Graphical abstract
Abbreviations: DPPH, di(phenyl)-(2,4,6-trinitrophenyl) iminoazanium; FRAP, Ferric reducing ability of plasma; ABTS, 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid); NAC, N-acetylcystein; TPTZ, 2,4,6-tri(2-pyridyl)-s-triazine; AOPP, advanced oxidation protein products
Keywords: Antioxidant property, Radiographic contrast media, Free radical
Highlights
-
•
Iobitridol (xenetix), iodixanol (visipaque), iohexol (omnipaque), ioxaglate (hexabrix), and isovue (iopamiro), iodinated radiographic contrast media: did not show in DPPH• radical-scavenging activity; did not show ferric reducing ability property, except for iobitridol at 200 mgI/mL and ioxaglate at 50–200 mgI/mL, showed ABTS•+ radical-scavenging activity.
-
•
In conclusion, iobitridol, iodixanol, iohexol, ioxaglate, and isovue exhibited weak in vitro antioxidant properties.
Abstract
This study reveals the antioxidant properties of iodinated radiographic contrast media to be used in diagnostic radiology. Di(phenyl)-(2,4,6-trinitrophenyl) iminoazanium (DPPH), ferric reducing ability of plasma (FRAP), and 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) assays were used for determining in vitro the antioxidant properties of five iodinated radiographic contrast media such as iobitridol (xenetix), iodixanol (visipaque), iohexol (omnipaque), ioxaglate (hexabrix), and isovue (iopamiro). An ascorbic acid and Trolox solution served as a positive control. The absorbance intensity of the colored product was recorded using a spectrophotometer. For DPPH and ABTS assay, the absorbance intensity at 533 and 752 nm, respectively was decreased when compared to control; it indicated an increase in antioxidant activity. For FRAP assay, the absorbance intensity at 593 nm was increased when compared to control; it indicated an increase in antioxidant activity. The results showed that five iodinated radiographic contrast media did not differ in DPPH• radical-scavenging activity when compared to a corresponding control. The ferric reducing ability of all of these iodinated radiographic contrast media also did not differ when compared to a corresponding control, except for iobitridol at 200 mgI/mL and ioxaglate at 50–200 mgI/mL. All iodinated radiographic contrast media showed ABTS•+ radical-scavenging activity. This finding suggested that iobitridol, iodixanol, iohexol, ioxaglate, and isovue exhibited weak in vitro antioxidant properties. The antioxidant ability depended on the type of free radical production and the concentration of iodinated radiographic contrast media.
1. Introduction
Iodinated radiographic contrast media is the most commonly used method in diagnostic radiology. It is a tri-iodinated derivative of benzoic acid [1]. Although iodinated radiographic contrast media is generally safe, side effects can still occur. However, the risk of side effects from nonionic iodinated radiographic contrast media is one-fifth that of ionic iodinated radiographic contrast media and the risk for mild side effects and severe side effects is one-tenth. Patients who receive nonionic iodinated radiographic contrast media that experience severe side effects occur in only 0.04% of cases, while 0.2% of those receiving ionic iodinated radiographic contrast media experience severe side effects. The risk of side effects from exposure to iodinated radiographic contrast media increases in high-risk patients, particularly in patients with asthma, or allergies. In addition, those suffering from dehydration, heart disease, preexisting renal disease, sickle cell anemia, polycythemia, and myeloma are also at risk [[2], [3], [4]]. Iodinated radiographic contrast media-induced nephropathy is one of the most common reasons for the occurrence acute renal failure in hospitalized patients [5]. It is possible that one of the possible mechanisms of iodinated radiographic contrast media-induced nephropathy is associated with renal oxidative stress which mediates the damage to cell membranes and mitochondria resulting in cellular apoptosis and necrosis [[5], [6], [7]]. However, N-acetylcystein (NAC), a thiol containing an antioxidant and a reactive oxygen radical scavenger, has been shown to prevent iodinated radiographic contrast media-induced nephropathy [7,8]. In addition, calcium, zinc, vitamin E, virgin olive oil and olive leaf extract have been shown to ameliorate renal damage in rat [9,10]. The role that iodinated radiographic contrast media has in oxidative stress is still worth investigating. However, the objective of this current study was to observe the reaction of iodinated radiographic contrast media with free radicals in in vitro. Three in vitro assays used in this study were di(phenyl)-(2,4,6-trinitrophenyl) iminoazanium (DPPH) assay, ferric reducing ability of plasma (FRAP) assay, and 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) assay, since those assays are the most common in vitro assays that are used to evaluate antioxidant activity and the action mechanism of non-enzymatic antioxidants. The reaction mechanism of DPPH, FRAP, and ABTS assays are based on single electron transfer/hydrogen atom transfer reactions, single electron transfer reaction, and hydrogen atom transfer reaction, respectively [[11], [12], [13]]. In this study, we evaluated the antioxidant activity of iodinated radiographic contrast media such as iobitridol (xenetix), iodixanol (visipaque), iohexol (omnipaque), ioxaglate (hexabrix), and isovue (iopamiro) that are currently used in diagnostic radiology.
