Abstract
Objective
Impaired repetitive movement in persons with Parkinson's disease (PD) is associated with reduced amplitude, paradoxical hastening and hesitations or arrest at higher movement rates. This study examined the effects of movement rate and medication on movement-related cortical oscillations in persons with PD.
Methods
Nine participants with PD were studied off and on medication and compared to nine control participants. Participants performed index finger movements cued by tones from 1 to 3 Hz. Movement-related oscillations were derived from electroencephalographic recordings over the region of the contralateral senorimotor cortex (S1/M1) during rest, listening, or synchronized movement.
Results
At rest, spectral power recorded over the region of the contralateral S1/M1 was increased in the alpha band and decreased in the beta band in participants with PD relative to controls. During movement, the level of alpha and beta band power relative to baseline was significantly reduced in the PD group, off and on medication, compared to controls. Reduced movement amplitude and hastening at movement rates near 2 Hz was associated with abnormally suppressed and persistent desynchronization of oscillations in alpha and beta bands.
Conclusion
Motor cortical oscillations in the alpha and beta bands are abnormally suppressed in PD, particularly during higher rate movements.
Significance
These findings contribute to the understanding of mechanisms underlying impaired repetitive movement in PD.
Keywords: Alpha Band, Beta Band, Desynchronization, Hastening, Hypokinesia
1. Introduction
Bradykinesia is a major source of functional disability in persons with Parkinson's disease (PD) and contributes to decreased quality of life (Muslimovic et al., 2008; Ellis et al., 2011). Clinical rating of the severity of this symptom is largely derived from the assessment of repetitive alternating movements performed “as quickly and as big as possible”, such as finger tapping. Moderate-to-severe impairment is associated with early and frequent interruptions, periods of prolonged arrest and a marked decrease in amplitude (hypokinesia) (Goetz et al., 2008). When repetitive movements are paced by an external cue, there is often a clear transition in performance as the rate changes from low to higher rate movements. Low rate movements are often associated with reduced velocity, but movement amplitude and phase are usually maintained (Stegemöller et al., 2009; Espay et al., 2009). At pacing rates near to and above 2 Hz, performance is often characterized by hypokinesia in concert with a paradoxical increase in movement frequency that exceeds the rate of the pacing cue (hastening) and an increase in the variability of the inter-movement interval (loss of phase) (Nakamura et al., 1978; Pastor et al., 1992; O'Boyle et al., 1996; Stegemöller et al., 2009). Interruptions and periods of arrest are usually preceded by hastening and a precipitous loss of amplitude (Nieuwboer et al., 2009; Vercruysse et al., 2012; Vercruysse et al. 2014). Similar manifestations of movement hastening and hypokinesia are seen prior to episodes of freezing of gait and speech arrest (Vercruysse et al., 2014). Each of these impairments, including the hastening and hypokinesia of repetitive finger movements (Pastor et al., 1992; Stegemöller et al., 2009), are often resistant to treatment with dopaminergic medications (Moreau et al., 2007; Nieuwboer et al., 2009), suggesting that extrastriatal mechanisms contribute to these disorders.
In healthy adults, externally paced movements show a transition in both movement timing and patterns of sensorimotor cortical activity at movement rates near 2 Hz (Mayville et al., 2002; Toma et al., 2002; Jantzen et al., 2005; Stegemöller et al., 2009). At low pacing rates, movement onset is usually in phase with the pacing cue and is preceded by desynchronization of sensorimotor cortical oscillations in the alpha (8-13 Hz) and beta (13-30 Hz) bands (as measured with scalp surface electroencephalography (EEG) recordings), followed by synchronization of oscillations in the interval between movements (Pfurtscheller & Lopes da Silva, 1999; Erbil & Ungan, 2007). This alternating sequence of desynchronization-synchronization of movement-related oscillations (MROs) is thought to reflect motor cortical activity associated with the suppression and release of movement respectively. At pacing rates near 2 Hz, movements become out of phase with the cue and cortical activity transitions to a pattern of near continuous desynchronization (Toma et al., 2002; Muthukumaraswamy, 2010). Local field potential recordings from deep brain stimulation electrodes implanted in the subthalamic nucleus (STN) of persons with PD have shown a similar pattern of changes in desynchronization-synchronization when transitioning from low to higher rate movements (Androulidakis et al., 2008; Hebb et al., 2012; Joundi et al., 2013). Movements near 2 Hz were associated with a reduction in beta band modulation and a concomitant worsening of finger tapping performance, suggesting that alterations in the capacity to modulate beta oscillations was linked to impairment (Androulidakis et al., 2008). However, since comparable subcortical recordings cannot be obtained in healthy adults, it remains unknown if the patterns of desynchronization-synchronization observed in PD were normal or abnormal.
This study examined differences in movement performance and MROs recorded over the region of the contralateral sensorimotor cortex (S1/M1) between participants with PD and matched control participants across a range of externally paced movement rates (1 to 3 Hz). In particular, we focused on the changes in MROs that precede and accompany the emergence of hypokinesia and hastening (~2 Hz), and the effects of medication on MROs. We hypothesized that the deterioration of movement impairment that occurs at pacing rates near 2 Hz would be associated with changes in the timing and relative magnitude of MROs in the beta band over the primary motor cortex in subjects with PD compared to matched healthy adults.
2. Methods
2.1 Participants
Nine participants with a diagnosis of idiopathic PD (age = 65 ± 8 years) and nine control participants (age = 65 ± 9 years) were tested (Table 1). Control participants were age (± 3 years), gender and handedness matched to participants with PD. Inclusion criteria for participants with PD included an akinetic-rigid presentation in the upper limb in conjunction with moderate-to-severe impairment in the performance of repetitive alternating movements based on the Unified Parkinson's Disease Rating Scale (UPDRS) items 23-25 (score on each item > 2). Exclusion criteria included excessive tremor (UPDRS resting or action tremor score > 2) and other neurological, cognitive, psychological, or musculoskeletal conditions that would confound the experiment. Patients were tested after a 12-hour overnight withdrawal from all antiparkinson medications and again one hour after taking 1 ½ times their normal morning dose of levodopa. The ON medication data from one participant with PD was unusable due to the development of dyskinesias. The UPDRS motor section was used to evaluate motor severity prior to performing the experiment OFF and ON medication. The Institutional Review Board of Northwestern University approved the procedures, and all participants gave their written informed consent according to the Declaration of Helsinki (BMJ 1991;302:1194).
Table 1.
