Abstract
Background
The main goal of this study was to assess the blood feeding behaviour and the contribution Anopheles coluzzii and Anopheles gambiae, 2 sibling species of An. gambiae sensu stricto. present and living in sympatry in 2 regions of northern Benin targeted for indoor residual spraying (IRS).
Methods
The study was carried out in 6 districts of 2 regions of Benin (Alibori and Donga). Human landing catches (HLC) performed inside and outside of the households and pyrethrum spray captures (PSC) carried out in bedrooms were used to sample vector populations (An. gambiae and An. coluzzii). Collected mosquitoes were analysed to estimate the human biting rate indoors and outdoors, the circumsporozoite antigen positivity, and the anthropophagic index using ELISA methodology. Polymerase chain reaction was used to estimate the frequency of the knockdown resistance (kdr) L1014F and the ace-1 mutations, 2 markers associated respectively with pyrethroids and carbamate/organophosphate insecticide resistance.
Results
A higher blood feeding rate was observed in An. gambiae compared to An. coluzzii as well as, a non-pronounced outdoor biting behavior in both species. The latter showed similar anthropophagic and sporozoite rates. However the analysis indicates a seasonal difference in the contribution of each species to malaria transmission associated with shifts in resting behaviour. Anopheles coluzzii females accounted for most of the detected infections: 86% in Alibori and 79% in Donga, during the dry season versus 14.4% and 21.2%, respectively for An. gambiae during the same period. This relationship was reversed in Donga during the rainy season (66% for An. gambiae against 34% for An. coluzzii). Results also indicated lower frequencies of kdr L1014F and ace-1 in An. coluzzii versus An. gambiae.
Conclusion
Despite similarity in some parameters related to malaria transmission in both surveyed species, An. coluzzii is potentially a more important malaria vector because of high density in the region. It is also characterized by lower frequencies of the ace-1 mutation than is An. gambiae. The ongoing use of pirimiphos methyl (organophosphate) for IRS should continue to show a good impact in Alibori and Donga because of the very low level of the ace-1 mutation in both species.
Keywords: Anopheles coluzzii, Anopheles gambiae, Sporozoite index, Entomological Inoculation Rate, Alibori, Donga, Benin
Background
Anopheles gambiae sensu lato (s.l.) is a complex of 8 species, which contribute differently to malaria transmission. Within the complex, An. gambiae sensu stricto (s.s.), the nominotypical member, is comprised of 2 species based on molecular evidence: Anopheles coluzzii (the An. gambiae molecular ‘M form’) and An. gambiae (the ‘S form’) [1].
Heterogeneity in the vector capacity of each species is due to a highly diverse bio-ecology: feeding on humans or cattle, resting indoors or outdoors. According to Akogbéto [2] and Awolola et al. [3], what makes An. gambiae an efficient vector is that it is highly anthropomorphic and endophilic, thereby frequently coming into contact with humans. In addition to its possibility of adaptation to various environments due to its genetic variants [4, 5], An. gambiae s.s. is a very efficient vector of malaria, especially in tropical Africa. Nonetheless, other sibling species of An. gambiae complex play a role, albeit a lesser role, in malaria transmission, for example, Anopheles melas in West Africa [6–8]. Studies on bio-ecology of An. gambiae s.l. have shown statistical differences of sporozoite index between different populations sibling species of the complex [9, 10]). Moreover, the main factor of vector competence of a mosquito to a parasite is its ability to offer a suitable physicochemical environment for the development of the parasite during the sporogonic cycle.
The objective of this study was to assess the blood feeding behaviour and the contribution in malaria transmission of each of the 2 sibling species (An. coluzzii and An. gambiae) present and living in sympatry in 2 regions of Benin, targeted for indoor residual spraying (IRS) on malaria transmission prior to the implementation of IRS. Given that the density of each of the 2 sibling species varies from one season to another, the more abundant species during the 3–6 months period following spraying when IRS residual efficacy operates, should be more influenced by this intervention.
The study is also evaluating, before spraying, the frequency of mutations associated with organophosphates resistance in An. coluzzii and An. gambiae, two sibling vector species living in sympatry in the study area and which were likely exposed to the same selection pressure for resistance. Indeed, if low frequencies of resistance mechanisms are observed in a given species, this may be sign that IRS with an organophosphate product could have a positive impact on the control of this species as previously observed by Aikpon et al. [11].
Detailed information on bio-ecology of vectors and their role in disease transmission are important for implementation of good vector control strategies or for their evaluation. In the study area (Alibori and Donga region, in the northern Benin) targeted for IRS campaign, where the 2 species are sympatric, the recorded data will be used as the comparison basis of the impact of IRS on each of the 2 Anopheles populations.
Study area
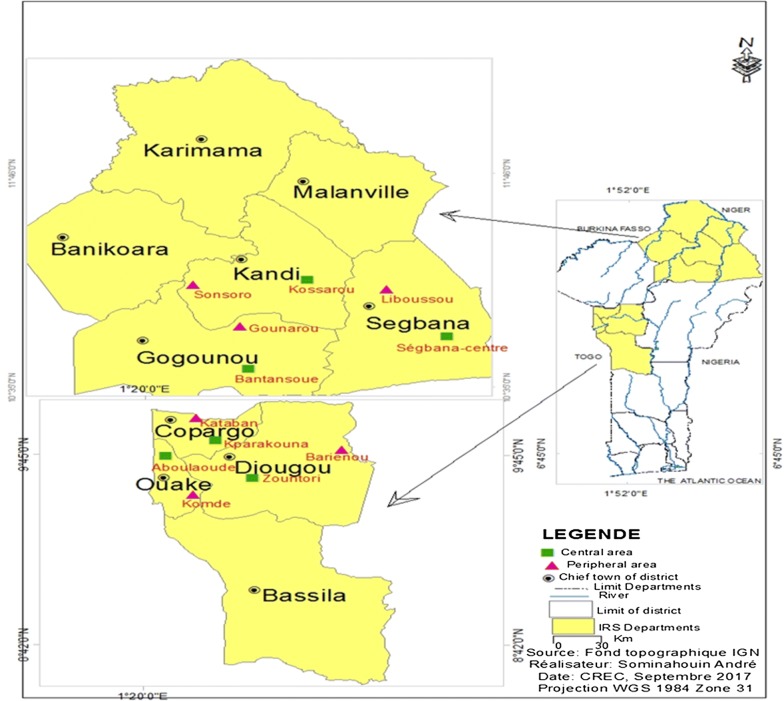
The study was conducted in 6 districts of the northern Benin: Kandi, Gogounou and Segbana in Alibori region, and Djougou, Copargo and Ouake in Donga region (Fig. 1). The 2 regions are characterized by one dry season (December to May) and one rainy season (June to November). The annual mean rainfall is 1300 mm and the mean monthly temperature varies between 23 and 40 °C. The Alibori region has more rivers than the Donga region. In Alibori, the soil is sandy type while in Donga, it is clayey type. In both regions the major economic activity is agriculture, including the production of cotton, maize and millet, where various classes of pesticides are used for pest control.
