Abstract
An intermittent fasting or calorie restriction diet has favorable effects in the mouse forms of multiple sclerosis (MS) and may provide additional anti-inflammatory and neuroprotective advantages beyond benefits obtained from weight loss alone. We conducted a pilot randomized controlled feeding study in 36 people with MS to assess safety and feasibility of different types of calorie restriction (CR) diets and assess their effects on weight and patient reported outcomes in people with MS. Patients were randomized to receive 1 of 3 diets for 8 weeks: daily CR diet (22% daily reduction in energy needs), intermittent CR diet (75% reduction in energy needs, 2 days/week; 0% reduction, 5 days/week), or a weight-stable diet (0% reduction in energy needs, 7 days/week). Of the 36 patients enrolled, 31 (86%) completed the trial; no significant adverse events occurred. Participants randomized to CR diets lost a median 3.4 kg (interquartile range [IQR]: −2.4, −4.0). Changes in weight did not differ significantly by type of CR diet, although participants randomized to daily CR tended to have greater weight loss (daily CR: −3.6 kg [IQR: −3.0, −4.1] vs. intermittent CR: −3.0 kg [IQR: −1.95, −4.1]; P=0.15). Adherence to study diets differed significantly between intermittent CR vs. daily CR, with lesser adherence observed for intermittent CR (P=0.002). Randomization to either CR diet was associated with significant improvements in emotional well-being/depression scores relative to control, with an average 8-week increase of 1.69 points (95% CI: 0.72, 2.66). CR diets are a safe/feasible way to achieve weight loss in people with MS and may be associated with improved emotional health.
Keywords: Multiple sclerosis, Dietary intervention, Weight loss intervention
1. Introduction
Multiple sclerosis (MS) is a disease of the central nervous system with a highly variable clinical course. Currently, it is unclear as to why certain individuals experience relatively little disability progression while others accumulate substantial physical and cognitive disability (Frohman et al., 2006). Interest in comorbidity as a potential factor that could explain some of heterogeneity in outcomes is rapidly rising (Marrie, 2017b). The metabolic syndrome and closely related vascular disorders like diabetes and hyperlipidemia are overrepresented in people with MS and are strongly associated with adverse MS outcomes (Marrie, 2017a). Aspects of diet are important determinants of the development of metabolic comorbidity in the general population and also directly influence biologic mechanisms potentially relevant to MS, such as immune and mitochondrial function, oxidative stress and the gut microbiota (Brown and Hu, 2001, Jangi et al., 2016, Bhargava and Calabresi, 2016, Bagur et al., 2017, Tremlett et al., 2017). As a result, dietary modification is emerging as a safe and interveneable approach to potentially modify disease course (Azary et al., 2017; Fitzgerald et al., 2017).
One aspect of diet that shows particular promise involves the timing and amount of caloric intake. Intermittent calorie restriction or intermittent fasting leads to a less aggressive disease course and has potential remyelinating properties in the mouse models of MS (Piccio et al., 2008, Choi et al., 2016). Recent studies of overweight healthy subjects have shown that an intermittent fasting diet in which subjects eat 500–600 calories on two days each week, with normal consumption on the other five days (5:2 diet) exhibit reductions in body weight and body fat, increased insulin sensitivity, and reductions in markers of oxidative stress and inflammation (Harvie et al., 2013, 2011). However, whether diets that incorporate fasting periods are feasible and beneficial in people with MS is unknown. Therefore, to assess the safety and feasibility of an intermittent fasting diet in people with MS, we conducted a pilot randomized controlled feeding study, denoted “Alternating the Timing and Amount of Calories in MS (ATAC-MS),” of two different types of calorie restriction (CR) diets (clinicaltrials.gov: NCT02647502) vs. placebo. Additional secondary outcomes explored the effects of CR diets on body weight and anthropometric characteristics as well as on patient-reported outcomes (PROs) including fatigue, sleep and mood.
2. Methods
2.1. Study population
Beginning in December 2015, people with MS aged 18 to 50 were recruited from the Johns Hopkins MS Center, which provides care to over 3000 people with MS annually. To be eligible, participants met the 2010 criteria for relapsing-remitting MS, had a new lesion or relapse in the past 2 years, had a disease duration ≤15 years, Expanded Disability Status Scale (EDSS)<6.0, were stable on an injectable MS therapy or not on any therapy, and were not planning to change vitamin D or thyroid medication for the next 48 weeks. Other eligibility criteria required participants to not smoke more than 1 cigarette/day (for at least 2 months), have a BMI of at least 23, maintain a stable weight (±8 lbs) for 3 months preceding the study and not be currently following a specialized diet for MS or other reasons. Exclusion criteria included a history of diabetes, eating disorders, kidney disease, warfarin use, major surgery (in previous 3 months), chemotherapy (in the past year), and pregnancy or breast feeding.
