Abstract
Background:
In patients with heart failure (HF), higher body mass index (BMI) has been associated with lower rates of hospitalization and mortality (obesity paradox). Symptoms are antecedents of hospitalizations, but little is known about the relationship between BMI and symptoms and gender differences.
Purpose:
To examine the association of BMI with symptoms in male and female patients with HF, controlling for covariates (sample characteristics, depressive symptoms, and sodium intake).
Methods:
In this cross-sectional correlational study, patients (N = 247) provided data on BMI, symptoms, and covariates. BMI was categorized into four groups: normal/underweight (< 25 kg/m2), overweight (25 – 29.9 kg/m2), obese I (30–34.9 kg/m2), and obese II/III (≥ 35 kg/m2). General linear regression was used to analyze the data.
Results:
The Obese II/III group had more severe HF symptoms than other groups only in male patients. In male patients, older age, Caucasian race, more comorbidities, and more severe depressive symptoms were also associated with more severe symptoms. In female patients, more severe depressive symptoms, more comorbidities, and higher sodium intake were associated with more severe symptoms.
Conclusions:
The obesity paradox does not fully extend to symptoms, and gender has a role in the relationship between obesity and symptoms.
Keywords: Heart failure, obesity, symptoms, depression, sodium
Introduction
Patients with heart failure (HF) experience a variety of HF-related physical symptoms (HF symptoms), including dyspnea, fatigue, sleeping difficulties, edema, chest pain, and dizziness.1–3 HF symptoms are strongly associated with health-related quality of life4–6 and are important antecedents of hospitalization in this population.7 About 75% of all hospitalizations in patients with HF are associated with exacerbation of HF symptoms.7 Therefore, it is critical to identify and manage factors affecting HF symptoms in these patients.
Obesity is one factor that may affect HF symptoms. Obesity is known to be a risk factor for development of HF.8, 9 In contrast, several studies and professional guidelines have suggested some positive effects of obesity on mortality and morbidity in established patients with HF or coronary disease, which has been known as obesity paradox.10–12 However, the details of the obesity paradox in the studies and guidelines are inconsistent. In one individual HF study13 and one HF meta-analysis,14 HF patients with high BMI and/or high waist circumference had lower rates of all-cause mortality than those who with low BMI and/or low waist circumference. In one HF study,11 four BMI quartiles were linearly associated with inhospital mortality rates, and the highest BMI group showed the lowest inhospital mortality rate compared to other BMI groups. However, in several HF studies, the relationships between obesity and mortality or hospitalization were non-linear. In a meta-analysis,15 risks for total and cardiovascular mortality were highest in the underweight group (relative risk [RR] = 1.27 [95% confidence interval: 1.17–1.37] and 1.20 [95% confidence interval: 1.01–1.43], respectively), but the risks for other groups did not differ (RR: from 0.71 to 0.82). At the same time, however, risks for hospitalization in that study were highest in the underweight group (RR = 1.19 [95% confidence interval: 1.09–1.30]) and lowest in the overweight group (BMI: 25–29.9 kg/m2, RR = 0.92 [95% confidence interval: 0.86–0.97]). In another study,16 which followed 4,109 HF patients with preserved left ventricular function for 49.5 months, the composite outcome of death or cardiovascular hospitalization was also lowest in overweight patients (BMI: 26.5 to 30.9 kg/m2). These two studies suggested a U-shaped relationship17 between obesity and hospitalization or death in patients with HF (low hospitalization or mortality rates in overweight patients and high hospitalization rates in both underweight and obese patients).
Several professional organizations reflect these findings in their guidelines. The European Society of Cardiology states that obesity should be managed, but does not specify any BMI levels and refers to follow the recommendations from “European Guidelines on Cardiovascular Disease Prevention in Clinical Practice.”18 In the guidelines recommended,19 the obesity paradox in established coronary disease is acknowledged without recommendations on specific BMI levels for these patients. The American College of Cardiology Foundation and the American Heart Association have noted a U-shaped relationship between obesity and mortality and suggest that patients with a BMI of 30 to 35 kg/m2 may have the best outcomes,20 even though no specific evidence for the recommendation was given. HF symptoms are prevalent and one major antecedent of hospitalization and mortality in patients with HF,7, 21 but little is known if obesity paradox or U-shaped relationship is extended to the relationship between obesity and HF symptoms. This information will be valuable to manage obesity in order to reduce HF symptoms, and, in turn, to reduce hospitalization rates in patients with HF.
