Abstract
Background
The importance of epigenetic alterations in psychiatric disorders is increasingly acknowledged and the use of DNA methylation patterns as markers of disease is a topic of ongoing investigation. Recent studies suggest that patients suffering from Borderline Personality Disorder (BPD) display differential DNA methylation of various genes relevant for neuropsychiatric conditions. For example, several studies report differential methylation in the promoter region of the brain-derived neurotrophic factor gene (BDNF) in blood. However, little is known about BDNF methylation in other tissues.
Results
In the present study, we analyzed DNA methylation of the BDNF IV promoter in saliva and blood of 41 BPD patients and 41 matched healthy controls and found significant hypermethylation in the BPD patient’s saliva, but not blood. Further, we report that BDNF methylation in saliva of BPD patients significantly decreased after a 12-week psychotherapeutic intervention.
Conclusions
Providing a direct comparison of BDNF methylation in blood and saliva of the same individuals, our results demonstrate the importance of choice of tissue for the study of DNA methylation. In addition, they indicate a better suitability of saliva for the study of differential BDNF methylation in BPD patients. Further, our data appear to indicate a reversal of disease-specific alterations in BDNF methylation in response to psychotherapy, though further experiments are necessary to validate these results and determine the specificity of the effect.
Electronic supplementary material
The online version of this article (10.1186/s13148-018-0544-6) contains supplementary material, which is available to authorized users.
Keywords: Epigenetics, Saliva, DNA methylation, BPD, DBT, Biomarker, Treatment outcome, BDNF
Background
Borderline personality disorder (BPD) is a severe mental disorder that is characterized by instability in affect, interpersonal relationships, and self-image, in addition to impulsivity, fear of abandonment, anger, and self-mutilating behavior [1]. The estimated lifetime prevalence of BPD is 1.6–5.9%, as estimated by two large nonclinical surveys in the USA [2, 3]. However, despite its high prevalence, the pathogenesis and underlying biological mechanisms of BPD are not fully understood. According to the biosocial developmental model of BPD proposed by M. Linehan in 1993, the susceptibility for the disorder is enhanced by an early emotional vulnerability, which is then potentiated across the life span. Initial vulnerability is mainly caused by environmental risk factors such as childhood abuse or neglect. The estimated contribution of genetic factors to the disorder is in the range of 42–68% [4, 5], while environmental factors account for the remaining variance.
Recent evidence indicates that the interplay of environmental and genetic factors in the development of psychiatric disorders is partially mediated by epigenetic regulation [6, 7]. Epigenetic modifications induce changes in gene expression without altering the DNA sequence. One of the most prominent and best studied epigenetic mechanisms is DNA methylation, a covalent modification of cytosine in a cytosine-guanine-dimer (CpG site). Although DNA methylation is generally described as a silencing epigenetic mark, it is increasingly acknowledged that its effect on gene expression is context-dependent. Hence, it may induce silencing of a gene, when found within its promoter region, but enhance expression, when found in the gene body [8, 9]. The degree of DNA methylation at a specific locus is determined by the underlying DNA sequence [10] and is to some extent dynamically regulated by DNA methyltransferase enzymes. As these act in response to environmental stimuli [11], DNA methylation provides the cell with a way to adapt to changes in the environment [12] and is an ideal candidate mechanism for studying the interplay of genetic and environmental signals on disease development [13]. A major challenge in epigenetic research is the cell type and tissue specificity of DNA methylation [14]. As access to brain tissue is limited, the great majority of epigenetic studies in psychiatry are conducted with blood as surrogate tissue [15].
In line with this, DNA methylation signatures have been analyzed in the peripheral blood of several BPD patient cohorts. Using targeted approaches aimed at well-known psychiatric candidate genes, epigenetic dysregulation in the blood of BPD patients has been reported e.g., for the serotonin receptor 2A (HTR2A), the monoamine oxidase A and B (MAOA and MAOB), the soluble catechol-o-methyltransferase (S-COMT), the glucocorticoid receptor (GR/NR3C1) (all reported by [16]), and the brain-derived neurotrophic factor (BDNF) [17]. Further, hypothesis-free epigenome-wide studies revealed a number of novel candidate genes to be differentially methylated in patients suffering from BPD [18, 19]. However, the findings for most of the above-mentioned studies are not fully consistent with each other, and their significance yet remains to be determined by replication in independent cohorts. For the role of BDNF methylation in BPD, support is already available from a study conducted by Thaler et al. [20], showing that increased BDNF methylation in patients with bulimic eating behavior is particularly prominent when associated with comorbid BPD. In addition, Thaler et al. and Perroud et al. [17] had found an association of BDNF methylation with childhood trauma. In line with these findings, several independent studies report a link between BDNF methylation, stress, and trauma [21–23]. Here, the most convincing evidence is available for animal models of early-life stress (ELS) [24–26]. For example, Roth et al. [26] report increased methylation of the BDNF gene in the prefrontal cortex of rats exposed to abusive mothers. In humans, post-traumatic stress disorder in combat veterans [22] and exposure to domestic violence in women [21] have been associated with increased BDNF methylation in peripheral blood. Further, a recent study found that DNA methylation within the BDNF gene moderates the association between childhood trauma and depressive symptoms [23]. It is hypothesized that the link between BDNF and psychological stress is mediated via the crosstalk of neurotrophin and glucocorticoid pathways [27], as BDNF signaling is a target of the glucocorticoid stress response [28]. Hence, the high prevalence of ELS among patients with BPD [29] makes it difficult to disentangle its effects on DNA methylation from BPD-specific effects. Another confounder for epidemiologic studies of BDNF methylation is smoking. Next to its reported global effects on DNA methylation [30, 31], there is evidence for the association of prenatal smoke exposure with changes in offspring BDNF methylation and expression [32]. These alterations may be long-lasting and promote vulnerability to psychiatric disease later in life, as suggested by human [33] and animal studies [34, 35]. With regard to direct effects of smoking on BDNF expression, most studies indicate increased peripheral BDNF protein in smokers as compared to non-smokers [36–38], but there are no reports of altered BDNF methylation.
Adding even further to the difficulty of studying BPD-specific effects, BDNF methylation was also found associated with a broad range of psychiatric symptoms and disorders other than BPD, such as bipolar disorder [39, 40], depression [41], schizophrenia [42], and suicidality [43, 44] (reviewed in [45, 46]). The ubiquitous role of BDNF methylation in psychiatry is presumably caused by the broad expression of the BDNF protein in the brain, its importance in learning and memory [47] and its key regulating function in neuronal differentiation, and neurite and synaptic growth [48]. BDNF promoter hypermethylation, as reported in the vast majority of studies, should lead to a decreased expression of the protein. Indeed, this is what independent studies of patient cohorts report for depression [49, 50], bipolar disorder [39, 51], schizophrenia [52] and, most interestingly, also for BPD [53] (for general review see [54]). In addition, an increasing number of studies suggest that antidepressant and mood-stabilizing substances increase BDNF expression in the blood [55–57] and brain [58, 59]. In line with this, D’Addario et al. [39] reported that mood-stabilizing medication decreases BDNF exon I promoter methylation. These findings provide further indication that low BDNF expression plays a role in the pathophysiology of BPD.
In 2013, Perroud et al. reported that psychotherapeutic treatment alone (dialectical behavior therapy) leads to a reversion of the initially increased DNA methylation of BDNF promoter I and IV in BPD patients [17]. The effect was specific for those patients that had responded with significant alleviation of symptoms to the therapy [17]. This indicates that BDNF methylation may serve as biomarker for symptom severity in BPD patients and as an indicator of treatment success. Epigenetic biomarker have already been proposed as both predictors and correlates of symptom improvement in PTSD patients [60] and would also be highly desirable for BPD patients, where they could pave the way towards personalized treatment. In addition to BDNF methylation, DNA methylation of APBA3 (amyloid beta A4 precursor protein-binding family A member 3) and MCF2 (oncogene MCF2) has recently been proposed as blood-based biomarker for BPD patients. In this case, methylation at the respective genes was proposed as predictor of therapy response [61].
However, recent evidence indicates that saliva might be a superior surrogate tissue to blood for the study of DNA methylation in psychiatric disorders. Cross-tissue comparisons show that saliva mirrors methylation levels in the brain to a greater extent than blood does [62]. This was explicitly shown for a number of CpG sites within BDNF [63], even though the explanatory power of the respective study is limited as data on the brain tissue did not originate from the same study cohort from which blood and saliva was sampled. In addition, salivary biomarkers display a much more convenient, non-invasive, and safe method for studying DNA methylation alterations. As such, saliva-based epigenetic biomarkers are universally applicable in in- and out-patient settings.