Fig. 1.
Map of Benin showing the Alibori and Donga regions and the sites of mosquito collections
Mosquito sampling and laboratory analysis
HLC carried out indoors and outdoors from 21.00 to 06.00 h and PSC performed inside houses from 06.00 to 09.00 were used to sample vector populations during the rainy (June–October 2016) and dry (November–May 2016) seasons in the 6 districts targeted for IRS implementation.
Human landing catches (HLC) and mosquito analysis
In each district, mosquito sampling was performed in 2 villages: one village in the central part of the district and one village at the periphery. In each village, mosquitoes were collected by adult volunteers in 2 houses for 2 consecutive nights, every month for 5 months per season, with one adult volunteer placed indoors and another placed outdoors. All Anopheles mosquitoes caught during the night were identified to species using the method described by Gilles and de Meillon [12]. To assess the infection status of each species, the head-thoraxes were tested using enzyme-linked immunosorbent analysis (ELISA) according to Wirtz et al. [13] to estimate circumsporozoite (CS) antigen of Plasmodium falciparum, the major malaria parasite occurring in the study area. Abdomens from females of the vector species were used for PCR analyses, to identify the sibling species of An. gambiae s.s. (An. gambiae, An. coluzzii). The indoor and outdoor human biting rate (HBR: also named ma) of each species was calculated to estimate the feeding behaviour of the 2 species.
In the study, malaria transmission in both the Alibori and Donga regions is expressed in terms of entomological inoculation rate (EIR = ma × s) due to both of the 2 species. The contribution of each species is the EIR calculated for the species divided by those of the 2 species × 100.
Indoor pyrethrum spray catches and mosquito analysis
In each village, 20 houses were selected for mosquito collection using indoor pyrethrum spray catches (PSC). Selected houses were sprayed with pyrethrum (mixed with water) and a white canvas was placed on the floor to collect the mosquitoes that were killed. After 10 min, all of the fallen mosquitoes were collected from the floor and placed in petri dishes. Fed, unfed, gravid, and half-gravid females of An. gambiae were counted to estimate the endophagy behaviour, defined as the proportion of each species resting in houses after blood feeding. Fed mosquitoes were also used to estimate the anthropophagic index in terms of the human blood meal rate using ELISA-blood-meal method. The anthropophagic index represents the proportion of blood meals derived from humans by mosquito vectors used to estimate human biting habit.
PCR detection of kdr L1014F and ace-1 mutations
The abdomens of An. coluzzii and An. gambiae females were used to identify the presence of L1014F (kdr) and G119S (ace-1) mutations. This was performed using PCR–RFLP [14] to estimate the frequency of kdr L1014F mutation in the sodium channel, which is associated with resistance to pyrethroid insecticides, and that of the ace-1 mutation, which is associated with carbamate and organophosphate insecticide resistance.
Statistical analysis
The human biting index (ma) (number of Anopheles per person and per night), room density (number of Anopheles collected in a room) and the circumsporozoite protein (CSP) positive rate were calculated for each species. The human blood-feeding rate was calculated using mosquitoes collected by PSC by dividing the number of fed and half-fed mosquitoes collected by the number of the total of mosquitoes collected. The CSP positive rate (% CS+) was calculated as the proportion of mosquitoes found to be positive for CSP. The EIR was defined as Anopheles density by the CSP and estimated as the number of infectious bites per human per night or per month. A Chi square test with the MINITAB statistical software (Version 12.2) was used to compare the proportions. The genotypic differentiation of kdr and ace-1 loci was tested using the Fischer exact test implemented in GenePop software [15], and the Fisher test was used to compare these frequencies. An analysis of variance (ANOVA) was performed to compare the entomological estimates (ma, EIR, CSP) among the 2 species.
Ethical consideration
Permission was sought from households to perform collections in their rooms. In addition, community consent had been obtained beforehand in all the villages. The volunteer mosquito collectors gave their consent before participating in the study. They were also subjected to regular medical check-ups with preventive malaria treatment. They were all vaccinated against yellow fever. This study received the approval of the Ethical Institutional Committee of the Centre for Entomological Research of Cotonou (CREC), Ministry of Health.
Results
Distribution of Anopheles gambiae complex in the two regions
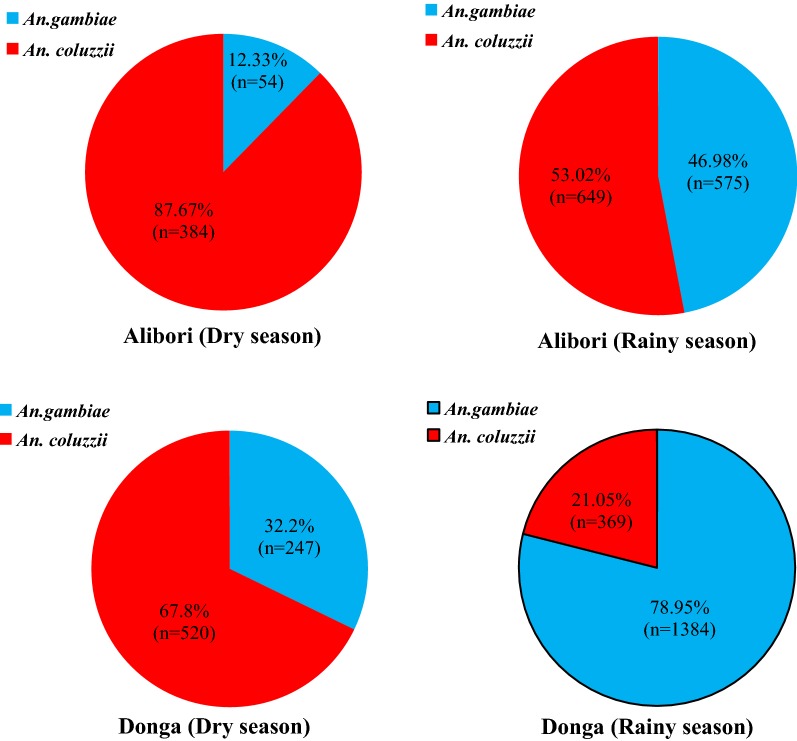
All samples of An. gambiae s.l. collected by HLC and PSC were used for species identification. In total, 4182 An. gambiae s.l. were analysed by PCR for species identification. Two species of the An. gambiae s.s. were identified: An. coluzzii and An. gambiae (Fig. 2). After 1 year of mosquito collection throughout both dry and rainy seasons, An. gambiae represented 54.0% (n = 2260) of the overall An. gambiae s.s. population compared to 46.0% (n = 1922) for An. coluzzii. However, the predominant species differed from one region to another. In Alibori, An. coluzzii represented 62.2% (n = 1033) of the overall An. gambiae s.s. population compared to 37.8% (n = 625) for An. gambiae. In Donga, An. gambiae was the most abundant at 64.7% (n = 1631).