2. Materials and methods
2.1. Chemicals
Five commercially available iodinated radiographic contrast media are iobitridol (xenetix; Guerbet, France), iodixanol (visipaque; GE Healthcare, Ireland), iohexol (omnipaque; GE Healthcare, China), ioxaglate (hexabrix; Guerbet, France), and isovue (iopamiro; Bracco, Italy). These iodinated radiographic contrast media are mainly used in diagnostic radiology. Di(phenyl)-(2,4,6-trinitrophenyl) iminoazanium (DPPH), ascorbic acid, 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), Trolox, and 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) were purchased from Sigma–Aldrich (St. Louis, MO, USA).
2.2. Di(phenyl)-(2,4,6-trinitrophenyl) iminoazanium (DPPH) assay
The DPPH assay was performed based on the work of Sun et al. [14] with some modifications. A brief summary of this process is as follows: DPPH was dissolved in ethanol to obtain a 0.2 mM concentration. The working solution (0.5 mL) composed of 1, 10, 50, 100, and 200 mgI/mL of iodinated radiographic contrast media in distilled water were allowed to react with 2.5 mL of 0.2 mM DPPH solution for 30 min at room temperature in the dark. Next, absorbance intensity of the colored product was recorded at 533 nm using a spectrophotometer (Agilent 8453, UV–vis spectrophotometer). The ascorbic acid was used as a positive control.
2.3. Ferric reducing ability of plasma (FRAP) assay
The FRAP assay was performed based on the method used by Benzie and Strain [15] with some modifications. In summary, the stock solutions included a 300 mM acetate buffer, pH 3.6, a 10 mM 2, 4, 6-tripyridyl-s-triazine (TPTZ) solution in 40 mM HCl, and a 20 mM FeCl3·6H2O solution. The working FRAP solution was freshly prepared by mixing an acetate buffer, TPTZ solution, and a FeCl3·6H2O solution in a 10:1:1 ratio. The working solution (0.1 mL) of 1, 10, 50, 100, and 200 mgI/mL of iodinated radiographic contrast media in distilled water were allowed to react with 3 mL of the FRAP solution for 8 min in the dark at room temperature. Afterward, the absorbance intensity of the colored product (ferrous tripyridyltriazine complex) was recorded at 593 nm using a spectrophotometer (Agilent 8453, UV–vis spectrophotometer). The Trolox was used as a positive control.
2.4. 2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) assay
The ABTS assay was performed based on the procedure done by Re et al. [16] with some modifications. Briefly, ABTS radical cation (ABTS•+) stock solution was produced by reaction of a 7 mM ABTS solution in ethanol with a 2.45 mM potassium persulfate (final concentration) and this mixture was permitted to react for 12 h at room temperature in the dark. Next, the ABTS•+ stock solution was diluted with ethanol to an absorbance intensity at 752 nm equal to 0.70 ± 0.02. The working solution (10 μL) of 1, 10, 50, 100, and 200 mgI/mL of iodinated radiographic contrast media in distilled water were allowed to react with 990 μL of ABTS•+ stock solution for 4 min at room temperature in the dark. Then, the absorbance intensity of the colored product was recorded at 752 nm using a spectrophotometer (Agilent 8453, UV–vis spectrophotometer).
