Abstract
A hallmark of the development of cancer is its ability to avoid detection and elimination by the immune system. There are many identified mechanisms of this immune evasion that can be measured both phenotypically and functionally. Functional studies directly show the ability of the tumor microenvironment to suppress immune responses, typically measured as lymphocyte proliferation, cytokine production or killing ability. While a direct measurement of function is ideal, these assays require ex vivo activation which may not accurately mimic in vivo conditions. Phenotypic assays can directly measure the distribution and activation of immune cell types rapidly after isolation, preserving the conditions present in the patient. While conventional flow cytometry is a rapid and well established assay, it currently allows for measurement of only 12–14 parameters. Mass spectrometry-based flow cytometry, or CyTOF, offers the ability to measure 3-fold more parameters than conventional optical-based modalities providing an advantage in depth of analysis that can be crucial for precious human samples. The goal of this report is to describe the system our group has developed to measure both the phenotype and function of immune cells in the bone marrow of patients with acute myeloid leukemia. We hope to explain our system in the context of previous studies aimed at measuring immune status in tumors and to inform the reader as to some experimental approaches our group has found useful in developing the basic data required to rationally pursue immune-based therapies for patients with cancer.
Keywords: Mass cytometry, AML, Microenvironment, Immunotherapy
1. Introduction
Immune evasion is intrinsic to the development of cancer and is also a mechanism that cancer cells utilize to avoid chemotherapeutic killing and propagate relapse (Hanahan and Weinberg, 2011; Son et al., 2017). Different mechanisms of immune evasion have been described in detail for solid tumors. These include expansion of suppressive cell types such as regulatory T cells (Tregs), M2 macrophages, and myeloid derived suppressor cells (MDSC), production of immunosuppressive cytokines, and alterations in the expression of stimulatory and inhibitory ligands, known as immune checkpoints. Any of these mechanisms may lead to the dysregulation and exhaustion of the immune cells normally responsible for tumor killing. Conversely, interventions aimed at blocking these pathways may support tumor clearance. Specifically, immune checkpoint inhibitors have shown remarkable efficacy in several solid and hematological malignancies (Topalian et al., 2012; Hodi et al., 2010; Ansell et al., 2015; Reck et al., 2016; Bellmunt et al., 2017; Nghiem et al., 2016; Swain et al., 2015). Measurement of the immune microenvironment (IME) of tumors holds the potential to rationally predict which type of immune-based therapy, or immunotherapy, may be efficacious in a specific tumor type. Because immunotherapies have been shown to be efficacious as a cancer treatment, there has been a push from both the clinical and pharmaceutical realms to initiate clinical trials as rapidly as possible. While exciting, this urgency may lead to a lack of mechanistic basis for the immune intervention being tested. Clinical trials with little or no pre-clinical rationale are destined to result in low response rates in tumors where only specific subset of patients may benefit. Poorly targeted trials risk low response rates or high rates of adverse effects that could negatively impact the field. Therefore, to design appropriate therapeutic interventions that target immune evasion, elucidating the IME of each cancer type is critical.
2. Mass cytometry to assess hematological malignancies
Since its development, the high parameter capabilities of mass spectrometry-based flow cytometry (mass cytometry) have been used to characterize the hematopoietic system (Bendall et al., 2011; Bendall et al., 2012; Newell et al., 2012; Whiting et al., 2015; Bandura et al., 2009; Nicholas et al., 2016). Defining the various subsets of developing and mature hematopoietic cell types requires the measurement of a large number of parameters due to the complex phenotypes of blood cells. For example, in order to identify hematopoietic stem cells in mice, a combination of 6 markers (e.g. Sca-1, c-KIT, CD34, FLT-3, CD48, CD150) plus a lineage marker “dump gate” of 5 more mature markers (e.g. CD11b, GR1, B220, CD3, TER119) are required. In order to identify myeloid and lymphoid precursors several more markers must be added to this combination (e.g. IL-7R, FcγR). In humans, a similar number of markers are needed to identify the same subsets (Chao et al., 2008; Kiel et al., 2005). Due to spectral overlap, this number of markers strains the capabilities of conventional fluorescence-based flow cytometry (commonly referred to as FACS, an acronym for “Florescence-Activated Cell Sorting”) and limits one’s ability to add additional phenotypic or functional parameters on top of cellular identification. An obstacle inherent to human research is the biologic heterogeneity between individuals. This difficulty is even more pronounced in the study of leukemia where many subclones may be present. These limitations make the expanded panels available in mass cytometry appealing for complex hematologic evaluations. Due to this advantage, several groups have used mass cytometry to assess the cellular and immune make up in patients with heme malignancies including lymphoma (Wogsland et al., 2017), multiple myeloma (Baughn et al., 2017), and CML (Gullaksen et al., 2017; Bandyopadhyay et al., 2017) (Table 1).
Table 1.
Publications using mass cytometry to study hematological malignancies.