Fig. 2.
Seasonal distribution of Anopheles coluzzii and Anopheles gambiae in Alibori and Donga, two regions of northern Benin
Both of the sibling species were present throughout the dry and rainy seasons in both regions (Fig. 2). During the dry season (January to June), An. coluzzii predominated in both the Alibori and Donga regions. However, during the rainy season, there were regional differences in the predominant species: during the rainy season in Donga, there was a high frequency (79.0%) of An. gambiae whereas during the rainy season in Alibori there was a relatively high frequency (53.0%) of An. coluzzii (Fig. 2).
Comparison of (CS) antigen index for Plasmodium falciparum in Anopheles gambiae and Anopheles coluzzii during the dry and rainy seasons
More than 4000 head-thoraxes of An. gambiae and An. coluzzii were tested using ELISA [13] for the presence of CS of P. falciparum. Various samples of An. gambiae (from HLC and PSC collection) were analysed in order to obtain enough mosquitoes (4182 head-thoraxes) for comparison.
During the dry season, rates of P. falciparum CS + were similar for An. gambiae (9.3%: 5 out of 54 thoraxes were CS+) and An. coluzzii (5.5%: 21 out of 384) (p = 0.426) in Alibori. Results were statistically different in Donga during the dry season with a higher rate of P. falciparum CS + for An. coluzzii (9.6%: 50 out of 520) compared to An. gambiae (4.9%: 12 out of 247) (p = 0.002) (Table 1).
Table 1.
Percentage of P. falciparum circumsporozoite antigen in An. coluzzii and An. gambiae during the dry and the rainy seasons
| Regions | Species | Dry season (May–Jun–Jan–Feb) | p-value | Rainy season (Jul–Aug–Oct) | p-value | Total | p-value | |
|---|---|---|---|---|---|---|---|---|
| Alibori | An. gambiae | Thorax | 54 | 0.426 | 575 | 0.096 | 629 | 0.55 |
| Thorax+ | 5 | 42 | 47 | |||||
| Is (%) | 9.26 | 7.30 | 7.47 | |||||
| IC-95% [IS %] | [3.07–20.30] | [5.31–9.75] | [5.54–9.81] | |||||
| An. coluzzii | Thorax | 384 | 649 | 1033 | ||||
| Thorax+ | 21 | 66 | 87 | |||||
| Is (%) | 5.47 | 10.17 | 8.42 | |||||
| IC-95% [IS %] | [3.41–8.24] | [7.95–12.76] | [6.80–10.28] | |||||
| Donga | An. gambiae | Thorax | 247 | 0.002 | 1384 | 1 | 1631 | 0.59 |
| Thorax+ | 12 | 132 | 144 | |||||
| Is (%) | 4.86 | 9.54 | 8.83 | |||||
| IC-95% [IS %] | [2.53–8.33] | [8.04–11.21] | [7.49–10.31] | |||||
| An. coluzzii | Thorax | 520 | 369 | 889 | ||||
| Thorax+ | 50 | 35 | 85 | |||||
| Is (%) | 9.62 | 9.49 | 9.56 | |||||
| IC-95% [IS %] | [7.22–12.48] | [6.69–12.94] | [7.7–11.69] | |||||
| Alibori-Donga | An. gambiae | Thorax | 301 | 0.252 | 1959 | 0.389 | 2260 | 0.607 |
| Thorax+ | 17 | 174 | 191 | |||||
| Is (%) | 5.65 | 8.88 | 8.45 | |||||
| IC-95% [IS %] | [3.32–8.89] | [7.65–10.23] | [7.33–9.67] | |||||
| An. coluzzii | Thorax | 904 | 1018 | 1922 | ||||
| Thorax+ | 71 | 101 | 172 | |||||
| Is (%) | 7.85 | 9.92 | 8.94 | |||||
| IC-95% [IS %] | [6.18–9.80] | [8.15–11.92] | [7.71–10.31] |
p = p-value of comparison of CircumSporozoite (CS) antigen index for Plasmodium falciparum of An. gambiae and An. coluzzii
Jun June, Jul July, Aug August, Oct October, Jan January, Feb February
During the rainy season, rates of P. falciparum CS + were similar for both species in Alibori (An. gambiae: 7.3%: 42 out of 575) thoraxes were CS + ; An. coluzzi: 10.2%, 66 out of 649) (p = 0.096) and in Donga (An. gambiae: 9.5%, 132 out of 1384; An. coluzzi: 9.5%, 35 out of 369) (p = 1) (Table 1).
No differences in CS + between species were observed in either region when data were cumulated over both seasons: 7.5%: 47 out of 629 thoraxes were CS + for An. gambiae and 8.4%: 87 out of 1033 for An. coluzzii in Alibori (p = 0.55). In Donga, CS + for An. gambiae and An. coluzzii were 8.8% (144 out of 1631) and 9.6% (85 out of 889), respectively (p = 0.59) (Table 1).
In total, 191 (8.5%) of 2260 thoraces of An. gambiae analysed in the two regions by ELISA-CSP were positive for P. falciparum CS antigen (Is = 8.5%). For An. coluzzii, 172 (8.9%) of 1922 thoraces were positive for P. falciparum CS antigen (p = 0.60) (Table 1).
