Abstract
Nanocrystals of magnetite (Fe3O4) in a meteorite from Mars provide the strongest, albeit controversial, evidence for the former presence of extraterrestrial life. The morphological and size resemblance of the crystals from meteorite ALH84001 to crystals formed by certain terrestrial bacteria has been used in support of the biological origin of the extraterrestrial minerals. By using tomographic and holographic methods in a transmission electron microscope, we show that the three-dimensional shapes of such nanocrystals can be defined, that the detailed morphologies of individual crystals from three bacterial strains differ, and that none uniquely match those reported from the Martian meteorite. In contrast to previous accounts, we argue that the existing crystallographic and morphological evidence is inadequate to support the inference of former life on Mars.
The startling report by McKay et al. (1) of evidence for former life on Mars has attracted major interest scientifically and with the general public. The evidence was based on four structural and chemical features in a meteorite, ALH84001, that originated on Mars. No single line of evidence was presented as individually compelling. However, the authors proposed that, when taken collectively, the separate observations provided a credible case for the past existence of life. This assumption has since been vigorously challenged but not disproved.
Data amassed since the McKay paper have resulted in the effective elimination of all but one of the original arguments. The case for former Martian life now rests on the identification of a small subset of the magnetite crystals in ALH84001 as biogenic in origin. A recent report by Friedmann et al. (2) interprets scanning electron microscope images of lines of small, bright objects in ALH84001 as magnetite crystals that are aligned in chains. Thomas-Keprta et al. (3–5), on the other hand, address the morphologies of individual magnetite grains and report that a minority (≈27%) of the magnetite crystals in ALH84001 are “indistinguishable” from those produced by a particular strain of terrestrial magnetotactic bacteria.
Both of these arguments are flawed. Friedmann et al. interpret contrast features that are at the limit of their available resolution, with no supporting chemical or microstructural evidence that the features they describe are indeed magnetite crystals. Moreover, magnetotactic bacteria are ubiquitous on Earth, and yet intact chains of nanosized magnetite crystals from bacteria are rarely found in terrestrial geological samples (5), suggesting that such chains are unlikely to survive geological processes. Friedmann et al. acknowledge that it is difficult to understand the intact occurrence of their hypothesized magnetite chains within fractures in which it is most unlikely that aquatic magnetotactic bacteria ever lived; the chains from dead bacteria would somehow have had to migrate intact into the fractures and remain there unbroken. In the absence of chemical and structural data, it is difficult to exclude the possibility of other semiperiodic features (such as serrated grain edges, possibly decorated selectively during sample coating).
Thomas-Keprta et al. (hereafter collectively referred to as T.-K.) focus on individual magnetite crystals in ALH84001 and conclude that “these Martian magnetites (are) physically and chemically identical to . . . magnetites produced by magnetotactic bacteria strain MV-1” (5). They cite six well known features of bacterial magnetite and conclude that “when taken collectively” these characteristics indicate a biogenic origin for the meteoritic magnetite. By their own reasoning, only a minority of the magnetite crystals in ALH84001 qualify for biogenic status on the basis of their sizes and shapes. It is therefore astonishing, and not widely appreciated, that the entire evidence for the former presence of life on Mars now rests on the shapes of a small fraction of the magnetite nanocrystals in ALH84001 (and their possible alignment in chains).
Much has been written about the biogenic Fe minerals in magnetotactic bacteria and about which of their features, if any, provide unambiguous evidence for former life [refs. 5, 6 (and references therein), 7, 8]. Given the importance of the papers and abstracts of T.-K., as well as the confidence with which their conclusions are presented, it is important to examine the reliability of their measurements. These measurements consist of the morphologies (3, 5) and aspect ratios (width/length; refs. 3 and 5) of a selection of magnetite crystals. We consider these issues below, as well as ambiguities in the terminology used to describe the morphologies of magnetite crystals from magnetotactic bacteria and the consequent confusion that results.