2.2. Study design
To determine energy needs precisely, at the study baseline, height and weight were recorded (used to calculate BMI), and all participants underwent indirect calorimetry. Each was randomized to 1 of 3 diets: a control diet (100% of calorie needs 7 days/week), a daily CR diet (78% of calorie needs 7 days/week) and intermittent CR (a “5:2” style diet; 25% of calorie needs 2 consecutive days/week; 100% of calorie needs 5 days/week). Weekly calorie deficits between the two CR diets were thus similar, and randomization was stratified by obesity status (BMI<30; BMI≥30). At study baseline and at week 8, body composition (lean, total and visceral fat) was determined using Dual X-ray Absorptiometry (DXA), and fasting glucose, triglycerides, and lipid levels were assessed using established clinical assays. At baseline and during weeks 4 and 8, participants also provided information onPROs including functional status (functional assessment of MS [FAMS]), fatigue (thinking/fatigue component of FAMS), mood (emotional well-being component of FAMS) and sleep quality (Pittsburgh Quality Sleep Index [PSQI]; at baseline and week 8). Visits during week 4 and 8 took place following a 25% intake day for individuals in the intermittent CR arm. All participants provided written consent at enrollment, and the study was approved by the Johns Hopkins School of Medicine Institutional Review Board.
2.3. Intervention
Prior to the initiation of the controlled feeding period, each participant completed three 24-h recalls (corresponding to 2 weekdays and 1 weekend day), which were collected and analyzed by a trained dietetic technician using Nutrition Data System for Research (NDSR) versions 2015–2016. The study diets were standardized to the US median for all macronutrient (carbohydrate, protein, and fat) content so as to avoid co-intervention due to substantial change in the diet components. For a period of 8 weeks, all meals were prepared by the US Department of Agriculture (USDA) Beltsville Human Nutrition Research Laboratory (Beltsville, MD) and shipped to participants’ homes twice per week. All meals were tailored to each individual’s specific calorie needs (determined by indirect calorimetry and by randomization status). Adherence to study diets was assessed via in-person 24-h recalls collected by study dieticians during study visits in weeks 4 and 8 (both following a 25% intake day for individuals in the intermittent CR arm).
2.4. Outcomes
The primary outcomes of the study were safety and feasibility of different types of CR diets in people with MS. Grade 3 events were specified as events which required hospitialization and limited the subject’s ability to care for himself or herself. We created a continuous measure of adherence as the difference between study-provided calorie intakes and participant reported intake collected during the 24-h recalls. Secondary outcomes included changes in weight, fasting glucose, lipid levels, and changes in PROs including fatigue, sleep and mood.
2.5. Statistical analysis
For the primary outcome, we assessed whether study adherence differed according to randomization status using mixed effects linear regression models to estimate average per-day difference in calorie intake between the diet provided by the study and what the person had actually consumed. For secondary outcomes, we applied a similar strategy assessing the effects of randomization to CR diets on changes in weight, BMI, body composition (both total and visceral fat mass and lean mass), fasting glucose and lipid levels and PROs, according to the intention to treat (ITT) principle. Further, as weight loss is associated with improvements in many PROs in the general population, (Alfaris et al., 2015, Rubin et al., 2013) in secondary, post hoc analyses, we also assessed whether there was an association between concomitant changes in anthropometric characteristics and in PROs. For these analyses, we calculated the correlation between the patient-specific rate of change in anthropometric characteristics and patient-specific rate of change in each PRO estimated from separate mixed-effects linear regression models.
3. Results
3.1. Characteristics of ATAC-MS study participants
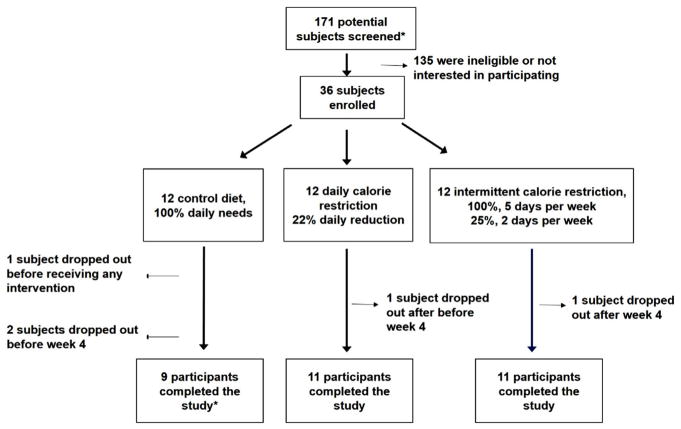
Baseline characteristics of all 36 enrolled subjects are provided in Table 1. At baseline, on average participants were aged 37.4 (standard deviation [SD]: 7.4), were 81% female, had an average BMI of 32.6 (SD: 7.8) kg/m2, and were 45.7% (SD: 7.5) body fat. At baselines, characteristics of study participants (with the exception of age) were generally comparable acros the three diets; control participants were on average slightly younger than those randomized to CR diets (P=0.04). The macronutrient composition of the trial diet was similar to what participants were consuming prior to enrollment, suggesting no average differences between pre-baseline diet and USDA-provided diets (Supplementary Fig. 1, all P>0.05). Of the 36 participants enrolled in the study, 31 (86%) completed the 8-week feeding portion of the study; three participants withdrew or were lost to follow-up in the control arm, one participant was lost in the intermittent CR arm, and one participant was lost in the daily CR arm (Fig. 1). These five indiviudals did not complete the feeding portion of the study and did not provide outcome data.
