Abstract
Purpose of review
The genetic basis of type 1 diabetes (T1D) is being characterized through DNA sequence variation and cell type specificity. This review discusses the current understanding of the genes and variants implicated in risk of T1D and how genetic information can be used in prediction, intervention, and components of clinical care.
Recent findings
Fine mapping and functional studies has provided resolution of the heritable basis of T1D risk, incorporating novel insights on the dominant role of HLA genes as well as the lesser impact of non-HLA genes. Evaluation of T1D-associated SNPs single nucleotide polymorphisms (SNPs), there is enrichment of genetic effects restricted to specific immune cell types (CD4+ and CD8+ T cells, CD19+ B cells, and CD34+ stem cells), suggesting pathways to improved prediction. In addition, T1D-associated SNPs have been used to generate genetic risk scores (GRS) as a tool to distinguish T1D from type 2 diabetes (T2D) and to provide pre-diagnostic data to target those for autoimmunity screening (e.g., islet autoantibodies) as a prelude for continuous monitoring and entry into intervention trials.
Summary
Genetic susceptibility accounts for nearly one-half of the risk for T1D. Although the T1D-associated SNPs in Caucasian populations account for nearly 90% of the genetic risk, with high sensitivity and specificity, the low prevalence of T1D makes the T1D GRS of limited utility. However, identifying those with highest genetic risk may permit early and targeted immune monitoring to diagnose T1D months prior to clinical onset.
Keywords: Genetics, markers, prediction, type 1 diabetes, diagnosis, risk
INTRODUCTION
Type 1 diabetes (T1D) is a complex autoimmune disorder that arises from the action of multiple genetic and environmental risk factors and can affect up to 1 in 300 children [1]. T1D arises from the autoimmune destruction of the insulin producing β cells of the pancreas, resulting in dependence on exogenously administered insulin to maintain glucose homeostasis. Based upon concordance of T1D in monozygotic (MZ, sharing 100% of genes) twin pairs, it has been estimated that the familial (heritable) risk for T1D is ~40%, with the remainder due to non-genetic causes [2]. The concordance of T1D in dizygotic (DZ, sharing 50% of genes) twin pairs is ~8%, similar to that risk in siblings of a person with T1D. The population prevalence of T1D varies by ethnicity, with highest rates in those of Northern European Caucasian ancestry (~4/1000), with particularly high rates in Finland. Given the higher rates of T1D in those of Caucasian ancestry, there are many families with two or more children with T1D; nonetheless, the prevalence of T1D in those of non-Caucasian ancestry remains significant [3].
GENETIC BASIS OF T1D
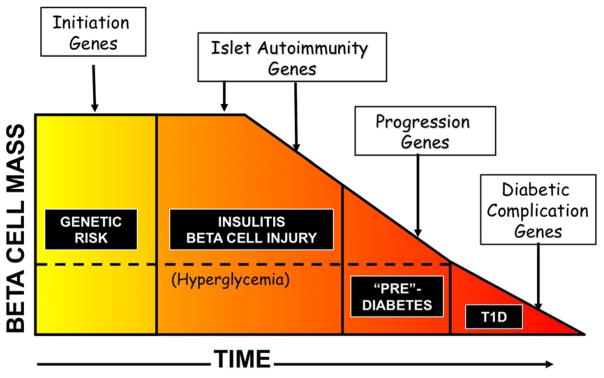
A conceptual framework of the progression to T1D was provided by George Eisenbarth, with the fundamental assumption that T1D develops based upon a singularly susceptible genetic background [4]. A compelling modification of this process is that there are sets of genes and variants that may play a role at each stage of the clinical course, leading to T1D (Fig. 1). Genome-wide linkage and genome-wide association studies (GWAS) have made great progress in the discovery of loci associated with risk of T1D. The first region of the genome implicated in risk for T1D, and the region with the greatest contribution to risk, includes the HLA genes on human chromosome 6p21 [5,6]. The HLA genes reside in the human Major Histocompatibility Complex (MHC), and the specific genes with variation associated with T1D risk are HLA-A, HLA-B and HLA-C (class I) and HLA-DR, HLA-DQ and HLA-DP (class II). Specific HLA alleles (that define amino acid residues) in each gene are at higher frequency in those with T1D than “controls” (susceptibility alleles) or at much lower frequency than controls (protective alleles). HLA contributes ~50% to the total genetic risk of T1D, with other, non-HLA, T1D loci having smaller effects on disease risk relative to HLA but comparable effect sizes to risk loci identified in other common human disorders.