Further, several studies suggest gender differences in obesity and its effects, symptom management, perceptions of HF symptoms, and medical treatment in patients with cardiac diseases, including HF.22–26 The effects of obesity, aging, and comorbidities on myocardial and vascular stiffness, myocardial hypertrophy, and skeletal muscle adaptation to HF in males and females were different.22 Female patients with cardiovascular diseases or diabetes mellitus were prescribed more medications, including different types of diuretics and short-acting nitroglycerin.23 In addition, male patients compared to female patients were better at interpreting their HF symptoms and initiate treatment.24 Thus, the relationship between obesity and HF symptoms may differ in male and female patients. Therefore, this study examined the association between obesity and HF symptoms in male and female patients with HF, controlling for covariates. Covariates were selected based on the literature and included sociodemographic and clinical characteristics (age,27 educational level,28 race,28 comorbidities,29 and left ventricular ejection fraction [LVEF]30), depressive symptoms,5, 27 and sodium intake.31, 32
Methods
Study design, Sample, and Procedure
This cross-sectional correlational analysis used baseline data from a longitudinal observational study (N = 302).33, 34 The purpose of the parent study was to examine the relationships among BMI, nutrition, inflammation, symptoms, and HF outcomes, including hospitalizations and health-related quality of life, in a convenience sample of patients with HF. We recruited eligible patients from outpatient clinics of six academic medical centers or community hospitals in three Mid-Western and Southern cities in the U.S from December, 2004 to March, 2009.33, 34
The sample size for the current study was calculated based on a prior study.29 In the prior study, a model including depressive symptoms, age, gender, BMI, comorbidities, and medications explained 35.5% of the variance in HF symptoms, and depressive symptoms, BMI, and comorbidities were significantly associated with HF symptoms. Thus, considering a medium effect size, a 5% significance level, 80% power, and 8 predictor variables, the sample size was 100. The sample sizes for males and females in the current study were 166 and 81, respectively (Figure 1).
Figure 1.
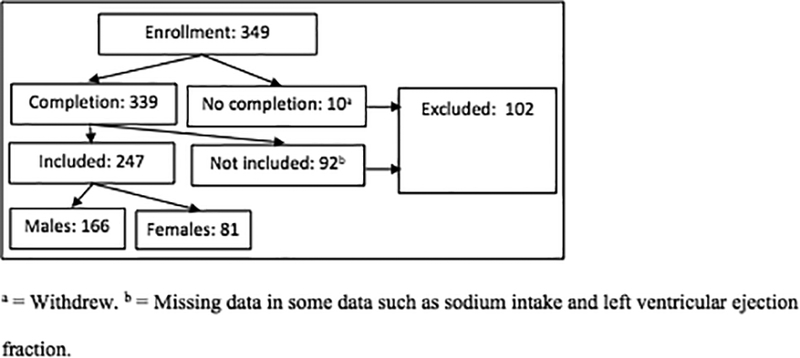
Enrollment and analysis
Patients who could speak and read English and who were stable on current HF medications for two clinic visits or for 3 months were included in the study. The research team confirmed the diagnosis of HF through medical record review. Patients were excluded if they had had a myocardial infarction or stroke within the previous 3 months, or had severe cognitive or psychiatric issues, or valvular or postpartum HF etiology. An approval for the study was obtained from three Internal Review Boards. Patients were recruited from outpatient clinics in three cities in the U.S, and all provided written informed consent. Of the 349 patients who enrolled in the study, 10 withdrew, and 92 had missing data such as left ventricular ejection fraction [LVEF]) and sodium intake (Figure 1). Thus, 247 patients were included in the current analysis.
Measures
HF symptoms referred common HF-related physical symptoms and measured by the Symptom Status Questionnaire-Heart Failure.29 This instrument assesses the presence, frequency, severity, and distress of 7 common HF symptoms: dyspnea during daily activities, dyspnea when lying down, fatigue, edema, sleeping difficulty, chest pain, and dizziness. The total symptom score ranges from 0 to 84, with higher scores indicating more severe HF symptom status. The reliability and validity of this instrument have been supported.29 In the current study, Cronbach’s alpha was .79.
BMI was calculated based on height in centimeter and weight in kilogram. Nurses in the General Clinical Research Centers at each site measured height and weight. BMI was calculated by dividing weight in kilograms by the square of height in meters. BMI was used as a continuous variable and also a categorical variable in two different models. As a categorical variable, patients were categorized into four groups based on the calculated BMI: normal/underweight (< 25 kg/m2), overweight (25 – 29.9 kg/m2), obese class I (30–34.9 kg/m2), and obese class II/III (≥ 35 kg/m2).35 We combined underweight group with normal weight group and obese class II with obese class III group because of small numbers in underweight group and obese III group.
Depressive symptoms were assessed by the Beck Depression Inventory II.36 Possible total scores range from 0 to 63, with higher scores indicating more severe depressive symptoms.36 The reliability and validity of the instrument have been supported with several populations, including patients with HF.36, 37 Cronbach’s α in the current study was .90.
