Abstract
Chromosome stability was maintained in mouse spermatozoa after freeze-drying or freezing without cryoprotection in a simple Tris⋅HCl buffer containing EGTA (50 mM) and NaCl (50 mM). The ability of spermatozoa to activate oocytes spontaneously was not destroyed by freeze-drying or freezing without cryoprotection in this solution. Embryos derived after injecting oocytes with sperm heads from rehydrated freeze-dried and from thawed spermatozoa developed normally. Provided the DNA integrity of the sperm nucleus is maintained, embryos can be generated by the intracytoplasmic sperm injection technique (ICSI) from severely damaged spermatozoa that are no longer capable of normal physiological activity. This procedure was effective for preserving spermatozoa from strains (C57BL/6J, 129/SvJ, and BALB/c) in which the fertility of spermatozoa frozen conventionally is extremely poor. The technique provides an effective means of storing mouse spermatozoa from many different inbred, mutant, and transgenic strains for biomedical research.
The mouse is the primary research animal in mammalian genetics, providing models for the analysis of embryonic development and human genetic diseases. Detailed study of the mouse genome by transgenesis and mutagenesis is leading to the generation of large numbers of new mouse lines worldwide (1, 2). Although >1,200 mutations covering a wide range of phenotypes have been described in the mouse, it represents just a small fraction (1–2%) of the total number of mouse genes. To close this so-called “phenotypic gap” several internationally renowned mouse genetic laboratories have commenced large chemical mutagenesis studies with chemicals such as ENU (N-ethyl-N-nitrosourea) (1, 3). It is not economically or practically possible to maintain all these unique stocks by conventional breeding, so alternative strategies are urgently needed to conserve these valuable genotypes for future study. The cryopreservation of embryos is not a realistic option because large numbers of animals are involved and while mouse oocytes can be successfully stored by freezing or vitrification (4, 5), difficulties in obtaining sufficient numbers of oocytes are similar to those for embryos. The banking of spermatozoa alone would be an efficient and cost-effective approach for the storage of transgenic and mutant stocks.
The major problem with cryopreserving mouse spermatozoa has been the sensitivity to damage during freezing and thawing (6), and it is only in the last few years that cryostorage of mouse spermatozoa has been achieved with any degree of success (2, 7–11). However, spermatozoa of some strains are very difficult to freeze—e.g., C57BL/6J (12) and BALB/c (2). Recently, live offspring were successfully obtained from hybrid mouse spermatozoa frozen without cryoprotection (13) or freeze-dried (14). On recovery from these procedures the spermatozoa are immotile and there is extensive damage to the sperm plasma membranes. Thus, it is necessary to inject them into oocytes by the intracytoplasmic sperm injection technique (ICSI) to achieve fertilization. As yet methods for freeze-drying are empirical, and information on genetic stability during storage in various media is unknown. In a preliminary study (unpublished work) comparing fresh and freeze-dried spermatozoa we found that structural chromosomal abnormalities are significantly higher in mouse oocytes injected with spermatozoa freeze-dried in CZB medium. Here, we show that modification of a simple Tris⋅HCl buffered solution with high concentrations of a calcium-chelating agent, normally used for the preparation of DNA from eukaryotic cells (15), can maintain chromosome integrity during freeze-drying. This procedure was also effective for storing spermatozoa from inbred strains where the fertility of spermatozoa frozen conventionally is very poor.
Materials and Methods
Animals.
Gametes were obtained from C57BL/6J, BALB/c, and 129/SvJ inbred and B6D2F1 hybrid mice aged between 8 and 12 weeks. Random-bred CD-1 females, 8–12 weeks old, mated with vasectomized males of the same strain were used as recipients for embryo transfer on the first day of pseudopregnancy. All animals were maintained according to the guidelines of the Laboratory Animal Service at the University of Hawaii and those prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (16). The protocol of animal handling and treatment was reviewed and approved by the Animal Care and Use Committee at the University of Hawaii.
Reagents and Media.
All chemicals were obtained from Sigma unless otherwise stated. The following solutions of 10 mM Tris⋅HCl buffer with various concentrations of NaCl and EGTA [ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid] were used for suspending spermatozoa for freezing and freeze-drying: (i) 20 mM NaCl and 50 mM EGTA, (ii) 50 mM NaCl and 50 mM EGTA, (iii) 50 mM NaCl and 10 mM EGTA, and (iv) 80 mM NaCl and 50 mM EGTA. These solutions were referred to as EGTA media and were prepared from stock solutions of 5 M NaCl, 0.5 M EGTA (pH 8.0, adjusted with NaOH), and 1 M Tris⋅HCl (pH 7.4) previously made up and diluted with ultrapure water (Millipore Systems). The pH of the final EGTA media was 8.2 to 8.4. The medium for oocyte collection and sperm injection was a modified CZB with 20 mM Hepes, 5 mM NaHCO3, and 0.1 mg/ml polyvinyl alcohol (PVA; cold water soluble; Mr 30,000–50,000) instead of BSA (Hepes-CZB; ref. 17). Oocytes were cultured after sperm injection in CZB medium (18, 19) supplemented with 5.56 mM glucose and 5 mg/ml BSA (fraction V, Calbiochem). CZB medium was maintained in 5% CO2 in air and Hepes-CZB in air.