Fig 1.
A model for the progression to type 1 diabetes (T1D)
The stage of progression in type 1 diabetes, shown with individuals having a baseline genetic risk (accounting for ~50% of total risk, with half of the genetic risk due to HLA gene variation), but with progression to the subsequent stages of disease defined by individual genetic factors (some that could be consistent across all stages, but with differing impact), leading to clinically defined T1D.
Source: adapted from [4].
Genes in the Major Histocompatibility Complex (HLA)
The allelic variation in the most strongly associated HLA region genes (HLA-DRB1, HLA-DQA1 and HLA-DQB1) alter specific amino acid residues that affect binding and presentation of foreign peptides (antigens) that underlies the autoimmune disease process. The well-established HLA-DQB1 position 57 residue [7] accounted for ~15% of the total risk of T1D, with HLA-DRB1 position 13 and HLA-DRB1 position 71 residues accounted for an additional 12% of risk [8**,9*]. In Caucasian populations, these three amino acid residues capture ~27% of T1D risk, or ~80% of the MHC-associated risk. In other populations, different HLA class I and HLA class II alleles contribute to risk by encoding different amino acid residues [10,11]. The relationship between genetic variant and amino acid residue with risk prediction is not simple, and the biology underlying the role of HLA and T1D risk remains an active area of research. Nonetheless, the impact of HLA on T1D risk is substantial.
Non-HLA region genes associated with T1D
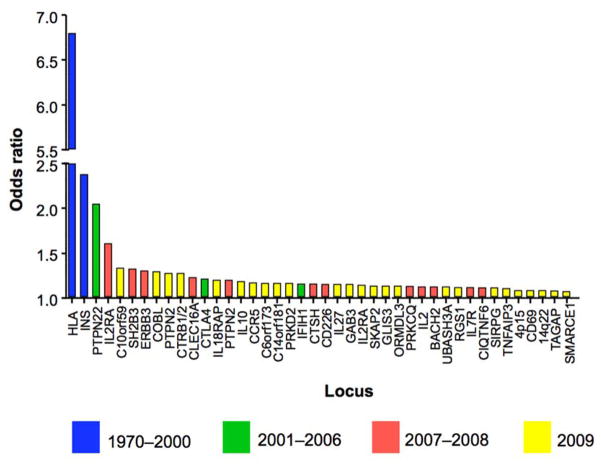
Until 10 years ago, few non-HLA candidate genes had been identified (e.g., INS [12], CTLA4 [13], PTPN22 [14], IL2RA [15]). The T1DGC identified a new locus (UBASH3A) [16] through linkage analysis and subsequently conducted the first robust T1D genome-wide association scan (GWAS) [17]. From the GWAS, over 40 loci attained genome-wide significance, 18 of which were novel (Fig. 2); however, these loci were large (~250kb) and contained a mean of 7 genes (range = 0–28) [18]. A custom genotyping array of ~196,000 SNPs was then used to interrogate 186 loci that were robustly associated with one or more of 12 autoimmune diseases [19**]. Evidence for T1D association in 44 of these regions was identified, 38 that had been discovered previously, four newly found loci (1q32.1, 2q13, 4q32.3, 5p13.2) and two loci (17q21.31 and 21q22.3) implicated in T1D through strong association with other autoimmune diseases. A Bayesian approach was used to establish a 99% credible set of SNPs (see Supplementary Table 1 [19**]) for each of the 44 T1D loci, which reduced the size of the loci and the number of potential candidate genes from an average of 7 to 2 per locus. This information can be used for construction of relevant genetic risk scores (GRS) predicting T1D, or for individual characterization of T1D-associated genetic variants with clinical correlates of T1D.