Contribution of Anopheles gambiae and Anopheles coluzzii to malaria transmission expressed in terms of the EIR
The intensity of the malaria transmission due to each species is expressed in terms of EIR, which is equal to ma times the sporozoite rate. As the ma parameter depends on the season, the EIR due to each species was assessed for the dry season when ma is expected to be low and for the rainy season when ma is expected to be high.
In both the Alibori and Donga regions, An. coluzzii accounts for most malaria transmission during the dry season. In Alibori, An. coluzzii accounts for 85.6% of malaria transmission during the dry season (EIR = 1.61 infected bite per man per month) compared to 14.3% (EIR = 0.27/month). In Donga, An. coluzzii and An. gambiae account for 78.8% (EIR = 3.20/month) and 20.2% (EIR = 0.81/month) of malaria transmission in the dry season, respectively (Fig. 3).
Fig. 3.
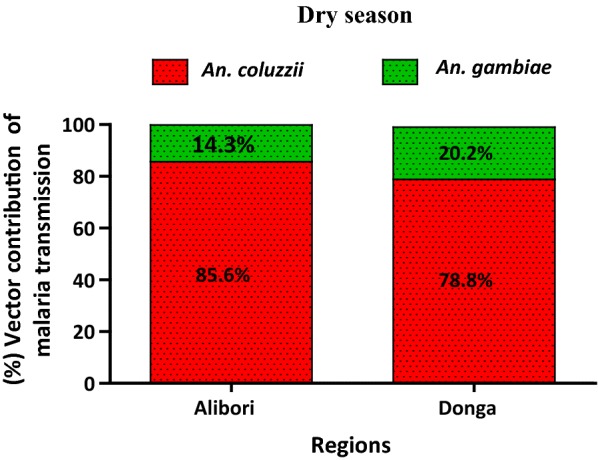
Contribution of An. coluzzii and An. gambiae expressed in the proportion of Entomological Inoculation Rate due to each species in Alibori and Donga during the dry season
During the rainy season, An. coluzzii continues to account for most malaria transmission in the Alibori region as occurred during the dry season: An. coluzzii accounts for 64.8% (14.37 infected bites) of malaria transmission compared to 35.2% (7.81 infected bites) for An. gambiae (Table 2). However, in the Donga region, inverted results were observed showing a higher EIR due to An. gambiae (77.7%: EIR = 26.25 infected bites/month) as compared to An. coluzzii (Fig. 4). When cumulated data, which were registered during the 7 months of the study, the observed EIR trend during the dry season was confirmed due to the high ma of An. coluzzii during this period: the EIR of An. coluzzii was 2 times higher than An. gambiae in Alibori (p = 0.0043) and 2 times lower in Donga (p = 0.000084) (Fig. 5).
Table 2.
Malaria transmission expressed in terms of Entomological Inoculation Rate (EIR) in An. coluzzii and An. gambiae in Alibori and Donga
| Regions | Species | Dry season (May–Jun–Jan–Feb) | p-value | Rainy season (Jul–Aug–Oct) | p-value | Total | P-value | |
|---|---|---|---|---|---|---|---|---|
| Alibori | An. gambiae | Thorax | 12 | 0.125 | 362 | 0.016 | 374 | 0.0043 |
| Thorax+ | 1 | 25 | 26 | |||||
| Is (%) | 8.33 | 6.91 | 6.95 | |||||
| ma/month | 3.21 | 113.12 | 53.94 | |||||
| EIR/month | 0.27 | 7.81 | 3.74 | |||||
| An. coluzzii | Thorax | 135 | 446 | 581 | ||||
| Thorax+ | 6 | 46 | 52 | |||||
| Is (%) | 4.44 | 10.31 | 8.95 | |||||
| ma/month | 36.16 | 139.37 | 83.79 | |||||
| EIR/month | 1.61 | 14.37 | 7.49 | |||||
| Donga | An. gambiae | Thorax | 147 | 0.03 | 846 | 0.000054 | 993 | 0.000084 |
| Thorax+ | 3 | 84 | 87 | |||||
| Is (%) | 2.04 | 9.93 | 8.76 | |||||
| ma/month | 1.31 | 264.37 | 143.22 | |||||
| EIR/month | 0.810 | 26.25 | 12.54 | |||||
| An. coluzzii | Thorax | 214 | 257 | 471 | ||||
| Thorax+ | 12 | 24 | 36 | |||||
| Is (%) | 5.607 | 9.339 | 7.643 | |||||
| ma/month | 57.32 | 80.31 | 67.93 | |||||
| EIR/month | 3.20 | 7.50 | 5.19 |
Italic values indicate significance of p value (p < 0.05)
p = p-value of comparison of CircumSporozoite (CS) antigen index for Plasmodium falciparum of An. gambiae and An. coluzzii
Jun June, Jul July, Aug August, Oct October, Jan January, Feb February
Fig. 4.
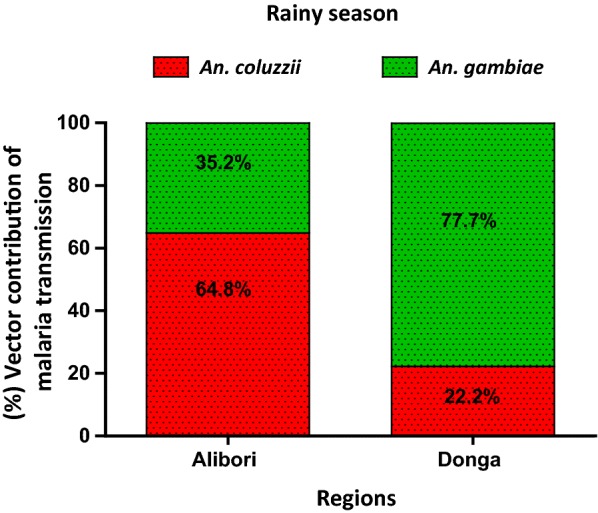
Contribution of An. coluzzii and An. gambiae expressed in the proportion of Entomological Inoculation Rate due to each species in Alibori and Donga during the rainy season
Fig. 5.
