q.C., Liu W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: a review. J. Sci. Food Agric. 2014;95(5):869–877. doi: 10.1002/jsfa.6789. [DOI] [PubMed] [Google Scholar]
- Bouayed J., Hoffmann L., Bohn T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: bioaccessibility and potential uptake. Food Chem. 2011;128(1):14–21. doi: 10.1016/j.foodchem.2011.02.052. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berse C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci. Technol. 1995;28(1):25–30. [Google Scholar]
- Chaitanya K.V., Rasineni G.K., Reddy A.R. Biochemical responses to drought stress in mulberry (Morus alba L.): evaluation of proline, glycine betaine and abscisic acid accumulationin five cultivars. Acta Physiol. Plant. 2009;31(3):437–443. [Google Scholar]
- Chu Y.F., Sun J.I.E., Wu X., Liu R.H. Antioxidant and antiproliferative activities of common vegetables. J. Agric. Food Chem. 2002;50(23):6910–6916. doi: 10.1021/jf020665f. [DOI] [PubMed] [Google Scholar]
- Chua I.Y.P., King P.J.H., Ong K.H., Sarbini S.R., Yiu P.H. Influence of light intensity and temperature on antioxidant activity in Premna serratifolia L. J. Soil Sci. Plant Nutr. 2015;15(3):605–614. [Google Scholar]
- Claussen W. Proline as a measure of stress in tomato plants. Plant Sci. 2005;168(1):241–248. [Google Scholar]
- Colla G., Kim H.-J., Kyriacouc M.C., Rouphael Y. Nitrate in fruits and vegetables. Sci. Hortic. 2018;237:221–238. [Google Scholar]
- Conesa E., Niñirola D., Vicente M., Ochoa J., Bañón S., Ferández A. The influence of nitrate/ammonium ratio on yield quality and nitrate, oxalate and vitamin C content of baby leaf spinach and bladder campion plants grown in a floating system. Acta Hortic. 2009;843:269–273. [Google Scholar]
- Costilow R.N., Cooper D. Identify of proline dehydrogenase and delta1-pyrroline-5-carboxylic acid reductase in Clostridium sporogenes. J. Bacteriol. 1978;134(1):139–146. doi: 10.1128/jb.134.1.139-146.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier A., Clifford M.N., Ashihara H. second ed. Blackwell Publishing; 2007. Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet. [Google Scholar]
- D'Souza M.R., Devaraj V.R. Biochemical responses of Hyacinth bean (Lablab purpureus) to salinity stress. Acta Physiol. Plant. 2010;32:341–353. [Google Scholar]
- Ghasemzadeh A., Jaafar H.Z.E., Rahmat A., Wahab P.E.M., Halim M.R.A. Effect of different light intensities on total phenolics and flavonoids synthesis and anti-oxidant activities in young ginger varieties (Zingiber officinale Roscoe) Int. J. Mol. Sci. 2010;11:3885–3897. doi: 10.3390/ijms11103885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat S., Hayat Q., Alyemeni M.N., Wani A.S., Pichtel J., Ahmad A. Role of proline under changing environments: a review. Plant Signal. Behav. 2012;7(11):1456–1466. doi: 10.4161/psb.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard L.R., Pandjaitan N., Morelock T., Gill M.I. Antioxidant capacity and phenolic content of spinach as affected by genetics and growing season. J. Agric. Food Chem. 2002;50(21):5891–5896. doi: 10.1021/jf020507o. [DOI] [PubMed] [Google Scholar]
- Ilic Z.S., Milenkovic L., Šunic L., Manojlovic M. Color shade nets improve vegetables quality at harvest and maintain quality during storage. Contemp. Agric. 2018;67(1):9–19. [Google Scholar]
- Ismail A., Marjan Z.M., Foong C.W. Total antioxidant activity and phenolic content in selected vegetables. Food Chem. 2004;87(4):581–586. [Google Scholar]