Patient information and UPDRS scores.
| Subject | Sex | Age | LED (mg/day) | UPDRS |
||
|---|---|---|---|---|---|---|
| Item 23 Hand Tested Off / On | Items 23-25 Hand Tested Off / On | Total Motor Score Off / On | ||||
| 1 | F | 58 | 1400 | 1 / 1 | 4 / 3 | 30 / 16 |
| 2 | M | 71 | 1100 | 1 / 1 | 4 / 4 | 28 / 24 |
| 3 | F | 66 | 2249 | 2 / 2 | 7 / 4 | 43 / 25 |
| 4 | M | 58 | 1100 | 2 / 2 | 7 / 6 | 28 / 21 |
| 5 | M | 67 | 1377 | 1 / 1 | 4 / 3 | 31 / 18 |
| 6 | M | 77 | 1078 | 3 / 3 | 7 / 7 | 43 / 37 |
| 7 | F | 53 | 875 | 3 / 3 | 8 / 8 | 32 / 28 |
| 8 | M | 56 | 630 | 2 / 1 | 7 / 4 | 26 / 22 |
| 9 | F | 66 | 900 | 2 /2 | 6 / 5 | 22/18 |
| Mean ± 1 SD | 64 ± 8 | 1190 ± 465 | 1.9 ± 0.8/ 1.8 ± 0.8 | 6.0± 1.6 / 4.9 ± 1.8 | 31.4 ± 7.2 / 23.2 ± 6.4 | |
| Statistics (Paired T-test) | t = 1.0, p = 0.347 | t = 2.86, p = 0.021 | t = 4.6, p = 0.002 | |||
SD = standard deviation, LED = levodopa equivalent dose
2.2 Movement Task
Participants sat in a chair with their forearm braced in a semi-pronated position with the palm facing downward. The thumb and fingers 3 to 5 were secured to the brace, restricting movement to the index finger. Each trial began with 38 seconds of rest (REST) followed by a series of acoustic tones (50 ms, 500 Hz, 80 dB) initially presented at a pacing rate of 1 Hz, maintained for 15 intervals, then increased by 0.25 Hz until reaching 3.0 Hz. Fifteen intervals were presented at each pacing rate (Figure 1A). Two task conditions were collected: (1) LISTEN, participants listened to the tone but did not move; (2) MOVE, participants were asked to synchronize movement with the tones. A total of eight LISTEN and MOVE trials were collected OFF and ON medication. The presentation order of LISTEN and MOVE trials was randomized across participants.
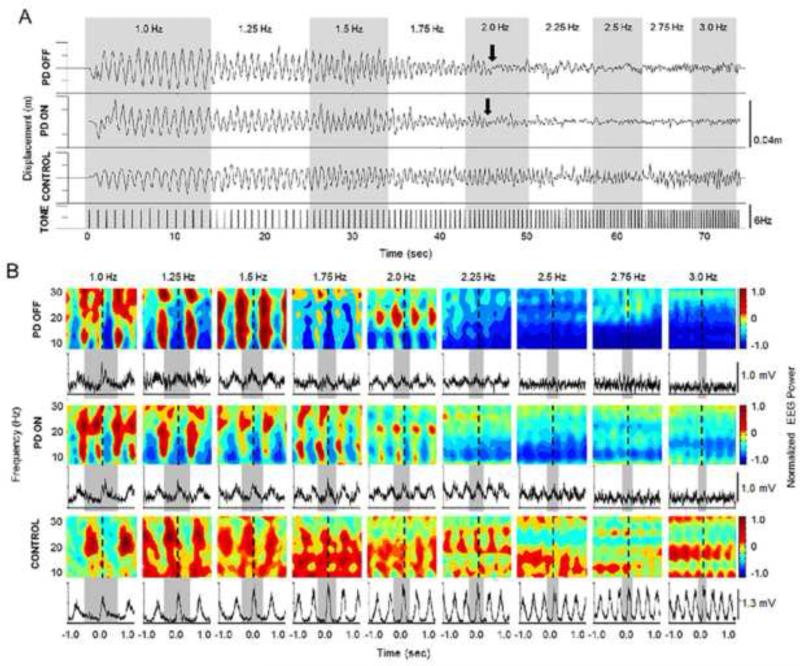
Figure 1.
(A) Paradigm example and position data shown for one trial from one participant with PD off and on levodopa, and one control participant. Arrows indicate the point where movement performance deteriorated. (B) Normalized EEG power from 8-30 Hz and normalized FDI EMG averages across trials for the same participants shown in Figure A. Dashed lines indicate movement onset. Gray bars indicated one movement cycle.
For MOVE trials, participants were instructed to start from a neutral position and quickly flex the index finger over as large a range of motion as possible, but were free to adjust movement amplitude to maintain a 1:1 stimulus/response ratio. To limit sources of tactile and visual feedback, the finger flexion was unconstrained, and participants were instructed to look straight ahead. Participants with PD performed the task with their most affected hand. Control participants performed the task with the same side as their matched counterpart. A two minute rest period was provided between trials to prevent fatigue. All participants were given practice trials to ensure task comprehension.
2.3 Data Collection
Finger kinematics were measured using an accelerometer (Measurement Specialties EGAXT3-15-/L2M) placed on the dorsum of the middle phalanx of the index finger. Bipolar surface electromyography (EMG) signals were recorded from the first dorsal interosseous (FDI) muscle, amplified and filtered (30-1000 Hz) (Grass P511, Grass Technologies) and collected at 2000 samples/second (Power 1401/ Signal 2 software, Cambridge Electronic Design, UK).
EEG signals were recorded from a montage of 74 scalp-surface electrodes. Electrode locations conformed to the international 10-20 system with increased density of electrodes over the right and left sensorimotor areas. The reference electrode was placed over the mastoid process ipsilateral to the moving finger. EEG signals were amplified with a gain of 2500, filtered (DC to 250 Hz) and sampled at a rate of 1000 samples/second (Neuroscan Syamps System/Neuroscan 4.1). Impedance was checked prior to beginning data collection and monitored throughout (Neuroscan Syamps System/Neuroscan 4.1). The trigger pulses controlling the onset of the trial and timing of the acoustic tones were collected simultaneously by the EMG and EEG data collection systems to ensure synchronization.
2.4 Data Analysis
2.4.1 Kinematics and Kinetics
The acceleration signal was notch (60 Hz) and high-pass filtered (2nd-order dual-pass Butterworth, 2 Hz cut-off) and double integrated to derive an estimate of finger displacement. Movement rate was calculated from the timing of each peak flexion displacement. To obtain a measure of hastening, the tone rate was subtracted from the actual movement rate performed (movement rate difference). Peak-to-peak displacement amplitude was also measured. The displacement data was normalized to the average peak-to-peak amplitude at 1 Hz in order to compare the relative change in movement amplitude across pacing rates and between-subjects. The FDI EMG signal was corrected for DC offset, rectified, averaged across movement epochs for each pacing interval and condition, and normalized to the peak EMG at 1Hz. All measures were averaged across participants within-group at each pacing rate (1-3 Hz).
2.4.2 EEG Spectral Power
The analysis of event-related power was focused on the signals obtained from electrodes overlying the region of the S1/M1 contralateral to the moving hand (electrodes C3 or C4). EEG signals were epoched relative to the movement onset of finger flexion. Flexion onset was manually marked using the acceleration signal in the flexion-extension plane as the primary criterion. When possible, EMG was used as a secondary marking criterion to screen for movements that were immediately preceded by a burst of activity in the FDI muscle. Movements at high rates in some participants with PD did not always demonstrate a clear EMG burst. In these cases, only the acceleration signal was used. To account for the effects of the acoustic stimulus on cortical oscillations, LISTEN data were epoched in an identical manner to MOVE data by superimposing the movement onset times from the corresponding movement trial. Similarly, movement onset times were superimposed on REST data. MOVE data was then normalized to the REST and LISTEN conditions allowing for better interpretation of movement related activity. EEG data were epoched into segments from 2500 ms before, to 2500 ms after each movement onset. Epoched data was low-pass filtered (dual-pass 2nd-order Butterworth filter, 62.5 Hz cut-off), and down-sampled by a factor of 4 for a sampling rate of 250 samples/second. A 2nd-order dual-pass Butterworth high-pass filter with a 1 Hz cut-off was applied to remove DC bias and drift. A Laplacian spatial filter using 8 neighboring electrodes was used to reduce the common activity from surrounding electrodes. Epochs containing eye blinks or saccades (determined from frontal electrodes), excessive scalp muscle activity, or other sources of noise that were greater than 50 μV were rejected. Epochs were then averaged across each pacing rate (1-3 Hz) and task condition (REST, LISTEN, MOVE), resulting in approximately 79 ± 21 (PD OFF), 86 ± 25 (PD ON), and 80 ± 16 (CONTROL) artifact free epochs across all pacing rates and all conditions.