Tilting Measurements
Many studies (3, 9–14) have shown that magnetite crystals from magnetotactic bacteria exhibit the cube {100}, octahedron {111}, and dodecahedron {110} crystallographic forms. However, T.-K. go considerably further in their interpretations, giving the relative development of the various forms greater significance than do previous authors. Although the identification of specific faces in undistorted macroscopic crystals is simple, it is far more complicated in nanometer-sized crystals, such as those that occur in bacterial strain MV-1. Careful and lengthy tilting experiments are required to determine the angles between the faces and thereby to identify them. The precise tilting of 50-nm crystals that are adjacent to one another is considerably more difficult than standard transmission electron microscope (TEM) crystal alignment, especially if images of a particular crystal are required along several specific zone axes.
Goniometric (angular) measurements require that one know the exact relative orientations of specific crystal faces. Such knowledge is difficult in the TEM because all one observes in a bright-field image is a crystal outline, much like a shadow. Although thick regions of a crystal are darker than thin regions, a bright-field image provides only highly limited thickness information. It is difficult, for example, to distinguish a crystal edge from a crystal face; there is a marked lack of morphological detail within the crystal periphery, and it is hard to perform precise tilting experiments about well defined crystallographic axes of nanocrystals. As a result, one can make guesses in favorable cases about three-dimensional crystal shapes, as is done by T.-K. (3–5), but fine details are certainly inaccessible, especially regarding faces in the projection direction of the electron beam. An electron-diffraction pattern defines the crystallographic orientation of an edge but not its morphology. Unless there is a distinct change in contrast from crystal periphery to center, it is impossible to know whether that periphery represents a face or an edge. Because of the insensitivity of a crystal outline to faces within its periphery, different three-dimensional models can yield almost identical projected outlines. For small crystals such as those in MV-1, it also can be difficult to distinguish between faces and rough surfaces or rounded edges that have no simple relation to internal symmetry.
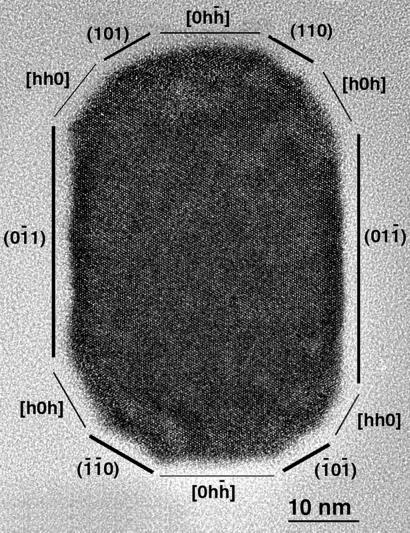
These points are illustrated by the <111> high-resolution image of a representative MV-1 magnetite crystal shown in Fig. 1. Simulations show that several different models that have identical angles around the crystal perimeter but different three-dimensional morphologies can be fitted to the image, even if it is assumed that this crystal has 3-fold symmetry about its axis of elongation and has faces of the forms {100}, {110}, and {111}.
Figure 1.
High-resolution [11̄1̄] image of a crystal from strain MV-1. The labels show one of the possible ways of interpreting the crystal outline. The heavy lines mark possible faces in projection, and the light lines mark possible crystal edges where faces intersect. Note the lack of thickness contrast within the center of the crystal (compare Figs. 1 and 2). The image was taken at 400 kV on a JEOL 4000EX TEM.
Determination of Crystal Morphology by Electron Microscopy
We know of no other groups that have done more work to study magnetite nanocrystals and to determine their shapes than those of McKay and T.-K. While respecting their careful efforts, we wish to illustrate more accurate methods for studying the morphologies of such crystals.