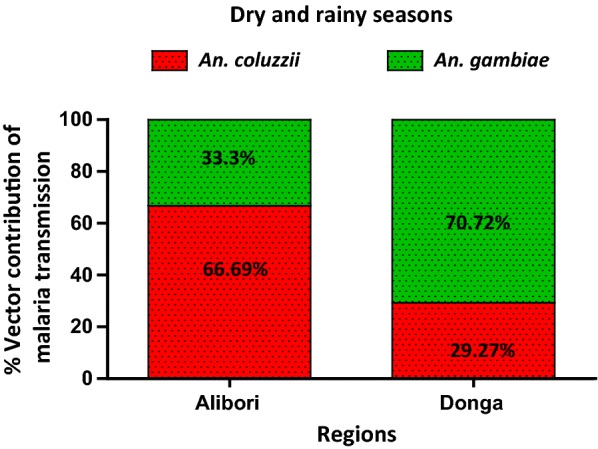
Contribution of An. coluzzii and An. gambiae expressed in the proportion of Entomological Inoculation Rate due to each species in Alibori and Donga during the two seasons
Indoor and outdoor biting behaviour of Anopheles gambiae and Anopheles coluzzii
More than 2400 An. gambiae collected by HLC and analysed by PCR showed that, the biting behaviour of An. gambiae and An. coluzzii was variable according to the location (indoors or outdoors). Out of 1302 An. gambiae collected by HLC in Alibori and Donga, 57.7% (752/1302) were collected indoors compared to 42.3% (550/1302) outdoors (p < 0.001) (Table 3). As for An. coluzzii, a similar biting behaviour was observed indoors and outdoors with respectively: 51.6% (575/1114) and 48.4% (539/1114) of specimens (p = 0.13), after cumulating data of both regions (Table 3). During the dry season, the biting behaviour of An. coluzzii was higher indoors with 56.4% (216/383) of specimens as compared to outdoors (43.6%) (p = 0.0004). In the same season, the biting behavior was similar indoors and outdoors (p = 0.25) for An. gambiae (Table 3).
Table 5.
Distribution of Knock-down resistance (Kdr) and Ace-1R frequencies in An. gambiae and An. coluzzii in Alibori and Donga
| Regions | Species | Number tested | Mutation KdrL1014F | Mutation Ace 1R | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | RS | SS | F (Kdr) | p-value | RR | RS | SS | F (Ace 1) | p-value | |||
| Alibori | An. gambiae | 170 | 123 | 34 | 13 | 0.82 | 0.0024 | 0 | 10 | 160 | 0.03 | 0.1381 |
| An. coluzzii | 271 | 143 | 90 | 38 | 0.69 | 0 | 7 | 264 | 0.01 | |||
| Donga | An. gambiae | 387 | 272 | 93 | 22 | 0.82 | 0.153 | 0 | 20 | 367 | 0.03 | 0.2981 |
| An. coluzzii | 152 | 102 | 34 | 16 | 0.78 | 0 | 4 | 148 | 0.01 | |||
| Total | An. gambiae | 557 | 395 | 127 | 35 | 0.82 | 0.0032 | 0 | 30 | 527 | 0.03 | 0.0483 |
| An. coluzzii | 423 | 245 | 124 | 54 | 0.73 | 0 | 11 | 412 | 0.01 | |||
Italic values indicate significance of p value (p < 0.05)
p = p-value of comparison of the frequencies Kdr and Ace-1 between An. gambiae and An. coluzzii
Table 3.
Number of An. gambiae and An. coluzzii collected indoors and outdoors by HLC
| Seasons | Species | Indoors | Outdoors | p-value | |
|---|---|---|---|---|---|
| Alibori | Dry season | An. gambiae (N) | 7 | 7 | – |
| An. coluzzii (N) | 59 | 74 | – | ||
| Rainy season | An. gambiae (N) | 212 | 164 | – | |
| An. coluzzii (N) | 256 | 176 | – | ||
| Donga | Dry season | An. gambiae (N) | 60 | 51 | – |
| An. coluzzii (N) | 157 | 93 | – | ||
| Rainy season | An. gambiae (N) | 472 | 329 | – | |
| An. coluzzii (N) | 103 | 196 | – | ||
| Both regions | Dry season | An. gambiae [% (N/T)] | 53.6% (67/125) | 46.4% (58/125) | 0.25 |
| An. coluzzii [% (N/T)] | 56.4% (216/383) | 43.60% (167/383) | 0.0004 | ||
| Rainy season | An. gambiae [% (N/T)] | 58.11% (684/1177) | 41.88% (493/1177) | < 0.001 | |
| An. coluzzii [% (N/T)] | 49.11% (359/731) | 50.88% (372/731) | 0.5 | ||
| Both regions | Both seasons | An. gambiae [% (N/T)] | 57.7% (751/1302) | 42.3% (551/1302) | < 0.001 |
| An. coluzzii [% (N/T)] | 51.6% (575/1114) | 48.4% (539/1114) | 0.13 |
Italic values indicate significance of p value (p<0.05)
N number of species collected in one location (indoors or outdoors), T total number of species collected in both locations (indoors + outdoors)
During the rainy season, the trend was reversed with a similar biting behaviour indoors and outdoors (p = 0.5) for An. coluzzii and, a higher indoor biting behaviour for An. gambiae (Table 3).
Blood feeding rate and anthropophagic index
With PSC performed in bedrooms, a significant higher blood feeding rate [96.68% (846 of fed and half-gravid females out of 875 total females)] was observed in An. gambiae as compared to An. coluzzii [90.20% (792/878)] (p < 0.0001) (Table 4). A high and similar anthropophagic index (0.97) was observed for both species with 34/35 An. gambiae specimens and 33/34 An. coluzzii having fed on humans (p = 0.98).
Table 4.
Blood feeding rate of An. gambiae and An. coluzzii collected by PSC method in Alibori and Donga
| Regions | An. gambiae | An. coluzzii | p-value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total collected | Unfed | Fed | Gravid | Half-gravid | Blood feeding rate (%) | Total collected | Unfed | Fed | Gravid | Half-gravid | Blood feeding rate (%) | ||
| Alibori | 235 | 5 | 216 | 3 | 11 | 96.6 | 460 | 20 | 358 | 35 | 47 | 88.04 | 0.0003 |
| Donga | 640 | 13 | 604 | 8 | 15 | 96.72 | 418 | 11 | 347 | 20 | 40 | 92.58 | 0.003 |
| Both regions | 875 | 18 | 820 | 11 | 26 | 96.68 | 878 | 31 | 705 | 55 | 87 | 90.20 | < 0.0001 |
Italic values indicate significance of p value (p<0.05)
p-value: p-value of comparison of the feeding rate of An. gambiae to that of An. coluzzii by region
Frequency of kdr mutation in Anopheles gambiae and Anopheles coluzzii populations
A total of 557 females of An. gambiae and 423 An. coluzzii were analysed for L1014F kdr mutation and G119S mutation (ace-1 gene) using PCR–RFLP [14]. The frequency of the 2 mutations is higher in An. gambiae (0.82 for kdr and 0.03 for ace-1) than in An. coluzzii (0.73 for kdr and 0.01 for ace-1) (Table 5).