Within-subject time-frequency power spectra profiles were obtained for each condition using the short-time Fourier transform method. This method provides the best compromise between temporal and frequency-domain resolution for this type of task (Allen & MacKinnon, 2010). Filtered signals were processed with a Short-Time Fourier transform (STFT) with a window length of 0.5 s (125 samples) and 124 sample overlap. Time-frequency spectral maps for the MOVE condition were then normalized to rest across all frequencies (8-40 Hz) in 1 Hz increments (Pfurtscheller & Lopes da Silva, 1999). To reduce the potential of temporal jitter between movements that may degrade the frequency-time characteristics of movements preceding and following of the center of the epoch, the analysis was confined to the cycle of a single movement centered on the epoch (e.g. +/− 0.5 s for 2 Hz movements, +/− 0.33 s for 3 Hz movements) and not the outlying movements.
To quantify differences in MROs between groups, the analysis focused on the alpha (9-14 Hz) and beta (20-25 Hz) bands. These band were chosen based on studies showing that the transition in movement-related power from synchronization to desynchronization is greatest in the “Rolandic mu rhythm” (alpha band from 9-14 Hz) and beta band from 20-24 Hz (Pfurtscheller & Lopes da Silva, 1999). In addition, we inspected each individual's movement-related power versus time plots to ensure that the synchronization-desynchronization bands were within the 9-14 and 20-25 H z ranges. Additional power spectral bands in the beta range (e.g. 15-20 Hz) were not analyzed further due to inconsistencies in the presence/absence of MROs across subjects. Normalized power in each frequency band was averaged for each group to obtain time-power plots for each pacing rate and condition. The amplitude of MROs was derived from measures of the peak-to-peak (maximum minus minimum) oscillations observed in the grand average waveform for each subject at each tone rate. To capture the overall magnitude of synchronization and desynchronization of MROs across a movement cycle relative to rest the area under the curve was calculated at each pacing rate and averaged across groups. Area values below zero reflect that the movement cycle was dominated by desynchronization relative to rest.
2.5 Statistical Analysis
Paired samples t-tests were conducted for UPDRS comparisons to evaluate differences in clinical motor skills OFF and ON medication. To examine differences in movement rate difference and peak-to-peak amplitude, a repeated measures analysis of variance model was estimated. The between-group factors were either PDOFF vs. PDON, PDOFF vs. controls, or PDON vs. controls. The within-subjects factor was pacing rate. Interaction effects were examined using Tukey's Honestly Significant Difference test.
Because power during the MOVE condition was normalized to rest, separate analysis completed to determine if differences in power at REST contributed to power during the MOVE condition. The REST condition was epoched into 1-second segments for each subject, and a fast Fourier transform was applied to each segment. The power spectrum for each segment was normalized so that total power in the spectrum was equal to 1 and then summed for each subject. To obtain the mean spectrum across subjects for each group, all epochs were summed resulting in a chi-square distribution. To compare spectra between groups, the mean spectrum of one group was divided by the mean spectrum of the second group resulting in an F distribution. This allowed for statistical comparison of spectrum between groups by obtaining the 95th percentile confidence limits from an F table using the total number of epochs in each group as the degrees of freedom. Any value below or above these limits was designated a significant difference between spectrums (Diggle, 1990).
Due to the non-Gaussian distribution of the MRO measures, a non-parametric Mann-Whitney-U test was used to examine differences in peak-to-peak amplitude and area under the curve between the PD and control groups. The Wilcoxon test was used to test for differences in these same measures between the PDOFF and PDON groups. Changes in the dependent variable across pacing rates were assessed using a Wilcoxon test. Statistical analysis was completed using SPSS and the level of significance for all tests was set at α < 0.05.
3. Results
3.1 Effects of medication on clinical motor ratings
Table 1 provides a summary of the patient characteristics and response to medication. There was a significant effect of levodopa on the UPDRS total motor score (t = 4.608, p = 0.002) and on the sum UPDRS items 23-25 (t = 2.86, p = 0.021), however there was no significant effect of medication on the UPDRS finger tapping score (Item 23; t = 1.000, p = 0.347; PDOFF = 1.9 ± 0.8; PDON = 1.8 ± 0.8).
3.2 Individual Data Examples
Figure 1A shows representative displacement data from one trial for one participant with PD in both medication states, and one control participant. In both medication states, the participant with PD showed deterioration in movement performance near a pacing rate of 2 Hz. At a pacing rate of 2.25 Hz, movements were markedly smaller, out of phase, and eventually hastened. In contrast, the control participant was able to maintain the appropriate movement rate, phase, and amplitude for the duration of the task. Specifically, the participant with PD demonstrated a decrease in amplitude (compared to 1 Hz) by 82% (off medication) and 88% (on medication) at the tone rate of 2.25 Hz. The control participant only demonstrated a decrease in amplitude (compared to 1 Hz) of only 10% at the rate of 2.25 Hz. At the tone rate of 3 Hz, decreases in amplitude were 55% (PDOFF), 64% (PDON), and 4% (CONTROL). For movement rate difference, the participant with PD moved 0.29 Hz (off medication) and 0.16 Hz (on medication) faster than the tone rate at 2.25 Hz compared to the control participant who moved 0.05 Hz faster than the tone rate at 2.25 Hz. At the tone rate of 3 Hz, movement rate difference was 0.60 Hz (PDOFF), 0.37 Hz (PDON), and 0.07 Hz (CONTROL).
Figure 1B shows representative EEG power spectra from the electrode recorded over the region of the contralateral S1/M1 and FDI EMG data from the same participant with PD in both medication states and the same control participant. At the pacing rate of 1 Hz, both the participant with PD (both medication states) and the control participant showed an attenuation of power in the alpha (~ 9-14 Hz) and beta (~ 20-25Hz) bands beginning shortly before movement onset and lasting the duration of the movement. Following movement, there was a rebound in power in these same bands. This general desynchronization-synchronization pattern was maintained up to a pacing rate of 2 Hz. In the participant with PD, the pattern transitioned to a state of persistent desynchronization with very little modulation in power at a pacing rate of 2.25 Hz which is the same rate at which movement became severely hypokinetic and FDI EMG lost a consistent modulation pattern. The control subject showed a similar transition to persistent desynchronization in the beta band, but maintained movement performance. However, note that in the participant with PD, the power was attenuated to a greater extent (darker blue) than the control participant. Moreover, the attenuation at higher pacing rates (> 2 Hz) ON medication was less than OFF medication. This is in contrast to the LISTEN and REST conditions in both the participant with PD and the control participant, where power remained consistent across all pacing rates (data not shown).