Fig 2.
T1D loci mapped by studies during a “genomic era”
The size of the effect (Odds ratio) of each locus on T1D risk using genome-wide association studies (GWAS), defined by the most significantly associated SNP and the location of that SNP (either in or near a likely candidate gene). The early era (blue, 1970–2000) is characterized by discovery of genes with large (Odds ratio > 2) effects that can be found with small sample sizes or in families. The second era (green, 2001–2006) included discovery of genes with smaller effect that required larger sample size (PTPN22, CTLA4) or scans of nonsynonymous SNPs (coding variation, IFIH1). The third era (red, 2007–2008) was the entry into large case-control studies with genome-wide coverage, led by the Wellcome Trust Case-Control Consortium (2,000 cases of 7 diseases compared to 3,000 common controls), in which more loci with small effects were identified. The recent era (yellow, 2009–present) established large consortia (Type 1 Diabetes Genetics Consortium) in which ever larger sample sizes permitted detection of loci with small effects (OR < 1.1) but potentially discovering important biology.
Source: adapted from [18]
Regulatory variation and cell specificities as contributors to T1D risk
The T1D credible SNP list (see Supplementary Table 1 [19**]) suggests that the T1D-associated genetic variants have few that map to coding regions of genes; in contrast, the majority of non-HLA genetic variation associated with T1D risk falls in DNA regulatory sequences. A total of 15 chromatin states across 127 tissues derived from the NIH Roadmap Epigenomics Mapping Consortium and ENCODE projects were interrogated using the 99% credible SNP list [19**]. A strong enrichment of T1D credible SNPs was found in enhancer chromatin states in immune-relevant tissues (thymus, CD4+ and CD8+ T cells, CD19+ B cells and CD34+ stem cells); further, there was less evidence of enrichment in promoter sequences, and there was no significant evidence of enrichment in pancreatic islet enhancers [19**].
PREDICTION OF ISLET AUTOIMMUNITY AND T1D
There is an urgent need to develop intervention and therapeutic strategies to delay or eliminate the clinical onset of T1D. A critical concept of intervention trials is the identification of those in the preclinical phase, marked by the presence of persistent islet autoantibodies, typically insulin (IAA), GAD, and protein tyrosine IA-2 (ICA512) autoantibodies. The Diabetes Autoimmunity Study in the Young (DAISY) followed two cohorts (n= 2,542) of young children at increased risk of diabetes based upon HLA genotype [20]. Children expressing at least two autoantibodies had ~70% progression to T1D by the 10-years; in contrast, 15% progressed to T1D with one antibody expressed over the same period. Further, the age of diagnosis of T1D was highly correlated with age of appearance of first autoantibody, emphasizing the impact of early detection and early screening.