Table 1.
Baseline characteristics of ATAC-MS participants.
| Control | Calorie restriction
|
P-value* | ||
|---|---|---|---|---|
| Daily | Intermittent | |||
| N | 12 | 12 | 12 | |
| Mean age, years (SD) | 33.3 (7.0) | 40.5 (5.4) | 38.5 (7.4) | 0.04 |
| Male sex (%) | 3 (25.0) | 2 (16.7) | 2 (16.7) | 0.84 |
| Waist circumference, cm (SD) | 101.1 (16.6) | 104.3 (21.4) | 96.4 (10.9) | 0.52 |
| Body Composition | ||||
| Lean mass, kg (SD) | 45.2 (9.7) | 45.0 (11.2) | 44.3 (7.8) | 0.97 |
| Fat mass, kg (SD) | 39.4 (12.0) | 44.5 (18.2) | 38.8 (11.4) | 0.57 |
| Visceral adipose tissue, kg (SD) | 0.68 (0.24) | 0.94 (0.48) | 0.78 (0.32) | 0.25 |
| %Body fat | 44.9 (4.6) | 47.4 (7.8) | 44.8 (7.3) | 0.63 |
| %Lean mass | 52.2 (7.4) | 49.8 (7.4) | 52.2 (6.9) | 0.66 |
| %VAT of total body fat | 1.7 (0.3) | 2.1 (0.4) | 2.0 (0.6) | 0.18 |
| Fasting glucose, mg/dL (SD) | 76.8 (8.5) | 82.9 (13.0) | 80.6 (12.5) | 0.42 |
| Triglycerides | 94.6 (65.4) | 89.9 (39.2) | 113.6 (76.1) | 0.63 |
| Total cholesterol, mg/dL (SD) | 181.3 (27.8) | 177.2 (27.1) | 177.6 (30.4) | 0.93 |
| HDL cholesterol, mg/dL (SD) | 57.8 (8.8) | 56.3 (15.2) | 55.3 (18.1) | 0.91 |
| LDL cholesterol, mg/dL (SD) | 104.7 (28.5) | 103.0 (25.3) | 99.7 (22.6) | 0.90 |
| Patient Reported Outcomes | ||||
| FAMSa,b total (SD) | 150.2 (11.5) | 138.2 (20.2) | 147.5 (22.0) | 0.63 |
| FAMS – emotional well-being/depressiona,b, (SD) | 26.4 (2.4) | 24.3 (4.0) | 25.6 (2.2) | 0.22 |
| FAMS – thinking/fatiguea,b, (SD) | 26.8 (5.2) | 24.3 (6.9) | 23.3 (10.1) | 0.53 |
| PSQI* (SD) | 4.9 (3.4) | 6.8 (3.7) | 6.8 (3.2) | 0.38 |
PSQI: Pittsburgh Quality Sleep Index.
P-values derived from univariate tests evaluating statistical differences between randomization arms and given baseline characteristic.
FAMS – Functional assessment of Multiple Sclerosis.
For FAMS scores, higher scores are preferred as they denote better health status.
Fig. 1.
ATAC-MS participant flow.
*Following a pre-screen review of medical charts by study team members.
3.2. Adherence to study diets
A quantitative measure of adherence was defined as the per-day difference in calorie intake between the diet provided by the study and what the person had actually consumed (recalls were conducted after a 25% day for individuals randomized to intermittent CR). We noted significant differences in adherence between the diets (Fig. 2; P for any difference between the diets=0.03). On average, individuals randomized to intermittent CR consumed on average 92 additional kilocalories than what was provided by study diets whereas individuals randomized to daily CR or control diets consumed respective 344 and 201 fewer kilocalories than what was provided by the study diets (P for intermittent vs. daily=0.002; P for intermittent vs. control=0.05). No significant difference in adherence was observed between individuals randomized to daily CR relative to control diets.