Sperm Collection, Freezing, and Freeze-Drying.
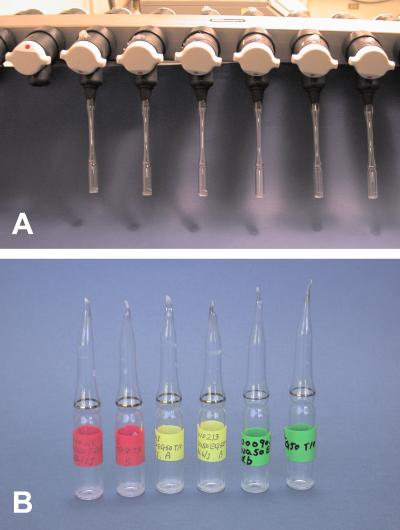
For each experiment two caudae epididymides of a male were removed and punctured with a sharply pointed forceps. Dense masses of spermatozoa squeezed from the epididymis were placed in the bottom of a 1.5-ml polypropylene microcentrifuge tube (flat top, Fisher) containing 1 ml of one of the NaCl/EGTA Tris⋅HCl-buffered solutions or 1 ml of CZB or Hepes-CZB culture medium. The tube was incubated for 10 min at 37°C to allow spermatozoa to disperse. The upper 700–800 μl of the sperm suspension in the tube was collected and 100-μl aliquots were transferred to either 1.5-ml polypropylene microcentrifuge tubes for freezing or 2-ml glass ampoules (Wheaton Scientific) for freeze-drying. The microcentrifuge tubes were placed directly in −20°C and −75°C freezers or plunged directly into liquid nitrogen and stored at these temperatures, respectively, until use. The glass ampoules with the sperm suspension were plunged into liquid nitrogen for 20 sec and then connected to a freeze-drying apparatus (Freeze-dry system, Labconco, Kansas City, MO) (Fig. 1A). After more than 4 h under lyophilization, each ampoule was flame-sealed (Fig. 1B). The inside pressure of ampoules at the time of sealing was 32–40 × 10−3 mbar (1 bar = 100 kPa). The ampoules were stored in the refrigerator at 4°C until use.
Figure 1.
Freeze-drying spermatozoa. (A) Six ampoules with spermatozoa, attached to a freeze-drying apparatus. Each ampoule is to be flame-sealed at the end of lyophilization. (B) White material at the bottom of each ampoule contains freeze-dried spermatozoa.
Preparation of Oocytes.
Female mice were superovulated with i.p. injections of 5 units of equine chorionic gonadotropin (eCG) and 5 units of human chorionic gonadotropin (hCG) (Calbiochem) given 48 h apart. Oocytes were collected from oviducts between 13 h and 16 h after hCG injection. They were freed from cumulus cells by treatment with 0.1% bovine testicular hyaluronidase (340 units/mg solid; Sigma) in Hepes-CZB medium. They were washed and kept in CZB medium at 37°C in 5% CO2 in air before sperm injection.
Preparation of Spermatozoa for ICSI.
The frozen sperm samples were thawed by warming the microcentrifuge tubes in a water bath at 37°C. The freeze-dried sperm samples were rehydrated by adding 100 μl of ultrapure water to each glass ampoule. A small volume (1–5 μl) of the sperm suspension from the thawed or rehydrated samples was thoroughly mixed with one drop (5–10 μl) of Hepes-CZB medium containing 12% (wt/vol) polyvinylpyrrolidone (PVP; Mr 360,000; ICN Pharmaceuticals). It was important to wash spermatozoa in another drop of the same medium containing 12% PVP before ICSI to minimize the introduction of EGTA into the oocyte cytoplasm because the EGTA might interfere with the normal activation of the oocyte (20, 21).
ICSI.
ICSI was carried out with modification of the technique originally described by Kimura and Yanagimachi (17); the sperm injections were performed at room temperature (25°C) rather than 17°C. A single spermatozoon was drawn tail first into the injection pipette and moved back and forth until the head-midpiece junction (the neck) was at the opening of the injection pipette. The head was separated from the midpiece and tail by applying one or more piezoelectric pulses (17). After the midpiece and tail had been discarded, the head was redrawn into the pipette and injected into an oocyte. The head and tail of many freeze-dried spermatozoa were separated, and therefore their separation by piezoelectric pulses was unnecessary in most cases. ICSI was completed within 1 h of rehydration of freeze-dried spermatozoa or thawing of frozen spermatozoa.