So far, differential BDNF methylation initially found in blood [39, 41], was confirmed to be also present in saliva for bipolar disorder [64], anxiety and depression [65, 66], but has not been investigated for BPD yet. For that reason, we assessed BDNF promoter IV methylation in both saliva and blood from the same BPD patients, thereby enabling a direct comparison of methylation levels in both tissues. Further, since Perroud et al. [17] had reported that dialectical behavior therapy (DBT), one of the most frequently applied psychotherapeutic intervention for BPD patients [67], leads to a decrease of previously elevated BDNF methylation levels in BPD patients, we sought to replicate this finding by reassessing the blood and salivary BDNF methylation in a subsample of patients after a 12-week DBT.
Results
Study population
BPD patients and healthy controls did not differ significantly in age, sex, and alcohol consumption. However, there were significantly more habitual smokers in the group of BPD patients (see Table 1 for details). Further, 85.36% of all BPD patients were under current psychopharmacological medication at the time of sampling, as opposed to 0% in the healthy control group. BPD patients scored significantly higher in the BSL23 (Borderline Symptom List 23), SCL90R (Symptom Checklist-90-revised), and Childhood Trauma Questionnaire (CTQ).
Table 1.
Comparison of BPD patient and healthy control cohorts
| BPD patients (T1) | Healthy controls | p value | |
|---|---|---|---|
| N | 41 | 41 | |
| Age | 30.4 ± 8.6 | 30.7 ± 9.3 | n.s. |
| Proportion of women (%) | 85.36% | 85.36% | n.s. |
| Proportion of individuals who are | |||
| Habitual smokers | 53.65% | 9.76% | < 0.001 |
| Habitual drinkers | 85.37% | 95.12% | n.s. |
| Under current medication* | 85.36% | 0% | < 0.001 |
| Psychiatric questionnaires | |||
| GSI score (SCL90R) | 2.1 ± 0.54 | 0.27 ± 0.24 | < 0.001 |
| PST score (SCL90R) | 69.88 ± 9.15 | 17.8 ± 13 | < 0.001 |
| BSL23 total score | 2.48 ± 0.76 | 0.17 ± 0.23 | < 0.001 |
| CTQ total score | 62.9 ± 24.4 | 33.93 ± 8.47 | < 0.001 |
Age and results from self-administered psychiatric questionnaires are displayed as mean ± standard deviation. p values derive from statistical analysis with independent t test or chi-square test for comparison of percentages
*Psychopharmacological medication only
n.s. not significant (p value > 0.05)
Higher BDNF IV promoter methylation levels in saliva, but not blood, of BPD patients as compared to healthy controls
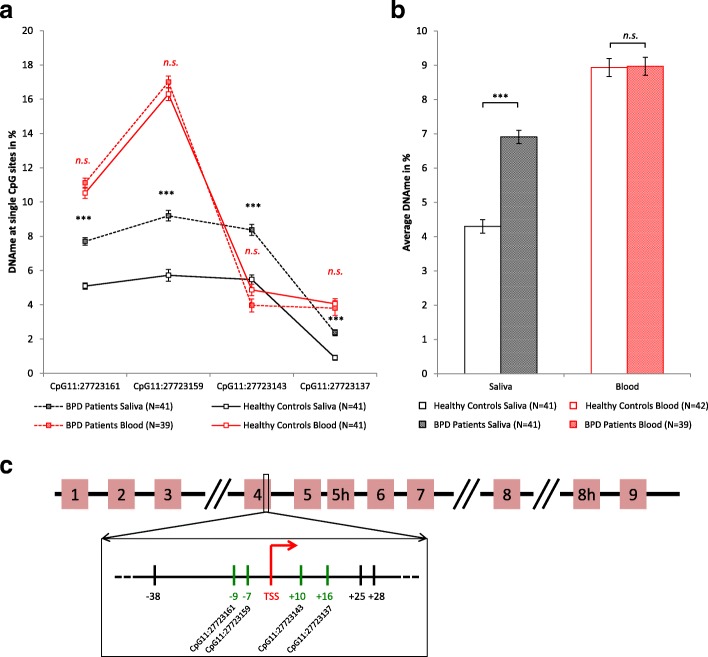
In saliva samples, DNA methylation was significantly higher in BPD patients than in healthy controls at all four analyzed CpG sites within the BDNF IV promoter (p < 0.001 for all sites, Fig. 1, see Table 2 for details). Further, the average methylation level calculated from all analyzed sites was higher in BPD patients than in healthy controls (M = 6.9%, SE = 0.19 vs. M = 4.3%, SE = 0.20, M = mean, SE = standard error). This difference, − 2.6%, 95% CI [− 3.163, − 2.061] was significant (t (80) = − 9.431, p value = 1.26 × 10−14) and represented a large effect (Cohen’s d = 2.1). These differences between BPD patients and healthy controls were also significant after including smoking behavior and experience of ELS as covariates into a general linear model to predict DNA methylation (b = 2.33, SE = 0.38, 95% CI [1.571, 3.058], β = 0.65, t (78) = 6.123, p value = 3.46 × 10−8 for average methylation). Neither covariate had a significant influence on DNA methylation in the model for any of the analyzed CpG sites (see Additional file 1: Table S2, for detailed results) and their addition to the model resulted in an average change in estimate (CIE) of 3.4% (smoking) and 10.2% (ELS). In DNA isolated from whole blood, BDNF methylation levels did not differ significantly between BPD patients and healthy controls neither for single CpG sites, nor for the average calculated from all sites (patient average 9.0% vs. healthy controls average 8.9%, detailed data in Additional file 1: Table S1). In line with this, multiple regression analysis showed no effect of group, smoking, or ELS on the blood DNA methylation at all analyzed CpG sites (see Additional file 1: Table S2 for detailed results).
Fig. 1.
a, b DNA methylation at the BDNF IV promoter in blood and saliva of BPD patients (T1) (N = 41 for saliva, N = 39 for blood) as compared to healthy controls (N = 41). Data shown for single CpG sites (a) and average methylation calculated over all analyzed CpG sites (b), error bars represent SEM. Three asterisks (***) indicate statistical significance at p value < 0.001. c Schematic drawing of the BDNF gene with exons 1–9. Analyzed CpG sites (marked in green) are within the exon IV promoter, in direct vicinity of the transcription start site (TSS)
Table 2.
Statistics for the comparison of saliva BDNF methylation in BPD patients and healthy controls
| CpG site | Mean difference | CI lower | CI upper | p value | Cohen’s d |
|---|---|---|---|---|---|
| CpG11:27723161 | − 2.6 | − 3.181 | − 2.047 | 3.966 × 10−14 | 2.1 |
| CpG11:27723159 | − 3.5 | − 4.392 | − 2.556 | 6.637 × 10−10 | 1.7 |
| CpG11:27723143 | − 2.9 | − 3.725 | − 2.064 | 9.387 × 10−9 | 0.9 |
| CpG11:27723137 | − 1.5 | − 1.922 | − 1.007 | 1.110 × 10−8 | 1.5 |
| Average | − 2.6 | − 3.163 | − 2.061 | 1.255 × 10−14 | 2.1 |
Results of independent t test for salivary BDNF IV promoter methylation in BPD patients (T1) and healthy controls. Results shown for individual CpG sites and average calculated from all sites
No correlation between the blood and salivary BDNF IV methylation
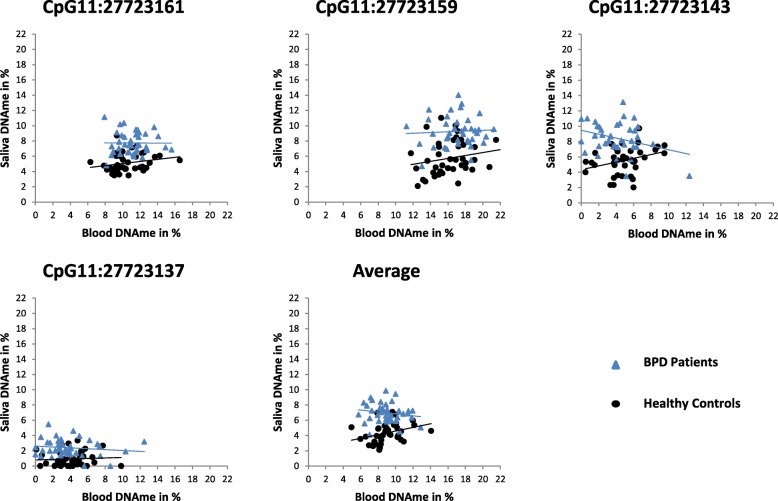
We compared BDNF IV promoter methylation in the saliva and blood of patients and controls and did neither find any significant correlation (Pearson’s correlation coefficient) in the combined cohort (N = 80) nor in the BPD patient cohort alone (N = 39). In the healthy cohort (N = 41), CpG11:27723143 and the average methylation were significantly but weakly correlated between both tissues (CpG11:27723143: r = 0.33, p = 0.035, 95% CI [0.088, 0.515]; average methylation: r = 0.33, p = 0.036, 95% CI [0.096, 0.574]) (Fig. 2, see Additional file 1: Table S4 for all data).