3.3 Kinematic Data
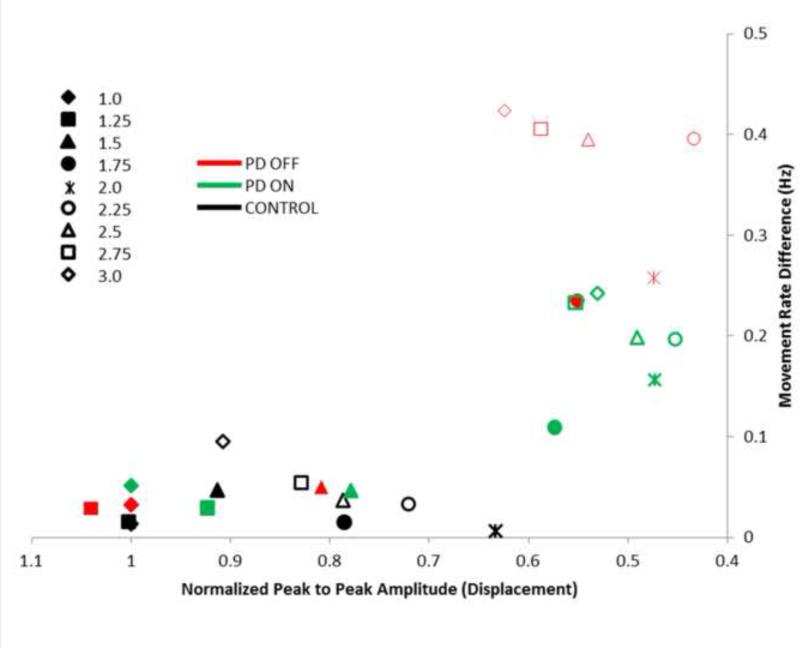
Movement rate difference (actual movement rate – tone rate) is plotted against normalized peak-to-peak amplitude in Figure 2. A positive movement rate difference reflects the presence of hastening (moving faster than the intended tone) whereas a negative difference indicates that movements were slower than the intended tone. Data points in the upper right quadrant of the plot indicate a decrease in amplitude and increase in movement rate. As in our previous study (Stegemöller et al., 2009), the participants with PD showed evidence of combined hypokinesia (low peak-to-peak amplitude) and hastening (high movement rate difference) at pacing rates ranging from 1.75 to 3 Hz both OFF (red data points) and ON (green data points) medication compared to the control participants (black data points). Participants with PD tested in this study did not demonstrate a slowing of movement rate.
Figure 2.
Mean normalized peak to peak amplitude plotted against movement rate difference for each group across each pacing rated. Closed symbols are pacing rates below 2 Hz and open symbols are pacing rates above 2 Hz.
Statistical analysis revealed that movement rate difference was significantly increased in the PDOFF group compared to the control group (F(1) = 3.304 , p = 0.045) but not between the PDON and control groups (F(1) = 2.567, p = 0.125). Analysis of interaction effects revealed that the PDOFF group significantly differed from the control group at pacing rates of 2.25 Hz and above (p < 0.05 for all pacing rates 2.25 Hz and above). To examine the pacing rate at which hastening was evident, the movement rate difference at 1 Hz was compared to the remaining pacing rates within the PDOFF and PDON groups. The PDOFF group showed a significant increase in movement rate difference at pacing rates of 2.5 Hz and above (F(1) > 5.601, p < 0.045 for all pacing rates of 2.5 Hz and above), while the PDON group showed significant differences at 2.75 Hz and above (F(1) > 7.398, p < 0.03 for all pacing rates of 2.75 Hz and above). See Supplementary Table S1 for group means and standard error across tone rates.
Statistical analysis of normalized peak-to-peak amplitude revealed a significant effect of pacing rate (F(8) > 9.674, p < 0.001), but no significant differences between groups (F(1) < 2.257, p > 0.152) or interaction effects (F(8) < 1.115, p > 0.358). To examine the pacing rate at which hypokinesia was evident, normalized peak-to-peak amplitude at 1Hz was compared to the remaining pacing rates within the PDOFF and PDON groups. Both the PDOFF and PDON group showed a significant decrease in movement amplitude compared to 1Hz at pacing rates of 1.75 Hz and above (F(1) > 5.601, p < 0.045 for all pacing rates of 1.75 Hz and above). There was no significant effect of levodopa on any of the kinematic measures. See Supplementary Table S1 for group means and standard error across tone rates.
3.4 Power Spectra at Rest
Figure 3 shows the difference in relative power in the electrode recorded over the region of contralateral S1/M1 at rest between the PD and control groups. The participants with PD showed an increase in relative power in the alpha band (~ 5-12 Hz) and suppression in the beta band (~ 13-30 Hz) in both medication states (Fig 3A) compared to controls. Figure 3B shows the ratio of the mean spectra between groups. The PD group, both medication states, had a significant increase in relative power in the alpha band and a significant decrease in relative power in the beta band (peak above or below the 95th lower confidence limit) compared to the control group. There were no significant differences between the spectra of the PDOFF and PDON groups across frequencies (Fig 3B).
Figure 3.
(A) Relative power spectra at rest recorded from the electrode over the region of the contralateral S1/M1. (B) Ratio of power spectra. Dashed lines represent 95th % confidence intervals. Any portion of the curve outside of the dashed lines represents significant difference in power spectra between groups. Red shaded bars indicate greater power and blue shaded bars indicate less power in the PD groups compared to the control group in the frequency bands of interest.
3.5 Movement Related Oscillations
Mean (averaged across all participants in each group) normalized time-frequency power spectra recorded from the electrode over the region of contralateral S1/M1 are shown in Figure 4. The control group showed an attenuation of power below resting levels prior to movement onset that lasted for the duration of movement. Following completion of movement, there was a rebound in power above resting levels. This general pattern was maintained across all pacing rates. A similar pattern of power attenuation and rebound was evident at lower pacing rates (< 2 Hz) in the PD group in both the OFF and ON medication states, but MROs at higher pacing rates (> 2 Hz) were characterized by a sustained suppression of power that exceeded the attenuation observed in the control group. Even at low movement rates (e.g. 1 Hz), there was reduced modulation of oscillations in the alpha and beta bands (Figure 5) in the PD group.
Figure 4.
Mean normalized power from 8-30 Hz recorded from the electrode over the region of the contralateral S1/M1 and mean normalized FDI EMG for each group across each pacing rate. Dashed lines indicate movement onset. Gray bars indicated one movement cycle.
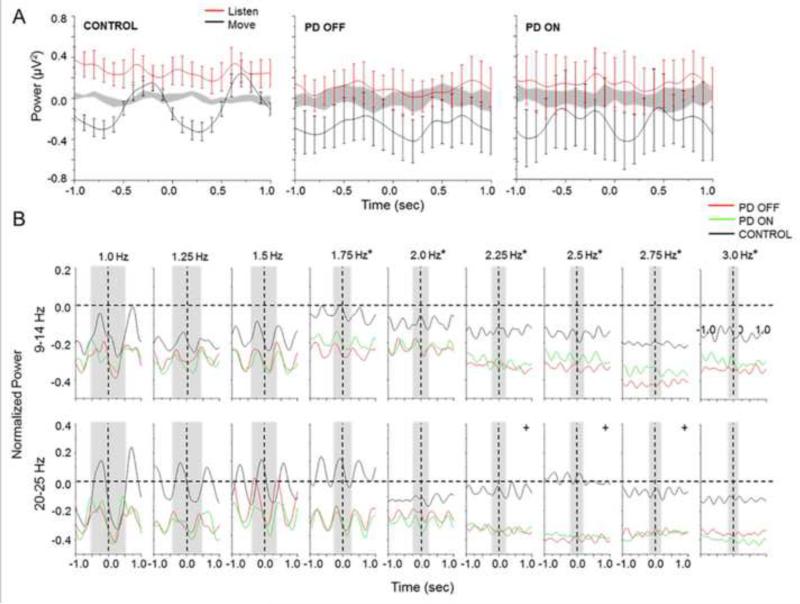
Figure 5.