Culture and Examination of Oocytes.
Sperm-injected oocytes were transferred into droplets (50–100 μl) of CZB medium under mineral oil (Squibb) and incubated at 37°C in a humidified atmosphere of 5% CO2 in air. Oocytes with a second polar body and two pronuclei at 5–6 h after ICSI were considered normally fertilized. The fertilized oocytes were either taken at 6–8 h after ICSI to incubate with vinblastine for chromosome analysis (see below) or left in culture to assess development to the blastocyst stage at 96 h after ICSI. Some of the embryos were transferred at the two-cell stage—i.e., 24 h after ICSI—to pseudopregnant recipients.
Embryo Transfer.
Two-cell embryos were transferred into oviducts of pseudopregnant CD-1 females (albino) that had mated with vasectomized CD-1 males (albino) the previous night. Postimplantation development was assessed on day 14 of pregnancy, when the number of implantation sites and normally developing fetuses were recorded. In practice this provides information on the extent of early embryonic loss after implantation, and normal fetuses at day 14 of pregnancy very rarely fail to develop to full term.
Chromosome Analysis.
Chromosome preparations were made according to procedures described previously (22, 23). At 6–8 h after ICSI, activated oocytes with two pronuclei and a second polar body were placed into a CZB medium containing 0.006 μg/ml vinblastine to arrest the metaphases of the first cleavage. After treatment with vinblastine for 19–21 h, the zona pellucida was removed with 0.5% Pronase (1,000 tyrosine units/mg; Kaken Pharmaceuticals, Tokyo) before placing the oocytes in a hypotonic citrate solution (1:1 mixture of 30% FBS and 1% sodium citrate). Fixation of the oocytes and spreading of chromosomes onto glass slides were performed according to the method of Kamiguchi and Mikamo (24). Structural chromosome aberrations were scored as outlined previously (25). The number of aberrations per oocyte was recorded without discriminating between paternal and maternal pronuclei. In this study, only oocytes with 40 chromosomes and without structural chromosome aberrations were recorded as karyotypically normal. Uncountable numbers of aberrations such as chromosome fragmentation and multiple exchanges were defined as >10 aberrations per oocyte.
Analysis of Data.
Treatment comparisons were made with the χ2 test using Yates' correction for continuity and Fisher's exact test where appropriate.
Results
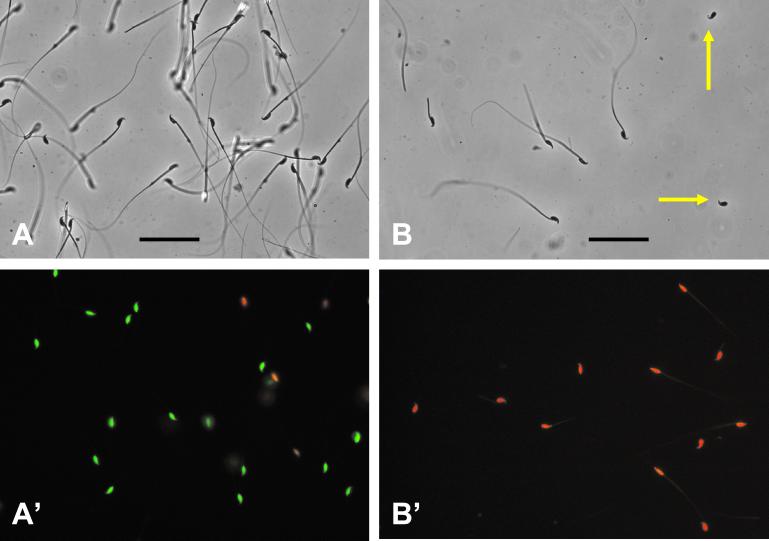
In experiments with B6D2F1 hybrid males, the spermatozoa swam very actively at first in the EGTA media, but motility was gradually lost and after 10 min they were virtually all immotile. Live/dead staining using a Live/Dead viability kit (FertiLight; Molecular Probes) showed that 70–90% (average 85%) of the spermatozoa were “alive”—i.e., plasma membranes were intact—after the 10-min incubation (Fig. 2). The sperm concentration in the dispersed samples was 1–2 × 106 spermatozoa per ml.
Figure 2.