Fig. 2.
Correlation of methylation levels between blood and saliva DNA samples of BPD patients (N = 39) and healthy controls (N = 41). Regression lines are displayed separately for healthy controls and BPD patients
Decrease of salivary DNA methylation levels in BPD patients following psychotherapeutic intervention
Following psychotherapeutic intervention, patients (N = 26) showed a significant reduction in general and BPD-specific psychiatric symptoms, as assessed by SCL90R and BSL23, respectively (Table 3).
Table 3.
Psychiatric symptoms of BPD patients before and after DBT
| Before treatment | After treatment | p value | |
|---|---|---|---|
| GSI score (SCL90R) | 2.03 ± 0.46 | 1.49 ± 0.67 | < 0.001 |
| PST score (SCL90R) | 69.0 ± 8.79 | 59.31 ± 14.29 | 0.001 |
| BSL23 total score | 2.29 ± 0.75 | 1.86 ± 0.77 | 0.012 |
Results from self-administered psychiatric questionnaires of 26 BPD patients before and after 12-week psychotherapeutic treatment (DBT) as means ± standard deviation. p values derive from statistical analysis with t test for paired samples
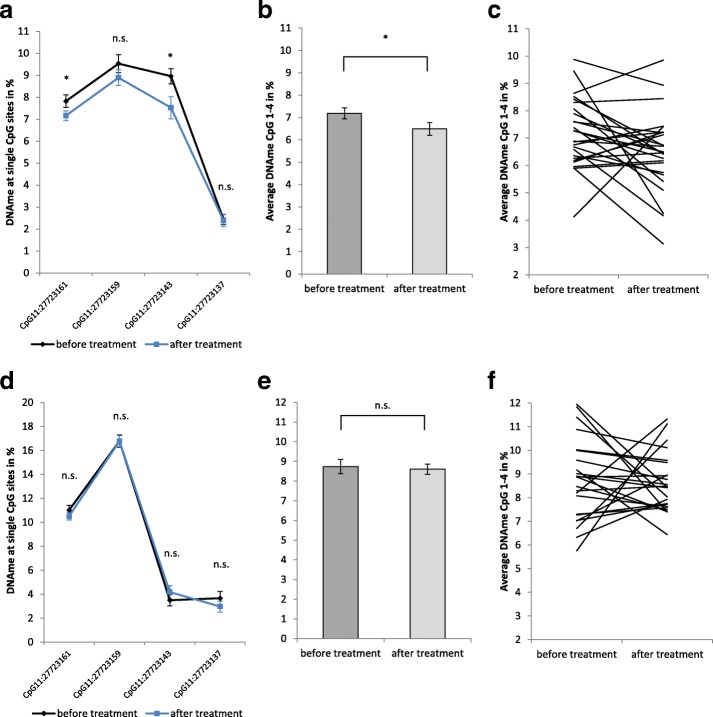
After treatment, salivary DNA methylation at BDNF IV promoter decreased at all analyzed CpG sites (Fig. 3a–c, Table 4), though the effect was significant only for CpG11:27723161, CpG11:27723143, and the average calculated from all sites, where BDNF methylation decreased from 7.2 to 6.5% (mean difference = − 0.7%, SE = 0.33, 95% CI [− 1.370,-0.019], t (25) = − 2.118), p value = 0.044, Cohen’s d = 0.4). Analysis of changes in individual patients revealed that DNA methylation levels remained unchanged (difference less than 0.5%) in seven out of 26 patients. Of the remaining 19 patients, 14 showed decreased, and five increased methylation levels. However, the observed change in DNA methylation did not correlate with symptom reduction in individual patients (Additional file 1: Table S5). Blood DNA methylation did not change from T1 to T2, neither for single CpG sites nor for the average calculated from all sites (8.7% vs. 8.6%, mean difference = − 0.1%, SE = 0.48, 95% CI [− 1.124,0.860], t (22) = − 0.276, p value = 0.785) (Fig. 3d–f, Additional file 1: Table S3).
Fig. 3.
BDNF IV promoter methylation in BPD patients before and after a 12-week psychotherapeutic treatment in saliva (a–c, N = 26) and blood (d–f, N = 23). Data shown for single CpG sites (a, d) and for the average calculated from all analyzed CpG sites (b, c, e, f), Data points are displayed as mean ± SEM of all patients at the two time points. Statistical analysis has been conducted with paired t tests. Asterisks indicate significance p < 0.05 (a, b, d, e). Individual differences in DNA methylation levels before and after treatment shown for every patient in saliva (c) and blood (f)
Table 4.
Statistics for the comparison of saliva BDNF methylation in BPD patients before and after therapy
| CpG site | Mean difference | CI lower | CI upper | p value | Cohen’s d |
|---|---|---|---|---|---|
| CpG11:27723161 | − 0.7 | − 1.303 | − 0.011 | 0.047 | 0.4 |
| CpG11:27723159 | − 0.6 | − 1.562 | 0.282 | 0.165 | n.a. |
| CpG11:27723143 | − 1.4 | − 2.542 | − 0.324 | 0.013 | 0.5 |
| CpG11:27723137 | − 0.0 | − 0.660 | 0.561 | 0.871 | n.a. |
| Average | − 0.7 | − 1.370 | − 0.019 | 0.044 | 0.4 |
Results of paired t tests for salivary BDNF IV promoter methylation in BPD patients before (T1) and after treatment (T2) (N = 26). Results shown for individual CpG sites and for the average calculated from all sites
n.a. not applicable (p value > 0.05)
Discussion
We assessed BDNF IV promoter methylation in blood and saliva of the same individuals and found no correlation between the tissues. This has been reported previously [63], even though there is evidence for a correlation between blood and saliva methylation on a genome-wide level [62].
When comparing BDNF methylation in BPD patients and healthy controls, we unexpectedly did not find differences in DNA extracted from blood, as had previously been reported [17] (Table 5). One reason for this discrepancy might be that Perroud et al. [17] had analyzed the average methylation level calculated from a longer section of the BDNF IV promoter and did not report individual CpG methylation. Therefore, our assay might not have covered the relevant CpG sites. Further, the different methods used for DNA methylation analysis (high resolution melt analysis in [17] vs. pyrosequencing in our study) may have contributed to the conflicting results. With regard to the absolute levels of BDNF, the low levels of DNA methylation observed in our study (3-17%) were still higher than previously described for the respective CpG sites (< 5%) [20, 42]. Similarly, this discrepancy may be partially due to methodological differences (EpiTYPER in [20]) and ethnical differences of the study cohort (Japanese cohort in [42] as compared to Caucasian cohort in the present study). Our finding of the unaltered blood DNA methylation of the BDNF IV promoter in BPD patients is supported by epigenome-wide studies, which also failed to provide evidence for differential BDNF promoter IV methylation in the blood of BPD patients [18, 19].
Table 5.
Genomic position of analyzed CpGs within BDNF IV promoter
| ID | Genomic position (hg19) | Differential DNAme in the context of psychiatric disorders previously analyzed in … |
|---|---|---|
| CpG11:27723161 | chr 11: 27,723,161–27,723,162 | Blood [17, 20]and saliva [68] |
| CpG11:27723159 | chr 11: 27,723,159–27,723,160 | Blood [17, 20]and saliva [68] |
| CpG11:27723143 | chr 11: 27,723,143–27,723,144 | Blood [17, 42], saliva [68], and brain [44] |
| CpG11:27723137 | chr 11: 27,723,137–27,723,138 | Blood [17, 42] and brain [44] |
In contrast to the results in blood, we found significant hypermethylation of the BDNF IV promoter in saliva of BPD patients as compared to healthy controls. While our study is the first to analyze BDNF methylation in saliva samples of BPD patients, few other studies have analyzed salivary DNA methylation at the same sites within the BDNF IV promoter in the context of other psychiatric conditions. These studies support an association of saliva BDNF hypermethylation with symptoms of psychiatric diseases [21, 65, 68]. Further, the low absolute levels of saliva BDNF DNA methylation observed in our study (2–10%) were comparable to those previously reported in the literature, though the exact percentage of reported methylation varies between studies (1–30%). While Moser et al. report DNA methylation levels ranging from 1 to 30% in the exon IV promoter region [21], Chagnon et al. observed levels around 2–3% [65] and Januar et al. report levels between 4 and 17% [68]. Since analysis methods and study cohorts are at least partially comparable, the most likely explanation for this discrepancy is the variability in the number of CpG sites analyzed and the method of summarization of these data into reported methylation scores. However, the observed range of the saliva DNA methylation at the analyzed CpG sites in the present study is similar to the levels reported by Keller et al. [44] in human post-mortem brain tissue at the same CpG sites (5–11%). This finding further supports the significance of saliva as surrogate tissue for the brain in the study of psychiatric disorders.