When data from DAISY were pooled with prospective cohort studies from Finland and Germany (a total of over 13,000 children), the progression to T1D at 10-year follow-up in 585 children with multiple islet autoantibodies was 69.7%, compared with 14.5% in children with a single islet autoantibody and 0.4% in those with no islet autoantibodies [21]. Progression to T1D in the children with multiple islet autoantibodies was faster for those with early (< 3 years) expression of islet autoantibodies and for those with HLA DR3/DR4-DQ8 genotype. This pattern has been replicated in many studies, most recently in The Environmental Determinants of Diabetes in the Young (TEDDY). TEDDY followed 8,503 children at increased genetic risk (based upon HLA genotype) with respect to IAA, GAD, and IA-2A autoantibodies measured every 3 months until 4 years of age and every 6 months thereafter; if results were positive, the autoantibodies were measured every 3 months. The number of autoantibodies, age at first persistently confirmed autoantibodies, and HLA genotypes were associated with an increased risk of T1D in those persistently autoantibody positive [22*]. Further, in TEDDY, presence of autoantibodies at 3 (0.1%) and 6 (0.2%) months of age were rare. Of the 549 participants with autoantibodies during follow-up, 43.7% only had IAA, 37.7% only had GADA, 13.8% only had IAA and GAD, 1.6% only had IA-2A, and 3.1% had other combinations [23]. The incidence of “IAA only” was early (within the first year of life) while the incidence in the “GAD only” group increased until the second year and remained relatively constant, with different HLA-DR frequencies. Thus, the autoantibodies that are predictive of conversion to clinical T1D occur at different times in the clinical course and are influenced by genetic factors (certainly HLA, but perhaps other genetic factors).
GENETICS CAN INCREASE PREDICTION OF ISLET AUTOIMMUNITY AND T1D
Multiple studies have shown the critical role of genetic variation in HLA-DR and HLA-DQ genes on expression of islet autoantibodies and progression/risk to T1D. Given the growing list of non-HLA genes and variants associated with T1D risk, several have been evaluated for association of the non-HLA variants with islet autoimmunity. In the DAISY cohort, single SNPs in 20 non-HLA genes were evaluated for association with islet autoimmunity and T1D risk [24], based upon the findings of the T1DGC GWAS [17]. The T1D-associated SNPs in UBASH3A and PTPN22 were associated with both islet autoimmunity and T1D risk, while the T1D-associated PTPN2 SNP was associated only with islet autoimmunity, and the INS T1D-associated SNP was associated only with T1D [24]. In a later study, SNPs in an additional seven non-HLA genes (ERBB3, CLEC16A, IL27, CTRB, C14orf64, GSDM, HORMAD2) were evaluated for association with islet autoimmunity and T1D risk, as well as examining their independent predictive value while controlling for the effects of HLA-DR, DQ genotypes [25]. The prediction of T1D and modeling genetic effects was addressed earlier using 6 HLA SNPs and 48 non-HLA SNPs for in ~4,000 T1D cases and ~4,000 controls [26[, demonstrating that the area under the ROC curve is 0.738, which increased slightly by the addition of the non-HLA SNPs. In DAISY [25], the genetic risk prediction model included only HLA class II (HLA DR3/4-DQ8), and risk SNPs in PTPN22 and UBASH3A, yet it significantly improved prediction of T1D for those in the high genetic risk group (45% by age 15 years) compared to those in the low genetic risk group (3% by age 15 years).
In TEDDY, a more comprehensive analysis of the T1D-associated SNPs was performed, with genotyping of 41 non-HLA SNPs from the T1DGC GWAS [17] in 5,164 Caucasian children [27**]. During the median follow-up time of 57 months, 350 children developed at least one persistent IA (GAD antibody, IA-2A, or IAA) and 84 of them progressed to T1D. After adjustment for multiple testing, SNPs in four non-HLA genes were associated with development of islet autoimmunity and T1D – PTPN22, ERBB3, SH2B3 and INS. In the Finnish DIPP study [28], 39 non-HLA SNPs were assessed for the development of autoantibody positivity and T1D progression in 521 autoantibody-positive and 989 control children. Similar to other studies, SNPs in the PTPN2, INS, PTPN22, IFIH1 and IKZF4/ERBB3 genes were associated with autoantibody positivity and progression to T1D. Although genes in the HLA region remain the most important genetic risk factors for development of islet autoimmunity and progression to overt T1D, other non-HLA genetic factors contribute to the disease process, a first step in understanding the pathogenesis of T1D.