| Author(s) (year) | Title | Summary of findings |
|---|---|---|
| Lakshmikanth et al. (2017) | Mass cytometry and topological data analysis reveal immune parameters associated with complications after allogeneic stem cell transplantation | This report identifies measurable parameters associated with complications post-allogeneic bone marrow transplant. |
| Baughn et al. (2017) | Phenotypic and functional characterization of a bortezomib-resistant multiple myeloma cell line by flow and mass cytometry | This study used mass cytometry to identify phenotypic changes in a cell line associated with drug resistance. |
| Bandyopadhyay et al. (2017) | Cholesterol esterification inhibition and imatinib treatment synergistically inhibit growth of BCR-ABL mutation-independent resistant chronic myelogenous leukemia | This paper used mass cytometry to identify the signaling pathway targeted in patient samples treated with two drugs. |
| Chretien et al. (2017) | Natural killer defective maturation is associated with adverse clinical outcome in patients with acute myeloid leukemia | Mass cytometry was used to identify specific subsets of NK cells associated with outcome in AML. |
| Gullaksen et al. (2017) | Single cell immune profiling by mass cytometry of newly diagnosed chronic phase chronic myeloid leukemia treated with nilotinib. | Mass cytometry was used to track ongoing changes in cell populations of CML patients receiving the TKI inhibitor nilotinib |
| Fisher et al. (2017) | Mass cytometry analysis reveals hyperactive NF Kappa B signaling in myelofibrosis and secondary acute myeloid leukemia | Myelofibrosis and secondary AML possess intracellular signaling phenotypes including the NFkB, MAP kinase, and PI3 kinase pathways, and JAK-STAT pathway exhibit constitutive signal activation and hypersensitivity to cytokine stimulation. |
| Wogsland et al. (2017) | Mass cytometry of follicular lymphoma tumors reveals intrinsic heterogeneity in proteins including HLA-DR and a deficit in nonmalignant plasmablast and germinal center B-cell populations | Use of mass cytometry to obtain deep profiling of cell subsets enabled identification of biologically important features, such as tumor heterogeneity and loss of nonmalignant B-cell subsets. |
| Saenz et al. (2017) | Novel BET protein proteolysis-targeting chimera exerts superior lethal activity than bromodomain inhibitor (BETi) against post-myeloproliferative neoplasm secondary (s)AML cells | In secondary AML cells utilizing mass cytometry coupled to SPADE algorithm clustering of the data, authors demonstrate treatment with ARV-825 (compared with OTX015), caused marked attenuation of BRD4, c-Myc and p-Rb, while inducing more p21 in the CD34 + stem/ progenitor cells. |
| Zhou et al. (2017) | Combined inhibition of β-catenin and Bcr-Abl synergistically targets tyrosine kinase inhibitor-resistant blast crisis chronic myeloid leukemia blasts and progenitors in vitro and in vivo | Combined inhibition of β-catenin and Bcr-Abl tyrosine kinase overcomes Bcr-Abl-dependent and -independent TKI resistance, targets BC-CML progenitors and BM niche components, and impairs engraftment potential of LSC. |
| Carter et al. (2016) | Combined targeting of BCL-2 and BCR-ABL tyrosine kinase eradicates chronic myeloid leukemia stem cells. | Mouse models of CML were treated with combined ABL inhibitor and Bcl-2 inhibitor. The authors used mass cytometry to track what cell type is the major target of inhibitor treatment. |
| Ferrell et al. (2016) | High-dimensional analysis of acute myeloid leukemia reveals phenotypic changes in persistent cells during induction therapy | Longitudinal assessment of the impact of treatment on the cellular milieu of the AML patient’s marrow and blood Using mass cytometry and computational analysis AML subpopulation dynamics in the early therapy response. Data indicated AML “persister” cells can become significantly less phenotypically stem-like immediately following treatment |
| Zeng et al. (2016) | MLN0128, a novel mTOR kinase inhibitor, disrupts survival signaling and triggers apoptosis in AML and AML stem/progenitor cells | Investigated mTOR inhibition on AML, using mass cytometry combined with SPADE and viSNE analyses to evaluate phenotypic heterogeneity of AML and AML stem/progenitor cells, and measure the altered intracellular molecules triggered by stimuli and inhibitors. Data indicate MLN0128 is a potent mTORCl/C2 inhibitor that selectively targeted the AKT/mTOR pathway in AML. |
| Romee et al. (2016) | Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia | Results indicate that memory-like NK cells are distinguishable from control NK cells from the same individual. IL-12, IL-15, and IL-18-induced memory-like NK cells exhibited enhanced triggering against AML regardless of KIR to KIR-ligand interactions, resulting in an expanded NK cell pool of AML-reactive effector cells. |
| Levine et al. (2015) | Data-driven phenotypic dissection of AML reveals progenitor-like cells that correlate with prognosis | Identified separate “functional” intracellular immaturity and surface phenotype in pediatric AML. The data shows that functional signaling immaturity correlates with poor outcome. |
| Hansmann et al. (2015) | Mass cytometry analysis shows that a novel memory phenotype B cell is expanded in multiple myeloma | Demonstrate high-dimensional cytometry data on the human immunologic landscape of peripheral blood cells across most of the known developmental stages of multiple myeloma (multiple myeloma, asymptomatic myeloma, MGUS, and healthy individuals). |
| Han et al. (2015) | Single-cell mass cytometry reveals intracellular survival/proliferative signaling in FLT3-ITD-mutated AML stem/progenitor cells | Using mass cytometry technique LSC surface markers and intracellular phosphoproteins in primary AML samples where characterized yielding multiple functional signaling pathways in antigen-defined subpopulations of AML. |
| Behbehani et al. (2015) | Mass cytometric functional profiling of acute myeloid leukemia defines cell-cycle and immunophenotypic properties that correlate with known responses to therapy | This study suggests that known chemotherapy sensitivities of common AML subsets are mediated by cell-cycle differences among LSCs and provides a basis for using in vivo functional characterization of AML cells to inform therapy selection. |
| Amir et al. (2013) | viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia | Evaluation of AML diagnosis and relapse samples supports that the cells first gain CD34 and subsequently acquire highly diverse abnormal combinations of lineage-specific markers without attenuation of CD34 |
Acute myeloid leukemia (AML) is the most common hematologic malignancy in adults with over 19,000 patients diagnosed each year. Only 25% of patients are cured of their disease at 5 years and this survival rate has plateaued despite intensive chemotherapeutic regimens (Deschler and Lubbert, 2006). This poor prognosis and the considerable side effects from conventional cytotoxic chemotherapies have created an impetus to explore novel treatments such as immunotherapies.
There have been several publications that have utilized mass cytometry to evaluate the heterogeneity and dynamic changes of the tumor phenotype in patients with AML, both before and after chemotherapy (Behbehani et al., 2015; Ferrell et al., 2016; Diggins et al., 2015). Mass cytometry has also been successfully utilized to investigate signaling pathways in AML tumor cells (Han et al., 2015; Fisher et al., 2017; Levine et al., 2015). For example, mass cytometry has been used to associate the functional maturity of AML cells to their surface phenotype and demonstrate that an “immature” signaling profile associates with poor outcome (Levine et al., 2015). An additional example of this type of analysis was recently reported by a group who was able to identify potential signaling pathway targets in patients with myelofibrosis and AML and overlay cell activation status into a hematopoietic map (Fisher et al., 2017). A similar strategy has been used to investigate the signaling impacts of mTOR and bromodomain inhibitors on leukemic stem cell populations in patients with AML (Zeng et al., 2016; Saenz et al., 2017). Despite these excellent investigations into AML cell phenotypes, there remain several questions regarding the role of the immune system in the development and relapse of AML in a large cohort of patients. What are the cell populations that make up the immune microenvironment in AML? Are T cells dysfunctional in AML? Is AML amenable to immune checkpoint inhibition? Our group set out to answer these questions by developing a comprehensive system that integrates mass cytometry with functional studies to define the IME and T-cell functional status in a large cohort of patients with AML. We present our data here as an example of how we have chosen to use mass cytometry to answer questions regarding the immune system in AML. The focus of this review is not from the perspective of a lab that developed techniques or innovates in the field of mass cytometry, but rather that of a general user navigating the issues that result from the large amounts of data generated by mass cytometry and how to integrate disparate data types.