Discussion
In Africa, vector surveillance is an integral component to the planning, implementation, monitoring, and the evaluation of vector control interventions. To assist with this goal, the current study was initiated in an area of Benin targeted for an IRS campaign to provide baseline data that could be used to help evaluate the impact and efficacy of IRS in the future.
Anopheles gambiae complex is the most important malaria vector in sub-Saharan Africa and has 4 species that occur in West Africa: An. coluzzii, An. gambiae, An. arabiensis and An. melas [4–8]. In the area investigated by the current study only 2 species maintain malaria transmission: An. gambiae and An. coluzzii. Both species are present year-round with a strong predominance of An. coluzzii in dry season. Despite the 2 study areas having a similar climate, there was a disparity in the distribution of the 2 species during the rainy season with a strong occurrence of An. coluzzii in the Alibori region compared with the Donga region. The low proportions of An. gambiae in the dry season may be due to temporary breeding sites that dry up, in addition to the presence of permanent and semi-permanent breeding sites more favourable to the development of An. coluzzii larvae.
Despite the large number of An. gambiae s.s. specimens (more than 4000) collected by HLC and PSC that were analysed by PCR, no An. arabiensis was found. Only one specimen of Anopheles nili was morphologically identified in Alibori and Donga. The absence of An. arabiensis in the study area could be due to the exophilic and zoophilic behaviour of this species and to its gradual disappearance in some environments in West Africa [4, 16], particularly in northern Benin [6]. Indeed, in 1992, Akogbeto [2] reported its presence in sympatry with An. gambiae s.s. in northern Benin, but in 2010, Aïkpon et al. [17] reported its disappearance in the same region.
The overall biting rate of An. gambiae and An. coluzzii was not higher outdoors than indoors in both the rainy and dry seasons, which suggests an important role for ITNs in the effort to prevent malaria. However, despite the presence of ITNs in houses in the study area, An. gambiae s.s. maintained a high index of human-vector contact as indicated by high human biting rates, which could be a result of its resistance to the pyrethroid insecticides used to treat bed nets.
The significant higher blood feeding rate observed in An. gambiae as compared to An. coluzzii indoors (p < 0.0001) would be likely due to a higher ability of An. gambiae to bite and feed on humans inside houses. Indeed, the collected HLC data show an overall more pronounced indoor man-vector contact in An. gambiae (56.6%: 751/1326) as compared to An. coluzzii (43.4%: 575/1326) (p < 0.0001). This result is similar to that obtained in Ghana by Tuno et al. [18] who showed that An. gambiae bit people inside dwellings more than did An. melas.
The fact that, the majority (≥ 90%) of An. coluzzii and An. gambiae collected indoors were blood-fed with a high and similar anthropophagic index (0.97), mirrors results from similar studies [17, 19–23]. As a result of both species having similar attractions to humans, An. gambiae and An. coluzzii had similar sporozoitic indexes at 8.5 and 8.9%, respectively (p = 0.60). This result is reminiscent of that of Carnevale et al. [24] who showed the same infection rate in An. coluzzii and An. gambiae in Lobito, Angola. Given the similar affinity for humans and the large number of specimens (more than 4000) examined through ELISA-CSP, it is likely that An. gambiae and An. coluzzii have the same ability to offer a physicochemical environment suitable for the development of the parasite during the sporogonic cycle.
By taking into account the fact that vector capacity of a population of Anopheles depends on several factors, in particular, the ability to foster the development of the sporogonic cycle, the contribution of An. coluzzii and An. gambiae to malaria transmission was expressed in terms of the sporozoitic index (s) and the frequency of human-vector contacts (ma). Based on the calculated EIRs, An. coluzzii is involved in 85.6% of malaria transmission in Alibori and 78.8% of transmission in the Donga during the dry season, compared to only 14.4 and 21.2% for An. gambiae in Alibori and Donga, respectively. Further, during the rainy season An. coluzzii continues to account for most malaria transmission in Alibori (64.8% compared to 35.2% for An. gambiae), but the role is reversed in Donga during the rainy season with a higher contribution toward malaria transmission from An. gambiae (65.7% compared to 34.3% for An. coluzzii). The variation in abundance of each vector species from one season to another could explain this. Indeed, Alibori and Donga regions are crossed by several rivers and water dams that create throughout the year (rainy and dry seasons), permanent and semi-permanent breeding sites suitable for the development of An. coluzzii larvae. In addition, the Donga region is characterized by a clayey type soil, which retains water, allowing then the formation of numerous temporary breeding sites favourable to the emergence of An. gambiae as leading vector of malaria transmission in the rainy season. By against, in Alibori, the sandy type soil causes the quick infiltration of water and as a result, fewer temporary larval habitats are formed, which justifies that An. coluzzii coming from permanent and semi-permanent breeding sites continues to play the major role in malaria transmission during the rainy season in this region. As observed in Donga from dry to rainy season, the switch of the mosquito species that leads malaria transmission has also been reported in Lobito, Angola from 2005 to 2006 and, the suspected causes were environmental anthropic modifications (installation of water cisterns that disrepair over time and are used as dumps, reduction of some artificial larval habitats types favourable to An. gambiae) [24].
There have been numerous reports of resistance to pyrethroid throughout Africa [25–30]. In Benin, the resistance of malaria vectors to pyrethroids observed first in Cotonou spread not only to central and southern regions of the country, but also to the northern parts [31–38]. The lower frequency of kdr L1014F mutation in An. coluzzii in Alibori could favour the impact of ITNs and result in better control of malaria in Alibori compared to Donga. However, if pirimiphos methyl (organophosphate) is used again for IRS in the study area as it was in previous years, a positive impact could be expected because of the very low level (0.01–0.03) of the ace-1 mutation in both species.