Fig. 2.
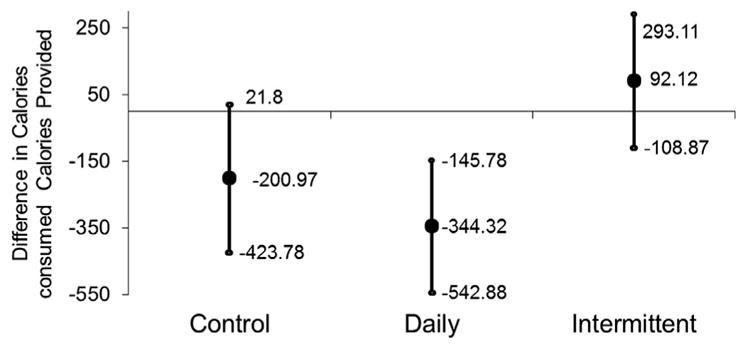
Adherence to study diets by randomization status.
Adherence to study diets was assessed by 24-h recalls collected during visits during weeks 4 and 8. For those in the intermittent calorie restriction arm, recalls during weeks 4 and 8 were completed following a 25% calorie intake day. Quantitative adherence was defined as the per-day difference in calorie intake between the diet provided by the study and what the person had actually consumed. We note a significant difference in mean adherence between intermittent vs. daily CR (P=0.002) and between intermittent vs. control (P=0.05). We observed no difference in mean adherence comparing daily vs. control (P=0.36).
3.3. Adverse effects and safety
We did not observe any adverse events of grade 3 or higher during the controlled feeding portion of the study. Of the 36 participants enrolled, 9 (25%) reported mild symptoms determined by an independent study monitor to be probably or certainly related to the provided diet, with hunger being the most common symptom experienced. Eight participants experienced symptoms determined to be “possibly” related to CR diets, with fatigue and headaches being the most common symptoms experienced (4/8 were randomized to intermittent CR; 2/8 were randomized to daily CR, 2/8 were randomized to control). A comprehensive metabolic panel was performed at baseline and at week 8 did not indicate any negative effects of either type of CR diet (Table 2).
Table 2.
Changes in anthropometric and lipid levels associated with randomization to calorie restriction diets.
| Control | Calorie restriction
|
P value
|
|||||
|---|---|---|---|---|---|---|---|
| Daily | Intermittent | Control vs. Daily CRa | Control vs. Intermittent CRb | Control vs. Either CRc | Daily vs. Intermittentd | ||
| Anthropometrice | |||||||
| Δ weight, kg/week | −0.17 (−0.34–0.00) | −0.53 (−0.69–−0.38) | −0.32 (−0.48–−0.17) | 0.003 | 0.21 | 0.02 | 0.15 |
| Δ BMI, kg/m2 per week | −0.05 (−0.11–0.01) | −0.19 (−0.24–−0.13) | −0.11 (−0.17–−0.06) | 0.003 | 0.15 | 0.01 | 0.15 |
| Δ waist circumference, cm/week | −0.18 (−0.67–0.31) | −0.24 (−0.70–0.21) | −0.20 (−0.64–0.23) | 0.86 | 0.94 | 0.89 | 0.41 |
| Δ hip circumferences, cm/week | −0.16 (−0.41–0.10) | −0.35 (−0.58–−0.11) | −0.48 (−0.71–0.25) | 0.07 | 0.30 | 0.09 | 0.41 |
| Δ Lean mass, g/week | −50.2 (−169.9–−50.2) | −144.6 (−253.0–−36.2) | −157.6 (−266.0–−49.3) | 0.26 | 0.21 | 0.17 | 0.96 |
| Δ Fat mass, g/week | −164.5 (−292.4–−36.6) | −330.1 (−445.8–−214.5) | −119.8 (−235.5–−4.2) | 0.07 | 0.62 | – | 0.03 |
| Δ VAT mass, g/week | 2.2 (−9.6–14.0) | −9.0 (−19.8–1.8) | −6.4 (−17.2–4.4) | 0.26 | 0.21 | 0.15 | 0.57 |
| %Δ in total body fat/week | −0.08 (−0.20–0.04) | −0.14 (−0.26–−0.03) | 0.00 (−0.12–0.10) | 0.52 | 0.37 | 0.88 | 0.12 |
| %Δ VAT of total fat/week | 0.01 (−0.01–0.03) | −0.005 (−0.02–0.01) | −0.01 (−0.03–0.01) | 0.17 | 0.06 | 0.05 | 0.77 |
| Fasting glucose mg/dL/week | 0.42 (−0.38–1.22) | −0.71 (−1.47–0.05) | −0.11 (−0.90–0.69) | 0.06 | 0.31 | 0.09 | 0.49 |
| Lipids | |||||||
| Δ triglycerides, mg/dL per week | −0.58 (−2.70–1.54) | −1.05 (−3.04–0.84) | −1.69 (−3.78–0.39) | 0.75 | 0.47 | 0.53 | 0.63 |
| Δ total cholesterol, mg/dL per week | −1.25 (−2.87–0.37) | −1.35 (−2.84–0.14) | −2.34 (−0.39–−3.90) | 0.93 | 0.35 | 0.55 | 0.60 |
| Δ HDL cholesterol, mg/dL per week | −0.46 (−1.15–0.22) | −0.61 (−1.24–0.04) | −0.72 (−1.39–−0.05) | 0.78 | 0.62 | 0.65 | 0.91 |
| Δ LDL cholesterol, mg/dL per week | −0.66 (−1.89–0.57) | −0.55 (−1.69–0.57) | −1.27 (−2.45–−0.09) | 0.91 | 0.49 | 0.74 | 0.52 |
Test for differential change between daily CR vs. control diet.