Data on other sociodemographic characteristics (age, education level, marital status, gender, and race) and clinical characteristics (etiology of HF, New York Heart Association [NYHA] functional class, comorbidity, and LVEF) were collected using a sociodemographic questionnaire and a clinical questionnaire. Data on comorbidities were collected through patient interview and medical record review using the Charlson Comorbidity Index,38 which was included in the clinical questionnaire. Sodium intake was assessed using 24-hour urine sodium, and patients were divided into two groups (< 3 g vs. ≥ 3 g per day) based on the recommendation from professional organizations.20
Data Analysis
We eliminated cases with missing data mainly due to sodium intake and left ventricular ejection fraction (Figure 1). The mean age, gender ratio, and obesity in the current study with 247 cases are the same or very similar to those in a prior study used the same data with 302 cases.34 Multiple regression analyses with Enter method were used to examine the association between obesity (as a continuous variable) and HF symptoms, controlling for age, education level, race, comorbidities, LVEF, depressive symptoms, and sodium intake. General linear regression analyses with Enter method were used to examine the same association between obesity (as a categorical variable) and HF symptoms, controlling for the same covariates. We used BMI as a continuous variable because it can be a more sensitive indicator of obesity than the categorical BMI groups. However, we also used BMI as a categorical variable for comparison because many studies used BMI as categorical variables.11, 15, 16 To compare the characteristics of the four obesity groups, analysis of variance and chi-square tests were used. To compare the characteristics and HF symptoms of males and females, independent t-test and chi-square test were used. For all analyses, a two-sided significance was set at p < .05.
Results
The mean age of the total sample was 61 years (standard deviation [SD]: ±11.6). The majority of the patients were Caucasian (74.1%), and 67.2% were males. More males than females were married (59% vs. 39.5%, p = .004), and more had ischemic-originated HF (56% vs. 28.4%, p < .001) and lower LVEF (32.5 [12.1] vs. 38.3[14.6], p = .001) (no table). There were no gender differences in age, education level, race, NYHA functional class, comorbidities, obesity, sodium intake, depressive symptoms, or HF symptoms (no table). Table 1 presents sample characteristics based on BMI groups in males and females. In males, the patients in the obese II/III group were younger, had higher LVEF, consumed more sodium, and had more severe HF symptoms than the normal/underweight group and/or the overweight group. In addition, more patients in this group were minority races than all other groups. In females, more patients in the obese II/III group were minorities and were at NYHA class III/IV than at least one of the other groups. In addition, patients in the obese II/III group consumed more sodium and had more severe HF symptoms than at least one of the other groups.
Table 1.
Sample Characteristics by Body Mass Index Group in Males and Females (N = 247)
| Characteristics | Males (n = 166) | Females (n = 81) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BMI Mean (± SD) or n (%) |
p value | BMI Mean (± SD) or n (%) |
p value | |||||||
| NW n = 39 |
OW n = 55 |
OB I n = 36 |
OB II/III n = 36 |
NW n = 22 |
OW n = 14 |
OB I n = 25 |
OB II/III n = 20 |
|||
| Mean age, years (SD) | 61.8(±12.7) | 64.2(±10.3) | 60.4(±13.3) | 55.2(±11.0) | .005 | 65.9(±11.9) | 58.4(±12.2) | 59.6(±8.2) | 60.4(±10.1) | .122 |
| Education, mean years (SD) | 13.9(±3.6) | 13.8(±3.4) | 14.3(±3.0) | 13.7(±2.8) | .881 | 14.4(±2.8) | 13.6(±3.0) | 12.8(±2.8) | 12.8(±3.4) | .209 |
| Marital status (% married) | 17.0(43.6) | 34.0(61.8) | 25.0(69.4) | 22.0(61.1) | .127 | 13.0(59.1) | 4.0(28.6) | 9.0(36.0) | 6.0(30.0) | .163 |
| Race (% Caucasian) | 34.0(87.2) | 46.0(83.6) | 28.0(77.8) | 20.0(55.6) | .005 | 17.0(77.3) | 13.0(92.9) | 12.0(48.0) | 13.0(65.0) | .023 |
| Etiology (% ischemic) | 22.0(56.4) | 35.0(63.6) | 20.0(55.6) | 16.0(44.4) | .353 | 8.0(36.4) | 6.0(42.9) | 4.0(16.0) | 5.0(25.0) | .248 |
| NYHA (% III/IV) | 16.0(41.0) | 19.0(34.5) | 18.0(50.0) | 19.0(52.8) | .287 | 7.0(31.8) | 9.0(64.3) | 9.0(36.0) | 15.0(75.0) | .012 |
| Comorbidities | 2.8(±1.7) | 2.7(±1.5) | 3.7(±2.5) | 3.5(±2.3) | .057 | 2.6(±1.5) | 3.5(±2.0) | 2.8(±1.8) | 3.6(±2.1) | .207 |
| LVEF | 31.1(±13.0) | 29.6(±10.4) | 34.8(±12.5) | 36.0(±12.3) | .042 | 32.2(±12.4) | 37.7(±13.3) | 44.6(±15.0) | 37.8(±15.3) | .034 |
| Sodium intake (% > 3 g /day) | 17(43.6) | 35(63.6) | 28(77.8) | 28(77.8) | .004 | 5(22.7) | 7(50.0) | 12(48.0) | 16(80.0) | .003 |
| Depressive symptoms | 10.5(±9.5) | 9.6(±7.2) | 11.4(±7.3) | 11.9(±10.0) | .588 | 10.2(±8.1) | 14.1(±10.1) | 8.6(±7.5) | 11.1(±7.4) | .257 |
| Physical symptoms | 18.9(±15.1) | 19.7(±12.2) | 26.7(±15.1) | 29.8(±19.9) | .003 | 20.2(±11.5) | 28.6(±16.4) | 22.1(±11.3) | 35.4(±20.9) | .007 |
LVEF = Left Ventricular Ejection Fraction, %. NW = normal/underweight (body mass index: < 25 kg/m2). NYHA = New York Heart Association functional class. OB I = obese class I (body mass index: 30–34.9 kg/m2). OB II/III = obese class II and III (body mass index: ≥ 35 kg/m2). OW = overweight (body mass index: 25–29.9 kg/m2).