GENETICS AND POPULATION SCREENING FOR T1D
There has been an increasing recognition that the genetic influence on development of autoantibodies relevant to the development of T1D is being clarified, the detection of autoantibodies that reflect initiation and progression of islet autoimmunity is being defined across multiple populations, and the staging of progression to T1D is being established [29*]. The fundamental question is no longer “should there be population screening for T1D” but “how best to conduct the screening”. The Fr1da Model Project Diabetes 2015 [30**] is a German effort to screen for multiple islet autoantibodies at “well child” visits at 3 and 4 years of age (~200,000 children). There is a two-step assay with replicate sampling/testing in situations of two positive autoantibodies for determination of family contact and follow-up. This process will permit detection of those subjects who have early evidence of islet autoimmunity.
An unanswered question is whether this screening can be made more targeted by using a genetic risk score (GRS) to focus on those with a high “genetic risk”. With the cost of genotyping declining, even a 96 SNP panel can be rapidly deployed for ~$7/person. The sensitivity and specificity of the GRS has been shown to have an area under the ROC curve approaching 0.9 for T1D; however, with the low prevalence of islet autoimmunity and T1D in the general population (~4/1000 in Northern Europeans, lower in those of African or Asian ancestry), the positive and negative predictive values remain poor. Our current concept is that genetics contributes ~50% to risk for T1D, with very little known about the non-genetic contribution to risk. As we accumulate the majority of genetic risk variants in multiple populations, the opportunity will come to test genetic screening, followed by autoantibody testing, in the general population. As shown by the DAISY, TEDDY and other studies, the GRS for T1D and islet autoimmunity does improve prediction, and having increased surveillance and monitoring reduces the risk of DKA, reduced hospitalizations, and lower HbA1c levels. There is little doubt that application of genetic and immunologic tools can enhance the detection of an at-risk population for islet autoimmunity and T1D.
CONCLUSIONS
The field of T1D genetics has progressed from HLA genotypes to a few non-MHC genes (INS, CTLA4, PTPN22) to the present situation of more than 40 risk loci with intriguing overlaps with other autoimmune diseases. We have shown evidence that the set of causal variants are enriched in regulatory (non-coding) regions of the genome, likely involved in gene regulation in specific cell types. The mechanism by which the genetic variation alters gene regulation and impacts development of islet autoimmunity and progression to T1D is unknown. Critically, further research will add to our understanding of genetic impact on the natural history of disease as well as identifying new point of intervention and targets of prevention. Even in absence of this biological understanding, the genetic variation that is associated with islet autoimmunity and progression to T1D can be used to better identify those in the population who are at risk. The combination of genetic risk and autoantibody screening can be used to form a basis for public health detection, better preservation of beta-cell function, and improved management of those with T1D.
KEY POINTS.
The risk of T1D is roughly one-half genetic and one-half environmental
Specific variants/amino acid residues in HLA class II genes (HLA-DR, HLA-DQ, HLA-DP) accounts for one-half of the genetic risk
The remainder of the genetic risk is being resolved, with the genetic variants appearing to be enriched in DNA regulatory regions (enhancers) in specific immune-relevant cell types
A group of genetic variants associated with T1D also are associated with autoantibody expression and timing of their appearance
Inclusion of T1D-associated genetic variants can improve the prediction of those at risk and should be considered in population screening for T1D
Acknowledgments
The author wishes to thank the many participants in the Type 1 Diabetes Genetics Consortium, the TEDDY cohort, and the many researchers and staff for their contributions.