Spermatozoa before and after freeze-drying. (A) Phase-contrast micrograph of spermatozoa in EGTA medium before freeze-drying, showing that most spermatozoa are structurally intact. (A′) Live/dead staining of spermatozoa in EGTA medium. Nuclei of “live” (plasma membrane-intact) spermatozoa fluoresce green, whereas those of “dead” (plasma membrane-disrupted) spermatozoa fluoresce red. (Bar equals 50 μm.) Spermatozoa in A and A′ are not in the same field of preparation. (B) Phase-contrast micrograph of freeze-dried spermatozoa, showing that many spermatozoa lost part or all of their tails (yellow arrows). (B′) Live/dead staining of freeze-dried spermatozoa, showing that all of them are “dead.”
The chromosome analysis of fertilized B6D2F1 oocytes generated by ICSI with B6D2F1 spermatozoa that were suspended in different media for freezing and freeze-drying is presented in Table 1. In the Tris⋅HCl-buffered medium, concentrations of 50 mM EGTA and 50 mM NaCl were found to be most effective at maintaining chromosome integrity after freezing to −75°C and −196°C and after freeze-drying. The proportion of normal karyotypes was similar to that obtained with fresh spermatozoa dispersed in either the EGTA medium or Hepes-CZB medium. Chromosomal aberrations increased significantly when spermatozoa were frozen in Hepes-CZB or freeze-dried in CZB medium. When spermatozoa were frozen in CZB medium there was only a slight reduction in the proportion of normal karyotypes compared with fresh spermatozoa in Hepes-CZB medium (92% vs. 81%). Variations in the concentrations of EGTA or NaCl above and/or below 50 mM had an adverse effect on chromosome integrity.
Table 1.
Chromosome analysis of B6D2F1 oocytes fertilized by ICSI with fresh, frozen, or freeze-dried B6D2F1 spermatozoa: Comparison of different media for sperm dispersion and storage
| Medium for sperm dispersion and storage | Sperm treatment | Final freezing temp., °C | Sperm storage
|
No. oocytes analyzed (No. exps.) | No. chromosome aberrations per oocyte | No. (%) oocytes with normal karyotype | |
|---|---|---|---|---|---|---|---|
| Temp., °C | Time, days | ||||||
| Hepes-CZB | Fresh | — | — | — | 145 (9) | 0.083 | 134 (92)a |
| Frozen | −196 | −196 | <1 | 58 (4) | 1.00 | 37 (64)a | |
| CZB | Frozen | −196 | −196 | <1 | 118 (7) | 0.41 | 96 (81)b |
| Freeze-dried | −196 | 4 | 2 to 63 | 63 (4) | 1.2 | 33 (52)b | |
| NaCl/EGTA* | |||||||
| 50 mM/50 mM | Fresh | — | — | — | 98 (4) | 0.07 | 91 (93)c |
| Frozen | −20 | −20 | Up to 14 | 51 (4) | 0.67 | 37 (73)c | |
| Frozen | −75 | −75 | Up to 14 | 63 (4) | 0.10 | 58 (92) | |
| Frozen | −196 | −196 | <1 | 63 (4) | 0.16 | 56 (89) | |
| Freeze-dried | −196 | 4 | Up to 21 | 105 (9) | 0.43 | 87 (83)d,e,f | |
| 20 mM/50 mM | Freeze-dried | −196 | 4 | Up to 28 | 89 (4) | 0.72 | 60 (67)d |
| 80 mM/50 mM | Freeze-dried | −196 | 4 | Up to 14 | 52 (4) | 1.2 | 28 (54)e |
| 50 mM/10 mM | Freeze-dried | −196 | 4 | Up to 21 | 65 (5) | 3.9 | 12 (18)f |
Statistically significant χ2 comparisons, comparing same letters:
,
,
, and
, P < 0.001;
, P < 0.01;
, P < 0.025.
Various concentrations of NaCl and EGTA in 10 mM Tris⋅HCl buffer, pH 8.2–8.4.
The chromosome analysis of fertilized B6D2F1 oocytes generated by ICSI from 129/SvJ, BALB/c, and C57BL/6J spermatozoa after freezing and freeze-drying is presented in Table 2. The proportion of normal karyotypes obtained with fresh 129/SvJ and BALB/c spermatozoa was significantly higher after dispersion in EGTA medium compared with Hepes-CZB medium. There was a significant reduction in chromosome normality with freeze-dried spermatozoa from both of these strains, and this was most pronounced in the 129/SvJ strain. Although fresh C57BL/6J spermatozoa were not examined in this study, a high proportion of normal karyotypes was obtained in oocytes injected with both frozen and freeze-dried spermatozoa.
Table 2.