Test for differential change between intermittent CR vs. control diet.
Test for differential change between any CR relative to control diet.
Test for differential change between daily vs. intermittent CR relative to control.
Values displayed are rate of change per week over the 8-week study period.
3.4. Anthropometric and biomarker outcomes
Changes in anthropometric and lipid measures are provided in Table 2. Participants randomized to CR diets lost a median 3.4 kg (interquartile range [IQR]: −2.4, −4.0). Changes in weight did not differ significantly by type of CR diet at a rate of −0.43 kg/week (Table 2 and Fig. 3; 95% CI: −0.54 to −0.31). Individuals randomized to daily CR tended to lose more weight than individuals randomized to intermittent CR, though this difference was not significant (Fig. 3; P=0.15). Those randomized to daily CR lost a median 3.6 kg (IQR: −3.0, −4.1) occurring at a rate of −0.53 kg/week (95% CI: −0.69 to −0.38), while those randomized intermittent CR lost a median 3.0 kg (IQR: −1.95, −4.1) at a rate of −0.32 kg/week (95% CI: −0.45 to −0.17). CR diets were also associated with significant reductions in fat and lean mass, and daily CR diets were associated with a significantly greater rate of reduction in fat mass when compared with intermittent CR (daily CR: −330.1 g/week; 95% CI: −445.8 to −214.5; intermittent CR: −119.8 g/week; 95% CI: −235.5 to −4.4; P for interaction=0.01). On average, intermittent CR diets were associated with significant rates of decline in cholesterol levels; however, differences over time between either CR diet vs. control diets were not statistically different (Table 2).
Fig. 3.
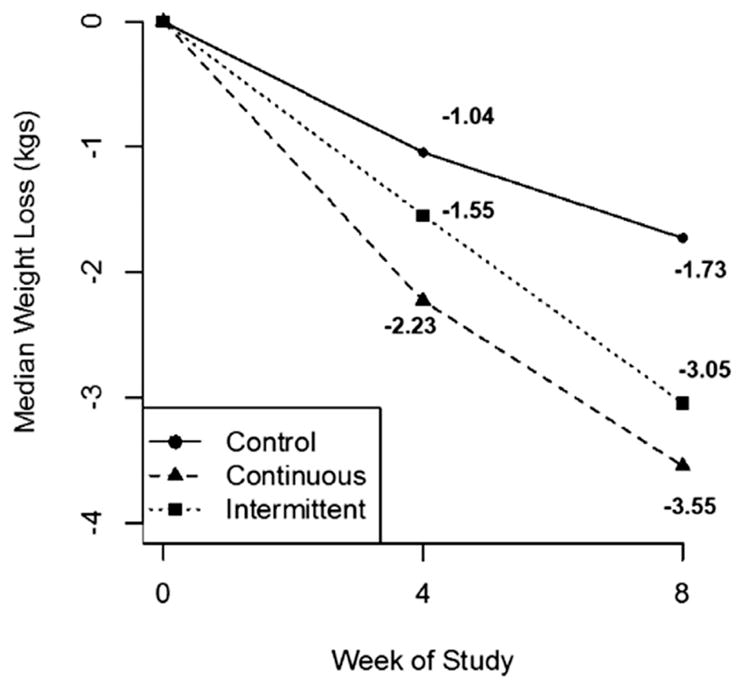
Changes in weight (kg) associated with randomization to calorie restriction diets.