3. Experimental design
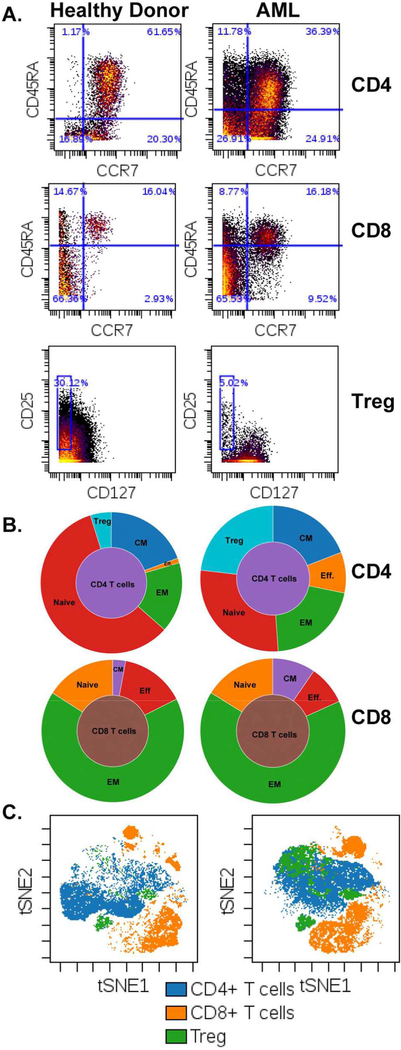
We developed a multifaceted study employing phenotypic and functional approaches to comprehensively examine the role of T-cell dysfunction in immune evasion and the development of AML. We have based our study design around mass cytometry as its ability for deep characterization is ideal to assess phenotypic characteristics that can serve as surrogates for mechanisms of T-cell dysfunction. These phenotypic characteristics include the activation profile, differentiation status, and checkpoint expression of T cells along with other associated lymphoid and myeloid immunosuppressive cell subsets. As an example, our group is interested in detailing the relative abundance of T cell subtypes resident in the bone marrow of patients with AML, specifically, naive and memory subtypes (identified by CD45RA and CCR7) along with the presence of Tregs. Tregs are critical for the initiation and maintenance of immune tolerance in both humans and mice (Josefowicz et al., 2012). It is hypothesized that Tregs are a central player in immune evasion in solid tumors and several studies have documented their presence and function in AML (Shenghui et al., 2011; Ustun et al., 2011; Szczepanski et al., 2009; Ersvaer et al., 2010; Kanakry et al., 2011). Tregs can be functionally identified based on their ability to suppress the proliferation of other T cells. Phenotypically, Tregs can be identified based on surface markers (i.e. CD3, CD4, CD25, CD 127) and/or by the presence of the nuclear transcription factor FOXP3 (Liu et al., 2006). The extended panels of mass cytometry are ideal for the identification of the complex phenotypes of T cells with several visualization tools that are available to aid in appreciating relative cellular proportions such as sunburst plots, clustering programs such as SPADE and methods that display high dimensional data in 2 parameter plots like viSNE (Diggins et al., 2015; Qiu et al., 2011) (Fig. 1).
Fig. 1.
An example of T cell differentiation Identification by mass cytometry. A. Plots Identifying T cell subtypes of a healthy donor (left) and patient with AML (right). Differentiation of CD4 T cells (top row) and CD8 T cells (middle row) can be further delineated into the following groups: Naïve (CCR7 + CD45RA +), effector (Eff CCR7 − CD45RA +), central memory (CM CCR7 − CD45RA +) and effector memory (CCR7 − CD45RA −). Treg (bottom row) gating scheme of CD4 T cells by expression of CD25 + CD127 −. B. Population Sunburst plots showing relative distributions of T cell subtypes based on gating shown In A (CD4 top. CD8 bottom). C. viSNE plots showing populations of CD4 and CD8 T cells and Tregs. All plots generated in Cytobank.
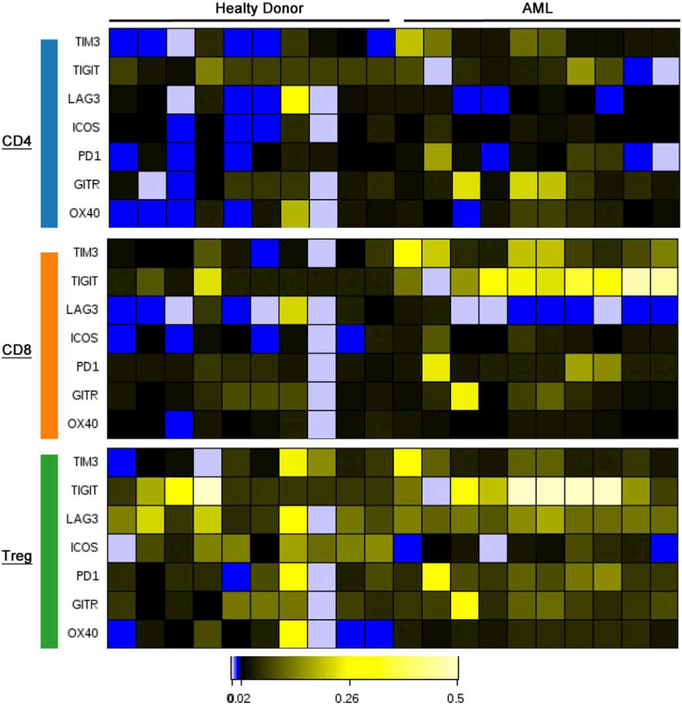
Mass cytometry is a powerful tool to profile a series of multiple markers across various cell types. We take advantage of this ability by characterizing the immune checkpoint receptors and their ligands, which play a role in immune evasion. These molecules are typically expressed in response to inflammatory stimuli as a normal negative feedback loop to limit T-cell activation during inflammation and maintain peripheral tolerance. Studies have shown that some cancers may be able to upregulate ligands that engage checkpoint receptors on T cells as a mechanism to inactivate T cells and lead to tumor escape. By targeting the immune checkpoint complex with blocking antibodies, the inhibitory signals can be disrupted, allowing T cells to perform their normal function. While CTLA-4 and PD-1 are the two most well-described checkpoint receptors, there are numerous others including TIM-3, VISTA, LAG3, BTLA. Given the heterogeneity of expression of these receptors and their ligands, the comprehensive panels available with mass cytometry are ideal for detailed single cell characterization (Fig. 2).