To characterize the morphology of a magnetite nanocrystal that has {100}, {110}, and {111} faces, there must be enough information to determine the 26 variable parameters that describe the relative sizes and positions of these faces. These parameters are the distances from the center of the crystal to the six {100}, eight {111}, and twelve {110} faces that are possible on an individual crystal. The projected thickness of a crystal contains far more morphological information than the crystal outline alone, but even if the projected thickness of the crystal has been measured at a known orientation it is difficult to determine the crystal morphology with confidence. For example, in the electron holographic phase image of four MV-1 crystals shown in Fig. 2, in which the contrast is proportional to projected thickness, the contours appear uniform along the tops of each crystal. However, it is difficult to tell from such an image whether the uniformity is a consequence of the presence of a large number of small faces or the lack of such faces. In the absence of such thickness information, a unique solution may not exist, and a best fit to one or more images in a tilt series may require all 26 parameters to be varied iteratively with a minimization algorithm. However, T.-K. have less information than the projected thickness. They present only the outlines of crystals at a small number of orientations. Unambiguous interpretation using this method is difficult at best. As we show below, the relative sizes and shapes of the faces that T.-K. illustrate are not only difficult to determine but can also vary widely among different bacterial strains, as well as between adjacent crystals in a chain.
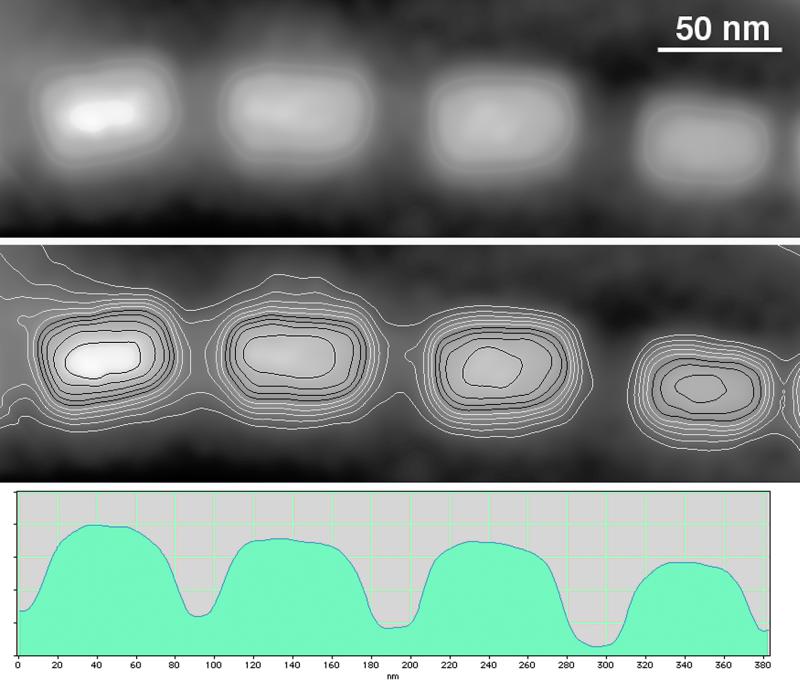
Figure 2.
An electron holographic phase image, in which the contrast is proportional to projected thickness, obtained from four representative MV-1 crystals both without (Top) and with (Middle) equally spaced contours. Each phase contour spacing of 0.47 radians corresponds to a thickness of 4.6 nm. (Bottom) Lengthwise cross sections of these crystals. The vertical scale is proportional to the measured phase shift in radians.
Instead of obtaining bright-field images of crystals and analyzing their outlines, it is possible to measure the projected thickness of a nanocrystal in the TEM in one of three ways: (i) by using energy-selected imaging to form three-window, background-subtracted chemical maps (as in ref. 15); (ii) by using high-angle annular dark-field (HAADF) imaging with the microscope in scanning TEM (STEM) mode; or (iii) by using electron holography (15–17). Method i is available in a TEM that has a postcolumn or in-column imaging spectrometer such as the Gatan imaging filter (GIF), although the resulting images can be noisy.‡‡ Methods ii and iii are available in many TEMs that have field-emission-gun (FEG) electron sources. The signal in method ii is especially useful; it is produced with electrons that are scattered at high angles (>50 mrad) and, therefore, are least affected by Bragg-diffraction, producing contrast that is primarily a function of the atomic number squared and, thus, is very sensitive to thickness.