Since differences in methylation between BPD patients and healthy controls were only evident in saliva, but not blood, our findings underline the importance of considering tissue-specificity of DNA methylation in biomarker studies. Salivary DNA derives from exfoliated epithelial cells and leukocytes, which migrate from the blood stream to the oral cavity [69]. Both cell types are known to express BDNF, though within leukocytes, all BDNF expression is driven by lymphocytes [70]. As there is indication for an enrichment of lymphocytes in oral samples as compared to blood [69], differential epigenetic regulation of this particular cell type may be more evident in saliva than in blood. In addition, the observed effects may also be driven by epithelial cells, as submandibular serous and ductal cells are sources of salivary BDNF protein [71] and may therefore be dynamically regulated. Further, BDNF overexpression derived from salivary glands was found to influence BDNF levels in the blood and hippocampus and exert anxiolytic effects on behavior [72]. This supports a functional role for BDNF methylation in salivary epithelial cells. Additional studies are necessary in order to determine whether the observed alterations in salivary BDNF IV promoter methylation are accompanied by changes in BDNF expression and how these relate to psychological symptoms of BPD. However, the past years of research have shown that even small alterations in DNA methylation (Δ < 5%), as have been observed in this study, can be functionally relevant, i.e., exert influence on gene transcription [73, 74]. In fact, previous studies report similarly small changes in BDNF methylation associated with psychiatric symptoms (Δ = 0.42% in [65], Δ = 5.4% in [68]), and these findings are in accordance with the understanding of the multifactorial origin of psychiatric disorders [75]. In addition, the subtle differences in methylation in BDNF DNA methylation may also reflect the “tip of the iceberg,” i.e., the measurable output of a complex, masked pattern of stronger, cell type-specific differential methylation. In this case, effects may be driven by buccal epithelial cells or different leukocyte subtypes contained in the saliva, as previously described in more detail. However, the detailed mechanisms underlying the differential BDNF IV promoter methylation in BPD patients are irrelevant to the validity of the epigenetic signal as biomarker for the disorder. With regard to that, it is important to note that despite the small absolute change, the difference in methylation between BPD patients and controls is significant and the effect size was large (Cohen’s d = 2.1).
BDNF mRNA and protein levels were not assessed in the present study, and are difficult to assess reliably in the saliva, where protein levels are below the detection limit [76]. However, there is evidence for reduced levels of serum BDNF protein in BPD patients from previous studies [53], which is what would be expected as consequence of BDNF promoter hypermethylation [77]. In particular, the CpG sites analyzed in the present study are in close vicinity (− 49, − 51, − 65 and − 74 bp) to the binding site of transcription factor cAMP response element binding protein (CREB, half consensus sequence “CGTCA” [78]). CREB controls BDNF transcription in a DNA methylation-dependent manner [77], indicating a plausible effect of the observed methylation difference on gene expression. Further support for the relevance of BDNF IV promoter hypermethylation for BPD is provided by animal experiments. These show that disruption of BDNF IV promoter-dependent expression results in deficits in prefrontal signaling [79–81], neurobiological changes which are also observed in BPD [82].
Lastly, we found that the level of salivary, but not blood BDNF IV methylation significantly decreases after patients underwent a 12-week psychotherapeutic treatment. This is particularly interesting, since the hereof predicted biological consequence, increased expression of BDNF, is also observed in response to antidepressant and mood-stabilizing pharmacological treatment and clinical improvement of BPD [55–59]. BPD patients did not experience any change of pharmacological treatment immediately before and during study participation. Therefore, the observed effects are unlikely to derive from psychopharmacological treatment and may present a true effect of psychotherapy. Our results are consistent with the data obtained by Perroud et al. [17], showing a decrease in BDNF IV methylation in BPD patients after the same psychotherapeutic intervention, though they observed the effect in the blood and not saliva. However, while Perroud et al. found the effect to be specific for treatment responders, we did not find differences in methylation change between patients with and without significant improvement of psychological symptoms after therapy. Still, the finding indicates psychotherapy-induced changes in DNA methylation. Therefore, it provides support for the conceptual premise that psychotherapeutic intervention alters biological mechanisms in a way that is comparable to pharmacological treatment [83]. Nevertheless, our results need to be interpreted with caution and the specificity of the observed effect remains to be elucidated. A major limitation of both the above-mentioned previous and the current study is the lack of appropriate control groups at the second time point of sampling, i.e., BPD patients and healthy controls without psychotherapeutic intervention. Further, potential bias may have been introduced by the cellular composition of our samples. This should be addressed in future experiments by analysis of isolated cell types, inclusion of cell counts, or application of post hoc statistical deconvolution algorithms if epigenome-wide methylation data is available [84]. One of the major confounders of cellular composition of the saliva is age [85] . However, as our sample of BPD patients and healthy control individuals was matched for age, we can exclude this as a confounder and assume that differences in cellular composition in the saliva introduced random noise rather than a systematic bias to the data. Variables that indeed differed significantly between patient and control group and have a known influence on BDNF IV methylation are smoking, experience of ELS, and intake of pharmacological medication. Smoking is known to exert a broad influence on genome-wide DNA methylation with so far limited understanding of gene-specific effects [30, 31]. We found only little influence of current smoking behavior on BDNF DNA methylation. However, we did not assess prenatal exposure to smoking, though it is reported to have an effect on BDNF methylation, as well as promote vulnerability to BPD later in life [86]. Consequently, we cannot exclude the influence of prenatal exposure to smoking on the observed differences in the saliva BDNF methylation of BPD patients. In addition, there is evidence that ELS, such as childhood maltreatment and abuse, influences BDNF IV promoter methylation specifically [24–26] and may therefore have introduced systematic bias in the results. In line with this, the experience of ELS was identified as confounder in our linear model to predict DNA methylation, even though its influence was relatively small (10.2% CIE, β < 0.1). Therefore, we are not able to fully disentangle the effects of ELS and BPD on BDNF IV promoter methylation. Further studies will be necessary to elucidate the potential role of BDNF methylation in mediating vulnerability to borderline personality traits conferred by ELS [29]. With regard to intake of medication, the majority of the literature points towards a positive effect of psychopharmacological treatment on BDNF levels both in serum of patients [55, 56] as well as in cell culture experiments [58, 59, 87]. According to the DNA methylation paradigm, this increase of BDNF protein levels would correspond to a decrease in promoter methylation. Since we observed increased methylation in pharmacologically treated subjects, we therefore assume that it is more likely that medication intake has masked potential differences, rather than produced them. Further, medication did not change between T1 and T2 and is therefore unlikely to have caused the observed decrease in DNA methylation in BPD patients in response to treatment. A limitation of our study is the undefined positive predictive power of our findings, since only a limited amount of data on effect sizes for differential BDNF methylation in BPD was available a priori. Therefore, even though the size of our sample is in the range of, if not higher than the sample sizes reported from comparable studies (see [16, 17, 19]), the biological relevance of our finding needs to be determined in future studies and the robustness of our findings need to be confirmed by replication in independent cohorts. Further, the so far limited understanding of the dynamics of DNA methylation patterns in human peripheral tissues is increasingly investigated [88] and future studies remain to determine the stability of the observed methylation differences over time and its potential correlation or predictive value for the long-term development of psychiatric symptoms.
Conclusions
We assessed DNA methylation levels at four sites within the BDNF IV promoter in blood and, for the first time, saliva of BPD patients and healthy controls and found significant hypermethylation in saliva, but not blood. Further, we found that the level of salivary, but not blood BDNF IV methylation significantly decreases after patients underwent a 12-week psychotherapeutic treatment. As such, our study adds to a growing body of evidence for an epigenetic dysregulation of BDNF in BPD, even though the previously reported differential methylation in blood [17] was not evident in our study population. Further, our results highlight the importance of considering tissue-specific differences in DNA methylation and suggest the exploration of saliva-based epigenetic biomarkers in psychiatry. Our study is the first to support the validity of BDNF IV promoter hypermethylation as a biomarker for BPD in a tissue other than the blood and provides additional indication for the reversal of disease-associated DNA methylation patterns in response to psychotherapy.