In individual HF symptom analyses, edema was the only variable that showed a gender difference, which females had higher total edema scores than males (3.37 vs. 2.46, p = .048), even though no significant differences in the presence, frequency, severity, and distress of this symptom (Table 2).
Table 2.
Symptoms in Males and Females
| Symptom | Total Score | Presence | Frequency | Severity | Distress | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | P value | Male | Female | P value | Male | Female | P value | Male | Female | P value | Male | Female | P value | |
| Dyspnea during daily activities | 4.26 | 4.58 | .510 | 65% | 72% | .304 | 55% | 53% | .756 | 15% | 5% | .061 | 20% | 16% | .468 |
| dyspnea when lying down | 2.42 | 2.24 | .720 | 37% | 36% | .885 | 53% | 41% | .326 | 12% | 17% | .452 | 13% | 11% | .728 |
| Fatigue | 5.61 | 630 | .127 | 84% | 90% | .177 | 56% | 63% | .333 | 21% | 11% | .071 | 25% | 33% | .187 |
| Edema | 2.46 | 3.37 | .048 | 43% | 54% | .088 | 44% | 46% | .851 | 10% | 16% | .335 | 8% | 14% | .208 |
| Sleeping difficulty | 3.91 | 4.69 | .171 | 53% | 59% | .354 | 68% | 69% | .945 | 31% | 33% | .751 | 18% | 27% | .077 |
| Chest pain | 1.64 | 1.60 | .915 | 30% | 30% | .986 | 33% | 24% | .441 | 10% | 12% | .768 | 7% | 9% | .567 |
| Dizziness | 2.93 | 3.20 | .541 | 57% | 56% | .803 | 36% | 33% | .743 | 8% | 9% | .941 | 11% | 16% | .246 |
Total score: Mean. Presence: presence of the symptom. Frequency: 3–5 times per week to almost daily. Severity: severe or very severe. Distress: quite a bit or very much.
In multiple regression analyses, race, comorbidities, depressive symptoms, and obesity as a continuous variable were associated with HF symptoms (R2 = .481, p < .001, Table 3). Patients who were minority race, had lower comorbidity burden, fewer depressive symptoms, and lower BMI had less severe HF symptoms. The interaction between obesity and gender was not a significant associate of HF symptoms, and obesity as a continuous variable was significantly associated with HF symptoms in both males and females. In male patients, age, race, comorbidities, depressive symptoms, and obesity as a continuous variable were associated with HF symptoms (R2 = .545, p < .001). Those who were younger and minority race, had lower comorbidity burden, fewer depressive symptoms, and lower BMI had less severe HF symptoms. In female patients, lower comorbidity burden, fewer depressive symptoms, lower sodium intake, and lower BMI were associated with less severe HF symptoms (R2 = .439, p < .001).
Table 3.
Association between Obesity (Continuous Variable) and Heart Failure Symptoms
| Variables | Total Sample | Males | Females | |||
|---|---|---|---|---|---|---|
| t Statistic | p Value | t Statistic | p Value | t Statistic | p Value | |
| Age | 1.674 | .095 | 2.166 | .032 | −.379 | .706 |
| Education level | −1.359 | .175 | −1.055 | .293 | −1.594 | .115 |
| Race | 1.990 | .048 | 2.976 | .003 | −.280 | .780 |
| Comorbidities | 4.170 | <.001 | 3.631 | < .001 | 2.120 | .037 |
| Left Ventricular Ejection Fraction | .178 | .859 | .654 | .514 | −.978 | .331 |
| Depressive symptoms | 10.534 | < .001 | 10.783 | < .001 | 3.377 | .001 |
| Sodium intake | 1.215 | .226 | −.157 | .875 | 2.020 | .047 |
| Gender | −.575 | .566 | N/A | N/A | N/A | N/A |
| Obesity*Gender | 1.009 | .314 | N/A | N/A | N/A | N/A |
| Obesity | 2.866 | .005 | 3.475 | .001 | 2.017 | .047 |
| R2 (adjusted R2) | .481 (.459) | .545 (.522) | .439 (.377) | |||
| F statistic | 21.846 | 23.540 | 7.047 | |||
| p value | < .001 | <.001 | < .001 | |||
In general linear regression analyses, comorbidities, depressive symptoms, and obesity as a categorical variable were associated with HF symptoms (R2 = .481, p < .001, Table 3). Patients who had lower comorbidity burden, fewer depressive symptoms, and lower BMI had less severe HF symptoms. The interaction between gender and obesity was not a significant associate of HF symptoms. In male patients, age, race, comorbidities, depressive symptoms, and obesity as a categorical variable were associated with HF symptoms (R2 = .568, p < .001). Those who were younger and minority race, had lower comorbidity burden, fewer depressive symptoms, and lower BMI had less severe HF symptoms. In female patients, lower comorbidity burden, fewer depressive symptoms, and lower sodium intake were associated with less severe HF symptoms (R2 = .441, p < .001), but obesity as a categorical variable was not associated with HF symptoms.