2.5. Statistical analysis
All experiments were performed in duplicate three times (n = 3). We expressed the results as mean ± standard deviation (SD). The Student’s t-test was used independently to evaluate any statistical differences in the mean values between each test group and the corresponding control. A p-value of less than 0.05 was considered as statistically significant.
3. Results
Fig. 1, Fig. 2, Fig. 3 summarize the results. The data in each figure represents the mean values from three times (duplicate experiments for each time) ± SD. The detailed results for each assay are shown below.
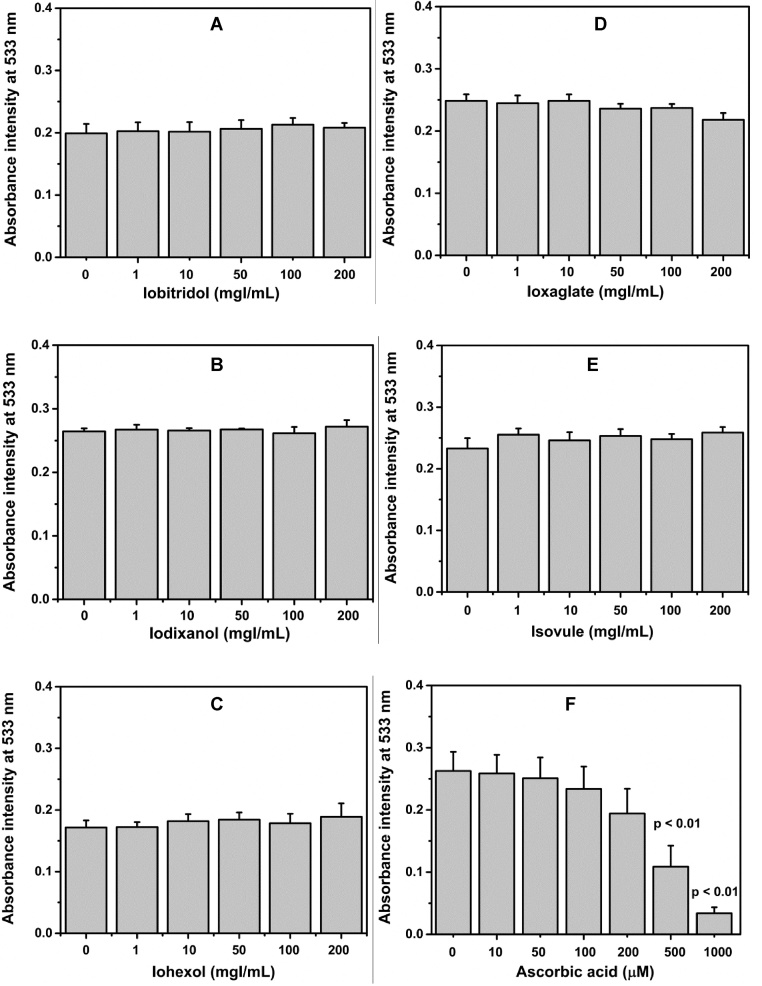
Fig. 1.
Concentration responses of iodinated radiographic contrast media (A–E) and ascorbic acid (F) in the DPPH reaction. Mean values ± SD are presented.
Fig. 2.
Concentration responses of iodinated radiographic contrast media (A–E) and ferrous sulfate (F) in the FRAP reaction. Mean values ± SD are presented.
Fig. 3.
Concentration responses of iodinated radiographic contrast media (A–E) and Trolox (F) in the ABTS reaction. Mean values ± SD are presented.