Methods
Study population
Forty-one currently hospitalized BPD patients and 41 healthy controls without any history of psychiatric disorders were included in the study. All subjects were of Caucasian origin and both groups were matched for age and sex. BPD patients were diagnosed according to the International Personality Disorder Examination (IPDE) and met at least five diagnostic criteria of BPD as defined in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). All study participants were phenotypically characterized with the following self-report questionnaires: Symptom Checklist 90 (SCL90R) [89], Borderline Symptom List 23 (BSL23) [90], and Childhood Trauma Questionnaire (CTQ) [91]. Further questionnaires assessed demographic information along with information about nicotine and alcohol consumption (AUDIT and Fagerström-Test). GSI (global severity index = average rating given to all items) and PST scores (positive symptom total = number of symptoms/items rated higher than zero) were calculated from SCL90R. Twenty-six BPD patients completed a 12-week psychotherapeutic treatment program (dialectical behavior therapy, DBT) and for these patients, psychological symptoms were assessed a second time after completion of the program using SCL90R and BSL23. Parts of the study cohort are identical to the cohort used in [61] but only those patients with available saliva samples were included and additional patients and controls were included in the present study.
Sampling and DNA extraction
Within the first week of hospital admission (T1), saliva was collected from 41 BPD patients using the Oragene Discover DNA Collection Kit (DNA Genotek, Ottawa, Canada). Saliva from 41 control individuals was collected immediately after study inclusion using the same method. All saliva samples were stored at − 20 °C until further analysis. Venous blood was drawn from 39 of the BPD patients at T1 and from all 41 controls, collected in ethylenediaminetetraacetic acid (EDTA) tubes and stored at − 80 °C until further analysis. From the 26 patients that completed the 12-week psychotherapeutic treatment (DBT), a second saliva sample was collected during the last week of the program (T2). A second blood sample (T2) was available from 23 of these 26 patients. DNA extraction was performed using the prepIT DNA extraction Kit (DNA Genotek) for the saliva and QIAamp DNA Blood Maxi-Kit (Qiagen, Hilden, Germany) for the blood samples.
DNA methylation analysis
Five hundred nanograms genomic DNA was bisulfite converted using the EpiTect Fast Bisulfite Conversion Kit (Qiagen) and the region of interest within the BDNF IV promoter was amplified using the PyroMark PCR Kit (Qiagen) according to the manufacturer’s instructions. PCR and sequencing primer (Metabion, Planegg, Germany) were as follows: PCR forward primer, 5′- TTT GTT GGG GTT GGA AGT GAA AAT-3′ PCR reverse primer, Biotin-5′-CCC ATC AAC TAA AAA CTC CAT TTA ATC TC-3′ (as in [92]); and sequencing primer, 5′-GTG GAT TTT TAT TTA TTT TTT TAT TTA T-3′.
Successful amplification as well as specificity of the PCR products was verified via agarose gel electrophoresis. Several PCR runs were performed as technical replicates for each sample (minimum two replications). Processing of the PCR amplicons for pyrosequencing analysis was performed according to the manufacturer’s protocol and PCR products were then sequenced using the PyroMark Q24 system and the PyroMark GoldReagents (Qiagen). The level of methylation in every sample was quantified using the PyroMark Q24 software version 2.0.6 (Qiagen). The pyrosequencing assay contained six CpG sites within the BDNF IV promoter, but only four sites passed pyrosequencing quality control and were used for further analysis (see Table 1 and Fig. 1c for genomic position). Only samples with standard deviation of < 3% between technical replicates were included in the analysis. DNA methylation standards with 0%, 25%, 50%, 75%, and 100% methylation (Qiagen) were used to generate a standard curve and all measurements were calibrated accordingly (see Additional file 1: Figure S1). In all steps of the DNA methylation analysis (bisulfite conversion, PCR, and pyrosequencing), samples were processed in balanced design in order to avoid batch effects.
Statistical analysis
Statistical analysis was performed with SPSS (IBM, version 26). Group mean comparisons were performed with Student’s t test. In addition, multiple regression analysis was performed to test the effect of group (BPD T1 vs. healthy controls) on DNA methylation, including smoking behavior (smoker vs. non-smoker) and ELS (CTQ total score) as covariates. Before and after treatment comparisons were performed using paired two-sided Student’s t test. Cohen’s effect size d for t test comparisons was calculated from the z-score. Differences in percentages between groups were assessed with chi-square test. Bivariate correlation analysis was performed using Pearson’s correlation coefficient and 95% percentile bootstrapping was performed.
Additional file
Table S1. Results of independent t test for blood BDNF IV promoter methylation in BPD patients (T1) and healthy controls. Results shown for individual CpG sites and average calculated from all sites. Table S2. Results of multiple regression analysis using group (BPD vs. healthy controls), smoking (smoker vs. non-smoker), and early-life stress (CTQ total score) as predictors and DNA methylation as dependent variable. Analysis was performed for each individual CpG site and for the average DNA methylation calculated from all analyzed CpG sites, as well as for saliva (SAL) and blood (BL). Table indicates regression coefficients b (b), standard error of b (SE b), lower and upper bound of 95% bootstrapped confidence intervals (CI lower b, CI upper b), standardized regression coefficient (β), p value (p value) and R2 of the model (R-squared). Table S3. Results of paired t test for the blood BDNF IV promoter methylation in BPD patients before (T1) and after treatment (T2). Results shown for individual CpG sites and average calculated from all sites. Table S4. Results of bivariate correlation analysis of the blood and salivary BDNF methylation at CpGs 1–4 and the average calculated from all sites. Ninety-five percentile bootstrapping was performed, and significant correlation (α=0.05) is marked in bold. Table S5. Correlation analysis of symptom reduction and change in salivary DNA methylation in BPD patients (N=26). Difference in scores of psychiatric questionnaires were correlated with difference in DNA methylation at all analyzed CpG sites using Pearson’s correlation coefficient. Table shows results of two-tailed significance test. Figure S1. Methylated DNA standards (0%, 25%, 50% 75%, 100%) plotted against measured methylation of BDNF-IV Pyrosequencing Assay. Regression line, formula, and coefficient of determination were produced with Excel 2010 and are shown in the graph. Raw methylation values were transformed using the linear equation. Resulting negative values were set to zero. (DOCX 93 kb)
Acknowledgements
The authors thank Gisbert Farger and Danuta Altpaß for their assistance with the experimental work and Dr. Daniel Bucher and Ariane Wiegand for proofreading of the manuscript. We acknowledge support by Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of University of Tübingen.
Funding
This work was funded by the NASARD Young Investigator grant (23494) from the Brain & Behavior Research Foundation to VN and by an IZKF grant (PK2015–1-11) to NK and VN. M.T. was supported with a fellowship of the German Academic Scholarship Foundation. The research group is furthermore supported by a grant from the Wilhelm-Schuler-Stiftung to VN.