Discussion
The findings of the current study did not support obesity paradox in the relationships between obesity and HF symptoms. In addition, the findings do not show the interaction effects between gender and obesity in the relationship to HF symptoms, even though the associates of HF symptoms in males and females slightly differed (multiple regression: males: R2 = .545, p < .001 and females: R2 = .439, p < .001; general linear regression: males: R2 = .568, p < .001 and females: R2 = .441, p < .001 ). In females, the relationship between obesity and HF symptoms differed depending on the levels of the measure (continuous vs. categorical). The findings of the current study showed additional associates of HF symptoms such as comorbidities and depressive symptoms in both genders, race in males, and sodium intake in females. Thus, the findings of the current study can provide valuable information for developing interventions to improve HF symptoms considering gender differences.
The relationships between obesity and HF symptoms slightly differed depending on gender and the levels of obesity measure. When BMI was used as a continuous variable, higher levels of BMI were associated with more severe HF symptoms in both males and females. Male patients in the obese II/III (BMI ≥ 35 kg/m2) group had more severe HF symptoms than the other groups regardless the levels of obesity measure. When BMI was used a categorical variable, female patients in the obese II/III group did not have more or less severe HF symptoms than the other groups. In addition, the study did not show negative effects of a lower BMI on HF symptoms. Thus, the findings in both males and females do not support the obesity paradox in the relationship between obesity and HF symptoms. However, we cannot compare the findings of this study with other findings, because no other HF studies have examined the relationship between obesity and HF symptoms and gender differences in the relationships. The significant relationship or no relationship observed between obesity and HF symptoms in the current study differed from the relationship between obesity and mortality in another study, which showed that higher BMIs were linearly associated with lower mortality.11 The relationship in the current study also differed from the relationship between obesity and hospitalization observed in other studies, which showed the lowest hospitalization rate in the overweight group compared with the normal/underweight and the obese groups (U-shaped relationship).15, 16
These differences in the relationships between obesity and HF symptoms and between obesity and hospitalization and mortality may reflect the fact that we did not separate the underweight group from the normal weight group because of the small sample. In one study,15 the relationships of obesity to all-cause mortality and to cardiovascular morality and HF-related hospitalization differed. Thus, it is also possible that the relationships of obesity to HF symptoms hospitalization, and mortality differ. Thus, further studies are needed to examine these relationships in patients with HF over time in order to determine the appropriate BMI levels based on not only longevity with the causes but also HF symptoms and hospitalization with the causes. Both Oga et al. and Lavie et al. acknowledged that obesity can be associated with better prognosis of HF, but concluded that further larger studies are needed to determine the effects of obesity on HF symptoms, hospitalization, and mortality and the mechanism.39, 40
In the current study, some other modifiable factors associated with HF symptoms were observed through examination of the effects of covariates on HF symptoms. For example, depressive symptoms were strongly associated with HF symptoms, consistent with the findings of other studies.5, 32 Thus, depressive symptoms need to be assessed and managed in patients with HF in order to improve HF symptoms. In the current study, more sodium intake was associated with more severe HF symptoms in female patients, and female patients also had higher mean edema scores than male patients. In other studies, sodium intake ≥ 3 g per day was associated with shorter event-free survival than normal sodium intake < 3 g per day,31 and more sodium intake was also associated with more severe HF symptoms.32 Thus, it may be beneficial to educate patients with HF to control sodium intake appropriately by teaching them ways to estimate dietary sodium intake and connect sodium intake to HF symptoms, such as edema, especially in female patients. For example, a patient with HF can enter his/her food consumption to any of several free Internet nutrition programs to monitor and manage sodium intake based on feedback on sodium intake.41 Then, the patient can be trained to observe how sodium intake each day is connected to changes in his/her body weight and also edema on ankles or legs.
In the current study, another common associate of HF symptoms was comorbidities. Thus, it is important to check comorbidities to manage HF symptoms. Age, educational level, race, comorbidities, etiology of HF, LVEF, depressive symptoms, sodium intake, and obesity explained 44% to 57% of the variance in HF symptoms, indicating that there may be other variables affecting HF symptoms. Thus, further studies are needed to explore and examine such variables.