3.1. DPPH assay
For DPPH assay, the absorbance intensity at 533 nm was decreased when compared to control; it indicated an increase in antioxidant activity. Fig. 1 shows the absorbance intensity at 533 nm of the colored product in iodinated radiographic contrast media groups and in the corresponding control. This data shows no changes occurring in the absorbance intensity of the colored product in iodinated radiographic contrast media groups, relative to the corresponding control at all concentrations. The absorbance intensity of the colored product in ascorbic acid decreased in a dose dependent relationship.
3.2. FRAP assay
For FRAP assay, the absorbance intensity at 593 nm was increased when compared to control; it indicated an increase in antioxidant activity. Fig. 2 shows the absorbance intensity at 593 nm of the colored product in iodinated radiographic contrast media groups and in the corresponding control. This data shows no change in the absorbance intensity of the colored product in iodixanol, iohexol and isovue in relation to the corresponding control at all concentrations. The absorbance intensity at 593 nm significantly increased in 200 mgI/mL of iobitridol and 50–200 mgI/mL of ioxaglate. The absorbance intensity of the colored product in ferrous sulfate increased as a dose dependent relationship.
3.3. ABTS assay
For ABTS assay, the absorbance intensity at 752 nm was decreased when compared to control; it indicated an increase in antioxidant activity. Fig. 3 shows the absorbance intensity at 752 nm of the colored product in iodinated radiographic contrast media groups and in the corresponding control. The absorbance intensity at 752 nm significantly decreased in 10–200 mgI/mL of iobitridol, isovue and 200 mgI/ml of iodixanol, iohexol, and ioxaglate. The absorbance intensity of the colored product in the Trolox group decreased in a dose dependent manner.
4. Discussion
It has been known that contrast-induced nephropathy is reversible acute renal failure occurred after usage of iodinated contrast media during medical procedures such as angiographic, urography [17,18]. Among the several risk factors that are related to contrast-induced nephropathy include such preexisting conditions as renal impairment, and diabetes mellitus, as well as the volume of radiographic contrast media administered [19]. The physico-chemical properties of iodinated radiographic contrast media i.e.; osmolality and ionic or nonionic, also have been reported to contribute to contrast-induced nephropathy [20,21]. Oxidative stress that results from an imbalance between free radicals and antioxidant agents, is one of risk factors that play an important role in contrast-induced nephropathy [22,23]. In addition, the authors have reported that ischemic patients who developed contrast-induced nephropathy were decrease in glutathione [24].
Recently, the antioxidant properties of radiographic contrast media have been studied in in vitro. Berg et al. investigated the potential properties of particular iodinated radiographic contrast media such as diatrizoate (urografin), ioxaglate (hexabrix), iohexol (omnipaque), and iodixanol (visipaque) in in vitro free radical generating reactions by mean of a xanthine oxidase reaction, a fenton reaction, the total antioxidant status, and acetyl-cholinesterase assays. These same researchers showed that high concentrations of ionic iodinated radiographic contrast media inhibited superoxide radical production, and that ionic media is more potent than non-ionic iodinated radiographic contrast media. Medium concentrations of iodinated radiographic contrast media reduced hydroxyl radical production, and both types of iodinated radiographic contrast media were equally potent in that regard. Low concentrations of non-ionic iodinated radiographic contrast media showed higher antioxidant capacity than their ionic counterparts when tested in the total antioxidant status assay [1].
Contrary to earlier findings, this present study results suggested that iodinated radiographic contrast media did not differ in DPPH• radical-scavenging activity when compared to a corresponding control. The ferric reducing ability of those all iodinated radiographic contrast media also did not differ when compared to a corresponding control, except for iobitridol at 200 mgI/ml and ioxaglate at 50–200 mgI/ml. However, all iodinated radiographic contrast media showed ABTS•+ radical-scavenging activity.
It should be noted that there was an important difference in the experimental designs between the present study and the study that was conducted by Berg et al. [[1] regarding the in vitro free radical generating assay used to investigate the antioxidant activity of iodinated radiographic contrast media. This present study used the DPPH, FRAP, and ABTS assays, while a xanthine oxidase reaction, a fenton reaction, the total antioxidant status, and acetyl-cholinesterase assays were used in the study conducted by Berg et al. [1].