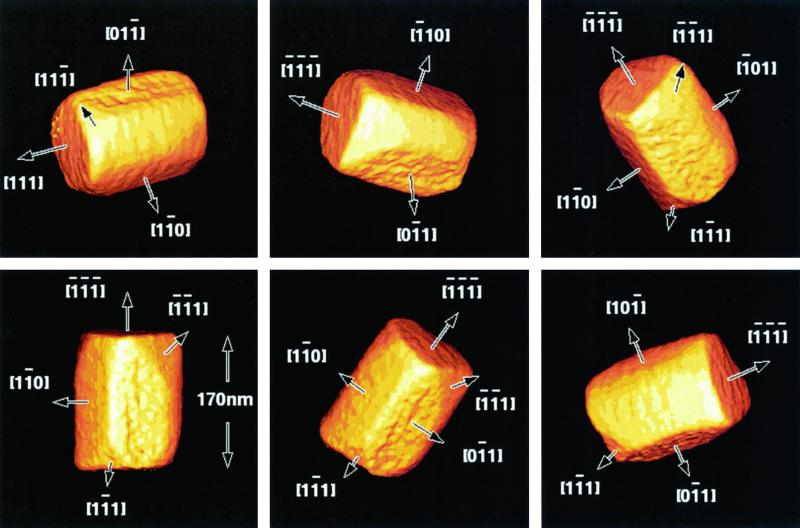
Whichever of these approaches is used, the three-dimensional morphology of a magnetite nanocrystal can be determined experimentally from a series of such projections obtained at a range of sample tilts by making use of algorithms developed for electron tomography (18). Tomographic reconstruction in the TEM was first used successfully in the biosciences, and the technique is now starting to be applied to problems in materials science (19, 20). No assumptions are required about the symmetry of the crystal or about the faces that may be present, and the tilt series can be obtained about an arbitrary axis without needing to tilt individual crystals to zone axes. Fig. 3 contains a tableau of images that show the three-dimensional morphology of a biogenic magnetite nanocrystal, which was determined experimentally from a tomographic reconstruction and is viewed from a range of directions. The figure was reconstructed from a series of 57 HAADF images obtained over a tilt range of ± 56° (one every 2° up to 56° in each direction). Six {110} faces along the length of the crystal and two {111} faces at its ends are labeled, as well as some smaller {111} corner faces. If a similar approach were applied to measure the three-dimensional morphologies of magnetite crystals in ALH84001, many of the problems associated with the bright-field approach used by T.-K. would be resolved.
Figure 3.
Tomographic reconstruction of a magnetite nanocrystal from an undescribed coccus collected from Sweet Springs Nature Reserve, Morro Bay, CA, reconstructed from a tilt series of STEM HAADF images obtained at 300 kV on a Philips CM300 FEG TEM over a range of ± 56°. The tableau shows the three-dimensional morphology of the crystal viewed from a range of directions.
Objectivity and Statistics
The small number of crystals for which tilting experiments were performed by T.-K. is understandable in light of the difficulty of the measurements. Nonetheless, the magnitude of the conclusions suggests that there should be concern that crystals closely similar to those in MV-1 could have inadvertently been selected for study from among the many dissimilar magnetite crystals in ALH84001. “Elongated prismatic” and “irregular” crystals fall within the same region of a scatter plot of aspect ratio shown as a function of crystal length (figure 10 in ref. 3). Thus, T.-K.'s “prismatic” crystals form a subset of all but the “whisker-like” magnetite grains described from ALH84001. As shown in Fig. 1 of T.-K. (3), their magnetite grains occur in aggregates in which many crystals overlap. Because it is unlikely that careful tilting experiments could have been carried out on all grains, the distinction between “prismatic” and “irregular” crystals based on two-dimensional projections is necessarily somewhat arbitrary. Additionally, in such samples there is likely to be considerable overlap between the sizes and morphologies of crystals that may, on average, be dissimilar. Although only a minority of the ALH84001 crystals qualify for biogenic status on the basis of their size and shape, the spread in those values is large enough that many could be matched to different well chosen biogenic crystals. Recognizing a subset of biogenic crystals among a larger set of abiogenic crystals, as T.-K. indicate they did, is clearly difficult, particularly without a measure of how objectively the crystals were chosen.