Conclusion
Anopheles coluzzii and An. gambiae have been observed to have the same anthropophagic and sporozoite rates in the 2 regions. However, An. coluzzii is highly involved in malaria transmission in Alibori and Donga during the dry season as compared to An. gambiae. During the rainy season An. coluzzii was still playing the leading role in Alibori whereas, in Donga, this role is reversed with a stronger participation of An. gambiae. Overall, the blood feeding rate was higher in An. gambiae than in An. coluzzii. Moreover, data collected reveal An. coluzzii is characterized by lower frequencies of the ace-1 mutation than is An. gambiae. But, a good impact on malaria control in Alibori and Donga is expected if pirimiphos methyl (organophosphate) is used again for IRS in Benin as in previous years, because of the very low level of the ace-1 mutation in both species.
Authors’ contributions
MCA, ASS, FD and RA designed the study. AS, ASS and RA carried out the experiments. MCA drafted the manuscript. FD, MS, MK and AS critically revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The research leading to these results was financially supported by The President’s Malaria Initiative of the US Government. We acknowledge The President’s Malaria Initiative through USAID Cotonou, Benin. We also thank Monica Patton, Peter Thomas and Raymond Beach of the US Centers of Disease Control and Prevention for providing technical support and proofreading the manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data used and/or analysed in this study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The protocol of this study was reviewed and approved by the Institutional Ethics Committee of CREC (IECC). Before mosquito collectors were involved in this study, they gave their consent to participate. They were vaccinated against yellow fever, regularly checked up by a medical doctor and taken care in case of confirmed malaria case.
Funding
This study was financially supported by the US President’s Malaria Initiative (PMI) thru the United States Agency for International Development (USAID) Africa Indoor Residual Spraying Project (AIRS) Project.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coetzee M, Hunt H, Wilkerson R, Della Tore A, Coulibali MB, Besansky NJ. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa. 2013;3619:246–274. doi: 10.11646/zootaxa.3619.3.2. [DOI] [PubMed] [Google Scholar]
- 2.Akogbéto M. Etude des aspects épidémiologiques sur la transmission du paludisme côtier lagunaire au Bénin, Afrique de l’Ouest. Thèse de doctorat ès-sciences, Université de Paris XI, Centre d’Orsay; 1992.
- 3.Awolola T, Oyewole I, Koekemoer L, Coetzee M. Identification of three members of the Anopheles funestus (Diptera: Culicidae) group and their role in malaria transmission. Trans R Soc Trop Med Hyg. 2005;99:525–531. doi: 10.1016/j.trstmh.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Coluzzi M, Sabatini A, Petrarca V, Di Deco MA. Chromosomal differentiation and adaptations to human environments in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg. 1979;73:483–497. doi: 10.1016/0035-9203(79)90036-1. [DOI] [PubMed] [Google Scholar]
- 5.Coluzzi M, Petrarca V, Di Deco MA. Chromosomal inversion intergradation and incipient speciation in Anopheles gambiae. Bollettino di Zoologia. 1985;52:45–63. doi: 10.1080/11250008509440343. [DOI] [Google Scholar]
- 6.Akogbéto M, Di Deco MA. Répartition des membres du complexe Anopheles gambiae et leurs variants chromosomiques au Bénin et au Togo, Afrique occidentale. J Afr Zool. 1995;109:443–454. [Google Scholar]
- 7.Akogbéto M, Laugé G, Di Deco MA. Observation sur la bioécologie de Anopheles melas dans un milieu côtier lagunaire, Bénin, Afrique occidentale. J Afr Zool. 1995;109:399–406. [Google Scholar]
- 8.Fontenille D, Traoré-Lamizana M, Cornet JP, Adam F, Lochouarn L. Activités du laboratoire ORSTOM de Zoologie Médicale. Rapport sur le fonctionnement technique de l’Institut Pasteur de Dakar; 1993.
- 9.Faye O, Gaye O, Fontenille D, Sy N, Konaté L, Bebrard G, et al. Comparaison de la transmission du paludisme dans deux faciès épidémiologiques au Sénégal: la zone côtière sahélienne et la zone méridionale soudanienne. Dakar Médical. 1995;40:201–207. [PubMed] [Google Scholar]
- 10.Salako SA, Aïkpon R, Akogbeto MC. Surveillance entomologique en prélude à la mise en œuvre de la pulvérisation intradomiciliaire dans l’Alibori et la Donga. USAID/CREC: Rapport Final; 2016. [Google Scholar]
- 11.Aïkpon R, Sèzonlin M, Tokponon F, Okè M, Oussou O, Oké-Agbo F, et al. Good performances but short lasting efficacy of Actellic 50 EC Indoor Residual Spraying (IRS) on malaria transmission in Benin, West Africa. Parasit Vectors. 2014;7:256. doi: 10.1186/1756-3305-7-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillies MT, de Meillon B. The anophelinae of Africa, south of the Sahara. Johannesburg: The South African Institute for Medical Research; 1968. p. 343.
- 13.Wirtz RA, Zavala F, Charoenvit Y, Campbell GH, Burkot TR, Schneider I, et al. Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull World Health Organ. 1967;65:39–45. [PMC free article] [PubMed] [Google Scholar]
- 14.Favia G, Lanfrancotti A, Spanos L, Siden-Kiamos I, Louis C. Molecular characterization of ribosomal DNA polymorphisms discriminating among chromosomal forms of Anopheles gambiae s.s. Insect Mol Biol. 2001;10:19–23. doi: 10.1046/j.1365-2583.2001.00236.x. [DOI] [PubMed] [Google Scholar]
- 15.Raymond M, Rousset F. GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Heredity. 1995;86:248–249. doi: 10.1093/oxfordjournals.jhered.a111573. [DOI] [Google Scholar]
- 16.Touré TY. Génétique écologique et capacité vectorielle des membres du complexe Anopheles gambiae au Mali. Thèse de doctorat d’Etat ès-science, Université Ex-Marseille, France; 1985.