3.5. Calorie restriction diets and patient-reported outcomes
In ITT analysis, randomization to either CR diets was not associated with improvements in overall FAMS scores relative to control; however, CR diets were associated with significant improvements in the emotional well-being/depression components of the FAMS score (Fig. 4; CR diets 0.21 point/week increase; 95% CI: 0.09 to 0.33; control diets −0.03; 95% CI: −0.21 to 0.16; P for difference in slope=0.04). Randomization to CR diets was not associated with changes in other PROs, including fatigue or sleep quality (all P>0.05). In post-hoc analyses, patient-specific rates of change in weight loss were also positively correlated with patient-specific rates of change in emotional well-being scores (Spearman r=0.39; P=0.03).
Fig. 4.
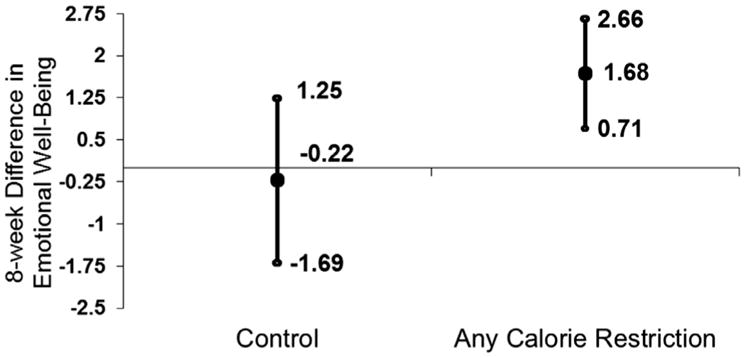
Change in emotional well-being/depression associated with randomization to calorie restriction diets.
4. Discussion
This randomized-controlled pilot feeding trial of different types of CR diets indicates that both intermittent and daily calorie restriction are unlikely to be harmful methods to reduce weight in people with MS, at least in the short term. The daily CR diet was associated with marginally greater weight loss than the intermittent CR diet. Differential adherence to study diets between intermittent vs. daily CR likely contributed to the observed difference in changes in anthropometric changes. Both CR diets were associated with trends toward improvements in cardiometabolic outcomes. With respect to PROs, we found that CR diets were associated with in improvements in emotional well-being.
In the mouse models of MS, intermittent fasting and “fasting mimicking diets” delay the onset of disease, lesser disease severity and decreases in markers of inflammation and relevant metabolic hormones, like leptin (Piccio et al., 2008, Choi et al., 2016). In humans, previous studies of fasting mimicking diets have predominantly concentrated on their effect on anthropometric and metabolic intermediates in healthy, but overweight adults. Results suggest favorable outcomes with respect to weight control and metabolic homeostasis for intermittent CR vs. daily CR (Harvie et al., 2013, Trepanowski et al., 2017, Hoddy et al., 2016, Barnosky et al., 2014). Follow-up analyses of ATAC-MS participants will assess the effects of different types of CR diets on relevant biologic intermediates like the metabolome (Bhargava and Calabresi, 2016) and the gut microbiota (Tremlett et al., 2017) as well as on immune and hormonal outcomes.
The results of our study assessing adherence are consistent with a large recent trial of alternate-day fasting vs. daily CR in 100 metabolically healthy but obese adults (Trepanowski et al., 2017). Specifically, the trial of a different fasting protocol tested whether daily CR (25% reduction in calories/day) vs. alternate-day fasting (2-day cycle of CR: 75% reduction in calories/day; 125% intake of calories/day) was associated with different anthropometric and cardiometabolic outcomes. After one year, change in weight did not differ significantly between the daily CR vs. alternate-day fasting (both were associated with a 5–6% decrease in body weight). Drop-out rates were also significantly higher in the alternate-day fasting group when compared with daily CR. While drop-out rates did not differ between CR arms in our study, adherence was lower in the intermittent CR arm relative to daily CR and suggests that drop-out could potentially differ following the conclusion of the controlled feeding portion of the study. Further studies may need to explore alternative forms of intermittent fasting and mechanisms to promote longer-term adherence to specific dietary recommendations in people with MS.
Several studies have suggested that in early MS, when physical disability is low, emotional health is one of the most burdensome symptoms for people with MS and may be associated with greater disability in the long term (Garg et al., 2016, Valet et al., 2017, Mowry et al., 2009). This study is promising in that even over a short period of time, randomization to calorie restriction diets was associated with a potential clinically meaningful improvement in emotional health; a clinically meaningful improvement in emotional well-being scores is a change in at least 0.97, as described in a much larger sample of people with MS (Mowry et al., 2009). Over the 8-week feeding period, ATAC-MS participants randomized to CR diets achieved an average improvement of 1.63 in emotional well-being scores, thus, suggesting a clinically meaningful improvement in emotional well-being. These results are consistent with a growing body of evidence implicating a role of diet in the etiology and severity of depression (Jacka et al., 2017) in the overall population as well in people with MS (Fitzgerald et al., 2017).