Fig. 2.
Demonstration of immune checkpoints and activation markers distributed over 10 healthy (left) and 10 AML (right) bone marrow samples. Heat maps are distributed by first gating on CD4 (blue), CD8 (orange), and Treg (green) populations. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.1. Antibody panel development
Commercially designed and manufactured panels are available for use but our group chose to design four unique panels tailored to our project’s specific goals. Each panel contains specific metal-conjugated antibodies as well as a viability stain (i.e. cisplatin or rhodium) to distinguish live from dead cells and a DNA intercalator (i.e. iridium) to identify cells and separate singlets from doublets.
In addition to commercially available panels, individual metal-conjugated antibodies are available for hundreds of different human and mouse markers. As mass cytometry use becomes more widespread, the list of markers available for purchase should continue to rapidly expand. Similar to FACS, there are several benefits to choosing commercially available antibodies. Specifically, these include quality assessments to determine the appropriate concentration and specificity by validating with control cell lines. It is not always possible to find markers that are commercially available and/or conjugated to the desired metal isotope. Custom conjugation kits and services are available and easy to use.
While mass cytometry allows for measurement of more parameters than FACS, multiple panels may still be needed to answer a particular question. Our first panel was designed to identify T cells and their major subtypes (naive, effector memory, central memory, effector, and Tregs) (Table 2). Our second panel was designed to further characterize the functional capacity of T cells by intracellular staining for several cytokines after overnight stimulation with anti-CD3 followed by inhibition of Golgi protein transport with brefeldin A. Our third panel is focused on myeloid cells and their major subsets (granulocytes, M1/M2 macrophages, monocytes, and MDSCs) along with the leukemic blast cells (Table 3). Additional markers were included in each panel to characterize the activation status of the cells of interest and quantify the surface density of immunologically relevant receptors and their ligands.
Table 2.
Mass cytometry panel designed to focus on T-cell phenotypes in human bone marrow samples.
| Marker | Common | Clone | Metal isotope |
Purpose |
|---|---|---|---|---|
| CD45 | LCA | HI30 | 141Pr | Hematopoietic marker |
| CD19 | HIB19 | 142Nd | B-cell marker | |
| CD 127 | IL-7R | A019D5 | 143Nd | T-cell subtyping |
| CD38 | ADPRC1 | ΗΓΤ2 | 144Nd | Myeloid marker |
| CD 4 | RPA-T4 | 145Nd | T-cell marker | |
| CD 8 | RPA-T8 | 146Nd | T-cell marker | |
| CD11c | Integrin | Bu 15 | 147Sm | Dendritic cell marker |
| CD16 | FcgRIII | 3G8 | 148Nd | Low affinity Fc receptor |
| CD25 | IL-2R | 2A3 | 149Sm | Tregulatory cell and activation marker |
| CD223 | LAG3 | 874501 | 150Nd | Checkpoint receptor |
| CD278 | ICOS | C398.4A | 151Eu | Activation marker |
| CD66b | 80H3 | 152Sm | Granulocyte marker | |
| CD45RA | HI100 | 153Eu | T-cell subtype | |
| TIM 3 | F38–2E2 | 154Sm | Checkpoint receptor | |
| CD27 | L128 | 155Gd | T-cell activation marker | |
| CD14 | HCD14 | 156Gd | Monocyte marker | |
| CD 134 | 0×40 | ACT35 | 158Gd | Checkpoint receptor |
| CD357 | GITR | 621 | 159Tb | T-cell activation marker |
| CD28 | CD28.2 | 159Tb | Activation marker | |
| CD 152 | CTLA4 | 14D3 | 161Dy | Checkpoint receptor |
| FoxP3 | 259D/C7 | 162Dy | T-regulatory cells | |
| CD272 | BTLA | MIH26 | 163Dy | Checkpoint receptor |
| CD 185 | CXCR5 | 51505 | 164Dy | T-follicular helper cells |
| CD40 | 5C3 | 165Ho | APC costimulatory protein | |
| CD44 | BJ18 | 166Er | T-cell activation marker | |
| CD 197 | CCR7 | G043H7 | 167Er | T-cell subtype |
| Ki-67 | Ki-67 | 168Er | Proliferation marker | |
| CD33 | WM53 | 169Tm | Myeloid marker | |
| CD 3 | UCHT1 | 170Er | T-cell marker | |
| CD20 | 2H7 | 171Yb | B-cell marker | |
| HLA-DR | MHCII | L243 | 173Yb | MHC class II receptor |
| TIGIT | MBSA43 | 174Yb | Checkpoint receptor | |
| CD279 | PD-1 | EH12.2H7 | 175Lu | Checkpoint receptor |
| CD56 | R19–760 | 176Yb | NK marker |
Table 3.
Mass cytometry panel designed to detect both normal and leukemic myeloid cell populations in the bone marrow of patients with AML.