There were some limitations to this study. The sample was relatively young. In older patients with HF, HF symptoms and the relationship of obesity to HF symptoms might differ from those reported here.27 We used stringent inclusion and exclusion criteria to avoid the confounding effects on the outcomes. Relatively younger age and stringent inclusion and exclusion criteria can limit the generalizability of the findings of the current study. This was a cross-sectional study, thus, we cannot examine causal relationships between obesity and HF symptoms. In the current study, not all obesity groups could be separated because of the small samples in some obesity groups in males and females, and the relationships between obesity and HF symptoms differed based on the levels of obesity measure. In addition, obesity can be assessed using several methods, including BMI, waist circumference, and combination of BMI and waist circumference.42, 43 Thus, researchers may test different obesity measures in larger samples to examine the relationship between obesity and HF symptoms more thoroughly. Nonetheless, the findings of this study provide important information about some modifiable factors, including obesity, depressive symptoms, and sodium intake, associated with HF symptoms in male and female patients with HF.
In conclusion, high BMI (> 35 kg / m2) was associated with more severe HF symptoms in male patients. Thus, obesity paradox on mortality and hospitalization did not extend to HF symptom status. There were gender differences in the relationship between obesity and HF symptoms and the associates of HF symptoms. In male patients, age, race, comorbidities, depressive symptoms, and obesity were associated with HF symptoms; while in female patients, comorbidity, depressive symptoms, and sodium intake were associated with less severe HF symptoms. Thus, it may be beneficial to consider these differences in developing interventions to improve HF symptoms in patients with HF. For example, clinicians may more focus on managing obesity, depressive symptoms, and comorbidities for male patients; while they may more focus on managing depressive symptoms, sodium intake, and comorbidities in female patients. Further studies are needed to examine the relationships among obesity, HF symptoms, hospitalization, and mortality over time to examine the causal relationships among these variables and to provide more rigorous and concrete evidence for appropriate BMI levels in male and female patients.
Table 4.
Association between Obesity (Categorical Variable) and Heart Failure Symptoms
| Variables | Total Sample | Males | Females | |||
|---|---|---|---|---|---|---|
| t Statistic | p Value | t Statistic | p Value | t Statistic | p Value | |
| Age | 1.896 | .059 | 2.664 | .009 | −.577 | .566 |
| Education level | −1.441 | .151 | −1.129 | .260 | −1.846 | .069 |
| Race | 1.894 | .059 | 3.379 | .001 | −.832 | .408 |
| Comorbidities | 3.933 | <.001 | 3.050 | .003 | 2.038 | .045 |
| Left Ventricular Ejection Fraction | .103 | .918 | .421 | .674 | −.716 | .477 |
| Depressive symptoms | 10.355 | < .001 | 10.985 | < .001 | 3.010 | .004 |
| Sodium intake | 1.386 | .167 | −.211 | .833 | 2.057 | .043 |
| Gender | .803 | .423 | N/A | N/A | N/A | N/A |
| Obesity*gender | .267 | .790 | N/A | N/A | N/A | N/A |
| Obesity | ||||||
| Normal/underweight | −3.419 | .001 | −4.264 | < .001 | −1.467 | .147 |
| Overweight | −3.438 | .001 | −3.837 | < .001 | −1.083 | .282 |
| Obese class I | −2.471 | .014 | −2.048 | .042 | −1.983 | .051 |
| R2 (adjusted R2) | .481 (.454) | .568 (.541) | .441 (.361) | |||
| F statistic | 18.062 | 20.412 | 5.524 | |||
| p value | < .001 | <.001 | < .001 | |||
The reference group for the obesity comparison was obese class II/III.
Acknowledgement
Source of Funding: Funding for this study came from an American Heart Association Postdoctoral Fellowship to Seongkum Heo; the National Institutes of Health (NIH), National Institute of Nursing Research (NINR) R01 NR009280 to Terry Lennie; Center grant NIH, NINR, 1P20NR010679 to Debra Moser, and, in part, PHS Grant M01 RR0039 from the General Clinical Research Center program, NINR, and PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program, NIH, National Center for Research Resources, PHS Grant M01 RR0039 from the General Clinical Research Center program, NINR, and PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program, NIH, National Center for Research Resources, to D. Stephens, and the Atlanta Veterans Administration Medical Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINR or the NIH.
Footnotes
Conflicts of Interest
For the remaining authors none was declared.
Contributor Information
Seongkum Heo, University of Arkansas for Medical Sciences, College of Nursing.
Debra K. Moser, University of Kentucky, College of Nursing.
Susan J. Pressler, Indiana University, School of Nursing.
Sandra B. Dunbar, Emory University, School of Nursing.
Kyoung Suk Lee, Chungnam National University, College of Nursing, Dea Jeon, South Korea.
JinShil Kim, Gachon University, College of Nursing, Incheon, South Korea.
Terry A. Lennie, University of Kentucky, College of Nursing.