Of note, a commercial iodinated radiographic contrast media that is ready-to-use contains an essential additive agent referred to as EDTA. It might be appropriate to discuss whether the additive agent in iodinated radiographic contrast media could have been what contributed to the antioxidant properties rather than any iodinated benzoic acid derivatives (active X-ray agents). The present study results suggested that iodinated radiographic contrast media exhibited weak antioxidant properties, but this finding provides hope that their antioxidant efficacy may be considerable in vivo.
There are several studies that have shown that iodinated radiographic contrast media increased the levels of free radicals and oxidative stress which ultimately can then mediate cell damage [[25], [26], [27]]. Those studies include reports concerning how iodinated radiographic contrast media increased superoxide in the thick ascending limbs of rats and the rate of cell death, as well. However, iodinated radiographic contrast media had no effect on the activity of superoxide dismutase [28]. The findings in that report were consistent with research done by other authors. They reported that iodinated radiographic contrast media might increase reactive oxygen species production, and renal injury in rats [29]. In addition, iodinated radiographic contrast media contributed to particular reactive oxygen species metabolites in rat kidneys, such as malondialdehyde, a lipid peroxidation end product that is an oxidative stress marker [20,30]. Authors found reversible oxidative stress after administration of iopromide solution, iodinated contrast media, in animal model [31]
Iodinated radiographic contrast media also increased cytotoxicity in human proximal renal tubular epithelial cells [32,33]. Other authors showed that iodinated radiographic contrast media caused severe and prolonged oxidative stress in hemodialysis patients. Moreover, iodinated radiographic contrast media increased advanced oxidation protein products (AOPP), catalase, 8-hydroxydeoxyguanosine, and malondialdehyde, which are oxidative stress markers, in hemodialysis patients [34].
In conclusion¸ the main findings of the present study were that the tested iodinated radiographic contrast media exhibited weak antioxidant properties in in vitro. However, this depended on the type of free radical production and the concentration of iodinated radiographic contrast media. In addition, there are several studies that show that these agents cause oxidative stress, particularly in hemodialysis patients, yet our results indicate weak radical scavenging ability and it does not appear that these compounds cause oxidative stress. The apparent discrepancies between the in vitro and in vivo work need to be more thoroughly explained.
Conflicts of interest
None.
Transparency document
Acknowledgment
This research was supported by a Faculty of Associated Medical Sciences, Chiang Mai University, Thailand grant that was awarded in 2017.
References
- 1.Berg K., Skarra S., Bruvold M., Brurok H., Karlsson J.O., Jynge P. Iodinated radiographic contrast media possess antioxidant properties in vitro. Acta Radiol. 2005;46:815–822. doi: 10.1080/02841850500336224. [DOI] [PubMed] [Google Scholar]
- 2.Namasivayam S., Kalra M.K., Torres W.E., Small W.C. Adverse reactions to intravenous iodinated contrast media: a primer for radiologists. Emerg. Radiol. 2006;12:210–215. doi: 10.1007/s10140-006-0488-6. [DOI] [PubMed] [Google Scholar]
- 3.Namasivayam S., Kalra M.K., Torres W.E., Small W.C. Adverse reactions to intravenous iodinated contrast media: an update. Curr. Probl. Diagn. Radiol. 2006;35:164–169. doi: 10.1067/j.cpradiol.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Thomsen H.S., Bush W.H., Jr. Adverse effects of contrast media: incidence, prevention and management. Drug Saf. 1998;19:313–324. doi: 10.2165/00002018-199819040-00006. [DOI] [PubMed] [Google Scholar]