- 17.Aïkpon R, Ossè R, Govoétchan R, Sovi A, Oké-Agbo F, Akogbéto M. Entomological baseline data on malaria transmission and susceptibility of Anopheles gambiae to insecticides in preparation for Indoor Residual Spraying (IRS) in Atacora, (Benin) J Parasitol Vector Biol. 2013;5:102–111. [Google Scholar]
- 18.Tuno N, Kjaerandsen J, Badu K, Kruppa T. Blood-Feeding Behavior of Anopheles gambiae and Anopheles melas in Ghana, Western Africa. J Med Entomol. 2010;47:28–31. doi: 10.1093/jmedent/47.1.28. [DOI] [PubMed] [Google Scholar]
- 19.Padonou GG, Gbedjissi G, Yadouleton A, Azondekon R, Razack O, Oussou O, et al. Decreased proportions of indoor feeding and endophily in Anopheles gambiae s.l. populations following the indoor residual spraying and insecticide-treated net interventions in Benin (West Africa) Parasit Vectors. 2012;5:262. doi: 10.1186/1756-3305-5-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ototo EN, Mbigi JP, Wanjala CL, Zhou G, Githeko AK, Yan G. Surveillance of malaria vector population density and biting behavior in Western Kenya. Malar J. 2015;14:244. doi: 10.1186/s12936-015-0763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Padonou GG, Sezonlin M, Gbedjissi GL, Ayi I, Azondekon R, Djenontin A, et al. Biology of Anopheles gambiae and insecticide resistance: entomological study for a large scale of indoor residual spraying in south east Benin. J Parasitol Vector Biol. 2012;4:59–68. [Google Scholar]
- 22.Aïkpon R, Agossa F, Ossè R, Oussou O, Aïzoun N, Oké-Agbo F, et al. Bendiocarb resistance in Anopheles gambiae s.l. populations from Atacora department in Benin, West Africa: a threat for malaria vector control. Parasit Vectors. 2013;6:192. doi: 10.1186/1756-3305-6-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gnanguenon V, Govoetchan R, Agossa FR, Ossè R, Oke-Agbo F, Azondekon R, et al. Transmission patterns of Plasmodium falciparum by Anopheles gambiae in Benin. Malar J. 2014;13:444. doi: 10.1186/1475-2875-13-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carnevale P, Toto JC, Besnard P, Dos Santos MA, Fortes F, Allan R, et al. Spatio-temporal variations of Anopheles coluzzii and An gambiae and their Plasmodium infectivity rates in Lobito. Angola. J Vect Ecol. 2015;40:172–179. doi: 10.1111/jvec.12147. [DOI] [PubMed] [Google Scholar]
- 25.Nwane P, Etang J, Chouaibou M, Toto JC, Kerah-hinzoumbe C, Mimpfoundi R, et al. Trends in DDT and pyrethroid resistance in Anopheles gambiae s.s. populations from urban and agro-industrial settings in southern Cameroon. BMC Infect Dis. 2009;9:163. doi: 10.1186/1471-2334-9-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camara S, Koffi AA, Ahoua Alou LP, Koffi K, Kabran JK, Koné A, et al. Mapping insecticide resistance in Anopheles gambiae s.l. from Côte d’Ivoire. Mapping insecticide resistance in Anopheles gambiae s.l. from Côte d’Ivoire. Parasit Vectors. 2018;11:19. doi: 10.1186/s13071-017-2546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathias DK, Ochomo E, Atieli F, Ombok M, Nabie Bayoh M, Olang G, et al. Spatial and temporal variation in the kdr allele L1014S in Anopheles gambiae s.s. and phenotypic variability in susceptibility to insecticides in western Kenya. Malar J. 2011;10:10. doi: 10.1186/1475-2875-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hien AS, Soma DD, Hema O, Bayili B, Namountougou M, Gnankiné O, et al. Evidence that agricultural use of pesticides selects pyrethroid resistance within Anopheles gambiae s.l. populations from cotton growing areas in Burkina Faso, West Africa. PLoS ONE. 2017;12:e0173098. doi: 10.1371/journal.pone.0173098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olé Sangba ML, Sidick A, Govoetchan R, Dide-Agossou C, Ossè RA, Akogbeto M, et al. Evidence of multiple insecticide resistance mechanisms in Anopheles gambiae populations in Bangui, Central African Republic. Parasit Vectors. 2017;13(10):23. doi: 10.1186/s13071-016-1965-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Djègbè I, Akoton R, Tchigossou G, Ahadji-Dabla KM, Atoyebi SM, Adéoti R, et al. First report of the presence of L1014S Knockdown-resistance mutation in Anopheles gambiae s.s. and Anopheles coluzzii from Togo. West Africa. Wellcome Open Res. 2018;3:30. doi: 10.12688/wellcomeopenres.13888.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sovi A, Govoétchan R, Tokponnon F, Hounkonnou H, Aïkpon R, Agossa F, et al. Impact of land-use on malaria transmission in the Plateau region, southeastern Benin. Parasit Vectors. 2013;6:352. doi: 10.1186/1756-3305-6-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gnanguenon V, Agossa FR, Badirou K, Govoetchan R, Anagonou R, Oke-Agbo F, et al. Malaria vectors resistance to insecticides in Benin: current trends and mechanisms involved. Parasit Vectors. 2015;8:223. doi: 10.1186/s13071-015-0833-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yahouédo GA, Cornelie S, Djègbè I, Ahlonsou J, Aboubakar S, Soares C, Akogbéto M, Corbel V. Dynamics of pyrethroid resistance in malaria vectors in southern Benin following a large scale implementation of vector control interventions. Parasit Vectors. 2016;9:385. doi: 10.1186/s13071-016-1661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akogbeto MC, Aikpon R, Azondekon R, Padonou G, Osse R, Agossa FR, et al. Six years of experience in entomological surveillance of indoor residual spraying against malaria transmission in Benin: lessons learned, challenges and outlooks. Malar J. 2015;14:242. doi: 10.1186/s12936-015-0757-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akogbéto M, Yakoubou S. Résistance des vecteurs du paludisme vis-à-vis des pyréthrinoïdes utilisés pour l’imprégnation des moustiquaires au Bénin, Afrique de l’Ouest. Bull Soc Path Exot. 1999;92:123–130. [PubMed] [Google Scholar]
- 36.Chandre F, Manguin S, Brengues C, DossouYovo J, Darriet F, Diabate A, et al. Current distribution of a pyrethroid resistance gene (kdr) in Anopheles gambiae complex from West Africa and further evidence for reproductive isolation of the Mopti form. Parassitologia. 1999;41:319–322. [PubMed] [Google Scholar]
- 37.Corbel V, N’guessan R, Brengues C, Chandre F, Djogbenou L, Martin T, et al. Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, West Africa. Acta Trop. 2007;101:207–216. doi: 10.1016/j.actatropica.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Yadouleton AW, Asidi A, Djouaka RF, Braïma J, Agossou CD, Akogbeto MC. Development of vegetable farming: a cause of the emergence of insecticide resistance in populations of Anopheles gambiae in urban areas of Benin. Malar J. 2009;8:103. doi: 10.1186/1475-2875-8-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used and/or analysed in this study are available from the corresponding author on reasonable request.