- Ismail H., Chan K., Mariod A., Ismail M. Phenolic content and antioxidant activity of cantaloupe (Cucumis melo) methanolic extracts. Food Chem. 2010;119(2):643–647. [Google Scholar]
- Jaganath I.B., Zainal A. Controlled environment for enhanced and consistent production of poly(pnenols) in speciality crops. In: Jaganath I.B., Zainal A., editors. Controlled Environment Agriculture - Production of Specialty Crops Providing Human Health Benefits Through Hydroponics. Agricultural Research and Development Institute, NOVA Science Publishers; Selangor: 2016. pp. 1–32. [Google Scholar]
- Kałużewicz A., Lisiecka J., Gąsecka M., Krzesiński W., Spiżewski T., Zaworska A., Frąszcza B. The effects of plant density and irrigation on phenolic content in cauliflower. Hortic. Sci. (Prague) 2017;44(4):178–185. [Google Scholar]
- Karimi E., Jaafar H.Z.E., Ghasemzadeh A., Ibrahim M.H. Light intensity effects on production and antioxidant activity of flavonoids and phenolic compounds in leaves, stems and roots of three varieties of Labisia pumila benth. Aust. J. Crop. Sci. 2013;7(7):1016–1023. [Google Scholar]
- Kiyosue T., Yoshida Y., Yamagushy-Shinozaki K. A nuclear gene encoding mitochondrial proline dehydrogenase, an enzyme involved in proline metabolism, is upregulated by proline but down regulated by dehydration in Arabidopsis. Plant Cell. 1996;8:1323–1335. doi: 10.1105/tpc.8.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klados E., Tzortzakis N. Effects of substrate and salinity in hydroponically grown Cichorium spinosum. J. Soil Sci. Plant Nutr. 2014;14(1):211–222. [Google Scholar]
- Kollia E., Markaki P., Zoumpoulakis P., Proestos C. Antioxidant activity of Cynara scolymus L. and Cynara cardunculus L. extracts obtained by different extraction techniques. Nat. Prod. Res. 2017;31(10):1163–1167. doi: 10.1080/14786419.2016.1219864. [DOI] [PubMed] [Google Scholar]
- Kyriacou M.C., Rouphael Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018;234:463–469. [Google Scholar]
- Lastra O.C. Derivate spectrophotometric determination of nitrate in plant tissue. J. AOAC Int. 2003;86(6):1001–1005. [PubMed] [Google Scholar]
- Leyva A., Jarillo J.A., Salinas J., Martinezzapater J.M. Low temperature induces the accumulation of phenylalanine ammonia lyase and chalcone synthase messenger RNAs of Arabidopsis thaliana in a light dependent manner. Plant Physiol. 1995;108:39–46. doi: 10.1104/pp.108.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.-G., Ding X.-J., Du P.-F. Hydrogen sulfide donor sodium hydrosulfide-improved heat tolerance in maize and involvement of proline. J. Plant Physiol. 2013;170(8):741–747. doi: 10.1016/j.jplph.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Roseburg N.J., Farr A.L., Randell R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Machuca A., Palma D., Tapia M.L., Lizana L.A., Escalona V. ASHS, Annual conference August; New Orleans, USA: 2015. Planting Densities Affect Nitrate Concentration in Spinach (Spinacia oleracea L.) Cultivated in Floating Hydroponic System Root. [Google Scholar]
- Marschner H. second ed. Academic Press; New York: 2012. Mineral Nutrition of Higher Plants. [Google Scholar]
- Mazzucotelli C.A., Gonzalez-Aguilar G.A., Villegas-Ochoa M.A., Domínguez-Avila A.J., Ansorena M.R., Di Scala K.C. Chemical characterization and functional properties of selected leafy vegetables for innovative mixed salads. J. Food Biochem. 2017;42 [Google Scholar]