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BDNF
Brain-derived neurotrophic factor
- BPD
Borderline personality disorder
- BSL23
Borderline Symptom List 23
- cAMP
Cyclic adenosine monophosphate
- CIE
Change in estimate
- CpG
Cytosine-phosphate-guanine
- CREB
cAMP response element binding protein
- CTQ
Childhood Trauma Questionnaire
- DBT
Dialectical behavior therapy
- DNA
Desoxyribonucleic acid
- DNAme
DNA methylation
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders IV
- EDTA
Ethylenediaminetetraacetic acid
- ELS
Early-life stress
- GR/NR3C1
Glucocorticoid receptor
- GSI
Global Severity Index
- HTR2A
Serotonin receptor 2A
- IPDE
International Personality Disorder Examination
- MAOA
Monoamine oxidase A
- MAOB
Monoamine oxidase B
- PCR
Polymerase chain reaction
- PST
Positive Symptom Total
- SCL90R
Symptom Checklist-90-revised
- S-COMT
Soluble catechol-O-methyltransferase
Authors’ contributions
AW and NK recruited study participants, performed pyrosequencing analyses, and contributed with scientific input to the design of the experiment. VN provided funding, designed, and supervised the study. KG performed pyrosequencing analysis, and FG and JBS recruited study participants. CB and MT supervised the experimental work. MT conducted the data analysis and wrote the first draft of the manuscript. VN and AW revised the manuscript critically and provided scientific input. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All research was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from each subject prior to participation in the study. Ethical approval was obtained from the research ethics committees of the University of Tuebingen (project number 90/2015BO2).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
- 2.Grant BF, Chou SP, Goldstein RB, Huang B, Stinson FS, Saha TD, Smith SM, Dawson DA, Pulay AJ, Pickering RP, Ruan WJ. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2008;69:533–545. doi: 10.4088/JCP.v69n0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lenzenweger MF, Lane MC, Loranger AW, Kessler RC. DSM-IV personality disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;62:553–564. doi: 10.1016/j.biopsych.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reichborn-Kjennerud T. The genetic epidemiology of personality disorders. Dialogues Clin Neurosci. 2010;12:103–114. doi: 10.31887/DCNS.2010.12.1/trkjennerud. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gunderson JG, Zanarini MC, Choi-Kain LW, Mitchell KS, Jang KL, Hudson JI. Family study of borderline personality disorder and its sectors of psychopathology. Arch Gen Psychiatry. 2011;68:753–762. doi: 10.1001/archgenpsychiatry.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nestler EJ, Pena CJ, Kundakovic M, Mitchell A, Akbarian S. Epigenetic basis of mental illness. Neuroscientist. 2016;22(5):447-63. [DOI] [PMC free article] [PubMed]
- 7.Mostafavi Abdolmaleky H. Horizons of psychiatric genetics and epigenetics: where are we and where are we heading? Iran J Psychiatry Behav Sci. 2014;8:1–10. [PMC free article] [PubMed] [Google Scholar]
- 8.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 9.Yang X, Han H, De Carvalho DD, Lay FD, Jones PA, Liang G. Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell. 2014;26:577–590. doi: 10.1016/j.ccr.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoemaker R, Deng J, Wang W, Zhang K. Allele-specific methylation is prevalent and is contributed by CpG-SNPs in the human genome. Genome Res. 2010;20:883–889. doi: 10.1101/gr.104695.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohli RM, Zhang Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature. 2013;502:472–479. doi: 10.1038/nature12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker-Andresen D, Ratnu VS, Bredy TW. Dynamic DNA methylation: a prime candidate for genomic metaplasticity and behavioral adaptation. Trends Neurosci. 2013;36:3–13. doi: 10.1016/j.tins.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Leenen FA, Muller CP, Turner JD. DNA methylation: conducting the orchestra from exposure to phenotype? Clin Epigenetics. 2016;8:92. doi: 10.1186/s13148-016-0256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones MJ, Moore SR, Kobor MS. Principles and challenges of applying epigenetic epidemiology to psychology. Annu Rev Psychol. 2018;69:459–485. doi: 10.1146/annurev-psych-122414-033653. [DOI] [PubMed] [Google Scholar]
- 15.Edgar RD, Jones MJ, Meaney MJ, Turecki G, Kobor MS. BECon: a tool for interpreting DNA methylation findings from blood in the context of brain. Transl Psychiatry. 2017;7:e1187. doi: 10.1038/tp.2017.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dammann G, Teschler S, Haag T, Altmuller F, Tuczek F, Dammann RH. Increased DNA methylation of neuropsychiatric genes occurs in borderline personality disorder. Epigenetics. 2011;6:1454–1462. doi: 10.4161/epi.6.12.18363. [DOI] [PubMed] [Google Scholar]
- 17.Perroud N, Salzmann A, Prada P, Nicastro R, Hoeppli ME, Furrer S, Ardu S, Krejci I, Karege F, Malafosse A. Response to psychotherapy in borderline personality disorder and methylation status of the BDNF gene. Transl Psychiatry. 2013;3:e207. doi: 10.1038/tp.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prados J, Stenz L, Courtet P, Prada P, Nicastro R, Adouan W, Guillaume S, Olie E, Aubry JM, Dayer A, Perroud N. Borderline personality disorder and childhood maltreatment: a genome-wide methylation analysis. Genes Brain Behav. 2015;14:177–188. doi: 10.1111/gbb.12197. [DOI] [PubMed] [Google Scholar]
- 19.Teschler S, Bartkuhn M, Kunzel N, Schmidt C, Kiehl S, Dammann G, Dammann R. Aberrant methylation of gene associated CpG sites occurs in borderline personality disorder. PLoS One. 2013;8:e84180. doi: 10.1371/journal.pone.0084180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thaler L, Gauvin L, Joober R, Groleau P, de Guzman R, Ambalavanan A, Israel M, Wilson S, Steiger H. Methylation of BDNF in women with bulimic eating syndromes: associations with childhood abuse and borderline personality disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. 2014;54:43–49. doi: 10.1016/j.pnpbp.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Moser DA, Paoloni-Giacobino A, Stenz L, Adouan W, Manini A, Suardi F, Cordero MI, Vital M, Sancho Rossignol A, Rusconi-Serpa S, et al. BDNF methylation and maternal brain activity in a violence-related sample. PLoS One. 2015;10:e0143427. doi: 10.1371/journal.pone.0143427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim TY, Kim SJ, Chung HG, Choi JH, Kim SH, Kang JI. Epigenetic alterations of the BDNF gene in combat-related post-traumatic stress disorder. Acta Psychiatr Scand. 2017;135:170–179. doi: 10.1111/acps.12675. [DOI] [PubMed] [Google Scholar]
- 23.Peng H, Zhu Y, Strachan E, Fowler E, Bacus T, Roy-Byrne P, Goldberg J, Vaccarino V, Zhao J. Childhood trauma, DNA methylation of stress-related genes, and depression: findings from two monozygotic twin studies. Psychosom Med. 2018. Publish Ahead of Print. [DOI] [PMC free article] [PubMed]
- 24.Kundakovic M, Gudsnuk K, Herbstman JB, Tang D, Perera FP, Champagne FA. DNA methylation of BDNF as a biomarker of early-life adversity. Proc Natl Acad Sci U S A. 2015;112:6807–6813. doi: 10.1073/pnas.1408355111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boersma GJ, Lee RS, Cordner ZA, Ewald ER, Purcell RH, Moghadam AA, Tamashiro KL. Prenatal stress decreases Bdnf expression and increases methylation of Bdnf exon IV in rats. Epigenetics. 2014;9:437–447. doi: 10.4161/epi.27558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daskalakis NP, De Kloet ER, Yehuda R, Malaspina D, Kranz TM. Early life stress effects on glucocorticoid-BDNF interplay in the hippocampus. Front Mol Neurosci. 2015;8:68. doi: 10.3389/fnmol.2015.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeanneteau F, Chao MV. Are BDNF and glucocorticoid activities calibrated? Neuroscience. 2013;239:173–195. doi: 10.1016/j.neuroscience.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pietrek C, Elbert T, Weierstall R, Muller O, Rockstroh B. Childhood adversities in relation to psychiatric disorders. Psychiatry Res. 2013;206:103–110. doi: 10.1016/j.psychres.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Lee KW, Pausova Z. Cigarette smoking and DNA methylation. Front Genet. 2013;4:132. doi: 10.3389/fgene.2013.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Philibert RA, Beach SR, Brody GH. The DNA methylation signature of smoking: an archetype for the identification of biomarkers for behavioral illness. Neb Symp Motiv. 2014;61:109–127. doi: 10.1007/978-1-4939-0653-6_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toledo-Rodriguez M, Lotfipour S, Leonard G, Perron M, Richer L, Veillette S, Pausova Z, Paus T. Maternal smoking during pregnancy is associated with epigenetic modifications of the brain-derived neurotrophic factor-6 exon in adolescent offspring. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1350–1354. doi: 10.1002/ajmg.b.31109. [DOI] [PubMed] [Google Scholar]
