Localization optoacoustic tomography provides superresolution imaging capability in 3D beyond the acoustic diffraction limit, which can be crucial for mapping microcirculation in cancers, brain functions, peripheral vascular diseases, etc.
Optoacoustic (also referred to as photoacoustic) tomography (OAT) has been gaining popularity for preclinical and clinical imaging during the past couple of decades1. OAT breaks the long-standing shallow imaging depth limitation of conventional optical imaging by forming an image using the optoacoustic (OA) effect. Through advances in ultrasound imaging technologies, OAT provides rich optical contrast while achieving high spatial resolution deep inside living subjects (up to several centimeters). Thanks to these hybrid technologies, the use of preclinical OAT to study cancer physiopathology, neural physiology, drug delivery, vascular diseases, etc., has spread globally to many laboratories. More importantly, the applications of OAT have been extended to include many clinical trials, such as early diagnosis and treatment monitoring of cancers, imaging of the bowel for diseases, human neuroimaging for diagnosing neurological defects, imaging of peripheral arteries and veins for detecting vascular disease, and intravascular imaging for characterizing plaque.
OAT is mainly implemented in two domains: the optical ballistic regime (<~1 mm in biological tissues) and the optical diffusive regime (>~1 mm in biological tissues). In the optical ballistic regime, the lateral resolution of OA imaging is determined by the tight optical focus, and the technology is referred to as optical-resolution OA microscopy (OAM). In the optical diffusive regime, the resolution is determined by the acoustic focus and/or ultrasound parameters, and this technology is referred to as acoustic-resolution OAT. The resolutions of OAT in both regimes are limited by either the optical or acoustic diffraction limit. Recently, several attempts were made by multiple researchers to exceed these diffraction limits to achieve superresolution imaging. In the optical ballistic domain, Lihong’s group explored subdiffraction OAM using either nonlinear optical saturation or photobleaching effects2, 3. In addition, Lee et al. developed superresolution photoactivated atomic force microscopy and improved the resolution to ~8 nm4. In the optical diffusive domain, Thomas et al. demonstrated superresolution OAT beyond the acoustic diffraction limit by either probing the fluctuations of OA signals with dynamic optical speckle excitation or detecting the fluctuating OA signals from flowing optical absorbers5, 6. Furthermore, Donald et al. applied a wavefront-shaping technology to squeeze the spatial resolution of OAT to smaller than the acoustic diffraction limit 7.
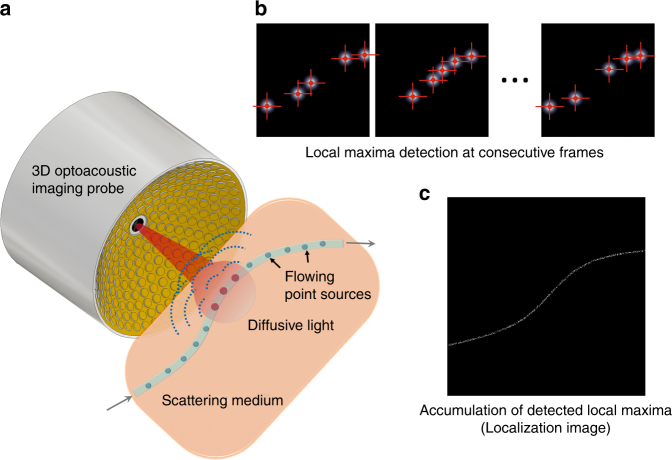
Compared to the previous results, Luís et al. applied the localization imaging approach to OAT to enhance the visualization of flowing particles that are embedded in an optical scattering medium in 3D and referred to this approach as localization optoacoustic tomography (LOT). In LOT, multiple OA images are obtained at the same location using a portable volumetric OAT system that is equipped with a spherical array probe (Fig. 1a)8. Then, the OA images are superimposed after filtering each image to obtain local maximum pixels (bright dots in Fig. 1b) that correspond to the particle’s OA signals. In the final superimposed image, the OA signals overlap and the final LOT image shows the path of the moving particle in 3D (Fig. 1c). Compared to the conventional OA images, the blurring effect of the point spread function disappears in the LOT images since only the locations of the point sources are used for localization of the signals. For high-resolution imaging of vasculatures, injection of exogenous agents into the blood stream is required. Table 1 compares the mechanisms and performances from previous reports with those of LOT.