(A) Mean and standard error for power across 20-25 Hz recorded from the electrode over the region of the contralateral S1/M1 for move, listen, and rest (shaded gray area) conditions. (B) Mean normalized time amplitude averaged across 9-14 Hz and 20-25 Hz for each pacing rate from the electrode recorded over the region of the contralateral S1/M1. Vertical dashed lines indicate movement onset. Gray bars indicate one movement cycle. Asterisks designate significant differences (<0.05) between peak to peak amplitude at 1 Hz and the remaining pacing rates in both frequency bands of interest. Crosses designated differences between both the PDOFF and PDON groups and Control group at the designated pacing rate.
To demonstrate differences in MROs within specific frequency bands of interest, Figure 5B shows the mean normalized time amplitude profiles for the PDOFF, PDON, and control groups averaged across 9-14 Hz and 20-25 Hz. Across all groups, peak-to-peak amplitude decreased from 1 to 3 Hz in both bands, with the greatest decrease at pacing rates of 2 Hz and above. Statistical analysis revealed that peak-to-peak amplitude in both bands significantly decreased relative to amplitude at 1 Hz for pacing rates of 1.75 Hz and above for all groups (Z(1) < −2.073, p < 0.038 for all pacing rates of 1.75 Hz and above). Moreover, significant group differences were revealed in both frequency bands between the PDOFF and control groups (Z(1) = −3.325, p < 0.001) and PDON and control groups (Z(1) = −2.102, p = 0.036). Across pacing rates, the PDOFF and PDON group significantly differed from the control group from 2.25 to 2.75 Hz and above in the 20-25 Hz band (Z(1) < −2.075, p < 0.038 for all pacing rates from 2.25 to 2.75 Hz). There was no significant effect of medication on peak-to-peak amplitude. See Supplementary Table S2 for group means and standard error across tone rates for the alpha and beta bands.
Across all pacing rates, the PDOFF and PDON groups show a greater amount of attenuation (desynchronization) in both bands of interest compared to the control group. Area under the curve was obtained as a measure of the difference in attenuation. Statistical analysis revealed there were no effects of pacing rate for all groups across both frequency bands of interest. However, significant differences were revealed between both PDOFF and PDON groups and the control group for both frequency bands (PDOFF vs. Control: Z(1) < −2.929, p < 0.003 for both bands; PDON vs. Control: Z(1) < −2.166, p < 0.03 for both bands). Medication significantly affected area under the curve in both bands (Z(1) < −2.542, p < 0.011 for both bands). Note that these differences reflect significant differences in the distribution of the data and not differences between means. See Supplementary Table S3 for group means and standard error across tone rates for the alpha and beta bands.
Figures 6 and 7 demonstrate the general relationships between changes in movement performance (movement rate and movement amplitude) and associated MROs (MRO amplitude and area under the curve) in both the alpha and beta bands. In general, for movement rates below pacing rates of 2 Hz, MRO amplitude is larger, and for movement rates above pacing rates of 2 Hz, MRO amplitude is smaller in both the alpha (Figure 6A) and beta (Figure 6C) bands. Moreover, as movement rate difference increases (i.e., as hastening emerges) in the PD groups, MRO amplitude in both the alpha (Figure 6A) and beta (Figure 6C) bands decrease. Likewise for movement amplitude at pacing rates below 2 Hz, MRO amplitude is larger, and for movement amplitude at pacing rate above 2 Hz, MRO amplitude is smaller in both alpha (Figure 6B) and beta (Figure 6D) bands. This effect appears to be more disparate in the PDOFF and PDON groups. For area under the curve (Figure 7), there is an observable difference between PD groups and the control group. In general across all pacing rates, it can be observed that the area under the curve is smaller in the PD groups. This observation is the same when comparing movement rate (figure 7A and 7C) and movement amplitude (Figure 7B and 7D) with area under the curve in both the alpha and beta bands.
Figure 6.
Mean movement rate difference and mean normalized peak to peak amplitude plotted against the mean peak to peak amplitude of MROs across groups for each pacing rate for 9-14 Hz (A and B) and 20-25 Hz bands (C and D). Closed symbols indicate pacing rates below 2 Hz. Open symbols indicate pacing rates above 2 Hz.
Figure 7.
Mean movement rate difference and mean normalized peak to peak amplitude plotted against the mean area under the curve of MROs across groups for each pacing rate for 9-14 Hz (A and B) and 20-25 Hz bands (C and D). Closed symbols indicate pacing rates below 2 Hz. Open symbols indicate pacing rates above 2 Hz.
4. Discussion
The main finding from this study was that MROs in the alpha and beta bands recorded over the region of the contralateral S1/M1 were associated with a marked increase in the relative amount of desynchronization and reduced modulation, particularly at higher movement rates. The movement rates at which the changes in modulation and desynchronization were most pronounced coincided with the rates where hypokinesia and hastening emerged. In addition, the abnormal oscillations in the participants with PD were not significantly altered by levodopa suggesting that non-dopaminergic mechanisms mediate this impairment.
4.1 Movement Performance Transition
In keeping with our previous study (Stegemöller et al., 2009), there was a distinct transition in behavior at pacing rates near 2 Hz in participants with PD. This transition was typically characterized by the emergence of hypokinesia and hastening. In healthy adults, a spontaneous transition in the phase between movement and the pacing cue typically occurs at this rate (Toma et al., 2002; Jantzen et al., 2005; Stegemöller et al., 2009). Thus, the decrement in movement amplitude and hastening that occurs near to and above 2 Hz in people with PD may reflect an inability to transition coordination patterns with an accompanying loss of movement stability. Two movements per second is also the rate at which movements typically change from discrete to continuous control (Huys et al., 2008; Sternad et al., 2013). Spencer and colleagues (2007) have hypothesized that discrete movement timing is predominantly controlled by the cerebellum whereas continuous movement timing may be dependent upon the basal-ganglia. Accordingly, the loss of movement control at tone rates near 2 Hz and corresponding abnormal suppression of MROs we observed in the participants with PD may reflect dysfunction of basal-ganglia thalamocortical networks that regulate continuous movement. However, the absence of significant change in performance with medications suggests that this control is predominantly mediated by non-dopaminergic mechanisms.