T.-K. rely heavily on the detailed morphologies of the magnetite crystals illustrated in their papers (3, 5). There are two problems with their approach. First, even if their interpretation were correct, it is not compelling, because the relative sizes of the faces depend on the growth conditions and can vary between strains, within a given strain (12), and even within given chains. Second, as shown above, it is extremely difficult to determine the morphological details of given nanocrystals from conventional projected TEM images; different models can be matched to given crystal outlines, and also, a different model may have been appropriate for a different choice of crystal.
Terminology: “Truncated Hexa-Octahedral” Magnetite Crystals
Standard names exist for the various sets of crystal faces that are related by symmetry. These names are given in most elementary textbooks on mineralogy (e.g., refs. 21 and 22) and are based on well established rules of nomenclature (23). It seems curious, therefore, to encounter a controversy over the names of crystallographic forms. As pointed out by Rogers over half a century ago (23), many of the names used by crystallographers were standard for decades before his paper. He reports that the name hexoctahedron (sometimes called hexaoctahedron, ref. 24) was introduced by the famous mineralogist, J. D. Dana, in 1850. Rogers (23) also states that “… for the sake of continuity with the past it seems advisable to use well established terms.”
The hexoctahedron is a form of type {hkl}, where h ≠ k ≠ l. It contains 48 faces and is the general form for the crystal class (point group) 4/m 3̄ 2/m (or m 3̄ m), the hexoctahedral class, which is the crystal class with the greatest symmetry. The foregoing is such basic mineralogical terminology that it hardly bears repeating, except that the literature on magnetite crystals from bacteria seems to ignore it. Earlier papers refer to hexagonal prisms (25) and prismatic magnetite crystals (3, 4), but prisms are not an isometric form (23).
T.-K. (3) recently called the ALH84001 and MV-1 bacterial magnetite crystals hexoctahedral and explained this name by stating (p. 4051) that “in a crystal with hexaoctahedral geometry, growth along all [111] axes should be equivalent.” No face exists perpendicular to [111] in a hexoctahedron; a crystal with equal growth along the <111> axes describes an octahedron rather than a hexoctahedron. In any case, unless growth was anisotropically constrained, under the influence of a directional feeder flux, or influenced by a defect such as a dislocation, isometric crystals normally have equivalent growth along symmetrically equivalent directions such as <111>, <100>, or <110>.
T.-K. (5) have most recently replaced their terminology by the report that biogenic magnetites are “truncated hexa-octahedral” crystals. By this expression, we believe that the authors still mean that the crystals display a combination of cube, octahedron, and dodecahedron faces. However, by assigning this new name, the crystals are given an apparently distinctive crystallographic identity that makes them seem unique.
Aspect Ratios and Size Distributions
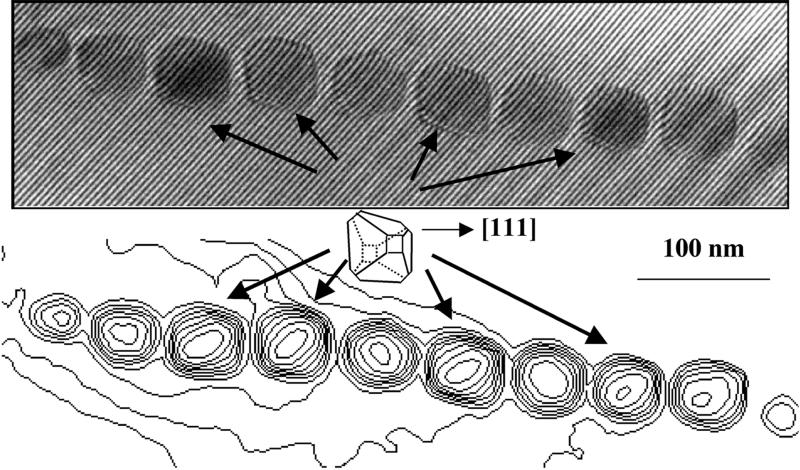
Many authors have pointed out that the magnetite crystals in some magnetotactic bacteria are elongated rather than having the equidimensional shapes typical of most isometric minerals. However, such elongation is not a distinctive or necessary feature of biogenesis. As shown in the electron hologram in Fig. 4, the dominantly octahedral shape of the magnetite crystals in Magnetospirillum magnetotacticum is equidimensional. The three-dimensional shapes of the nanocrystals in the three strains of bacteria shown in Figs. 1–4 clearly differ from one another. Those in strain MV-1 (Figs. 1 and 2) and an unidentified magnetotactic coccus collected in California (Fig. 3) are elongated, whereas those in M. magnetotacticum (Fig. 4) are not (26). Fig. 4 also demonstrates the difficulty of determining the three-dimensional morphology of a crystal from its projected outline, especially if it is tilted away from an advantageous zone axis.