| Marker | Common Name |
Clone | Metal isotope |
Purpose |
|---|---|---|---|---|
| CD45 | LCA | HI30 | 141Pr | Hematopoietic marker |
| CD19 | HIB19 | 142Nd | B-cell marker | |
| CD117 | cKIT | 104D2 | 143Nd | Immature marker |
| CD38 | ADPRC1 | HIT2 | 144Nd | Myeloid marker |
| CD4 | RPA-T4 | 145Nd | T-cell marker | |
| CD8 | RPA-T8 | 146Nd | T-cell marker | |
| CD11c | Integrin | Bu 15 | 147Sm | Dendritic cell marker |
| CD16 | FcgRIII | 3G8 | 148Nd | Low affinity Fc receptor |
| CD34 | 581 | 149Sm | Stem cell marker | |
| CD86 | B7–2 | IΤ2.2 | 150Nd | T-cell costimulatory marker |
| CD123 | IL-3R | 6H6 | 151Eu | Myeloid marker |
| CD66b | 80H3 | 152Sm | Granulocyte marker | |
| TIM3 | F38–2E2 | 153Eu | Checkpoint receptor | |
| CD163 | GHI/61 | 154Sm | M2 macrophage marker | |
| CD14 | HCD14 | 156Gd | Monocyte marker | |
| CD135 | FLT3 | BV10A4H2 | 158Gd | Myeloid marker |
| CD115 | CSF1R | 9–4D2–1E4 | 159Tb | Myeloid marker |
| CD13 | WM15 | 159Tb | Myeloid marker | |
| CD80 | B7–1 | 2D10.4 | 162Dy | T-cell costimulatory marker |
| TGF | 658922 | 163Dy | Immunosuppressive cytokine |
|
| Arginase | MHN2–25 | 164Dy | Immunosuppressive enzyme |
|
| Notch2 | JES3–9D7 | 165Ho | Cell fate molecule | |
| IL-10 | ICRF44 | 166Er | Immunosuppressive cytokine |
|
| CD11b | 18/P-Stat6 | 167Er | Myeloid marker | |
| pStat6 | 2D10.4 | 168Er | Signaling molecule | |
| CD33 | WM53 | 169Tm | Myeloid marker | |
| CD3 | UCHT1 | 170Er | T-cell marker | |
| CD20 | 2H7 | 171Yb | B-cell marker | |
| CD15 | W6D3 | 172Yb | Myeloid marker | |
| HLA-DR | L243 | 173Yb | MHC class II receptor | |
| CD274 | PD-L1 | 29E.2A3 | 175Lu | Checkpoint ligand |
| CD56 | R19–760 | 176Yb | NK marker |
Stromal components in the bone marrow are critical for normal hematopoietic stem cell function (Morrison and Scadden, 2014). In recent years, a large body of literature has developed showing that the non-hematopoietic stromal components of tumors have a major impact on tumor development and survival (Turley et al., 2015; Hanahan and Coussens, 2012). The contribution of stromal components has been well-studied in leukemia where they appear to be important for leukemic cell proliferation, survival and chemotherapeutic resistance (Tabe and Konopleva, 2014). For these reasons, we designed a fourth panel to identify stromal components present in the bone marrow of patients with AML. Staining with this panel was initially performed on the fresh bone marrow aspirates. However, due to the low recovery of stromal cells, our group is in the process of utilizing cultured stromal cells isolated from patient bone marrow samples.
When creating unique panels, it is critical to plan markers and their paired metal isotopes in advance for several reasons. Background noise, isotope impurity, and signal detection optimization are all technical issues that can arise during development and lead to inaccurate results. Experts and online tools are available to prevent and/or reduce these issues (https://www.fluidigm.com/productsupport/cytof-helios). We begin our panel design by identifying which markers are commercially available. These markers serve as a framework that can be filled in with custom conjugated markers. For those that are not commercially available we then either have them conjugated and tested by Fluidigm or conjugate and test ourselves. Optimization and troubleshooting of antibody metal conjugates is similar to the procedures used for conventional flow cytometry antibodies. After conjugation antibodies should be tested on samples where expression of the target is known. Ideally, new conjugates should be tested on biological tissues with expression levels similar to those to be stained in the actual experiments as this will yield realistic estimation of staining intensity. If staining intensity is low, rare or unknown we will often use AML cell lines with known expression of the antigen of interest. Finally, for antigens where the expression pattern in actual patient tissues or cell lines is unknown we have used expression via mammalian expression plasmids to determine a positive signal. More details of issues to be considered around panel design have been published and should be referred to when starting to design a new panel (Leipold et al., 2015).
3.2. Functional correlates and mechanistic studies
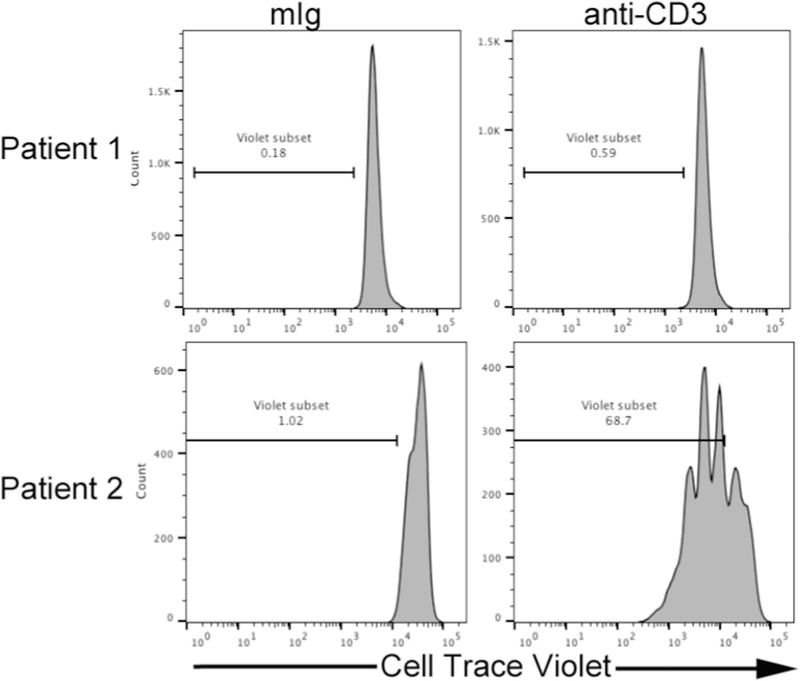
A hallmark of tumor-mediated suppression of adaptive immune responses is impaired proliferative and cytokine production capacity by T cells. We have developed an ex vivo system to test these functional parameters. For our functional assays, total bone marrow mononuclear cells are labeled with Cell Trace Violet (Life Technologies). The labeled cells are then incubated in anti-CD3-coated plates. We intentionally leave out CD28 agonistic antibodies, which are common in T cell proliferation assays, so that the cumulative co-stimulatory or co-inhibitory signals are supplied by the surrounding tumor cells. For our assays, cells are harvested after 5 days of culture and the T-cell proliferative capacity is assessed by FACS. At the time of harvest, the supernatant from the culture is removed and frozen. A multiplex bead-based assay is performed on these supernatants to measure the presence of various cytokines. These cytokines help profile the skewing of T-cell phenotypes in the context of the IME. To further define the mechanism behind any immune suppression, these same assays are performed in the presence of immune checkpoint inhibitors (e.g. antibodies against PD-1, CTLA-4).
Our preliminary results suggest that samples can be subcategorized based on the proliferative capacity of their T cells (Fig. 3). Samples in which T cells have reduced proliferation and or cytokine production may represent immune dysfunction. We designed our study with the goal of correlating the phenotypic data generated from mass cytometry with the functional data. Mechanisms that can be identified by mass cytometry and may correlate with our functional findings include T-cell exhaustion, skewed T-cell differentiation, checkpoint expression or increased immunosuppressive subsets. Analyses to establish these correlates are currently underway.