4.2 Suppression of Movement Related Oscillations
Much like the transition in movement behavior, we observed a distinct transition in the pattern of MROs at the pacing rate of 2 Hz in both the participants with PD and healthy adults (Figure 5B). During low rate movements alpha and beta band oscillations were modulated with a desynchronization-synchronization pattern similar to that observed during single discrete movements (Pfurtscheller & Lopes da Silva, 1999). At pacing rates near to and above 2 Hz the pattern of desynchronization-synchronization was markedly attenuated and there was a persistent suppression of alpha and beta oscillations below baseline levels. Although this transition in MRO pattern from lower (< 2 Hz) to higher rate (> 2 Hz) movements has been described for both scalp surface EEG recordings in healthy adults (Toma et al., 2002) and local field potential recordings of the subthalamic nucleus (STN) in patients with PD (Joundi et al, 2013), this study is the first to show distinct differences between participants with PD and matched control participants. The principal difference in MROs between PD and control groups was the mean level of power relative to baseline (rest) in both the alpha and beta bands. The pattern of beta band oscillations in the control participants during low rate movements (e.g., 1 to 1.75 Hz) was characterized by peak synchronization (+10 to 20%) and desynchronization (−10 to −30%) comparable to those reported in healthy adults (Toma et al., 2002). In contrast, the average peak amplitude of beta band synchronization at low movement rates in participants with PD remained below baseline and the average peak desynchronization reached approximately 40% below baseline. These modulation patterns were most distinct in the 20-25 Hz band and were not significantly changed by levodopa. A similar pattern of reduced movement-related synchronization and increased desynchronization in the beta band (13-30 Hz) at low movement rates (0.5 – 0.67 Hz) in patients with PD has been reported previously (Praamstra and Pope, 2007; te Woerd et al., 2014). In the present study, we also observed a pattern of increased desynchronization and decreased peak synchronization was observed in the alpha band of participants with PD (Figure 5B). The magnitudes of desynchronization-synchronization we observed were in keeping with the local field potential pattern recorded from the STN during low rate movements (0.5 or 1.0 Hz) on medication (Joundi et al., 2013). Thus, MROs in the PD group, both off and on medication, were characterized by a bias towards increased desynchronization and a reduced capacity for rebound synchronization between movements compared to control participants at pacing rates below 2 Hz.
A persistent relative desynchronization of alpha and beta oscillations was observed in both participants with PD and control participants at pacing rates near to and above 2 Hz, however, the level of desynchronization in the PD group was markedly increased. On average, beta oscillations in the PD group (off and on medication) were suppressed nearly two-fold compared to the control group (PD ~ 40%, controls ~ 20%). Alpha band oscillations were also consistently suppressed (~ 20%) at pacing rates of 2 Hz and above in the PD group (off and on medication), while the peak desynchronization observed in the control group did not exceed 20%. Joundi and colleagues (2013) reported similar levels of beta and alpha desynchronization in the STN for movements at a pacing rate of 2 Hz. Taken together, these data suggest that alpha and beta oscillations at the motor cortical and STN levels are abnormally desynchronized in people with PD compared to healthy older adults, particularly at movement rates above 2 Hz.
Currently, the physiological roles of alpha and beta desynchronization are poorly understood. Periods of alpha and beta desynchronization are associated with a transition from rhythmic burst firing to single spike firing with a resulting suppression of local field potential oscillations (Steriade, 2006). During this period, motor cortical excitability is increased, intracortical inhibition is decreased, and sensory input to motor cortical areas is suppressed (Seki & Fetz, 2012; Takemi et al., 2013). Jenkinson and Brown (2011) have proposed that the level of suppression of sensorimotor beta oscillations reflects the likelihood that a motor action is to be performed. Accordingly, the persistent desynchronization of beta oscillations that is observed during movements above 2Hz may reflect overlapping neuronal activity associated with the preparation of the forthcoming movement in concert with changes in motor cortical excitability, intracortical inhibition, and sensory gating that mediate the execution of the present movement.
Increased responsiveness of the basal ganglia to cortical input may explain abnormal desynchronization of alpha and beta oscillations. In addition to increased phasic firing during slow wave oscillations (Magill et al., 2001), subthalamic and globus pallidus internus neurons also show an abnormally prolonged period of inhibition (firing rate suppression) in response to motor cortical stimulation in the dopamine depleted state (Kita & Kita, 2011). Thus, a prolonged volley of motor cortical input to the basal ganglia in the parkinsonian state might result in abnormal silencing of pallidal input to thalamocortical neurons, resulting in increased facilitation of motor output. When movement rates are increased, the periods of prolonged pallidal suppression between movements may begin to overlap resulting in a reduced capacity to phasically modulate thalamo-motor cortical activity, particularly a stop signal between movements and corresponding rebound synchronization. Appropriate electrophysiological experiments will be required to test this hypothesis.
4.3 Resting State Oscillations
Beta oscillations recorded over the region of contralateral S1/M1 in the resting state were also suppressed in the participants with PD compared to control subjects. Several previous studies have similarly shown suppressed cortical beta oscillations at rest, in conjunction with increased theta and alpha oscillations, in people with comparable severity of PD to the cohort tested in the present study (Bosboom et al., 2006; Stoffers et al., 2007; Heinrichs-Graham et al., 2014). In contrast, Pollok and colleagues (2012) showed that beta power was increased in both de novo (unmedicated) and early stage medicated patients with PD compared to controls. The average age of the group in this study was 55 years and the mean UPDRS III scores was 11. Thus, the differences in our findings might be explained by stage of disease. This raises the interesting possibility that exaggerated cortical and basal ganglia oscillations are present in early stage disease but with increasing disease severity either compensatory or additional neuropathological changes contribute to a suppression of cortical beta oscillations.
At first glance, the finding of reduced beta oscillations at rest in PD seems to be at odds with literature from animal and human studies showing that beta band oscillations in the basal ganglia are enhanced at rest in the parkinsonian state (Brown, 2003; Wichmann & Delong, 2003) and correlate with improvements in motor performance, suggesting that enhanced resting state oscillations have an anti-kinetic effect (Brown, 2003; Kuhn et al, 2006). However, our data challenge the assumptions that either hypersynchrony originates in the cortex and is transmitted to the basal ganglia or that excessive basal ganglia oscillations are translated to thalamocortical pathways. The balance of evidence suggests that the emergence of exaggerated beta oscillations in the subthalamic nucleus and globus pallidus following dopaminergic denervation results, in part, from alterations in the response of STN neurons to cortical input (via the hyperdirect pathway) (Magill et al., 2001; Mallet et al., 2008a,b; Holgado et al., 2010; Kita & Kita, 2011; Cruz et al., 2011; Chu et al., 2015). In this state, the reciprocal connections of the STN-globus pallidus externus circuit tends to amplify the beta oscillations that are transmitted from cortex to the STN via the hyperdirect pathway. In this manner, low amplitude oscillations at the level of the cortex will be converted to exaggerated synchrony within and between the nuclei of the basal ganglia, thus reinforcing an akinetic state.
4.4 Limitations
To avoid confounding kinematic and EEG signals associated with large amplitude tremor, the inclusion/exclusion criteria for this study restricted the cohort to those with a predominantly akinetic-rigid syndrome, so interpretation to the broader population of people with PD should be done with caution. The focus of this paper was on MROs recorded over the region of S1/M1 contralateral to movements. A source localization approach to verify the source of the signal was beyond the scope of this study, thus it is possible that other cortical (e.g. dorsal premotor cortex, primary sensorimotor cortex) or subcortical regions may have contributed to the signal recorded over the region of the contralateral S1/M1. The suppression of MROs at higher movement rates may also be attributed, in part, to spectral smearing due to the time-frequency method used to generate the spectral power. The STFT method used in this study has been shown to have a temporal resolution of 7 samples (28 ms at 250 samples/s) and 4 samples (16 ms at 250 samples/s) for pure 10 Hz and 20 Hz oscillations respectively (Allen and MacKinnon, 2010). However, this resolution is reduced with the addition of noise and for lower frequency components to the spectra. Thus, we cannot rule out the possibility that some of the suppression of MROs was due to temporal overlap, particularly for lower frequency oscillations at higher movement rates. The lack of a significant medication effect on motor behavior and MROs might be explained by a relatively poor response to medication in our patient cohort. Nonetheless, there was an 8 point (28%) decrease in the total UPDRS motor score which is in keeping with the expected change produced by medication within the first few hours following overnight withdrawal. The lack of change in measures of bradykinesia (items 23, 24 and 25 of the UPDRS) with medication is consistent with our quantitative behavioral findings.