Chromosome analysis of B6D2F1 oocytes fertilized by ICSI with spermatozoa from 129/SvJ, BALB/c, and C57BL/6J inbred strains after freezing and/or freeze-drying
| Sperm treatment | Medium for dispersion and storage | Mouse strain | Final freezing temp., °C | Sperm storage
|
No. oocytes analyzed (No. exps.) | No. chromosome aberrations per oocyte | No. (%) oocytes with normal karyotype | |
|---|---|---|---|---|---|---|---|---|
| Temp., °C | Time, days | |||||||
| None (fresh) | Hepes-CZB | 129/SvJ | — | — | — | 78 (5) | 1.0 | 53 (68)a |
| BALB/c | — | — | — | 72 (5) | 0.64 | 47 (65)b | ||
| EGTA* | 129/SvJ | — | — | — | 76 (4) | 0.3 | 64 (84)a,c | |
| BALB/c | — | — | — | 61 (5) | 0.066 | 58 (95)b,d | ||
| Frozen | EGTA* | C57BL/6J | −75 | −75 | Up to 14 | 64 (4) | 0.19 | 55 (86) |
| Freeze-dried | EGTA* | C57BL/6J | −196 | 4 | Up to 56 | 92 (5) | 0.55 | 67 (73)e |
| 129/SvJ | −196 | 4 | Up to 42 | 78 (6) | 1.1 | 42 (54)c,e,f | ||
| BALB/c | −196 | 4 | Up to 42 | 60 (5) | 0.25 | 49 (82)d,f | ||
Statistically significant χ2 comparisons, comparing same letters:
,
,
, and
, P < 0.001;
, P < 0.025;
, P < 0.05.
Composition of EGTA medium: 50 mM NaCl, 50 mM EGTA, and 10 mM Tris⋅HCl.
The development of embryos generated from B6D2F1 oocytes injected with fresh, frozen, or freeze-dried B6D2F1 spermatozoa is presented in Table 3. Activation of the oocytes was reduced after injection with freeze-dried spermatozoa, but the proportion was still extremely high. There was also a slight reduction in the number of two-cell embryos developing to the blastocyst stage, and this may reflect the higher proportion of abnormal karyotypes observed at first cleavage after injecting freeze-dried spermatozoa (≈10%).
Table 3.
In vitro development of B6D2F1 oocytes fertilized by ICSI with fresh, frozen, or freeze-dried B6D2F1 spermatozoa
| Sperm treatment | Sperm freezing medium | Sperm storage time, days | No. oocytes injected (No. exps.) | No. (%) oocytes activated* | No. (%) 2-cell embryos | No. (%) blastocysts |
|---|---|---|---|---|---|---|
| Fresh | Hepes-CZB | — | 71 (4) | 71 (100) | 71 (100) | 61 (86)a |
| Frozen and kept at −75°C | EGTA† | Up to 112 | 96 (4) | 95 (99) | 94 (99) | 75 (80) |
| Freeze-dried and kept at 4°C | EGTA | Up to 28 | 158 (7) | 135 (85) | 133 (99) | 96 (72)a |
Statistically significant χ2 comparison:
, P < 0.05.
Activated oocytes with two pronuclei and the second polar body.
Composition of EGTA medium: 50 mM NaCl, 50 mM EGTA, and 10 mM Tris⋅HCl.
A summary of the postimplantation development of two-cell embryos generated by ICSI of fresh, frozen, and freeze-dried spermatozoa from the various strains is shown in Table 4. In all cases the two-cell embryos were derived from oocytes and spermatozoa of the same strain. Morphologically normal live 14-day-old fetuses were obtained in all strains with fresh, frozen, and freeze-dried spermatozoa. Overall, the proportion of transferred embryos implanting was high. Only with embryos derived from fresh 129/SvJ and freeze-dried C57BL/6J spermatozoa was there a significant reduction in implantation. In all strains early embryonic loss after implantation was similar for embryos from fresh and frozen spermatozoa but greater after freeze-drying. However, the number of fetuses was reduced only with B6D2F1 and C57BL/6J embryos derived from freeze-dried spermatozoa.
Table 4.
Postimplantation development of oocytes fertilized by ICSI with fresh, frozen, or freeze-dried spermatozoa
| Sperm treatment | Medium for sperm dispersion and storage | Mouse strain | Sperm storage time, days | No. 2-cell embryos* transferred (No. exps.) | No. recipients† | Examination on day 14 post coitum
|
||
|---|---|---|---|---|---|---|---|---|
| No. (%) implants | No. (%) normal fetuses | Range | ||||||
| Fresh | Hepes-CZB | B6D2F1 | — | 94 (8) | 12 | 75 (80) | 54 (57)b | 0–83 |
| C57BL/6J | — | 48 (6) | 6 | 36 (75) | 26 (54)c | 29–73 | ||
| 129/SvJ | — | 54 (4) | 4 | 37 (69) | 22 (41) | 13–58 | ||
| BALB/c | — | 65 (4) | 4 | 36 (55) | 16 (25) | 14–40 | ||
| Frozen and kept at −75°C | EGTA‡ | B6D2F1 | Up to 28 | 66 (4) | 8 | 46 (70) | 31 (47) | 14–70 |
| C57BL/6J | Up to 28 | 76 (7) | 8 | 68 (89)a | 42 (54)d | 30–90 | ||
| 129/SvJ | Up to 42 | 43 (4) | 4 | 31 (72) | 19 (44) | 38–67 | ||
| Freeze-dried and kept at 4°C | EGTA‡ | B6D2F1 | Up to 14 | 98 (5) | 8 | 72 (73) | 37 (38)b | 11–56 |
| C57BL/6J | Up to 56 | 176 (17) | 18 | 112 (64)a | 43 (24)c,d | 0–69 | ||
| 129/SvJ | Up to 56 | 60 (6) | 6 | 46 (77) | 24 (40) | 27–50 | ||
| BALB/c | Up to 35 | 75 (6) | 6 | 58 (77) | 16 (21) | 6–40 | ||
Statistically significant χ2 comparisons between treatments within strains:
,
, and
, P < 0.001;
, P < 0.01.