Fig. 3.
Functional measurement of AML bone marrow T cells showing proliferation of CD3-gated cells after 5 days of culture via dilution of Cell Trace Violet. Histograms represent Individual AML bone marrow samples with T cells that are non-responsive (top row) or responsive to anti-CD3 stimulation (bottom row).
3.3. Strategies to overcome obstacles related to evaluating hematologic malignancies with mass cytometry
Many mass cytometry studies of patient samples (peripheral blood or bone marrow) successfully utilize protocols that yield good results from viably frozen cell samples. A well-developed method for studying AML cells by mass cytometry on frozen samples is available from Zeng et al. (2017). While assessing banked frozen patient samples with mass cytometry permits greater convenience with regard to planning and batching experiments, the focus on fresh cells likely provides a more accurate snapshot of the in vivo biology being measured. Beyond reduced cell recovery, studies have shown that assessment of frozen samples via cytometry leads to variable cell subset representation based on surface marker expression (Lemieux et al., 2016). Improved cell recovery from frozen samples can be achieved by optimal sample preparation (Gaudilliere et al., 2014).
Cell numbers can remain an issue even with fresh samples due to variable and unpredictable sample procurement. Our group has optimized procedures using a first generation mass cytometer and has found cell recovery is one of the biggest limitations. Using mass cytometry, other researchers have found cell numbers as low as 10,000 are sufficient to identify the representative distribution of plentiful cells (Yao et al., 2014). While it is possible to identify highly representative cell phenotypes in small samples, the reality remains that rare cell populations of interest require a large number of input events for valid interpretation. This can be an issue when it comes to a limited resource such as the human bone marrow that we study.
Up to 70% of cells can be lost during data acquisition in a first generation mass cytometry instrument due to the imprecise nature of nebulizer sample spray patterns. Taking this loss into account, our protocol calls for a starting cell count of 3 × 106 per panel. Helios instruments have claimed improved efficiency up to 60%. These numbers may be further improved by using third party fluidics upgrades. An additional strategy that can be implemented to increase cell recovery is designing panels that are limited to surface markers and consolidating intracellular or intra-nuclear markers to a single panel, similar to our T-cell cytokine panel. Staining protocols for these latter conditions require harsher buffers and additional washes that can further reduce cell recovery.
An additional obstacle created by restricting our study to fresh samples is the logistical issues involved in clinical acquisition, especially over long-term projects. Staining and running samples independently over a multi-year project provides the advantage of capturing the biology more closely associated with each individual sample but it also leads to the potential for increased variability in staining and acquisition. Studies involving banked samples can overcome some of this variability by using barcoding technology. Using mixes of palladium isotopes or cisplatin, up to 20 samples can be stained and analyzed together in one tube and the individual identity of each sample can be retrieved during data analysis (Zunder et al., 2015; McCarthy et al., 2017).
Signal intensity of mass cytometry instruments are variable over long runs of analysis. This can lead to difficulties in comparisons over long-term studies. Calibration beads are included with each sample to allow for normalization during runs (Finck et al., 2013). For another level of quality control (QC), we have a banked sample obtained via leukapheresis with a defined immunophenotype that is stained with a limited panel (i.e. CD45, CD3, CD4) and serves as a standard to compensate for further machine drift over multi-year projects. We chose our three markers based on high (CD3), intermediate (CD3), and low (CD45) staining intensity of our banked sample. This permits measurement of variability across staining intensity ranges. Limiting our control sample stain to 3 markers was purely an economic decision, in cases where more markers are economically feasible it would be preferable to include as many as possible. Finally, during panel development, our group utilized a strategy used in FACS and incorporated a core group of 14 well-established phenotypic markers in each panel. This allows for identification of basic cell types within each panel and serves as an additional QC for stains between panels.
3.4. Computational strategies to ensure rigor and reproducibility
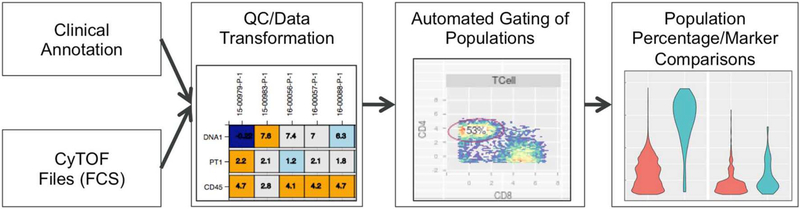
Critical factors that must be considered in the analysis of mass cytometry data include: 1) management of multiple panels, 2) collection protocol, 3) disease heterogeneity, which contributes to technical variability, and 4) reproducibility in gating. To this end, we have developed a metadata-centric pipeline that merges both information from FCS files and patient-based clinical annotation into a single file manifest. Based on this manifest, files can be batch-processed by panel type, and files that do not pass QC procedures can be flagged at each step of analysis. The pipeline currently handles panel reconciliation, scale transformation, quality control, automatic gating, and visualization of both fluorescence and percentage within populations of interest (Fig. 4). Where possible, we have implemented best practices that have been suggested by FlowCAP (Aghaeepour et al., 2016; Aghaeepour et al., 2013). Each step is visualized and each visualization can be faceted by clinical annotation to allow for exploration of questions based on the clinical annotation. We leverage existing Bioconductor packages for analysis, utilizing the flowCore (for loading FCS Files), flowWork-space/openCyto (for developing automated gating schema), and ncdfFlow (for on-disk processing of FCS files) packages (Finak et al., 2014; Hahne et al., 2009; Finak et al., 2016).
Fig. 4.
Mass cytometry pipeline. FCS files are processed, visualized and associated with clinical annotation. Each step of analysis (QC/data transformation, automated gating using the openCyto pipeline, and comparison of population percentages/marker expression) is visualized and assessed for potential impact on analysis.
With respect to QC, we initially assess staining variability by visually comparing staining for a marker within a panel for the entire set of samples. Violin plots are used because they allow for rapid visual comparison of distributions across many samples (Hintze and Nelson, 1998). By comparing MFIs using a z-score, samples with abnormal staining can be flagged and identified early in the pipeline. Additionally, we compare the core marker expression across panels to assess potential intra-patient staining variability.