The current study has several limitations. It was short in duration, while sustained benefits of a dietary change in MS will require long term adoption thereof. Future studies assessing stragtegies to maximize adherence to dietary intervention trials are also important, as establishing efficacy is moot if participants are unable to follow such a diet in the long term. Generalizabiliity of the results may be reduced because the study included only relapsing-remitting patients on injectable MS therapies and because it is likely that the patients who are willing to participate in such a trial are different than those who are not. Although the study provided all food for all participants (to exact calorie levels), its remote design relies on participant self-report to track adherence, whereas in a more traditional feeding study in which participants consume all meals at the research center (uneaten foods can be registered on-site). However, the remote design is a practical necessity in order to feasibly recruit people with MS to participate in a feeding study (i.e., an effectiveness study). Finally, an important secondary outcome of our study was to quantify whether intermittent CR relative to daily CR has additional benefits beyond weight loss, as indicated by the laboratory studies. Because adherence was reduced in the intermittent CR when compared with the daily CR, residual confounding resulting from differential adherence patterns may bias comparisons between patient-reported outcome differences between the CR arms. Despite these limitations, our study is strengthened by its randomized and tightly controlled design. Further, our study was a controlled feeding study, and therefore, provided all meals for a period of 8-weeks, which has been shown to promote adherence to the specified foods (regardless of randomization arm) in several previous dietary intervention studies (Trepanowski et al., 2017, Hall and Most, 2005).
Our trial results suggest that even if there are no associated benefits related to traditional MS clinical or radiological outcomes, calorie restriction and weight loss represent a low-tech intervention for clinically relevant symptoms due to MS, such as emotional well-being, without adding meaningful risks or adverse outcomes. In follow-up studies, we are currently assessing the effects of long-term adherence to intermittent CR in this population. Future effectiveness studies are needed to evaluate how to help individuals follow dietary protocols without the aid of shipped and portioned foods for the long term and whether other potentially more amenable fasting-mimicking diet protocols are more easily adopted and have similar benefits with respect to anthropometric and patient-reported outcomes.
Supplementary Material
Acknowledgments
The authors would like to acknowledge advice and guidance provided the late Dr. John Milner of the United States Department of Agriculture in developing the design of the study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or National Institutes of Health (NIH). This work was performed while ALH was a full-time employee of Johns Hopkins University. This publication was made possible by the Johns Hopkins Institute for Clinical and Translational Research (ICTR) which is funded in part by Grant Number UL1 TR 001079 from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, NCATS or NIH. This study was supported in part by the Intramural Research Program of the National Institute on Aging.
Funding
This study was supported by a Harry Weaver Neuroscience Scholar Award from the National MS Society (NMSS) to Ellen M. Mowry. Kathryn Fitzgerald is supported by postdoctoral fellowships from the National MS Society and the Consortium of MS Centers. This study was also supported in part by the Intramural Research Program of the National Institute on Aging.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.msard.2018.05.002.
Disclosures
Dr. Fitzgerald has nothing to disclose.
Ms. Vizthum has nothing to disclose.
Ms. Henry-Barron has nothing to disclose.
Dr. Schweitzer has nothing to disclose.
Dr. Cassard has nothing to disclose.
Dr. Kossoff reports serving on scientific advisory boards for Atkins Nutritionals and for Nutricia, outside the submitted work
Dr. Hartman has nothing to disclose.
Dr. Kappogiannis has nothing to disclose
Dr. Mattson has nothing to disclose.
Mr. Sullivan has nothing to disclose.
Dr. Baer has nothing to disclose.
Dr. Appel reports that Healthways, Inc. developed the website for two weight loss interventions used in the POWER trial in collaboration with Johns Hopkins investigators and provided coaching effort for the transtelephonic intervention. Healthways also provided some research funding to supplement NIH support. Under an institutional consulting agreement with Healthways, the Johns Hopkins University received fees for advisory services to Healthways during the POWER trial. Faculty members who participated in the consulting services received a portion of the University fees. On the basis of POWER trial results, Healthways developed and is commercializing a weight-loss intervention program called Innergy. Under an agreement with Healthways, Johns Hopkins faculty monitor the Innergy program’s content and process (staffing, training, and counseling) and outcomes (engagement and weight loss ) to ensure consistency with the corresponding arm of the POWER Trial. Johns Hopkins receives fees for these services and faculty members who participate in the consulting services receive a portion of these fees. Johns Hopkins receives royalty on sales of the Innergy program.