- Miljus-Djukic J., Stanisavljevic N., Radovic S., Jovanovic Z., Mikic A., Maksimovic V. Differential response of three contrasting pea (Pisum arvense, and P. fulvum) species to salt stress: assessment of variation in antioxidative defence and miRNA expression. Aust. J. Crop. Sci. 2013;7(13):2145. [Google Scholar]
- Oh M.-M., Carey E.E., Rajashekar C.B. Environmental stresses induce health promoting phytochemicals in lettuce. Plant Physiol. Biochem. 2009;47:578–583. doi: 10.1016/j.plaphy.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Ou B., Huang D., Hampsch-Woodill M., Flanagan J.A., Deemer E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: a comparative study. J. Agric. Food Chem. 2002;50(11):3122–3128. doi: 10.1021/jf0116606. [DOI] [PubMed] [Google Scholar]
- Per T.S., Khan N.A., Reddy P.S., Masood A., Hasanuzzaman M., Khan M.I.R., Anjum N.A. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. 2017;115:126–140. doi: 10.1016/j.plaphy.2017.03.018. [DOI] [PubMed] [Google Scholar]
- Pérez-López U., Miranda-Apodaca J., Muñoz-Rueda A., Mena-Petite A. Lettuce production and antioxidant capacity are differentially modified by salt stress and light intensity under ambient and elevated CO2. J. Plant Physiol. 2013;170:1517–1525. doi: 10.1016/j.jplph.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Pérez-López U., Miranda-Apodaca J., Muñoz-Rueda A., Mena-Petite A. Interacting effects of high light and elevated CO2 on the nutraceutical quality of two differently pigmented Lactuca sativa cultivars (Blonde of Paris Batavia and Oak Leaf) Sci. Hortic. 2015;191:38–48. [Google Scholar]
- Pérez-López U., Sgherrib C., Miranda-Apodaca J., Micaelli F., Lacuestac M., Mena-Petitea A., Quartacci M.F., Muñoz-Rueda A. Concentration of phenolic compounds is increased in lettuce grown under high light intensity and elevated CO2. Plant Physiol. Biochem. 2018;123:233–241. doi: 10.1016/j.plaphy.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Petropoulos S.A., Pereira C., Barros L., Ferreira I.C.F.R. Leaf parts from Greek artichoke genotypes as a good source of bioactive compounds and antioxidants. Food Funct. 2017;8:2022–2029. doi: 10.1039/c7fo00356k. [DOI] [PubMed] [Google Scholar]
- Prazeres A.O. Programa e Livro de Resumos do 1º Congresso Nacional de Rega e Drenagem. Instituto Politécnico de Beja; Beja, Portugal: 2005. Comparação de metodologias laboratoriais para determinação de azoto nítrico e amoniacal em solos e águas; pp. 59–71. [Google Scholar]
- Proteggente A.R., Pannala A.S., Paganga G., van Buren L., Wagner E., Wiseman S., van de Put F., Dacombe C., Rice-Evans C.A. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radic. Res. 2002;36(2):217–223. doi: 10.1080/10715760290006484. [DOI] [PubMed] [Google Scholar]
- Qiu W., Wang Z., Huang C., Chen B., Yang R. Nitrate accumulation in leafy vegetables and its relationship with water. J. Soil Sci. Plant Nutr. 2014;14(4):761–768. [Google Scholar]
- Reddy P.S., Jogeswar G., Rasineni G.K., Maheswari M., Reddy A.R., Varshney R.K., Kishor P.K. Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum (Sorghum bicolor (L) Mohench) Plant Physiol. Biochem. 2015;94:104–113. doi: 10.1016/j.plaphy.2015.05.014. [DOI] [PubMed] [Google Scholar]
- Salandanan K., Bunning M., Stonaker F., Külen O., Kendall P., Stushnoff C. Comparative analysis of antioxidant properties and fruit quality attributes of organically and conventionally grown melons (Cucumis melo L.) Hortscience. 2009;44(7):1825–1832. [Google Scholar]