- 33.Talati A, Odgerel Z, Wickramaratne PJ, Weissman MM. Brain derived neurotrophic factor moderates associations between maternal smoking during pregnancy and offspring behavioral disorders. Psychiatry Res. 2016;245:387–391. doi: 10.1016/j.psychres.2016.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao L, Kish VL, Benders KM, Wu ZX. Prenatal and early postnatal exposure to cigarette smoke decreases BDNF/TrkB signaling and increases abnormal behaviors later in life. Int J Neuropsychopharmacol. 2016;19(5):pyv117. [DOI] [PMC free article] [PubMed]
- 35.Yochum C, Doherty-Lyon S, Hoffman C, Hossain MM, Zelikoff JT, Richardson JR. Prenatal cigarette smoke exposure causes hyperactivity and aggressive behavior: role of altered catecholamines and BDNF. Exp Neurol. 2014;254:145–152. doi: 10.1016/j.expneurol.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jamal M, Van der Does W, Elzinga BM, Molendijk ML, Penninx BW. Association between smoking, nicotine dependence, and BDNF Val66Met polymorphism with BDNF concentrations in serum. Nicotine Tob Res. 2015;17:323–329. doi: 10.1093/ntr/ntu151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colle R, Trabado S, Rotenberg S, Brailly-Tabard S, Benyamina A, Aubin HJ, Hardy P, Falissard B, Becquemont L, Verstuyft C, et al. Tobacco use is associated with increased plasma BDNF levels in depressed patients. Psychiatry Res. 2016;246:370–372. doi: 10.1016/j.psychres.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Bus BA, Molendijk ML, Penninx BJ, Buitelaar JK, Kenis G, Prickaerts J, Elzinga BM, Voshaar RC. Determinants of serum brain-derived neurotrophic factor. Psychoneuroendocrinology. 2011;36:228–239. doi: 10.1016/j.psyneuen.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 39.D'Addario C, Dell'Osso B, Palazzo MC, Benatti B, Lietti L, Cattaneo E, Galimberti D, Fenoglio C, Cortini F, Scarpini E, et al. Selective DNA methylation of BDNF promoter in bipolar disorder: differences among patients with BDI and BDII. Neuropsychopharmacology. 2012;37:1647–1655. doi: 10.1038/npp.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carlberg L, Scheibelreiter J, Hassler MR, Schloegelhofer M, Schmoeger M, Ludwig B, Kasper S, Aschauer H, Egger G, Schosser A. Brain-derived neurotrophic factor (BDNF)-epigenetic regulation in unipolar and bipolar affective disorder. J Affect Disord. 2014;168:399–406. doi: 10.1016/j.jad.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 41.Fuchikami M, Morinobu S, Segawa M, Okamoto Y, Yamawaki S, Ozaki N, Inoue T, Kusumi I, Koyama T, Tsuchiyama K, Terao T. DNA methylation profiles of the brain-derived neurotrophic factor (BDNF) gene as a potent diagnostic biomarker in major depression. PLoS One. 2011;6:e23881. doi: 10.1371/journal.pone.0023881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ikegame T, Bundo M, Sunaga F, Asai T, Nishimura F, Yoshikawa A, Kawamura Y, Hibino H, Tochigi M, Kakiuchi C, et al. DNA methylation analysis of BDNF gene promoters in peripheral blood cells of schizophrenia patients. Neurosci Res. 2013;77:208–214. doi: 10.1016/j.neures.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Kim JM, Kang HJ, Kim SY, Kim SW, Shin IS, Kim HR, Park MH, Shin MG, Yoon JH, Yoon JS. BDNF promoter methylation associated with suicidal ideation in patients with breast cancer. Int J Psychiatry Med. 2015;49:75–94. doi: 10.1177/0091217415574439. [DOI] [PubMed] [Google Scholar]
- 44.Keller S, Sarchiapone M, Zarrilli F, Videtic A, Ferraro A, Carli V, Sacchetti S, Lembo F, Angiolillo A, Jovanovic N, et al. Increased BDNF promoter methylation in the Wernicke area of suicide subjects. Arch Gen Psychiatry. 2010;67:258–267. doi: 10.1001/archgenpsychiatry.2010.9. [DOI] [PubMed] [Google Scholar]
- 45.Mitchelmore C, Gede L. Brain derived neurotrophic factor: epigenetic regulation in psychiatric disorders. Brain Res. 2014;1586:162–172. doi: 10.1016/j.brainres.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 46.Zheleznyakova GY, Cao H, Schioth HB. BDNF DNA methylation changes as a biomarker of psychiatric disorders: literature review and open access database analysis. Behav Brain Funct. 2016;12:17. doi: 10.1186/s12993-016-0101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cunha C, Brambilla R, Thomas KL. A simple role for BDNF in learning and memory? Front Mol Neurosci. 2010;3:1. doi: 10.3389/neuro.02.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bus BA, Molendijk ML, Tendolkar I, Penninx BW, Prickaerts J, Elzinga BM, Voshaar RC. Chronic depression is associated with a pronounced decrease in serum brain-derived neurotrophic factor over time. Mol Psychiatry. 2015;20:602–608. doi: 10.1038/mp.2014.83. [DOI] [PubMed] [Google Scholar]
- 51.Fernandes BS, Molendijk ML, Kohler CA, Soares JC, Leite CM, Machado-Vieira R, Ribeiro TL, Silva JC, Sales PM, Quevedo J, et al. Peripheral brain-derived neurotrophic factor (BDNF) as a biomarker in bipolar disorder: a meta-analysis of 52 studies. BMC Med. 2015;13:289. doi: 10.1186/s12916-015-0529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiatry. 2011;16:960–972. doi: 10.1038/mp.2010.88. [DOI] [PubMed] [Google Scholar]
- 53.Koenigsberg HW, Yuan P, Diaz GA, Guerreri S, Dorantes C, Mayson S, Zamfirescu C, New AS, Goodman M, Manji HK, Siever LJ. Platelet protein kinase C and brain-derived neurotrophic factor levels in borderline personality disorder patients. Psychiatry Res. 2012;199:92–97. doi: 10.1016/j.psychres.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64:238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ricken R, Adli M, Lange C, Krusche E, Stamm TJ, Gaus S, Koehler S, Nase S, Bschor T, Richter C, et al. Brain-derived neurotrophic factor serum concentrations in acute depressive patients increase during lithium augmentation of antidepressants. J Clin Psychopharmacol. 2013;33:806–809. doi: 10.1097/JCP.0b013e3182a412b8. [DOI] [PubMed] [Google Scholar]
- 56.Tunca Z, Ozerdem A, Ceylan D, Yalcin Y, Can G, Resmi H, Akan P, Ergor G, Aydemir O, Cengisiz C, Kerim D. Alterations in BDNF (brain derived neurotrophic factor) and GDNF (glial cell line-derived neurotrophic factor) serum levels in bipolar disorder: the role of lithium. J Affect Disord. 2014;166:193–200. doi: 10.1016/j.jad.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 57.Molendijk ML, Bus BA, Spinhoven P, Penninx BW, Kenis G, Prickaerts J, Voshaar RC, Elzinga BM. Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment. Mol Psychiatry. 2011;16:1088–1095. doi: 10.1038/mp.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De-Paula VJ, Gattaz WF, Forlenza OV. Long-term lithium treatment increases intracellular and extracellular brain-derived neurotrophic factor (BDNF) in cortical and hippocampal neurons at subtherapeutic concentrations. Bipolar Disord. 2016;18:692–695. doi: 10.1111/bdi.12449. [DOI] [PubMed] [Google Scholar]
- 59.Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang DM. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol Psychiatry. 2009;14:51–59. doi: 10.1038/sj.mp.4002099. [DOI] [PubMed] [Google Scholar]
- 60.Yehuda R, Daskalakis NP, Desarnaud F, Makotkine I, Lehrner AL, Koch E, Flory JD, Buxbaum JD, Meaney MJ, Bierer LM. Epigenetic biomarkers as predictors and correlates of symptom improvement following psychotherapy in combat veterans with PTSD. Front Psychiatry. 2013;4:118. doi: 10.3389/fpsyt.2013.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knoblich N, Gundel F, Bruckmann C, Becker-Sadzio J, Frischholz C, Nieratschker V. DNA methylation of APBA3 and MCF2 in borderline personality disorder: potential biomarkers for response to psychotherapy. Eur Neuropsychopharmacol. 2018;28:252–263. doi: 10.1016/j.euroneuro.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 62.Langie SAS, Moisse M, Declerck K, Koppen G, Godderis L, Vanden Berghe W, Drury S, De Boever P. Salivary DNA methylation profiling: aspects to consider for biomarker identification. Basic Clin Pharmacol Toxicol. 2017;121(Suppl 3):93–101. doi: 10.1111/bcpt.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith AK, Kilaru V, Klengel T, Mercer KB, Bradley B, Conneely KN, Ressler KJ, Binder EB. DNA extracted from saliva for methylation studies of psychiatric traits: evidence tissue specificity and relatedness to brain. Am J Med Genet B Neuropsychiatr Genet. 2015;168B:36–44. doi: 10.1002/ajmg.b.32278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rao JS, Keleshian VL, Klein S, Rapoport SI. Epigenetic modifications in frontal cortex from Alzheimer's disease and bipolar disorder patients. Transl Psychiatry. 2012;2:e132. doi: 10.1038/tp.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chagnon YC, Potvin O, Hudon C, Preville M. DNA methylation and single nucleotide variants in the brain-derived neurotrophic factor (BDNF) and oxytocin receptor (OXTR) genes are associated with anxiety/depression in older women. Front Genet. 2015;6:230. doi: 10.3389/fgene.2015.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song Y, Miyaki K, Suzuki T, Sasaki Y, Tsutsumi A, Kawakami N, Shimazu A, Takahashi M, Inoue A, Kan C, et al. Altered DNA methylation status of human brain derived neurotrophis factor gene could be useful as biomarker of depression. Am J Med Genet B Neuropsychiatr Genet. 2014;165B:357–364. doi: 10.1002/ajmg.b.32238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reddy MS, Vijay MS. Empirical reality of dialectical behavioral therapy in borderline personality. Indian J Psychol Med. 2017;39:105–108. doi: 10.4103/IJPSYM.IJPSYM_132_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Januar V, Ancelin ML, Ritchie K, Saffery R, Ryan J. BDNF promoter methylation and genetic variation in late-life depression. Transl Psychiatry. 2015;5:e619. doi: 10.1038/tp.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Theda C, Hwang SH, Czajko A, Loke YJ, Leong P, Craig JM. Quantitation of the cellular content of saliva and buccal swab samples. Sci Rep. 2018;8:6944. doi: 10.1038/s41598-018-25311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Edling AE, Nanavati T, Johnson JM, Tuohy VK. Human and murine lymphocyte neurotrophin expression is confined to B cells. J Neurosci Res. 2004;77:709–717. doi: 10.1002/jnr.20176. [DOI] [PubMed] [Google Scholar]