Figure 4.
Electron hologram of a chain of crystallites in M. magnetotacticum. The fine lines are holographic interference fringes, which bend as they pass through each crystal. Below the hologram are thickness contours that are derived from a phase image reconstructed from the hologram. The inset indicates the approximate orientation of the dominantly octahedral magnetosomes in this chain. Crystals in the chain that are not well oriented have outlines and projected thicknesses that are indistinct, and therefore their three-dimensional morphology is indeterminate.
It is certainly interesting that some ALH84001 crystals show similar elongations to those in certain biogenic crystals (3, 14). Although the lengthened shapes of some of the magnetite crystals are intriguing, it is also true that many other nonbiogenic isometric minerals can grow with elongated shapes. For example, cuprite (Cu2O, Pn3m) can form fibrous, hair-like crystals with the mineral name chalcotrichite (27). Many of the 73% of the magnetite crystals from ALH84001 that do not qualify for the hypotheses of T.-K. are themselves extended rather than equidimensional. Bradley et al. (28) report greatly elongated magnetite crystals from both ALH84001 and terrestrial origins. It seems that although a study of aspect ratios is a good approach to use, particular aspect ratios do not necessarily suggest a biogenic origin.
The crystal-size distribution (CSD), commonly shown as a plot of size vs. frequency, was not included among the six criteria listed by T.-K. (3) as a distinguishing feature of biogenic magnetite. In our view, the CSD may be one of the best indicators of the bacterial origin of magnetite. All CSDs of magnetite from magnetotactic bacteria that have been studied to date are negatively skewed (12–14), and some have sharp cutoffs toward larger sizes. In contrast, the CSDs of inorganic magnetite are typically lognormal and tail off toward larger sizes. Although plots of size vs. frequency for the ALH84001 magnetite grains are not given by T.-K. (3), the shapes of the CSDs can be estimated from their figure 10b. The CSD of MV-1 crystals in their plot appears to have a distinct upper-size cutoff, whereas the “prismatic” magnetite crystals from ALH84001 have a more gradual upper-size distribution. If this observation is correct, it suggests that the CSDs of the biogenic and meteoritic magnetite crystals differ.
Conclusions
The reconstructed three-dimensional shapes of magnetite nanocrystals from three strains of magnetotactic bacteria differ from one another. Those in strain MV-1 and the magnetotactic coccus are slightly elongated, whereas those in M. magnetotacticum are equidimensional. The CSDs of bacterial magnetite show sharp discontinuities at the upper ends of their size distributions.
We believe that there is insufficient evidence to support many of the published interpretations of the morphologies of magnetite crystals from ALH84001 and their recently proposed identity (5) to magnetite from bacterial strain MV-1. The projected shapes of bacterial crystals have long been known, and the meteoritic shapes have been pointed out many times in recent years. The three-dimensional shapes of magnetite nanocrystals differ among different strains of magnetotactic bacteria, whereas the spread in the sizes and shapes of the crystals in ALH84001 is large enough that many could be matched to different well chosen biogenic crystals and, by extension, to nonbiogenic crystals. In addition, the crystal size distributions of bacterial and meteoritic magnetite seem to be different. The recent work also obscures the central observation of the higher than expected aspect ratios of many of the bacterial and ALH84001 magnetite crystals. Although the similarities are intriguing, we believe that they do not provide strong evidence that meteoritic magnetite crystals are “Martian magnetofossils” or that they “constitute evidence of the oldest life yet found.” We suggest that current knowledge about the magnetite crystals in ALH84001, when examined critically, is inadequate to support the proposed former existence of extraterrestrial life, i.e., for the ancient Martian-life hypothesis of McKay et al. (1).