In all strains two-cell embryos were derived from syngeneic gametes.
CD-1 females (albino) mated with vasectomized CD-1 males (albino). Two-cell embryos transferred on the first day of pseudopregnancy.
Composition of EGTA medium: 50 mM NaCl, 50 mM EGTA, and 10 mM Tris⋅HCl.
Discussion
We have shown that chromosome integrity of mouse spermatozoa can be maintained during freeze-drying or freezing without cryoprotection when the spermatozoa are suspended in a simple Tris⋅HCl-buffered solution containing 50 mM EGTA and 50 mM NaCl. This somewhat “unphysiological” solution affords sufficient protection to the DNA to enable normal development of embryos generated from spermatozoa suspended in it for storage. We also have preliminary evidence indicating that this EGTA medium can maintain the chromosomal integrity of human and rabbit spermatozoa after freeze-drying (unpublished observations).
Overall a high proportion of oocytes (≈90%) activated spontaneously after sperm head injection (data not shown), indicating that the sperm-activating molecule (26) was not destroyed by freeze-drying or freezing. The proportion of chromosome aberrations is similar to that found after ICSI with fresh spermatozoa (≈9%; ref. 23). Since almost 100% of mouse oocytes fertilized in vivo are karyologically normal (27, 28), it would appear that the ICSI procedure per se, independently of freezing or freeze-drying, has some adverse affect upon chromosome stability. For mouse ICSI, the sperm neck region is damaged (17) or heads are separated from tails (29) before injection into the oocytes, and it would appear that the resulting damage to the plasma membrane increases the chance of chromosome aberrations occurring in the resulting embryo. Sonication and freezing of human spermatozoa without cryoprotection has been reported to increase chromosome structural aberrations (30, 31). However, it is more likely that prolonged exposure of membrane-damaged spermatozoa to culture media rather than sonication or cryopreservation themselves is detrimental to chromosomal stability (23).
Freezing and thawing per se does not appear to damage DNA (32). The DNA integrity of mammalian sperm cells (hamster, ref. 33; human, ref. 34) and somatic cells (human blood, ref. 35) is maintained during freezing and thawing by methods that achieve optimal rates of cell survival. The comet assay (36, 37) shows that cryopreserved human lymphocytes elicit the same response to the induction of DNA damage by treatment with H2O2 as similarly treated freshly isolated human lymphocytes (38). This suggests that DNA damage is induced by mechanical or oxidative stress not during freezing or freeze-drying but during the holding period before ICSI after thawing or rehydration. Enriching the suspending media for isolated sperm heads with K+, to simulate the intracellular environment, maintained chromosome integrity for longer periods than in Na+-rich culture media (29). The release of endogenous nucleases from plasma membrane-damaged spermatozoa after sperm head isolation, freeze-drying, or freezing without cryoprotection is the most likely cause of the structural chromosome aberrations. Maione et al. (39) reported the existence of Ca2+-dependent endogenous nucleases in mouse spermatozoa. The presence of the chelating agent EDTA and the absence of Ca2+ and Mg2+ from K+-rich media for sperm head isolation probably contributed to the improvement in chromosome stability (23, 29). Surprisingly, the greatest stability of chromosome structure was obtained with an extremely high concentration of EGTA (50 mM). The other Tris⋅HCl-buffered media with lower and higher levels of Na+ (20 and 80 mM) or a lower concentration of EGTA (10 mM) were much less effective in maintaining sperm chromosome integrity (Table 1). Our initial experience with EGTA media with low pH showed that it negatively affected chromosome stability. Why high pH (8.2–8.4) is important is presently not known, but it may assist in repressing the activity of the endogenous endonucleases. Sperm chromosome aberrations might be further reduced if Ca2+ is removed from the injection medium. Previous reports have not examined the individual effect of Ca2+, Mg2+, Na+, or K+ in the sperm injection medium on sperm chromosome stability.