References
- 1.Albert N, Trochelman K, Li J and Lin S. Signs and symptoms of heart failure: Are you asking the right questions? American Journal of Critical Care 2010; 19: 443–52. [DOI] [PubMed] [Google Scholar]
- 2.Jurgens CY, Hoke L, Byrnes J and Riegel B. Why do elders delay responding to heart failure symptoms? Nursing Research 2009; 58: 274–82. [DOI] [PubMed] [Google Scholar]
- 3.Reeder KM, Ercole PM, Peek GM and Smith CE. Symptom perceptions and self-care behaviors in patients who self-manage heart failure. Journal of Cardiovascular Nursing 2015; 30: E1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heo S, Moser DK, Lennie TA, Zambroski CH and Chung ML. A comparison of health-related quality of life between older adults with heart failure and healthy older adults. Heart Lung 2007; 36: 16–24. [DOI] [PubMed] [Google Scholar]
- 5.Bekelman DB, Havranek EP, Becker DM et al. Symptoms, depression, and quality of life in patients with heart failure. Journal of Cardiac Failure 2007; 13: 643–8. [DOI] [PubMed] [Google Scholar]
- 6.Blinderman CD, Homel P, Billings JA, Portenoy RK and Tennstedt SL. Symptom distress and quality of life in patients with advanced congestive heart failure. Journal of Pain and Symptom Management 2008; 35: 594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giamouzis G, Kalogeropoulos A, Georgiopoulou V et al. Hospitalization epidemic in patients with heart failure: Risk factors, risk prediction, knowledge gaps, and future directions. Journal of Cardiac Failure 2011; 17: 54–75. [DOI] [PubMed] [Google Scholar]
- 8.Abate N Obesity and cardiovascular disease. Pathogenetic role of the metabolic syndrome and therapeutic implications. J Diabetes Complications 2000; 14: 154–74. [DOI] [PubMed] [Google Scholar]
- 9.Bjorck L, Novak M, Schaufelberger M, Giang KW and Rosengren A. Body weight in midlife and long-term risk of developing heart failure-A 35-year follow-up of the primary prevention study in Gothenburg, Sweden. BMC Cardiovasc Disord 2015; 15: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis JP, Selter JG, Wang Y et al. The obesity paradox: Body mass index and outcomes in patients with heart failure. Archives of Internal Medicine 2005; 165: 55–61. [DOI] [PubMed] [Google Scholar]
- 11.Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB and Lopatin M. An obesity paradox in acute heart failure: Analysis of body mass index and inhospital mortality for 108,927 patients in the Acute Decompensated Heart Failure National Registry. American Heart Journal 2007; 153: 74–81. [DOI] [PubMed] [Google Scholar]
- 12.Shah R, Gayat E, Januzzi JL Jr. et al. Body mass index and mortality in acutely decompensated heart failure across the world: A global obesity paradox. J Am Coll Cardiol 2014; 63: 778–85. [DOI] [PubMed] [Google Scholar]
- 13.Clark AL, Fonarow GC and Horwich TB. Waist circumference, body mass index, and survival in systolic heart failure: The obesity paradox revisited. Journal of Cardiac Failure 2011; 17: 374–80. [DOI] [PubMed] [Google Scholar]
- 14.Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM and McAlister FA. Body mass index and mortality in heart failure: A meta-analysis. American Heart Journal 2008; 156: 13–22. [DOI] [PubMed] [Google Scholar]
- 15.Sharma A, Lavie CJ, Borer JS et al. Meta-analysis of the relation of body mass index to all-cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol 2015; 115: 1428–34. [DOI] [PubMed] [Google Scholar]
- 16.Haass M, Kitzman DW, Anand IS et al. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: Results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail 2011; 4: 324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habbu A, Lakkis NM and Dokainish H. The obesity paradox: Fact or fiction? American Journal of Cardiology 2006; 98: 944–8. [DOI] [PubMed] [Google Scholar]
- 18.McMurray JJ, Adamopoulos S, Anker SD et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. European Journal of Heart Failure 2012; 14: 803–69. [DOI] [PubMed] [Google Scholar]
- 19.Perk J, De Backer G, Gohlke H et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). European Heart Journal 2012; 33: 1635–701. [DOI] [PubMed] [Google Scholar]
- 20.Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147–239. [DOI] [PubMed] [Google Scholar]
- 21.Zaharias E, Cataldo J, Mackin L and Howie-Esquivel J. Simple measures of function and symptoms in hospitalized heart failure patients predict short-term cardiac event-free survival. Nurs Res Pract 2014; 2014: 815984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regitz-Zagrosek V, Brokat S and Tschope C. Role of gender in heart failure with normal left ventricular ejection fraction. Progress in Cardiovascular Diseases 2007; 49: 241–51. [DOI] [PubMed] [Google Scholar]