The technology needed to address the question of the detailed shapes of nanocrystals, and the proposed similarity between magnetite crystals from bacteria and the ALH84001 meteorite, is available. The problem could be solved by using one or more of the methods mentioned above for measuring the projected thicknesses of crystals to reconstruct their three-dimensional morphologies. In this way, a more definitive and unambiguous study could be performed than has been carried out to date. Such measurements also could be made on the products of experiments that indicate magnetite crystals similar to those from ALH84001 have been grown inorganically (29). Because it seems that the magnetite nanocrystals in ALH84001 are the only remaining, potentially definitive indicators of former life on Mars, such careful work is justified and, indeed, demanded.
Acknowledgments
We thank T. Sharp for helpful comments. This work was supported in part by grants from the National Science Foundation, the National Aeronautics and Space Administration, and the Royal Society.
Abbreviations
- TEM
transmission electron microscope
- CSD
crystal-size distribution
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Energy-selected imaging to form chemical maps that reflect crystal thickness involves using a GIF or similar device to acquire three images at different energy losses. Two are obtained at lower energy losses than a core-loss edge such as the Fe L2,3 edge, and one is acquired at a higher energy-loss. Once the three images are aligned, for each pixel a power-law background as a function of energy loss is fitted to the intensities in the preedge images and extrapolated to the energy of the postedge image. This intensity is then subtracted from the original postedge image to provide the chemical signal, which is approximately proportional to sample thickness for “thin” (below ≈100 nm) samples (30). The spatial resolution can be better than 1 nm, although, in practice, noise usually limits the resolution to a few nm.
References
- 1.McKay D S, Gibson E K, Jr, Thomas-Keprta K L, Vali H, Romanek C S, Clemett S J, Chillier X D F, Maechling C R, Zare R N. Science. 1996;273:924–930. doi: 10.1126/science.273.5277.924. [DOI] [PubMed] [Google Scholar]
- 2.Friedmann E I, Wierzchos J, Ascaso C, Winklhofer M. Proc Natl Acad Sci USA. 2001;98:2176–2181. doi: 10.1073/pnas.051514698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas-Keprta K L, Bazylinski D A, Kirschvink J L, Clemett S J, Wentworth D S, Vali H, Gibson E K, Jr, Romanek C S. Geochim Cosmochim Acta. 2000;64:4049–4081. doi: 10.1016/s0016-7037(00)00481-6. [DOI] [PubMed] [Google Scholar]
- 4.Thomas-Keprta K L, Clemett S J, Bazylinski D A, Kirschvink J L, McKay D S, Wentworth S J, Vali H, Gibson E K., Jr Meteoritics Planet Sci Suppl. 2000;35:A156–A157. [Google Scholar]
- 5.Thomas-Keprta K L, Clemett S J, Bazylinski D A, Kirschvink J L, Wentworth D S, Vali H, Gibson E K, Jr, McKay M F, Romanek C S. Proc Natl Acad Sci USA. 2001;98:2164–2169. doi: 10.1073/pnas.051500898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frankel R B, Buseck P R. Curr Opin Chem Biol. 2000;4:171–176. doi: 10.1016/s1367-5931(99)00072-1. [DOI] [PubMed] [Google Scholar]