Fig. 1. Imaging principles of localization optoacoustic tomography.
a A spherical array of ultrasound transducers is used to acquire a three-dimensional optoacoustic image of flowing absorbers in an optical scattering medium for each laser pulse. b The positions of sparsely distributed absorbers are measured (localized) in a sequence of images. c An image is formed by superimposing the localized positions
Table 1.
Comparison of the imaging domain, superresolution mechanism, image formation mechanism, and dimensions from previous reports and of LOT
| Imaging domain | Superresolution mechanism | Image formation mechanism | Dimensions | |
|---|---|---|---|---|
| Lee et al.4 | Surface | OA/photothermal detection using AFM tips | Point-by-point scanning | 2D in x and y |
| Danielli et al.2 | Ballistic | Nonlinear optical excitation using optical saturation | Point-by-point scanning | 3D |
| Yao et al.3 | Nonlinear optical excitation using photobleaching | |||
| Chaigne et al.5, 6 | Diffusive | Fluctuations of OA signals with dynamic optical speckle excitation | Ultrasound beamforming using a linear array probe | 2D in x and z |
| Fluctuations of OA signals with flowing particles | ||||
| Conkey et al.7 | Optical excitation with wavefront shaping | Point-by-point scanning | 3D | |
| Luís et al.8 | Fluctuations of OA signals with flowing particles | Ultrasound beamforming using a spherical array probe | 3D |
LOT is suitable for imaging any structure with the flow of optical absorbers. For biomedical applications, imaging of vascular structures with high resolution is very attractive for studying cancers, brain activities, peripheral vascular diseases, etc.
Acknowledgements
This work was supported by the MSIT (Ministry of Science and ICT), Korea, under the ICT Consilience Creative program (IITP-2018-2011-1-00783) supervised by the IITP (Institute for Information & communications Technology Promotion), the grant of the Korea Health Technology R&D Project (HI15C1817) through the KHIDI (Korea Health Industry Development Institute), funded by the Ministry of Health & Welfare, Republic of Korea, the NRF (National Research Foundation) grant (2011-0030075) funded by MSIT, Republic of Korea.
Conflict of interest
Chulhong Kim has a financial interest in OPTICHO, which did not support this work.
References
- 1.Wang LV, Hu S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science. 2012;335:1458–1462. doi: 10.1126/science.1216210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danielli A, et al. Label-free photoacoustic nanoscopy. J. Biomed. Opt. 2014;19:086006. doi: 10.1117/1.JBO.19.8.086006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao JJ, Wang LD, Li CY, Zhang C, Wang LV. Photoimprint photoacoustic microscopy for three-dimensional label-free subdiffraction imaging. Phys. Rev. Lett. 2014;112:014302. doi: 10.1103/PhysRevLett.112.014302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee S, et al. Super-resolution visible photoactivated atomic force microscopy. Light Sci. Appl. 2017;6:e17080. doi: 10.1038/lsa.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaigne T, et al. Super-resolution photoacoustic fluctuation imaging with multiple speckle illumination. Optica. 2016;3:54–57. doi: 10.1364/OPTICA.3.000054. [DOI] [Google Scholar]
- 6.Chaigne T, Arnal B, Vilov S, Bossy E, Katz O. Super-resolution photoacoustic imaging via flow-induced absorption fluctuations. Optica. 2017;4:1397–1404. doi: 10.1364/OPTICA.4.001397. [DOI] [Google Scholar]
- 7.Conkey DB, et al. Super-resolution photoacoustic imaging through a scattering wall. Nat. Commun. 2015;6:7902. doi: 10.1038/ncomms8902. [DOI] [PubMed] [Google Scholar]
- 8.Deán-Ben XL, Razansky D. Localization optoacoustic tomography. Light Sci. Appl. 2018;7:18004. doi: 10.1038/lsa.2018.4. [DOI] [PMC free article] [PubMed] [Google Scholar]