Dr. Mowry reports receiving free glatiramer acetate for the investigator-initiated vitamin D trial, of which she is the PI from Teva Neuroscience provides. She is also the PI of investigator-initiated studies funded by Biogen, Sanofi-Genzyme. She is also a site investigator of trials sponsored by Sun Pharma, Biogen and royalties from Up-to-date.
References
- Alfaris N, et al. Effects of a 2-year behavioral weight loss intervention on sleep and mood in obese individuals treated in primary care practice. Obesity (Silver Spring) 2015;23:558–564. doi: 10.1002/oby.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azary S, et al. Contribution of dietary intake to relapse rate in early paediatric multiple sclerosis. J Neurol Neurosurg Psychiatry. 2017 doi: 10.1136/jnnp-2017-315936. [DOI] [PMC free article] [PubMed]
- Bagur MJ, et al. Influence of diet in multiple sclerosis: a systematic review. Adv Nutr. 2017;8:463–472. doi: 10.3945/an.116.014191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnosky AR, Hoddy KK, Unterman TG, Varady KA. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: a review of human findings. Transl Res. 2014;164:302–311. doi: 10.1016/j.trsl.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Bhargava P, Calabresi PA. Metabolomics in multiple sclerosis. Mult Scler. 2016;22:451–460. doi: 10.1177/1352458515622827. [DOI] [PubMed] [Google Scholar]
- Brown AA, Hu FB. Dietary modulation of endothelial function: implications for cardiovascular disease. Am J Clin Nutr. 2001;73:673–686. doi: 10.1093/ajcn/73.4.673. [DOI] [PubMed] [Google Scholar]
- Choi IY, et al. A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep. 2016;15:2136–2146. doi: 10.1016/j.celrep.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KC, et al. Diet quality is associated with disability and symptom severity in multiple sclerosis. Neurology. 2017 doi: 10.1212/WNL.0000000000004768. [DOI] [PubMed]
- Frohman EM, Racke MK, Raine CS. Multiple sclerosis–the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- Garg H, Bush S, Gappmaier E. Associations between fatigue and disability, functional mobility, depression, and quality of life in people with multiple sclerosis. Int J MS Care. 2016;18:71–77. doi: 10.7224/1537-2073.2015-013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DM, Most MM. Dietary adherence in well-controlled feeding studies. J Am Diet Assoc. 2005;105:1285–1288. doi: 10.1016/j.jada.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Harvie MN, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond), International journal of obesity, International journal of obesity (2005), International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2011;35:714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvie M, et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr. 2013;110:1534–1547. doi: 10.1017/S0007114513000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoddy KK, Bhutani S, Phillips SA, Varady KA. Effects of different degrees of insulin resistance on endothelial function in obese adults undergoing alternate day fasting. Nutr Healthy Aging. 2016;4:63–71. doi: 10.3233/NHA-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacka FN, et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial) BMC Med. 2017;15:23. doi: 10.1186/s12916-017-0791-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangi S, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7:12015. doi: 10.1038/ncomms12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie RA. Comorbidity in multiple sclerosis: implications for patient care. Nat Rev Neurol. 2017a;13:375–382. doi: 10.1038/nrneurol.2017.33. [DOI] [PubMed] [Google Scholar]
- Marrie RA. Comorbidity in multiple sclerosis: implications for patient care. Nat Rev Neurol. 2017b doi: 10.1038/nrneurol.2017.33. advance online publication. [DOI] [PubMed] [Google Scholar]
- Mowry EM, et al. Quality of life in multiple sclerosis is associated with lesion burden and brain volume measures. Neurology. 2009;72:1760–1765. doi: 10.1212/WNL.0b013e3181a609f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccio L, Stark JL, Cross AH. Chronic calorie restriction attenuates experimental autoimmune encephalomyelitis. J Leukoc Biol. 2008;84:940–948. doi: 10.1189/jlb.0208133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin RR, et al. Patient-reported outcomes in the practice-based opportunities for weight reduction (POWER) trial. Qual Life Res. 2013;22:2389–2398. doi: 10.1007/s11136-013-0363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremlett H, Bauer KC, Appel-Cresswell S, Finlay BB, Waubant E. The gut microbiome in human neurological disease: a review. Ann Neurol. 2017;81:369–382. doi: 10.1002/ana.24901. [DOI] [PubMed] [Google Scholar]
- Trepanowski JF, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177:930–938. doi: 10.1001/jamainternmed.2017.0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valet M, et al. Fatigue and physical fitness of mildly disabled persons with multiple sclerosis: a cross-sectional study. Int J Rehabil Res. 2017 doi: 10.1097/MRR.0000000000000238. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.