- 5.Chang C.F., Lin C.C. Current concepts of contrast-induced nephropathy: a brief review. J. Chin. Med. Assoc. 2013;76:673–681. doi: 10.1016/j.jcma.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Pisani A., Riccio E., Andreucci M., Faga T., Ashour M., Di Nuzzi A., Mancini A., Sabbatini M. Role of reactive oxygen species in pathogenesis of radiocontrast-induced nephropathy. Biomed. Res. Int. 2013 doi: 10.1155/2013/868321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanaei-Ardekani M., Movahed M.-R., Movafagh S., Ghahramani N. Contrast-induced nephropathy: a review. Cardiovasc. Revascularization Med. 2005;6:82–88. doi: 10.1016/j.carrev.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Colbay M., Yuksel S., Uslan I., Acarturk G., Karaman O., Bas O., Mollaoglu H., Yagmurca M., Ozen O.A. Novel approach for the prevention of contrast nephropathy. Exp. Toxicol. Pathol. 2010;62:81–89. doi: 10.1016/j.etp.2009.02.119. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Gayoum A.A., Al-Hassan A.A., Ginawi I.A., Alshankyty I.M. The ameliorative effects of virgin olive oil and olive leaf extract on amikacin-induced nephrotoxicity in the rat. Toxicol. Rep. 2015;2:1327–1333. doi: 10.1016/j.toxrep.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adi P.J., Burra S.P., Vataparti A.R., Matcha B. Calcium, zinc and vitamin E ameliorate cadmium-induced renal oxidative damage in albino Wistar rats. Toxicol. Rep. 2016;3:591–597. doi: 10.1016/j.toxrep.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang N., Kitts D.D. Antioxidant property of coffee components: assessment of methods that define mechanisms of action. Molecules. 2014;19:19180–19208. doi: 10.3390/molecules191119180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prior R.L., Wu X., Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 13.Thaipong K., Boonprakob U., Crosby K., Cisneros-Zevallos L., Hawkins Byrne D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006;19:669–675. [Google Scholar]
- 14.Sun J., Yao J., Huang S., Long X., Wang J., García-García E. Antioxidant activity of polyphenol and anthocyanin extracts from fruits of Kadsura coccinea (Lem.) A.C. Smith. Food Chem. 2009;117:276–281. [Google Scholar]
- 15.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 16.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 17.Heyman S.N., Rosen S., Khamaisi M., Idee J.M., Rosenberger C. Reactive oxygen species and the pathogenesis of radiocontrast-induced nephropathy. Invest. Radiol. 2010;45:188–195. doi: 10.1097/RLI.0b013e3181d2eed8. [DOI] [PubMed] [Google Scholar]
- 18.Mamoulakis C., Tsarouhas K., Fragkiadoulaki I., Heretis I., Wilks M.F., Spandidos D.A., Tsitsimpikou C., Tsatsakis A. Contrast-induced nephropathy: basic concepts, pathophysiological implications and prevention strategies. Pharmacol. Ther. 2017;180:99–112. doi: 10.1016/j.pharmthera.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Soma V.R., Cavusoglu E., Vidhun R., Frishman W.H., Sharma S.K. Contrast-associated nephropathy. Heart Dis. 2002;4:372–379. doi: 10.1097/00132580-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Cetin M., Devrim E., Serin Kilicoglu S., Erguder I.B., Namuslu M., Cetin R., Durak I. Ionic high-osmolar contrast medium causes oxidant stress in kidney tissue: partial protective role of ascorbic acid. Ren. Fail. 2008;30:567–572. doi: 10.1080/08860220802064739. [DOI] [PubMed] [Google Scholar]