5. Conclusion
Our finding of abnormal suppression of MROs in both the alpha and beta bands at rest and during repetitive movement in participants with PD suggests that the mechanisms contributing to movement-related desynchronization and synchronization are impaired in the parkinsonian state. We propose that this impairment results in excessive desynchronization and/or the inability to generate rebound synchronization that contributes to the emergence of hypokinesia and hastening at movement rates near 2 Hz. The absence of significant change in the level or pattern of MROs during movement performance after the administration of levodopa suggests that non-dopaminergic mechanisms contribute to this impairment.
Supplementary Material
Highlights.
Impairments in the performance of repetitive movement in Parkinson's disease is associated with excessive and prolonged desynchronization of motor cortical oscillations.
This impairment is most marked at movement rates that are considered to be mediated by continuous or automatic control processes.
The absence of significant change in motor performance and motor cortical oscillations with dopaminergic drugs suggests that non-dopaminergic mechanisms contribute to this disorder.
Acknowledgements
We thank Christopher Robinson and Di Zhang for their assistance with construction of the finger movement device, Dr. Lance Myers for the development of signal processing software, and Dr. Mack Shelley for statistical advice. This work was supported by National Institutes of Health grant number RO1 NS054199-01A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None.
References
- Allen DP, MacKinnon CD. Time-frequency analysis of movement-related spectral power in EEG during repetitive movements: a comparison of methods. J Neurosci Methods. 2010;186:107–5. doi: 10.1016/j.jneumeth.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androulidakis AG, Brucke C, Kempf F, Kupsch A, Aziz T, Kuhn AA, Brown P. Amplitude modulation of oscillatory activity in the subthalamic nucleus during movement. Eur J Neurosci. 2008;27:1277–84. doi: 10.1111/j.1460-9568.2008.06085.x. [DOI] [PubMed] [Google Scholar]
- Bosboom JL, Stoffers D, Stam CJ, van Dijk BW, Verbunt J, Berendse HW, Wolters ECh. Resting state oscillatory brain dynamics in Parkinson's disease: an MEG study. Clin Neurophysiol. 2006;117:252–31. doi: 10.1016/j.clinph.2006.06.720. [DOI] [PubMed] [Google Scholar]
- Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophisiology of Parkinson's disease. Move Disord. 2003;18:357–63. doi: 10.1002/mds.10358. [DOI] [PubMed] [Google Scholar]
- Chu HY, Atherton JF, Wokosin D, Surmeier DJ, Bevan MD. Hetersynaptic regulation of external globus pallidus inputs to the subthalamic nucleus by the motor cortex. Neuron. 2015;85:364–76. doi: 10.1016/j.neuron.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz AV, Mallet N, Magill PJ, Brown P, Averbeck BB. Effects of dopamine depletion on information flow between the subthalamic nucleus and external globus pallidus. J. Neurophysiol. 2011;106:2012–23. doi: 10.1152/jn.00094.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle PJ. A point process modelling approach to rasied incidence of a rare phenomenon in the vicinity of a pre-specified point. J R Stat Soc. 1990;160:491–505. [Google Scholar]
- Ellis T, Cavanaugh JT, Earhart GM, Ford MP, Foreman KB, Dibble LE. Which measures of physical function and motor impairment best predict quality of life in Parkinson's disease? Parkinsonism Relat Disord. 2011;17:693–697. doi: 10.1016/j.parkreldis.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbil N, Ungan P. Changes in the alpha and beta amplitudes of the central EEG during the onset, continuation, and offset of long-duration repetitive hand movements. Brain Res. 2007;1169:44–56. doi: 10.1016/j.brainres.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Espay AJ, Giuffrida JP, Chen R, Payne M, Mazzella F, Dunn E, et al. Differential response of speed, amplitude, and rhythm to dopaminergic medications in Parkinson's disease. Mov Disord. 2011;26:2504–8. doi: 10.1002/mds.23893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement disorder society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- Grimbergen YA, Langston JW, Roos RA, Bloem BR. Postural instability in Parkinson's disease: the adrenergic hypothesis and the locus coeruleus. Expert Rev Neurother. 2009;9:279–90. doi: 10.1586/14737175.9.2.279. [DOI] [PubMed] [Google Scholar]
- Hebb AO, Darvas F, Miller KJ. Transient and state modulation of beta power in human subthalamic nucleus during speech production and finger movement. Neurosci. 2012;202:218–33. doi: 10.1016/j.neuroscience.2011.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham H, Kurz MJ, Becker KM, Santamaria PM, Gendelman HE, Wilson TW. Hypersynchrony despite pathologically reduced beta oscillations in patients with Parkinson's disease: a pharmo-magnetoencephalography study. J Neurophysiol. 2014;112:1739–1747. doi: 10.1152/jn.00383.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Castro-Alamancos MA. Neocortex network activation and deactivation states controlled by the thalamus. J Neurophysiol. 2010;103:1147–57. doi: 10.1152/jn.00955.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgado AJ, Terry JR, Bogacz R. Conditions for the generation of beta oscillations in the subthalamic nucleus-globus pallidus network. J. Neurosci. 2010;30:12340–52. doi: 10.1523/JNEUROSCI.0817-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huys R, Studenka BE, Rheaume NL, Zelaznik HN, Jirsa VK. Distinct timing mechanisms produce discrete and continuous movements. PLoS Comput Biol. 2008;4(4):e1000061. doi: 10.1371/journal.pcbi.1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen KJ, Steinberg FL, Kelso JA. Functional MRI reveals the existence of modality and coordination-dependent timing networks. Neuroimage. 2005;25:1031–42. doi: 10.1016/j.neuroimage.2004.12.029. [DOI] [PubMed] [Google Scholar]
- Jenson O, Goel P, Kopell N, Pohja M, Hari R, Ermentrout B. On the human sensorimotor-cortex beta rhythm: sources and modeling. Neuroimage. 2005;26:347–55. doi: 10.1016/j.neuroimage.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Joundi RA, Brittain JS, Green AL, Aziz TZ, Brown P, Jenkinson N. Persistent suppression of subthalamic beta-band activity during rhythmic finger tapping in Parkinson's disease. Clin Neurophysiol. 2013;124:565–73. doi: 10.1016/j.clinph.2012.07.029. [DOI] [PubMed] [Google Scholar]
- Karachi C, Grabli D, Bernard FA, Tande D, Wattiez N, Belaid H, et al. Cholinergic mesencephalic neurons are involved in gait and postural disturbances in Parkinson's disease. J Clin Invest. 2010;120:2745–54. doi: 10.1172/JCI42642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Kita T. Cortical stimulation evokes abnormal responses in the dopamine-depleted rat basal ganglia. J Neurosci. 2011;31:10311–22. doi: 10.1523/JNEUROSCI.0915-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn AA, Kupsch A, Schneider GH, Brown P. Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson's disease. Eur J Neurosci. 2006;23:1956–60. doi: 10.1111/j.1460-9568.2006.04717.x. [DOI] [PubMed] [Google Scholar]