As previously mentioned, our experimental design requires fresh patient samples to be run immediately upon collection. For this reason, standardization methods such as barcoding cannot be used, as they require multiple samples to be mixed and barcoded, which is not possible with this experimental protocol. Additionally, because manual gating is highly subjective, we elected to utilize automated gating algorithms to identify cellular populations in our cohort. Automated gating is done leveraging the openCyto framework (Finak et al., 2014). Automated gating has been shown to be dependable and reproducible through a number of flow Cap challenges (https://www.ncbi.nlm.nih.gov/pubmed/23396282, https://www.ncbi.nlm.nih.gov/pubmed/25755118, https://www.ncbi.nlm.nih.gov/pubmed/26447924, https://www.ncbi.nlm.nih.gov/pubmed/26861911). We used several auto-gating algorithms including flowClust (for singlet gating), mindensity (for 1 dimensional +/−), tailgate (for tails of populations), and flowDensity (for finding quadrant boundaries) for the gating scheme. The output of all algorithms was confirmed visually to ensure that they were appropriate. Due to inter-patient heterogeneity in AML, some subpopulations may not be present in each patient sample. To this end, we have incorporated error handling into the openCyto package, which allows for conditional gating of child populations when the parent population is not found. Selection of autogating algorithms and their associated parameters in openCyto requires multiple iterations of testing and visual comparison of the automatic gates. We compare our autogated populations with manual expert gating in order to assess the suitability of our parameters as an additional QC step.
Once populations are identified, we explore novel markers within a population to identify new phenotypes that exist within a subpopulation. Due to technical variability, we need to estimate positive and negative populations within a marker by automated gating on 1-channel histograms within a patient sample. Within a cell population, such as T cells, we examine the presence of subtypes.
One drawback to using mass cytometry rather than FACS is that side scatter is not available to identify blast cells. To this end, we have developed a simple discrimination method using t-SNE to compare AML patient samples with our set of healthy donor (HD) samples. For each HD sample, we sample 5% from each FCS file and concatenate these sampled rows to the AML sample in question. Graphed t-SNE is then run on this concatenated file. HD and normal immunologic cells from the AML samples group together, allowing for identification of the AML cells by drawing a contour gate around the HD and normal immunologic cells.
4. Conclusion
Ex vivo research on rare human leukemia samples can provide valuable insight into the pathogenesis of the disease and potential therapeutic targets. Integrating functional studies with deep immune profiling gives a broad insight into the status of IME in patients with hematological malignancies. The ability to utilize the expanded panels available in mass cytometry is a strength over FACS that has led to our group successfully adopting this modality as our primary means of phenotypic analysis. We believe our strategy is generalizable to other hematological malignancies and we have begun applying the same techniques to acute lymphoblastic leukemia and chronic myelogenous leukemia. The pipeline, including autogating parameters and transformation scripts, as well as our protocols will be freely disseminated for others to use.
Acknowledgments
The authors would like to thank the OHSU Flow Cytometry Shared Resource Directed by Philip Streeter PhD and directed by Miranda Gilchrist for support with instrumentation.
Footnotes
Conflict of interest
The authors have no conflict of interest to report.
References
- Aghaeepour N, et al. , 2013. Critical assessment of automated flow cytometry data analysis techniques. Nat. Methods 10, 228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghaeepour N, et al. , 2016. A benchmark for evaluation of algorithms for identification of cellular correlates of clinical outcomes. Cytometry A 89, 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir el-A.D., et al. , 2013. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat. Biotechnol 31, 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell SM, et al. , 2015. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med 372, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura DR, et al. , 2009. Mass cytometry: technique for real time single cell multi target immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Anal. Chem 81, 6813–6822. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, et al. , 2017. Cholesterol esterification inhibition and imatinib treatment synergistically inhibit growth of BCR-ABL mutation-independent resistant chronic myelogenous leukemia. PLoS One 12, e0179558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughn LB, et al. , 2017. Phenotypic and functional characterization of a bortezomib-resistant multiple myeloma cell line by flow and mass cytometry. Leuk. Lymphoma 58, 1931–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behbehani GK, et al. , 2015. Mass cytometric functional profiling of acute myeloid leukemia defines cell-cycle and immunophenotypic properties that correlate with known responses to therapy. Cancer Discov. 5, 988–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellmunt J, et al. , 2017. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med 376, 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall SC, et al. , 2011. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332, 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall SC, Nolan GP, Roederer M, Chattopadhyay PK, 2012. A deep profiler’s guide to cytometry. Trends Immunol. 33, 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BZ, et al. , 2016. Combined targeting of BCL-2 and BCR-ABL tyrosine kinase eradicates chronic myeloid leukemia stem cells. Sci. Transl. Med 8, 355ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MP, Seita J, Weissman IL, 2008. Establishment of a normal hematopoietic and leukemia stem cell hierarchy. Cold Spring Harb. Symp. Quant. Biol 73, 439–449. [DOI] [PubMed] [Google Scholar]
- Chretien AS, et al. , 2017. Natural killer defective maturation is associated with adverse clinical outcome in patients with acute myeloid leukemia. Front. Immunol 8, 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschler B, Lubbert M, 2006. Acute myeloid leukemia: epidemiology and etiology. Cancer 107, 2099–2107. [DOI] [PubMed] [Google Scholar]