- 23.Brannstrom J, Hamberg K, Molander L, Lovheim H and Gustafson Y. Gender disparities in the pharmacological treatment of cardiovascular disease and diabetes mellitus in the very old: an epidemiological, cross-sectional survey. Drugs and Aging 2011; 28: 993–1005. [DOI] [PubMed] [Google Scholar]
- 24.Riegel B, Dickson VV, Kuhn L, Page K and Worrall-Carter L. Gender-specific barriers and facilitators to heart failure self-care: A mixed methods study. International Journal of Nursing Studies 2010; 47: 888–95. [DOI] [PubMed] [Google Scholar]
- 25.Vest AR, Wu Y, Hachamovitch R, Young JB and Cho LS. Reply: Concerning the role of gender difference in obesity paradox in patients with heart failure. JACC Heart Fail 2016; 4: 236. [DOI] [PubMed] [Google Scholar]
- 26.Emami A, Dos Santos MR, Anker SD, von Haehling S and Sandek A. Concerning the role of gender difference in obesity paradox in patients with heart failure. JACC Heart Fail 2016; 4: 235–6. [DOI] [PubMed] [Google Scholar]
- 27.Heo S, Doering LV, Widener J and Moser DK. Predictors and effect of physical symptom status on health-related quality of life in patients with heart failure. Am J Crit Care 2008; 17: 124–132. [PubMed] [Google Scholar]
- 28.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ and Croft JB. State of disparities in cardiovascular health in the United States. Circulation 2005; 111: 1233–41. [DOI] [PubMed] [Google Scholar]
- 29.Heo S, Moser DK, Pressler SJ, Dunbar SB, Mudd-Martin G and Lennie TA. Psychometric properties of the Symptom Status Questionnaire-Heart Failure. Journal of Cardiovascular Nursing 2015; 30: 136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacob Rodriguez J, Herrero Puente P, Martin Sanchez FJ et al. [EAHFE (Epidemiology Acute Heart Failure Emergency) study: Analysis of the patients with echocardiography performed prior to an emergency visit due to an episode of acute heart failure]. Rev Clin Esp 2011; 211: 329–37. [DOI] [PubMed] [Google Scholar]
- 31.Son YJ, Lee Y and Song EK. Adherence to a sodium-restricted diet is associated with lower symptom burden and longer cardiac event-free survival in patients with heart failure. Journal of Clinical Nursing 2011; 20: 3029–38. [DOI] [PubMed] [Google Scholar]
- 32.Heo S, Moser DK, Lennie TA, Fischer M, Smith E and Walsh MN. Modifiable correlates of physical symptoms and health-related quality of life in patients with heart failure: A cross-sectional study. International Journal of Nursing Studies 2014; 51: 1482–90. [DOI] [PubMed] [Google Scholar]
- 33.Heo S, Moser DK, Pressler SJ et al. Dose-dependent relationship of physical and depressive symptoms with health-related quality of life in patients with heart failure. European Journal of Cardiovascular Nursing 2013; 12: 454–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lennie TA, Song EK, Wu JR et al. Three gram sodium intake is associated with longer event-free survival only in patients with advanced heart failure. Journal of Cardiac Failure 2011; 17: 325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horwich TB, Leifer ES, Brawner CA, Fitz-Gerald MB and Fonarow GC. The relationship between body mass index and cardiopulmonary exercise testing in chronic systolic heart failure. American Heart Journal 2009; 158: S31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Storch EA, Roberti JW and Roth DA. Factor structure, concurrent validity, and internal consistency of the Beck Depression Inventory-Second Edition in a sample of college students. Depress Anxiety 2004; 19: 187–9. [DOI] [PubMed] [Google Scholar]
- 37.Hammash MH, Hall LA, Lennie TA et al. Psychometrics of the PHQ-9 as a measure of depressive symptoms in patients with heart failure. European Journal of Cardiovascular Nursing 2013; 12: 446–53. [DOI] [PubMed] [Google Scholar]
- 38.Charlson ME, Pompei P, Ales KL and MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987; 40: 373–83. [DOI] [PubMed] [Google Scholar]
- 39.Oga EA and Eseyin OR. The Obesity Paradox and Heart Failure: A Systematic Review of a Decade of Evidence. J Obes 2016; 2016: 9040248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavie CJ, Sharma A, Alpert MA et al. Update on Obesity and Obesity Paradox in Heart Failure. Prog Cardiovasc Dis 2016; 58: 393–400. [DOI] [PubMed] [Google Scholar]
- 41.Hwang KO, Ning J, Trickey AW and Sciamanna CN. Website usage and weight loss in a free commercial online weight loss program: retrospective cohort study. J Med Internet Res 2013; 15: e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puig T, Ferrero-Gregori A, Roig E et al. Prognostic value of body mass index and waist circumference in patients with chronic heart failure (Spanish REDINSCOR Registry). Rev Esp Cardiol (Engl Ed) 2014; 67: 101–6. [DOI] [PubMed] [Google Scholar]
- 43.Clark AL, Chyu J and Horwich TB. The obesity paradox in men versus women with systolic heart failure. American Journal of Cardiology 2012; 110: 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]