- Magill PJ, Bolam JP, Bevan MD. Dopamine regulates the impact of the cerebral cortex on the subthalamic nucleus-globus pallidus network. Neurosci. 2001;106:313–30. doi: 10.1016/s0306-4522(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Marton LF, Bolam JP, Brown P, Magill PJ. Parkinsonian beta band oscillations in the external globus pallidus and their relationship with subthalamic nucleus activity. J Neurosci. 2008a;28:14245–58. doi: 10.1523/JNEUROSCI.4199-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Sharott A, Csicsvari J, Bolam JP, Brown P, Magill PJ. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J. Neurosci. 2008b;28:4795–806. doi: 10.1523/JNEUROSCI.0123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayville JM, Jantzen KJ, Fuchs A, Steinburg FL, Kelso JA. Cortical and subcortical networks underlying syncopated and synchronized coordination revealed using fMRI. Functional magnetic resonance imaging. Hum Brain Mapp. 2002;17:214–29. doi: 10.1002/hbm.10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau C, Ozsancak C, Blatt JL, Deramure P, Destee A, Defebvre L. Oral festination in Parkinson'd disease: biomechanical analysis and correlation with festination and freezing of gait. Mov Disord. 2007;22:1503–6. doi: 10.1002/mds.21549. [DOI] [PubMed] [Google Scholar]
- Muslimovic D, Speelman JD, Schmand B, de Haan RJ, CARPA Study Group Determinants of disability and quality of life in mild to moderate Parkinson disease. Neurology. 2008;70:2241–247. doi: 10.1212/01.wnl.0000313835.33830.80. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD. Functional properties of human primary motor cortex gamma oscillations. J Neurophysiol. 2010;104:2873–85. doi: 10.1152/jn.00607.2010. [DOI] [PubMed] [Google Scholar]
- Nakamura R, Nagasaki H, Narabayashi H. Disturbances of rhythm formation in patients with Parkinson's disease: part I. Characteristics of tapping response to periodic signals. Percept Mot Skills. 1978;46:63–75. doi: 10.2466/pms.1978.46.1.63. [DOI] [PubMed] [Google Scholar]
- Ni Z, Bahl N, Gunraj CA, Mazzella F, Chen R. Increased motor cortical facilitation and decreased inhibition in Parkinson's disease. Neurology. 2013;80:1746–53. doi: 10.1212/WNL.0b013e3182919029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwboer A, Vercruysse S, Feys P, Levin O, Spildooren J, Swinnen S. Upper limb movement interruptions are correlated to freezing of gait in Parkinson's disease. Eur J Neurosci. 2009;29:1422–30. doi: 10.1111/j.1460-9568.2009.06681.x. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10:734–44. doi: 10.1016/S1474-4422(11)70143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Boyle D, Freeman JS, Cody FW. The accuracy and precision of timing or self-paced, repetitive movements in subjects with Parkinson's disease. Brain. 1996;119:51–70. doi: 10.1093/brain/119.1.51. [DOI] [PubMed] [Google Scholar]
- Pastor MA, Jahanshahi M, Artieda J, Obeso JA. Performance of repetitive wrist movements in Parkinson's disease. Brain. 1992;115:875–91. doi: 10.1093/brain/115.3.875. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–57. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Pollok B, Krause V, Martsch W, Wach C, Schnitzler A, Südmeyer M. Motor-cortical oscillations in early stages of Parkinson's disease. J Physiol. 2012;590:3203–32-12. doi: 10.1113/jphysiol.2012.231316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praamstra P, Pope P. Slow brain potential and oscillatory EEG manifestations of impaired temporal preparation in Parkinson's disease. J Neurophysiol. 2007;98:2848–2857. doi: 10.1152/jn.00224.2007. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Inzelberg R, Rothwell JC. Changes in excitability of motor cortical circuitry in patients with Parkinson's disease. Ann Neurol. 1995;37:181–8. doi: 10.1002/ana.410370208. [DOI] [PubMed] [Google Scholar]
- Seki K, Fetz EE. Gating of sensory input at spinal and cortical levels during preparation and execution of voluntary movement. J Neurosci. 2012;32:890–902. doi: 10.1523/JNEUROSCI.4958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RM, Verstynen T, Brett M, Ivry R. Cerebellar activation during discrete and not continuous timed movements: an fMRI study. Neuroimage. 2007;26:378–87. doi: 10.1016/j.neuroimage.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegemöller EL, Simuni T, MacKinnon CD. Timing and frequency barriers during repetitive finger movements in patients with Parkinson's disease. Mov Disord. 2009;24:1162–69. doi: 10.1002/mds.22535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. Grouping of brain rhythms in corticothalamic systems. Neurosci. 2006;137:1087–106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Sternad D, Marino H, Charles SK, Duarte M, Dipietro L, Hogan N. Transitions between discrete and rhythmic primitives in a unimanual task. Front Comput Neurosci. 2013;7:90. doi: 10.3389/fncom.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffers D, Bosboom JL, Deijen JB, Wolters EC, Berendse HW, Stam CJ. Slowing of oscillatory brain activity is a stable characteristic of Parkinson's disease without dementia. Brain. 2007;130(Pt 7):1847–60. doi: 10.1093/brain/awm034. [DOI] [PubMed] [Google Scholar]
- Takemi M, Masakado Y, Liu M, Ushiba J. Event-related desynchronization reflects downregulation of intracortical inhibition in human primary motor cortex. J Neurophysiol. 2013;110:1158–66. doi: 10.1152/jn.01092.2012. [DOI] [PubMed] [Google Scholar]
- te Woerd ES, Oostenveld R, de Lange FP, Praamstra P. A shift from prospective to reactive modulation of beta-band oscillations in Parkinson's disease. Neuroimage. 2014;100:507–519. doi: 10.1016/j.neuroimage.2014.06.039. [DOI] [PubMed] [Google Scholar]
- Toma K, Mima T, Matsuoka T, Gerloff C, Ohnishi T, Koshy B, et al. Movement rate effect on activation and functional coupling of motor cortical areas. J Neurophysiol. 2002;88:3377–85. doi: 10.1152/jn.00281.2002. [DOI] [PubMed] [Google Scholar]
- Vercruysse S, Spildooren J, Heremans E, Vandenbossche J, Wenderoth N, Swinnen SP, et al. Abnormalitites and cue dependence of rhythmical upper-limb movements in Parkinson patients with freezing of gait. Neurorehabil Neural Repair. 2012;26:636–45. doi: 10.1177/1545968311431964. [DOI] [PubMed] [Google Scholar]
- Vercruysse S, Gilat M, Shine JM, Heremans E, Lewis S, Nieubower A. Freezing beyond gait in Parkinson's disease: a review of current neurobehavioral evidence. Neurosci Biobehav Rev. 2014;43:213–27. doi: 10.1016/j.neubiorev.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Delong MR. Pathophysiology of Parkinson's disease: the MPTP primate model of the human disorder. Ann N Y Acad Sci. 2003;991:199–213. doi: 10.1111/j.1749-6632.2003.tb07477.x. [DOI] [PubMed] [Google Scholar]
- Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol. 2003;60:337–41. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.