- Diggins KE, Ferrell PB Jr., Irish JM, 2015. Methods for discovery and characterization of cell subsets in high dimensional mass cytometry data. Methods 82, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersvaer E, Liseth K, Skavland J, Gjertsen BT, Bruserud O, 2010. Intensive chemotherapy for acute myeloid leukemia differentially affects circulating TCI, TH1, TH17 and TREG cells. BMC Immunol. 11, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell PB Jr., et al. , 2016. High-dimensional analysis of acute myeloid leukemia reveals phenotypic changes in persistent cells during induction therapy. PLoS One 11, e0153207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finak G, et al. , 2014. OpenCyto: an open source infrastructure for scalable, robust, reproducible, and automated, end-to-end flow cytometry data analysis. PLoS Comput. Biol 10, el003806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finak G, et al. , 2016. Standardizing flow cytometry immunophenotyping analysis from the human immunophenotyping consortium. Sci. Rep 6, 20686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck R, et al. , 2013. Normalization of mass cytometry data with bead standards. Cytometry A 83, 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DA, et al. , 2017. Mass cytometry analysis reveals hyperactive NF Kappa B signaling in myelofibrosis and secondary acute myeloid leukemia. Leukemia 31, 1962–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudilliere B, et al. , 2014. Clinical recovery from surgery correlates with single-cell immune signatures. Sci. Transl. Med 6, 255ra131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullaksen SE, et al. , 2017. Single cell immune profiling by mass cytometry of newly diagnosed chronic phase chronic myeloid leukemia treated with nilotinib. Haematologica 102, 1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahne F, et al. , 2009. flowCore: a Bioconductor package for high throughput flow cytometry. BMC Bioinf. 10, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, et al. , 2015. Single-cell mass cytometry reveals intracellular survival/proliferative signaling in FLT3-ITD-mutated AML stem/progenitor cells. Cytometry A 87, 346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Coussens LM, 2012. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA, 2011. Hallmarks of cancer: the next generation. Cell 144, 646–674. [DOI] [PubMed] [Google Scholar]
- Hansmann L, et al. , 2015. Mass cytometry analysis shows that a novel memory phenotype B cell is expanded in multiple myeloma. Cancer Immunol. Res 3, 650–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintze JL, Nelson RD, 1998. Violin plots: a box plot-density trace synergism. Am. Stat 52, 181–184. [Google Scholar]
- Hodi FS, et al. , 2010. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med 363, 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu LF, Rudensky AY, 2012. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol 30, 531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanakry CG, et al. , 2011. Early lymphocyte recovery after intensive timed sequential chemotherapy for acute myelogenous leukemia: peripheral oligoclonal expansion of regulatory T cells. Blood 117, 608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, et al. , 2005. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121. [DOI] [PubMed] [Google Scholar]
- Lakshmikanth T, et al. , 2017. Mass cytometry and topological data analysis reveal immune parameters associated with complications after allogeneic stem cell transplantation. Cell Rep. 20, 2238–2250. [DOI] [PubMed] [Google Scholar]
- Leipold MD, Newell EW, Maecker HT, 2015. Multiparameter phenotyping of human PBMCs using mass cytometry. Methods Mol. Biol 1343, 81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux J, Jobin C, Simard C, Neron S, 2016. A global look into human T cell subsets before and after cryopreservation using multiparametric flow cytometry and two-dimensional visualization analysis. J. Immunol. Methods 434, 73–82. [DOI] [PubMed] [Google Scholar]
- Levine JH, et al. , 2015. Data-driven phenotypic dissection of AML reveals progenitor-like cells that correlate with prognosis. Cell 162, 184–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, et al. , 2006. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4 -I-T reg cells. J. Exp. Med 203, 1701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy RL, Mak DH, Burks JK, Barton MC, 2017. Rapid monoisotopic cisplatin based barcoding for multiplexed mass cytometry. Sci. Rep 7, 3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Scadden DT, 2014. The bone marrow niche for haematopoietic stem cells. Nature 505, 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell EW, Sigal N, Bendall SC, Nolan GP, Davis MM, 2012. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity 36, 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem PT, et al. , 2016. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N. Engl. J. Med 374, 2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas KJ, et al. , 2016. Multiparameter analysis of stimulated human peripheral blood mononuclear cells: a comparison of mass and fluorescence cytometry. Cytometry A 89, 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu P, et al. , 2011. Extracting a cellular hierarchy from high-dimensional cytometry data with SPADE. Nat. Biotechnol 29, 886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck M, et al. , 2016. Pembrolizumab versus chemotherapy for PD-L1 -positive non-small-cell lung cancer. N. Engl. J. Med 375, 1823–1833. [DOI] [PubMed] [Google Scholar]
- Romee R, et al. , 2016. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med 8, 357ra123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz DT, et al. , 2017. Novel BET protein proteolysis-targeting chimera exerts superior lethal activity than bromodomain inhibitor (BETi) against post-myeloproliferative neoplasm secondary (s) AML cells. Leukemia 31, 1951–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenghui Z, et al. , 2011. Elevated frequencies of CD4( + ) CD25( + ) CD1271o regulatory T cells is associated to poor prognosis in patients with acute myeloid leukemia. Int. J. Cancer 129, 1373–1381. [DOI] [PubMed] [Google Scholar]
- Son B, et al. , 2017. The role of tumor microenvironment in therapeutic resistance. Oncotarget 8, 3933–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SM, et al. , 2015. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med 372, 724–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski MJ, et al. , 2009. Increased frequency and suppression by regulatory T cells in patients with acute myelogenous leukemia. Clin. Cancer Res 15, 3325–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabe Y, Konopleva M, 2014. Advances in understanding the leukaemia micro environment. Br. J. Haematol 164, 767–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, et al. , 2012. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med 366, 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley SJ, Cremasco V, Astarita JL, 2015. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol 15, 669–682. [DOI] [PubMed] [Google Scholar]
- Ustun C, Miller JS, Munn DH, Weisdorf DJ, Blazar BR, 2011. Regulatory T cells in acute myelogenous leukemia: is it time for immunomodulation? Blood 118, 5084–5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting CC, et al. , 2015. Large-scale and comprehensive immune profiling and functional analysis of normal human aging. PLoS One 10, eOl 33627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wogsland CE, et al. , 2017. Mass cytometry of follicular lymphoma tumors reveals intrinsic heterogeneity in proteins including HLA-DR and a deficit in nonmalignant plasmablast and germinal center B-cell populations. Cytometry B Clin. Cytom 92, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, et al. , 2014. CyTOF supports efficient detection of immune cell subsets from small samples. J. Immunol. Methods 415, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z, et al. , 2016. MLN0128, a novel mTOR kinase inhibitor, disrupts survival signaling and triggers apoptosis in AML and AML stem/progenitor cells. Oncotarget 7, 55083–55097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z, Konopleva M, Andreeff M, 2017. Single-cell mass cytometry of acute myeloid leukemia and leukemia stem/progenitor cells. Methods Mol. Biol 1633 75–86. [DOI] [PubMed] [Google Scholar]
- Zhou H, et al. , 2017. Combined inhibition of beta-catenin and Bcr-Abl synergistically targets tyrosine kinase inhibitor-resistant blast crisis chronic myeloid leukemia blasts and progenitors in vitro and in vivo. Leukemia 31, 2065–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunder ER, et al. , 2015. Palladium-based mass tag cell barcoding with a doublet-filtering scheme and single-cell deconvolution algorithm. Nat. Protoc 10, 316–333. [DOI] [PMC free article] [PubMed] [Google Scholar]