- 21.Davidson C., Stacul F., McCullough P.A., Tumlin J., Adam A., Lameire N., Becker C.R. Contrast medium use. Am. J. Cardiol. 2006;98:42k–58k. doi: 10.1016/j.amjcard.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 22.Naziroglu M., Yoldas N., Uzgur E.N., Kayan M. Role of contrast media on oxidative stress, Ca(2+) signaling and apoptosis in kidney. J. Membr. Biol. 2013;246:91–100. doi: 10.1007/s00232-012-9512-9. [DOI] [PubMed] [Google Scholar]
- 23.Persson P.B., Tepel M. Contrast medium-induced nephropathy: the pathophysiology. Kidney Int. Suppl. (2011) 2006:S8–S10. doi: 10.1038/sj.ki.5000367. [DOI] [PubMed] [Google Scholar]
- 24.Tsarouhas K., Tsitsimpikou C., Papantoni X., Lazaridou D., Koutouzis M., Mazzaris S., Rezaee R., Mamoulakis C., Georgoulias P., Nepka C., Rentoukas E., Kyriakides Z., Tsatsakis A., Spandidos D.A., Kouretas D. Oxidative stress and kidney injury in trans-radial catheterization. Biomed. Rep. 2018;8:417–425. doi: 10.3892/br.2018.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yesildag A., Ozden A., Yilmaz H.R., Uz E., Agackiran Y., Yesildag M., Yilmaz N., Sirmali R., Vural H., Naziroglu M. Erdosteine modulates radiocontrast-induced hepatotoxicity in rat. Cell Biochem. Funct. 2009;27:142–147. doi: 10.1002/cbf.1546. [DOI] [PubMed] [Google Scholar]
- 26.Yesilyurt A., Aydin Erden I., Bilgic I., Erden G., Albayrak A. The protective effect of erdosteine on radiocontrast induced nephrotoxicity in rats. Environ. Toxicol. 2011;26:395–402. doi: 10.1002/tox.20691. [DOI] [PubMed] [Google Scholar]
- 27.Zager R.A., Johnson A.C., Hanson S.Y. Radiographic contrast media-induced tubular injury: evaluation of oxidant stress and plasma membrane integrity. Kidney Int. 2003;64:128–139. doi: 10.1046/j.1523-1755.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- 28.Liu Z.Z., Schmerbach K., Lu Y., Perlewitz A., Nikitina T., Cantow K., Seeliger E., Persson P.B., Patzak A., Liu R., Sendeski M.M. Iodinated contrast media cause direct tubular cell damage, leading to oxidative stress, low nitric oxide, and impairment of tubuloglomerular feedback. Am. J. Physiol. Renal Physiol. 2014;306:F864–872. doi: 10.1152/ajprenal.00302.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu S.P., Tsai T.J., Chien C.T. Ioxitalamate induces renal tubular apoptosis via activation of renal efferent nerve-mediated adrenergic signaling, renin activity, and reactive oxygen species production in rats. Toxicol. Sci. 2010;114:149–158. doi: 10.1093/toxsci/kfp290. [DOI] [PubMed] [Google Scholar]
- 30.Devrim E., Cetin M., Namuslu M., Erguder I.B., Cetin R., Durak I. Oxidant stress due to non ionic low osmolar contrast medium in rat kidney. Indian J. Med. Res. 2009;130:433–436. [PubMed] [Google Scholar]
- 31.Tsamouri M.M., Rapti M., Kouka P., Nepka C., Tsarouhas K., Soumelidis A., Koukoulis G., Tsatsakis A., Kouretas D., Tsitsimpikou C. Histopathological evaluation and redox assessment in blood and kidney tissues in a rabbit contrast-induced nephrotoxicity model. Food Chem. Toxicol. 2017;108:186–193. doi: 10.1016/j.fct.2017.07.058. [DOI] [PubMed] [Google Scholar]
- 32.Liu G.L., Lei R., Duan S.B., Tang M.M., Luo M., Xu Q. Atorvastatin alleviates iodinated contrast media-induced cytotoxicity in human proximal renal tubular epithelial cells. Exp. Ther. Med. 2017;14:3309–3313. doi: 10.3892/etm.2017.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu N., Lei R., Tang M.M., Cheng W., Luo M., Xu Q., Duan S.B. Autophagy is activated to protect renal tubular epithelial cells against iodinated contrast mediainduced cytotoxicity. Mol. Med. Rep. 2017;16:8277–8282. doi: 10.3892/mmr.2017.7599. [DOI] [PubMed] [Google Scholar]
- 34.Hwang S.D., Kim Y.J., Lee S.H., Cho D.K., Cho Y.H., Moon S.J., Lee S.C., Yoon S.Y. Iodinated contrast media can induce long-lasting oxidative stress in hemodialysis patients. Yonsei Med. J. 2013;54:1438–1446. doi: 10.3349/ymj.2013.54.6.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.