Preservation of spermatozoa from inbred strains that have previously proved difficult to conserve by conventional techniques of freezing and in vitro fertilization is a great advance for the preservation of inbred lines carrying mutations and transgenes. In this study we have shown that with relatively few oocytes a sufficient number of viable progeny to re-establish the strains can be generated after ICSI with freeze-dried and frozen spermatozoa.
The storage of mouse spermatozoa in the freeze-dried state offers many advantages over cryostorage at −196°C where a constant supply of liquid nitrogen is difficult or liquid nitrogen storage containers may break down or become contaminated. In addition, storage at ambient temperatures is cheap and also facilitates transport of samples between countries. We have already found that samples can be transported over long distances at ambient temperatures without any increase in chromosome damage or loss of developmental potential (unpublished observations). Spermatozoa frozen without cryoprotection to −75°C generated viable progeny, and this may be a convenient way to store spermatozoa for the short term when liquid nitrogen is unavailable, but permanent storage at −75°C is not recommended because biological activity does not cease at low temperatures until the temperature falls below −130°C.
In conclusion, the preservation of spermatozoa by freeze-drying or freezing in a simple EGTA/Tris⋅HCl-buffered medium provides an important means of maintaining a whole spectrum of mouse strains at low cost without incurring heritable damage.
Acknowledgments
We thank Dr. H. Tateno, Asahikawa Medical College, Asahikawa, Japan, for giving us invaluable advice. This material is based on work done as part of the National Cooperative Program on Mouse Sperm Cryopreservation, which is funded by the National Institute of Child Health and Human Development and the National Center for Research Resources, National Institutes of Health Cooperative Agreement U01 HD38205.
Abbreviations
- EGTA
ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid
- ICSI
intracytoplasmic sperm injection technique
Footnotes
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on May 1, 2001.
References
- 1.Nolan P M, Peters J, Vizor L, Strivens M, Washbourne R, Hough T, Wells C, Glenister P, Thornton C, Martin J, et al. Mamm Genome. 2000;11:500–506. doi: 10.1007/s003350010096. [DOI] [PubMed] [Google Scholar]
- 2.Thornton C E, Brown S D M, Glenister P H. Mamm Genome. 1999;10:987–992. doi: 10.1007/s003359901145. [DOI] [PubMed] [Google Scholar]
- 3.Brown S D M, Peters J. Trends Genet. 1996;12:433–435. doi: 10.1016/0168-9525(96)30094-2. [DOI] [PubMed] [Google Scholar]
- 4.Carroll J, Wood M J, Whittingham D G. Biol Reprod. 1993;48:606–612. doi: 10.1095/biolreprod48.3.606. [DOI] [PubMed] [Google Scholar]
- 5.Wood M J, Barros C, Candy C J, Carroll J, Melendez J, Whittingham D G. Biol Reprod. 1993;49:489–495. doi: 10.1095/biolreprod49.3.489. [DOI] [PubMed] [Google Scholar]
- 6.Mazur P, Katkov I I, Katkova N, Critser J K. Cryobiology. 2000;40:187–209. doi: 10.1006/cryo.2000.2238. [DOI] [PubMed] [Google Scholar]
- 7.Tada N, Sato M, Yamanoi J, Mozorogi T, Kasai K, Ogawa S. J Reprod Fertil. 1990;89:511–516. doi: 10.1530/jrf.0.0890511. [DOI] [PubMed] [Google Scholar]
- 8.Nakagata N. Mamm Genome. 2000;11:572–576. doi: 10.1007/s003350010109. [DOI] [PubMed] [Google Scholar]
- 9.Nakagata N, Takeshima T. Exp Anim. 1993;42:317–320. doi: 10.1538/expanim1978.42.3_317. [DOI] [PubMed] [Google Scholar]
- 10.Sztein J M, Farley J S, Young A F, Mobraaten L E. Cryobiology. 1997;35:46–52. doi: 10.1006/cryo.1997.2024. [DOI] [PubMed] [Google Scholar]
- 11.Sztein J M, Farley J S, Mobraaten L. Biol Reprod. 2000;63:1774–1780. doi: 10.1095/biolreprod63.6.1774. [DOI] [PubMed] [Google Scholar]