- Samuoliené G., Sirtautas R., Brazaitytė A., Viršilė A., Duchovskis P. Supplementary red-LED lighting and the changes in phytochemical content of two baby leaf lettuces varieties during three seasons. J. Food Agric. Environ. 2012;10:701–706. [Google Scholar]
- Santamaria P. Nitrate in vegetables: toxicity, content, intake and EC regulation. J. Sci. Food Agric. 2006;86:10–17. [Google Scholar]
- Santamaria P., Elia A., Serio F., Todaro E. A survey of nitrate and oxalate content in fresh vegetables. J. Sci. Food Agric. 1999;79:1882–1888. [Google Scholar]
- Shimomachi T., Kawahara Y., Kobashigawa C., Omoda E., Hamabe K., Tamaya K. Effect of residual salinity on spinach growth and nutrient contents in polder soil. Acta Hortic. 2008;797:419–424. [Google Scholar]
- Sokal R.R., Rohlf F.J. third ed. W.H. Freeman; New York: 1997. Biometry. [Google Scholar]
- Sreeramulu D., Reddy C.V.K., Chauhan A., Balakrishna N., Raghunath M. Natural antioxidant activity of commonly consumed plant foods in India: effect of domestic processing. Oxid. Med. Cell Longev. 2013 doi: 10.1155/2013/369479. Hindawi Publishing Corporation. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratil P., Klejdus B., Kubáň V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006;54(3):607–616. doi: 10.1021/jf052334j. [DOI] [PubMed] [Google Scholar]
- Sucupira N., Silva A., Pereira G., Costa J. Métodos para determinação da atividade antioxidante de frutos. UNOPAR Cient. Ciênc. Biol. Saúde. 2012;14(4):263–269. [Google Scholar]
- Telesiñski A., Nowak J., Smolik B., Dubowska A., Skrzypiec N. Effect of soil salinity on activity of antioxidant enzymes and content of ascorbic acid and phenols in bean [Phaseolus vulgaris L.] plants. J. Elem. 2008;13(3):401–409. [Google Scholar]
- Tian B., Qiao Z., Zhang L., Li H., Pei Y. Hydrogen sulfide and proline cooperate to alleviate cadmium stress in foxtail millet seedlings. Plant Physiol. Biochem. 2016;109:293–299. doi: 10.1016/j.plaphy.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Valdés R., Ochoa J., Sánchez-Blanco M.J., Franco J.A., Bañón S. Irrigation volume and the number of emitters per pot affect root growth and saline ion contents in weeping fig. Agric. Agric. Sci. Proc. 2015;4:356–364. [Google Scholar]
- Verbruggen N., Hermans C. Proline accumulation in plants: a review. Amino Acids. 2008;35(4):753–759. doi: 10.1007/s00726-008-0061-6. [DOI] [PubMed] [Google Scholar]
- Wang H., Gu M., Cui J., Shi K., Zhou Y., Yu J. Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, and expression of Calvin cycle genes and carbohydrate accumulation in Cucumis sativus. J. Photochem. Photobiol. B. 2009;96(1):30–37. doi: 10.1016/j.jphotobiol.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Wang W.-G., Li R., Liu B., Li L., Wang S.-H., Chen F. Effects of low nitrogen and drought stresses on proline synthesis of Jatropha curcas seedling. Acta Physiol. Plant. 2011;33(5):1591–1595. [Google Scholar]
- Yoon Y.-E., Kuppusamy S., Cho K.M., Kim P.J., Kwac Y.-B., Lee Y.B. Influence of cold stress on contents of soluble sugars, vitamin C and free amino acids including gamma-aminobutyric acid (GABA) in Spinach (Spinacia oleracea) Food Chem. 2017;215:185–192. doi: 10.1016/j.foodchem.2016.07.167. [DOI] [PubMed] [Google Scholar]
- Yosefi Z., Tabaraki R., Gharneh H.A., Mehrabi A.A. Variation in antioxidant activity, total phenolics, and nitrate in spinach. Int. J. Veg. Sci. 2010;16(3):233–242. [Google Scholar]