Financial support and sponsorship
The sponsor of the Type 1 Diabetes Genetics Consortium was the National Institutes of Health (NIH) through the National Institute of Diabetes, Digestive and Kidney Diseases by cooperative agreement U01-DK062418, the National Institute of Allergy and Infectious Diseases, the National Human Genome Research Institute, the National Institute of Child Health and Human Development, the Wellcome Trust through grant WT061858/09115 to the Diabetes and Inflammation Laboratory of Cambridge University, and the Juvenile Diabetes Research Foundation International. Part of this contribution was supported by the Strategic Investment Fund grant from the University of Virginia. The contents of this Article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the JDRF. Stephen Rich is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Abbreviations
- DAISY
Diabetes Autoimmunity Study in the Young
- GAD
glutamic acid decarboxylase (autoantibody)
- GRS
genetic risk score
- GWAS
genome-wide association study
- HLA
Human Leukocyte Antigen
- IAA
insulin autoantibody
- IA-2A
insulinoma-2-associated (autoantibody)
- ICA512
islet cell autoantibody
- SNP
single nucleotide polymorphism
- TEDDY
The Environmental Determinants of Diabetes in the Young
- T1D
type 1 diabetes
- T1DGC
Type 1 Diabetes Genetics Consortium
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383(9911):69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rich SS. Mapping genes in diabetes. Genetic epidemiological perspective Diabetes. 1990;39(11):1315–1319. doi: 10.2337/diab.39.11.1315. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014. [Google Scholar]
- 4.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358(9277):221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 5.Nerup J, Platz P, Andersen OO, et al. HL-A antigens and diabetes mellitus. Lancet. 1974;2(7885):864–866. doi: 10.1016/s0140-6736(74)91201-x. [DOI] [PubMed] [Google Scholar]
- 6.Noble JA, Erlich HA. Genetics of type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2(1):a007732. doi: 10.1101/cshperspect.a007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Todd JA, Bell JI, McDevitt HO. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987;329(6140):599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- 8**.Hu X, Deutsch AJ, Lenz TL, et al. Additive and interaction effects at three amino acid positions in HLA-DQ and HLA-DR molecules drive type 1 diabetes risk. Nat Genet. 2015;47(8):898–905. doi: 10.1038/ng.3353. Detailed analysis of common SNPs and haplotypes in the HLA genes, with characterization of the resulting amino acid residues that are most associated with T1D risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9*.Lenz TL, Deutsch AJ, Han B, et al. Widespread non-additive and interaction effects within HLA loci modulate the risk of autoimmune diseases. Nat Genet. 2015;47(9):1085–1090. doi: 10.1038/ng.3379. An evaluation of interactions between HLA SNPs and haplotypes in T1D and other autoimmune diseases, suggesting both common and unique interactions within the MHC that account for differential risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noble JA, Johnson J, Lane JA, Valdes AM. HLA class II genotyping of African American type 1 diabetic patients reveals associations unique to African haplotypes. Diabetes. 2013;62(9):3292–3299. doi: 10.2337/db13-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black MH, Lawrence JM, Pihoker C, et al. HLA-associated phenotyeps in youth with autoimmune diabetes. Pediatr Diabetes. 2013;14(2):121–128. doi: 10.1111/j.1399-5448.2012.00905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barratt BJ, Payne F, Lowe CE, et al. Remapping the insulin gene/IDDM2 locus in type 1 diabetes. Diabetes. 2004;53(7):1884–1889. doi: 10.2337/diabetes.53.7.1884. [DOI] [PubMed] [Google Scholar]
- 13.Nistico L, Buzzetti R, Pritchard LE, et al. The CTLA-4 gene region of chromosome 2q33 is linked to, and associated with, type 1 diabetes. Belgian Diabetes Registry Hum Mol Genet. 1996;5(7):1075–1080. doi: 10.1093/hmg/5.7.1075. [DOI] [PubMed] [Google Scholar]