- 71.Saruta J, Fujino K, To M, Tsukinoki K. Expression and localization of brain-derived neurotrophic factor (BDNF) mRNA and protein in human submandibular gland. Acta Histochem Cytochem. 2012;45:211–218. doi: 10.1267/ahc.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saruta J, To M, Sugimoto M, Yamamoto Y, Shimizu T, Nakagawa Y, Inoue H, Saito I, Tsukinoki K. Salivary gland derived BDNF overexpression in mice exerts an anxiolytic effect. Int J Mol Sci. 2017;18(9):1902. [DOI] [PMC free article] [PubMed]
- 73.Murphy SK, Adigun A, Huang Z, Overcash F, Wang F, Jirtle RL, Schildkraut JM, Murtha AP, Iversen ES, Hoyo C. Gender-specific methylation differences in relation to prenatal exposure to cigarette smoke. Gene. 2012;494:36–43. doi: 10.1016/j.gene.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Breton CV, Marsit CJ, Faustman E, Nadeau K, Goodrich JM, Dolinoy DC, Herbstman J, Holland N, LaSalle JM, Schmidt R, et al. Small-magnitude effect sizes in epigenetic end points are important in children's environmental health studies: the Children's Environmental Health and Disease Prevention Research Center's Epigenetics Working Group. Environ Health Perspect. 2017;125:511–526. doi: 10.1289/EHP595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sullivan PF, Daly MJ, O'Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet. 2012;13:537–551. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vrijen C, Schenk HM, Hartman CA, Oldehinkel AJ. Measuring BDNF in saliva using commercial ELISA: results from a small pilot study. Psychiatry Res. 2017;254:340–346. doi: 10.1016/j.psychres.2017.04.034. [DOI] [PubMed] [Google Scholar]
- 77.Zheng F, Zhou X, Moon C, Wang H. Regulation of brain-derived neurotrophic factor expression in neurons. Int J Physiol Pathophysiol Pharmacol. 2012;4:188–200. [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang X, Odom DT, Koo SH, Conkright MD, Canettieri G, Best J, Chen H, Jenner R, Herbolsheimer E, Jacobsen E, et al. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc Natl Acad Sci U S A. 2005;102:4459–4464. doi: 10.1073/pnas.0501076102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sakata K, Woo NH, Martinowich K, Greene JS, Schloesser RJ, Shen L, Lu B. Critical role of promoter IV-driven BDNF transcription in GABAergic transmission and synaptic plasticity in the prefrontal cortex. Proc Natl Acad Sci U S A. 2009;106:5942–5947. doi: 10.1073/pnas.0811431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sakata K, Jin L, Jha S. Lack of promoter IV-driven BDNF transcription results in depression-like behavior. Genes Brain Behav. 2010;9:712–721. doi: 10.1111/j.1601-183X.2010.00605.x. [DOI] [PubMed] [Google Scholar]
- 81.Sakata K, Duke SM. Lack of BDNF expression through promoter IV disturbs expression of monoamine genes in the frontal cortex and hippocampus. Neuroscience. 2014;260:265–275. doi: 10.1016/j.neuroscience.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 82.Ruocco AC, Carcone D. A neurobiological model of borderline personality disorder: systematic and integrative review. Harv Rev Psychiatry. 2016;24:311–329. doi: 10.1097/HRP.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 83.Barsaglini A, Sartori G, Benetti S, Pettersson-Yeo W, Mechelli A. The effects of psychotherapy on brain function: a systematic and critical review. Prog Neurobiol. 2014;114:1–14. doi: 10.1016/j.pneurobio.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 84.Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, Wiencke JK, Kelsey KT. DNA methylation arrays as surrogate measures of cell mixture distribution. Bmc Bioinformatics. 2012;13 [DOI] [PMC free article] [PubMed]
- 85.Eipel M, Mayer F, Arent T, Ferreira MR, Birkhofer C, Gerstenmaier U, Costa IG, Ritz-Timme S, Wagner W. Epigenetic age predictions based on buccal swabs are more precise in combination with cell type-specific DNA methylation signatures. Aging (Albany NY) 2016;8:1034–1048. doi: 10.18632/aging.100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schwarze CE, Hellhammer DH, Frieling H, Mobascher A, Lieb K. Altered DNA methylation status (BDNF gene exon IV) associated with prenatal maternal cigarette smoking in borderline patients and healthy controls. Psychoneuroendocrinology. 2015;61:29. doi: 10.1016/j.psyneuen.2015.07.468. [DOI] [Google Scholar]
- 87.Dwivedi T, Zhang H. Lithium-induced neuroprotection is associated with epigenetic modification of specific BDNF gene promoter and altered expression of apoptotic-regulatory proteins. Front Neurosci. 2014;8:457. doi: 10.3389/fnins.2014.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Forest M, O'Donnell KJ, Voisin G, Gaudreau H, MacIsaac JL, McEwen LM, Silveira PP, Steiner M, Kobor MS, Meaney MJ, Greenwood CMT. Agreement in DNA methylation levels from the Illumina 450K array across batches, tissues, and time. Epigenetics. 2018;13(1):19-32. [DOI] [PMC free article] [PubMed]
- 89.Franke GH. Symptom-Checkliste von L.R. Derogatis - Deutsche Version (SCL-90-R) Göttingen: Beltz Test; 2002. [Google Scholar]
- 90.Wolf M, Limberger MF, Kleindienst N, Stieglitz RD, Domsalla M, Philipsen A, Steil R, Bohus M. Short version of the borderline symptom list (BSL-23): development and psychometric evaluation. Psychother Psychosom Med Psychol. 2009;59:321–324. doi: 10.1055/s-0028-1104598. [DOI] [PubMed] [Google Scholar]
- 91.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–190. doi: 10.1016/S0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 92.Stenz L, Zewdie S, Laforge-Escarra T, Prados J, La Harpe R, Dayer A, Paoloni-Giacobino A, Perroud N, Aubry JM. BDNF promoter I methylation correlates between post-mortem human peripheral and brain tissues. Neurosci Res. 2015;91:1–7. doi: 10.1016/j.neures.2014.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Results of independent t test for blood BDNF IV promoter methylation in BPD patients (T1) and healthy controls. Results shown for individual CpG sites and average calculated from all sites. Table S2. Results of multiple regression analysis using group (BPD vs. healthy controls), smoking (smoker vs. non-smoker), and early-life stress (CTQ total score) as predictors and DNA methylation as dependent variable. Analysis was performed for each individual CpG site and for the average DNA methylation calculated from all analyzed CpG sites, as well as for saliva (SAL) and blood (BL). Table indicates regression coefficients b (b), standard error of b (SE b), lower and upper bound of 95% bootstrapped confidence intervals (CI lower b, CI upper b), standardized regression coefficient (β), p value (p value) and R2 of the model (R-squared). Table S3. Results of paired t test for the blood BDNF IV promoter methylation in BPD patients before (T1) and after treatment (T2). Results shown for individual CpG sites and average calculated from all sites. Table S4. Results of bivariate correlation analysis of the blood and salivary BDNF methylation at CpGs 1–4 and the average calculated from all sites. Ninety-five percentile bootstrapping was performed, and significant correlation (α=0.05) is marked in bold. Table S5. Correlation analysis of symptom reduction and change in salivary DNA methylation in BPD patients (N=26). Difference in scores of psychiatric questionnaires were correlated with difference in DNA methylation at all analyzed CpG sites using Pearson’s correlation coefficient. Table shows results of two-tailed significance test. Figure S1. Methylated DNA standards (0%, 25%, 50% 75%, 100%) plotted against measured methylation of BDNF-IV Pyrosequencing Assay. Regression line, formula, and coefficient of determination were produced with Excel 2010 and are shown in the graph. Raw methylation values were transformed using the linear equation. Resulting negative values were set to zero. (DOCX 93 kb)
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.