- 12.Nakagata N, Okamoto M, Ueda O, Suzuki H. Biol Reprod. 1997;57:1050–1055. doi: 10.1095/biolreprod57.5.1050. [DOI] [PubMed] [Google Scholar]
- 13.Wakayama T, Whittingham D G, Yanagimachi R. J Reprod Fertil. 1998;112:11–17. doi: 10.1530/jrf.0.1120011. [DOI] [PubMed] [Google Scholar]
- 14.Wakayama T, Yanagimachi R. Nat Biotechnol. 1998;16:639–641. doi: 10.1038/nbt0798-639. [DOI] [PubMed] [Google Scholar]
- 15.Davis L G, Dibner M D, Battey J F. Basic Methods in Molecular Biology. New York: Elsevier; 1986. p. 47. [Google Scholar]
- 16.Committee on Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. Bethesda: Natl. Inst. Health; 1985. , DHHS Publ. No. (NIH) 85–23. [Google Scholar]
- 17.Kimura Y, Yanagimachi R. Biol Reprod. 1995;52:709–720. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- 18.Chatot C L, Ziomek A, Bavister B D, Lewis J L, Torres I. J Reprod Fertil. 1989;86:679–688. doi: 10.1530/jrf.0.0860679. [DOI] [PubMed] [Google Scholar]
- 19.Chatot C L, Lewis J L, Torres I, Ziomek C A. Biol Reprod. 1990;42:432–440. doi: 10.1095/biolreprod42.3.432. [DOI] [PubMed] [Google Scholar]
- 20.Izant J G. Chromosoma. 1983;88:1–10. doi: 10.1007/BF00329497. [DOI] [PubMed] [Google Scholar]
- 21.Groigno L, Whitaker M. Cell. 1998;92:193–204. doi: 10.1016/s0092-8674(00)80914-9. [DOI] [PubMed] [Google Scholar]
- 22.Kishikawa H, Tateno H, Yanagimachi R. Biol Reprod. 1999;61:809–812. doi: 10.1095/biolreprod61.3.809. [DOI] [PubMed] [Google Scholar]
- 23.Tateno H, Kimura Y, Yanagimachi R. Biol Reprod. 2000;63:341–346. doi: 10.1095/biolreprod63.1.341. [DOI] [PubMed] [Google Scholar]
- 24.Kamiguchi Y, Mikamo K. Am J Hum Genet. 1986;38:724–740. [PMC free article] [PubMed] [Google Scholar]
- 25.Kusakabe H, Yamakage K, Tanaka N. Mutat Res. 1996;369:51–58. doi: 10.1016/s0165-1218(96)90047-6. [DOI] [PubMed] [Google Scholar]
- 26.Parrington J, Lai F A, Swann K. Curr Topics Dev Biol. 1998;39:215–243. doi: 10.1016/s0070-2153(08)60457-3. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka N, Katoh M, Iwahara S. Cytogenet Cell Genet. 1981;31:145–152. doi: 10.1159/000131640. [DOI] [PubMed] [Google Scholar]
- 28.Katoh M, Tanaka N, Iwahara S. Jpn J Genet. 1981;56:357–363. [Google Scholar]
- 29.Kuretake S, Kimura Y, Hoshi K, Yanagimachi R. Biol Reprod. 1996;55:789–795. doi: 10.1095/biolreprod55.4.789. [DOI] [PubMed] [Google Scholar]
- 30.Martin R H, Ko E, Rademaker A. J Reprod Fert. 1988;84:179–186. doi: 10.1530/jrf.0.0840179. [DOI] [PubMed] [Google Scholar]
- 31.Rybouchkin A V, De Sutter P, Dhont M. Zygote. 1996;4:263–268. doi: 10.1017/s0967199400003208. [DOI] [PubMed] [Google Scholar]
- 32.Whittingham D G, Lyon M F, Glenister P H. Genet Res. 1977;29:171–181. doi: 10.1017/s0016672300017237. [DOI] [PubMed] [Google Scholar]
- 33.Ohsako S, Nagano R, Sugimoto Y, Goto K. J Vet Med Sci. 1997;59:1085–1088. doi: 10.1292/jvms.59.1085. [DOI] [PubMed] [Google Scholar]
- 34.Steele E K, McClure N, Lewis S E. Fertil Steril. 2000;74:450–453. doi: 10.1016/s0015-0282(00)00680-4. [DOI] [PubMed] [Google Scholar]
- 35.Ross K S, Haites N E, Kelly K F. J Med Genet. 1990;27:569–570. doi: 10.1136/jmg.27.9.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh N P, McCoy M T, Tice R R, Schneider E L. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 37.Fairbairn D W, Olive P L, O'Neill K L. Mutat Res. 1995;339:37–59. doi: 10.1016/0165-1110(94)00013-3. [DOI] [PubMed] [Google Scholar]
- 38.Visvardis E E, Tassiou A M, Piperakis S M. Mutat Res. 1997;383:71–80. doi: 10.1016/s0921-8777(96)00047-x. [DOI] [PubMed] [Google Scholar]
- 39.Maione B, Pittoggi C, Achene L, Lorenzini R, Spadafora C. DNA Cell Biol. 1997;16:1087–1097. doi: 10.1089/dna.1997.16.1087. [DOI] [PubMed] [Google Scholar]