- 14.Bottini N, Musumeci L, Alonso A, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type 1 diabetes. Nat Genet. 2004;36(4):337–338. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- 15.Vella A, Cooper JD, Lowe CE, et al. Localization of a type 1 diabetes locus in the IL2RA/CD25 region by use of tag single-nucleotide polymorphisms. Am J Hum Genet. 2005;76(5):773–779. doi: 10.1086/429843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Concannon P, Onengut-Gumuscu S, Todd JA, et al. A human type 1 diabetes susceptibility locus maps to chromosome 21q22. 3. Diabetes. 2008;57(10):2856–2861. doi: 10.2337/db08-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrett JC, Clayton DG, Concannon P, et al. Genome-wide association study and meta-analysis find that over 40 loci affect the risk of type 1 diabetes. Nat Genet. 2009;41(6):703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med. 2009;360(16):1646–1654. doi: 10.1056/NEJMra0808284. [DOI] [PubMed] [Google Scholar]
- 19**.Onengut-Gumuscu S, Chen WM, Burren O, et al. Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat Genet. 2015;47(4):381–386. doi: 10.1038/ng.3245. Results of fine-mapping the 40+ non-HLA loci implicated in T1D, with confirmation of loci (but with different, most-associated SNPs), discovery of new loci, and recognition that the most likely (credible) set of T1D-associated SNPs are in DNA regulatory regions (enhancers, not promoters) restricted to immune cell types (CD4+ and CD8+ T cells, CD19+ B cells, CD34+ stem cells) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steck AK, Johnson K, Barriga KJ, et al. Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA-2 autoantibodies, predict age at diagnosis of type 1 diabetes: diabetes autoimmunity study in the young. Diabetes Care. 2011;34(6):1397–1399. doi: 10.2337/dc10-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309(23):2473–2479. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22*.Steck AK, Vehik K, Bonifacio E, et al. Predictors of progression from the appearance of islet autoantibodies to early childhood diabetes: The Environmental Determinants of Diabetes in the Young (TEDDY) Diabetes Care. 2015;38(5):808–813. doi: 10.2337/dc14-2426. A major international study (TEDDY) of children followed from 3 months with high genetic (HLA) risk of T1D shows the significance of multiple autoantibodies on risk and the timing of the appearance and persistence of autoantibodies on prediction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krischer JP, Lynch KF, Schatz DA, et al. Diabetologia. 2015;58(5):980–987. doi: 10.1007/s00125-015-3514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steck AK, Wong R, Wagner B, et al. Effects of non-HLA gene polymorphisms on development of islet autoimmunity and type 1 diabetes in a population with high-risk HLA-DR, DQ genotypes. Diabetes. 2012;61:753–758. doi: 10.2337/db11-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steck AK, Dong F, Wong R, et al. Improving prediction of type 1 diabetes by testing non-HLA genetic variants in addition to HLA markers. Pediatr Diabetes. 2014;15(5):355–362. doi: 10.1111/pedi.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clayton DG. Prediction and interaction in complex disease genetics: experience in type 1 diabetes. PLoS Genet. 2009;5(7):e1000540. doi: 10.1371/journal.pgen.1000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27**.Torn C, Hadley D, Lee HS, et al. Role of type 1 diabetes-associated SNPs on risk of autoantibody positivity in the TEDDY study. Diabetes. 2015;64(5):1818–1829. doi: 10.2337/db14-1497. The evaluation of SNPs associated with T1D from the genome-wide association scan (reference 17) in TEDDY with respect to autoantibodiy positivity, implicating several T1D risk genes/variants with immune-related function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lempainen J, Laine AP, Hammais A, et al. Non-HLA gene effects on the disease process of type 1 diabetes: from HLA susceptibility to overt disease. J Autoimmun. 2015;61:45–53. doi: 10.1016/j.jaut.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 29*.Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38(10):1964–1974. doi: 10.2337/dc15-1419. Developing a new process of staging the development of T1D to facilitate early detection and population screening. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30**.Ziegler AG, Bonifacio E, Powers AC, et al. Type 1 diabetes prevention: a goal dependent on accepting a diagnosis of an asymptomatic disease. Diabetes. 2016;65(11):3233–3239. doi: 10.2337/db16-0687. An in-depth discussion of promise and issues related to population screening in children under the age of 5 year for islet autoantibodies, the process as currently implemented in Germany (Fr1da) and the potential for early detection, the reduction in risk of DKA at presentation, and better clinical management. [DOI] [PMC free article] [PubMed] [Google Scholar]