- 7.Pósfai M, Cziner K, Márton E, Márton P, Buseck P R, Frankel R B, Bazylinski D A. Eur J Mineral. 2001;13:691–703. [Google Scholar]
- 8.Taylor A P, Barry J C, Webb R I. J Microsc (Oxford) 2001;201:84–106. doi: 10.1046/j.1365-2818.2001.00760.x. [DOI] [PubMed] [Google Scholar]
- 9.Matsuda T, Endo J, Osakabe N, Tonomura A, Arii T. Nature (London) 1983;302:411–412. [Google Scholar]
- 10.Mann S, Frankel R B. In: Biomineralization: Chemical and Biochemical Perspectives. Mann S, Webb J, Willams R J P, editors. New York: VCH; 1989. pp. 389–426. [Google Scholar]
- 11.Sparks N H C, Mann S, Bazylinski D A, Lovley D R, Jannasch H W, Frankel R B. Earth Planet Sci Lett. 1990;98:14–22. [Google Scholar]
- 12.Meldrum F C, Heywood B R, Mann S, Frankel R B, Bazylinski D A. Proc R Soc London Ser B. 1993;251:237–242. [Google Scholar]
- 13.Meldrum F C, Heywood B R, Mann S, Frankel R B, Bazylinski D A. Proc R Soc London Ser B. 1993;251:231–236. [Google Scholar]
- 14.Devouard B, Pósfai M, Hua X, Bazylinski D A, Frankel R B, Buseck P R. Am Mineral. 1998;83:1387–1398. [Google Scholar]
- 15.Dunin-Borkowski R E, McCartney M R, Frankel R B, Bazylinski D A, Pósfai M, Buseck P R. Science. 1998;282:1868–1870. doi: 10.1126/science.282.5395.1868. [DOI] [PubMed] [Google Scholar]
- 16.McCartney M R, Lins U, Farina M, Buseck P R, Frankel R B. Eur J Mineral. 2001;13:685–689. [Google Scholar]
- 17.Dunin-Borkowski R E, McCartney M R, Pósfai M, Frankel R B, Bazylinski D A, Buseck P R. Eur J Mineral. 2001;13:671–684. [Google Scholar]
- 18.Koster A J, Grimm R, Typke D, Hegerl R, Stoschek A, Walz J, Baumeister W. J Struct Biol. 1997;120:276–308. doi: 10.1006/jsbi.1997.3933. [DOI] [PubMed] [Google Scholar]
- 19.Koster A J, Ziese U, Verkleij A J, Janssen A H, de Jong K P. J Phys Chem B. 2000;104:9368–9370. [Google Scholar]
- 20.Midgley P A, Weyland M, Thomas J M, Johnson B F G. Chem Commun. 2001;10:907–908. [Google Scholar]
- 21.Klein C, Hurlbut C S., Jr . Manual of Mineralogy. New York: Wiley; 1993. [Google Scholar]
- 22.Bloss F D. Crystallography and Crystal Chemistry. Rinehart and Winston, New York: Holt; 1971. [Google Scholar]
- 23.Rogers A F. Am Mineral. 1935;20:838–851. [Google Scholar]
- 24.Hahn T, editor. International Tables for Crystallography: Vol. A. Space-Group Symmetry. Dordrecht, The Netherlands: D. Reidel; 1983. [Google Scholar]
- 25.Mann S, Sparks N H C, Blakemore R P. Proc R Soc London Ser B. 1987;231:477–487. [Google Scholar]
- 26.Mann S, Frankel R B, Blakemore R P. Nature (London) 1984;310:405–407. [Google Scholar]
- 27.Veblen D R, Post J E. Am Mineral. 1983;68:790–803. [Google Scholar]
- 28.Bradley J P, Harvey R P, McSween H Y., Jr Geochim Cosmochim Acta. 1996;60:5149–5155. doi: 10.1016/s0016-7037(96)00383-3. [DOI] [PubMed] [Google Scholar]
- 29.Golden D C, Ming D W, Schwandt C S, Lauer H V, Socki R A, Morris R V, Lofgren G E, McKay G A. Am Mineral. 2001;86:370–375. [Google Scholar]
- 30.Egerton R F. Electron Energy-Loss Spectroscopy in the Electron Microscope. New York: Plenum; 1996. [Google Scholar]